Abstract
Biofilm formation is a key factor in microbial survival and persistence, often contributing to reduced antimicrobial susceptibility. This systematic literature review investigates whether increased biofilm formation correlates with decreased antibiotic susceptibility. The literature search was conducted in the Pubmed database and we identified and screened 328 studies, with 35 ultimately meeting the inclusion criteria for detailed analysis. Findings reveal that the relationship between biofilm size and antimicrobial susceptibility is highly variable and influenced by multiple factors, including microbial species, strain-specific traits, antibiotic type, and experimental methodologies. While some studies report a positive correlation between biofilm biomass and reduced susceptibility, others show weak or no such relationships, and statistical support for a correlation is often lacking (also due to small sample sizes). The lack of standardized biofilm quantification methods and susceptibility metrics further complicates cross-study comparisons. These findings underscore the need for standardized protocols and more comprehensive datasets to clarify the complex interplay between biofilm formation and antibiotic susceptibility. Regardless of these difficulties, the available data clearly indicate that ‘bigger’ biofilms are not by definition less susceptible. Future research should prioritize diverse and sufficiently large strain collections and consistent methodologies to better understand and address biofilm-associated antimicrobial tolerance.
1. Introduction
The majority of chronic and/or medical device-related infections in humans are biofilm-associated. Biofilms are aggregates of surface-associated or tissue-embedded microbial cells that are enclosed within an extracellular polymeric substance matrix [1,2]. One of the hallmarks of biofilm-related infections is the difficulty of successful antimicrobial treatment, as biofilm cells frequently exhibit reduced susceptibility [3,4,5]. There are several factors involved in the reduced susceptibility of biofilms to antibiotics, including the blocking and repelling of antibiotics by the extracellular matrix [2,3]. An example of this is the reduced penetration of the glycopeptide antibiotic vancomycin into Staphylococcus epidermidis biofilms, which is mediated by an increase in the extracellular DNA (eDNA) concentration [6]. Similarly, binding of the negatively charged aminoglycoside antibiotic tobramycin to alginate (an important polysaccharide component in the Pseudomonas aeruginosa biofilm matrix) can reduce tobramycin concentrations in deeper layer of the biofilm [7]. In addition, the accumulation of antibiotic-degrading enzymes in the matrix (e.g., β-lactamase in P. aeruginosa biofilms [8]) could contribute to reduced biofilm susceptibility. Other factors involved in reduced susceptibility of biofilms include changes in metabolism that lead to reduced production of antibiotic-induced reactive oxygen species as well as the presence of biofilm-specific efflux pumps [5,9,10,11,12]. In addition, the structural and metabolic heterogeneity often observed in biofilms leads to gradients (e.g., of oxygen and nutrients) that can affect physiology of organisms (e.g., by inducing a state of dormancy) and affect antimicrobial susceptibility [13,14].
While many studies have investigated mechanisms of biofilm formation and how biofilms respond to exposure to antibiotics under different conditions, it remains unclear whether there is a correlation between the amount of biofilm formed (i.e., biofilm ‘size’ or ‘thickness’) and antimicrobial susceptibility. While this may seem like a simple question at first sight, a combination of several factors determines the biofilm phenotype, including the species involved and the environmental conditions under which the biofilm is formed and/or exposed to antibiotics [15,16,17,18]. In addition, biofilms are quantified using a wide range of approaches and it is important to keep in mind that these different approaches frequently measure/quantify different aspects of biofilms and each come with their own advantages and limitations [19]. For example, the frequently used crystal violet and resazurin-based staining approaches allow quantification of total biomass (live and dead cells as well as some matrix components) and the number of metabolically active cells, respectively, and results obtained with these different approaches may yield very different outcomes. Moreover, results obtained with these indirect quantification approaches based on various chemical stainings may yield results that are not necessarily in line with those obtained by determining the number of culturable cells (i.e., determination of the number of colony forming units [CFU]) [19,20].
In the present study, we report on a systematic literature review that was conducted to investigate whether the amount of biofilm formed correlates with antibiotic susceptibility, or phrased differently: are biofilms with greater biomass or thickness less susceptible to antibiotics?
2. Materials and Methods
2.1. Search Strategy
This systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [21]. Publications were retrieved from the PubMed electronic database (https://pubmed.ncbi.nlm.nih.gov/) using free text words. The search was restricted to articles published up to 31 July 2025. The final input for the search was ‘biofilm thickness OR biofilm quantification OR biofilm biomass AND antibiotic susceptibility’ to maximize the number of publications for subsequent screening. This review was not registered and a protocol was not prepared.
2.2. Study Selection and Exclusion Criteria
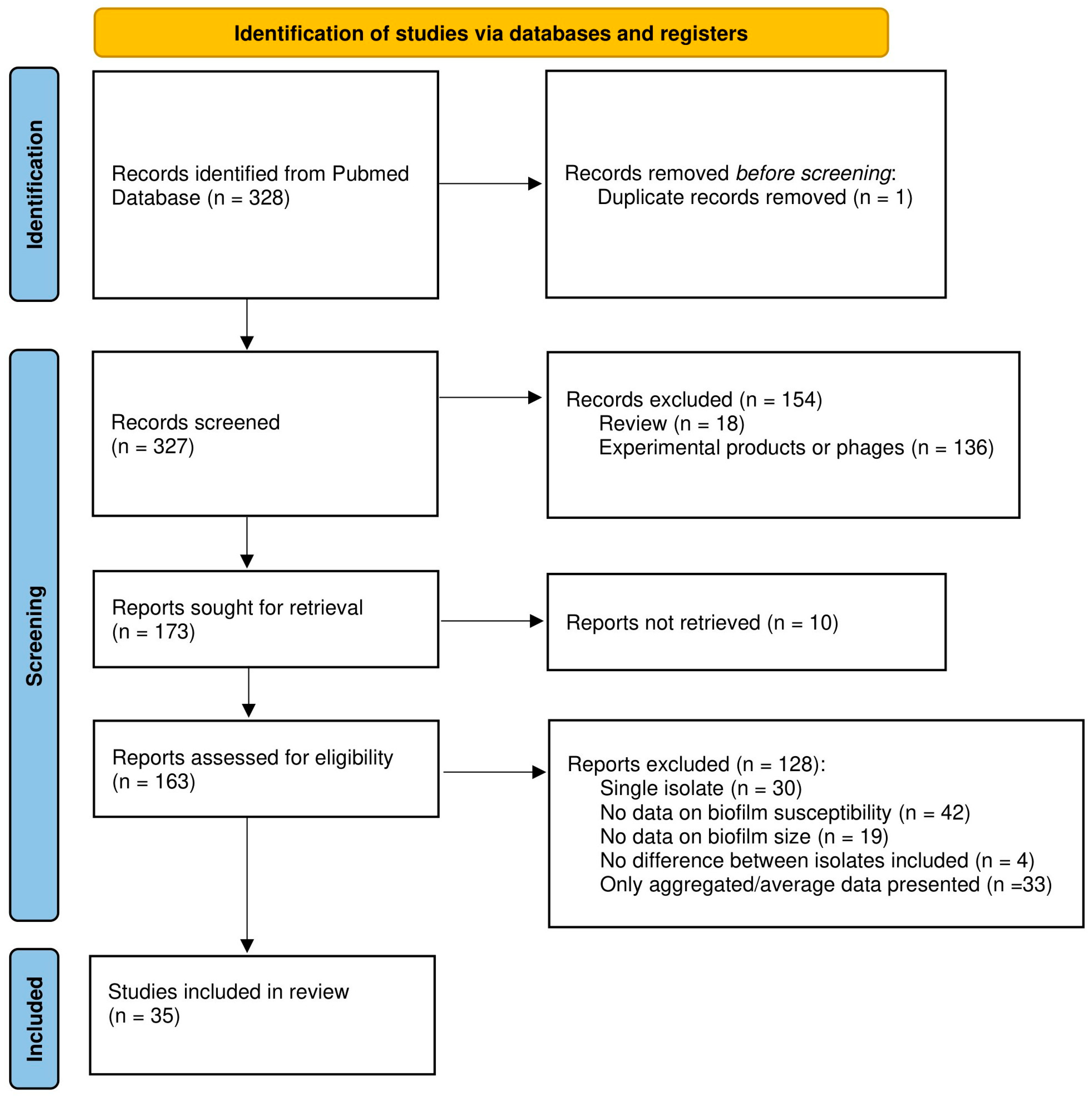
Abstracts and full texts from articles were screened by two independent reviewers (A.M. and T.C., or L.V. and T.C.) to collect data on organism(s), biofilm model system(s), quantification method(s), antimicrobial compound(s), and data on biofilm size and susceptibility. Reviews, publications for which we could not access the full text at the time of the search, and studies using phages or experimental products instead of conventional antibiotics were excluded. As it was our goal to compare data obtained with biofilms of different sizes, studies in which only a single isolate and/or a single time point was included, studies in which no relevant data on (biofilm) susceptibility and/or biofilm size were reported (e.g., studies reporting only the minimum inhibitory concentration (MIC) and/or minimum bactericidal concentration (MBC) data were excluded as these values do not accurately reflect biofilm susceptibility [4]), and studies lacking data for the individual strains tested (i.e., studies in which only average or otherwise aggregated data were presented) were excluded. Finally, for several studies data reported indicate that the isolates included had virtually identical biofilm biomass and susceptibility. As answering the question whether increased biofilm biomass correlates with decreased antibiotic susceptibility requires isolates with different biomass and/or different susceptibility, these studies were also excluded. To avoid bias, no other selection criteria were used. Due to lack of formal criteria to assess the quality of non-clinical biofilm studies and the lack of widely accepted guidelines for reporting on such studies, these criteria were not used for selecting studies (i.e., quality of, and potential bias in, the different studies were not assessed and were not used as selection criteria). The study selection process is illustrated in the PRISMA flowchart (Figure 1).
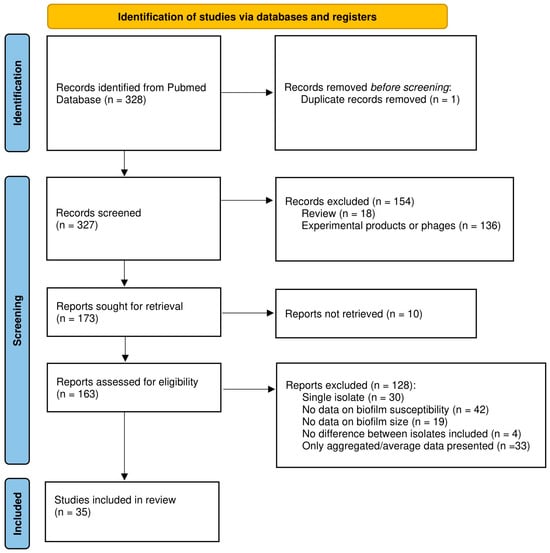
Figure 1.
PRISMA flowchart of the study selection process.
2.3. Statistical Analysis
When raw data were included in the main text and/or supplementary data of the studies retrieved, these were used for statistical analysis. However, as many publications retrieved in the framework of this systematic review did not include raw data, we frequently had to estimate data on susceptibility and/or biofilm biomass from figures provided in the publications (e.g., by measuring the height of bars in bar charts). Goodness of fit between two parameters was assessed by calculating the Pearson correlation coefficient (r2) as well as the significance of the correlation. The latter allowed to determine whether the slope of the linear regression curve significantly differed from zero based on a two-tailed t-test (p-values < 0.05 were considered significant). Statistical analyses were carried out using GraphPad Prism (v10.5.0).
3. Results
3.1. Results of the Literature Search
From the 328 studies initially identified, 293 were excluded for various reasons or not retrieved, leaving 35 papers that were analyzed in detail, as outlined in the PRISMA flowchart (Figure 1). Most studies identified during the initial screening were removed because they pertained to experimental products or phages (n = 136), because no data on biofilm susceptibility were included (n = 42), or because only average or otherwise aggregated data were presented (n = 33). The complete list of the publications analyzed, along with the number of strains included, biofilm model system(s), biofilm quantification method, and antibiotic(s) tested is presented in Table A1 (note that some studies include data on multiple species and are listed several times).
3.2. General Overview of Results
The diversity in terms of species investigated, model systems and methods for biofilm quantification used, number of strains included, and/or antibiotics tested makes it difficult to make in-depth and/or across the board comparisons between studies; however, a selection of publications is discussed in more detail below. The studies that included appropriate (raw) data on a sufficient number of strains to allow for the calculation of r2 values between two relevant parameters are listed in Table 1. While the majority of these studies pertain to Staphylococcus aureus, the table also includes studies with Candida albicans, Candida glabrata, Candida krusei, and Helicobacter pylori. Out of the 35 studies analyzed for this review, only one study had this systematic review’s topic as its primary focus [22]. Overall, a wide range of r2 values (and corresponding p-values) is found based on the data reported in different studies. There are a number of factors potentially influencing the antimicrobial susceptibility of microbial biofilms of different sizes. These are discussed below and illustrated with representative examples.

Table 1.
Summary of results obtained in studies in which r2 values between two relevant parameters were calculated. Specific concentrations of antibiotics and/or durations of exposure are only mentioned if, in a study, the same antibiotic was used at different concentrations or for different durations. Abbreviations: CV, crystal violet; CFU, colony forming units; MBEC, minimal biofilm eradication concentration; MIC, minimal inhibitory concentration. * indicates correlations that are statistically significant (p < 0.05).
3.3. Species- and Strain-Dependent Differences
A first hypothesis that we addressed in this systematic review is that the relationship between biofilm size and susceptibility is species-dependent (i.e., there is a relationship between size and susceptibility in species A, but no—or an inverse—correlation is found in species B). Example of studies that supports this are the study by Oschmann-Kadenbach et al. [31] and Silva et al. [23]. In these studies a strong correlation was found between biofilm size and susceptibility towards amikacin for Mycobacteroides abscessus [31], while low r2 values were found for S. aureus [23] (Table 1) (it should, however, be noted that statistical analysis indicated this correlation in both cases was not significant; this is discussed further down). A second example is amphotericin B: while a low r2 value is observed for C. albicans, a high value is reported for C. krusei (Table 1) [28]. These results seem to suggest that species-specific differences indeed may occur.
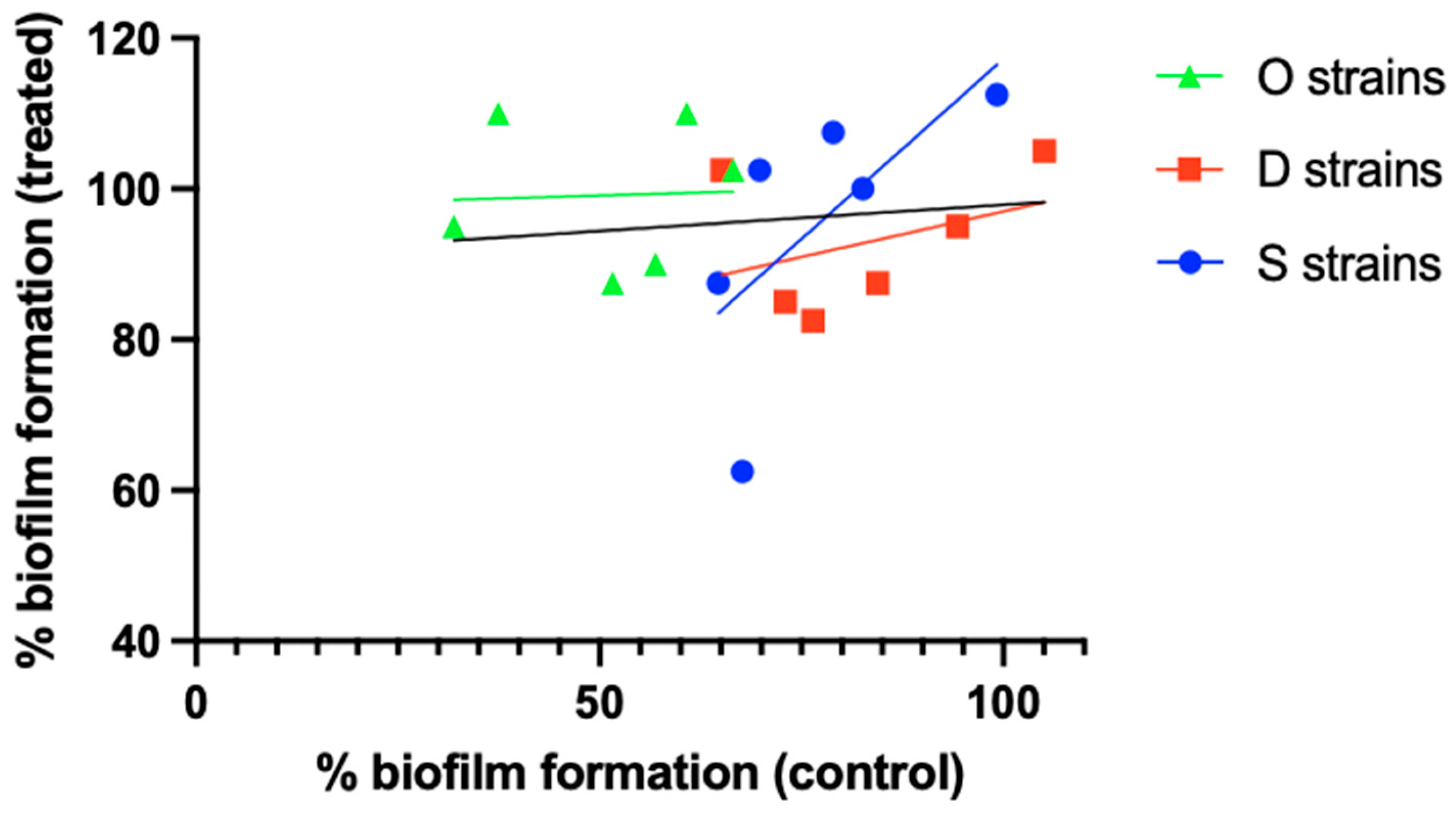
A second hypothesis is that finding a correlation between biofilm size and susceptibility is strain-dependent (i.e., there is a relationship between size and susceptibility in a particular strain or set of strains of a certain species, but no—or an inverse—correlation is found in a different set of strains belonging to the same species). The results obtained by Silva et al. [24] illustrate this. When comparing relative biofilm formation (i.e., crystal violet absorbance relative to that of S. aureus ATCC 25923) in the absence of antibiotic to that after exposure to 4.5 µg/mL ciprofloxacin, a low r2 value (0.011) was observed for the overall set of 18 S. aureus (MRSA) isolates. However, when these 18 isolates were divided into 3 groups based on their isolation source (osteomyelitis [O strains], diabetic foot [D strains], or bacteremia [S strains]), a different picture emerged (Figure 2), with r2 values of 0.002, 0.142, and 0.450 for the O, D, and S strains, respectively. Although these results suggest that the relationship between biofilm formation and antibiotic susceptibility can depend on the (sub)set of S. aureus isolates studied, statistical analysis of the full dataset as well as the subsets revealed that none of the correlations is significant, despite considerable differences in p-values (Table 2).
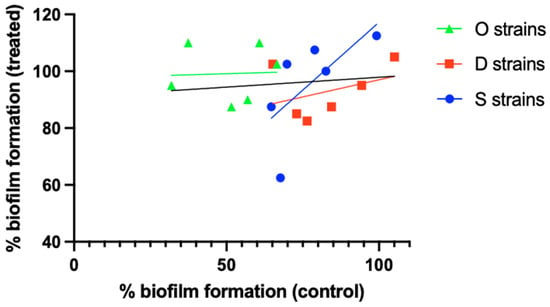
Figure 2.
Differences in the relationship between biofilm formation and biofilm ciprofloxacin susceptibility between different S. aureus strain sets. O strains (green), D strains (red), and S strains (blue) were isolated from cases of osteomyelitis, diabetic foot ulcers, or bacteremia, respectively. Individual data points and linear correlation curves per subset are shown. The black line indicates the correlation curve for the entire strain collection. Based on the data reported by Silva et al. [24]. Statistical analysis of these data is presented in Table 2.

Table 2.
Summary of statistical analysis of linear regression performed for selected studies. * indicates correlations that are statistically significant (p < 0.05).
3.4. Antibiotic-Dependent Differences
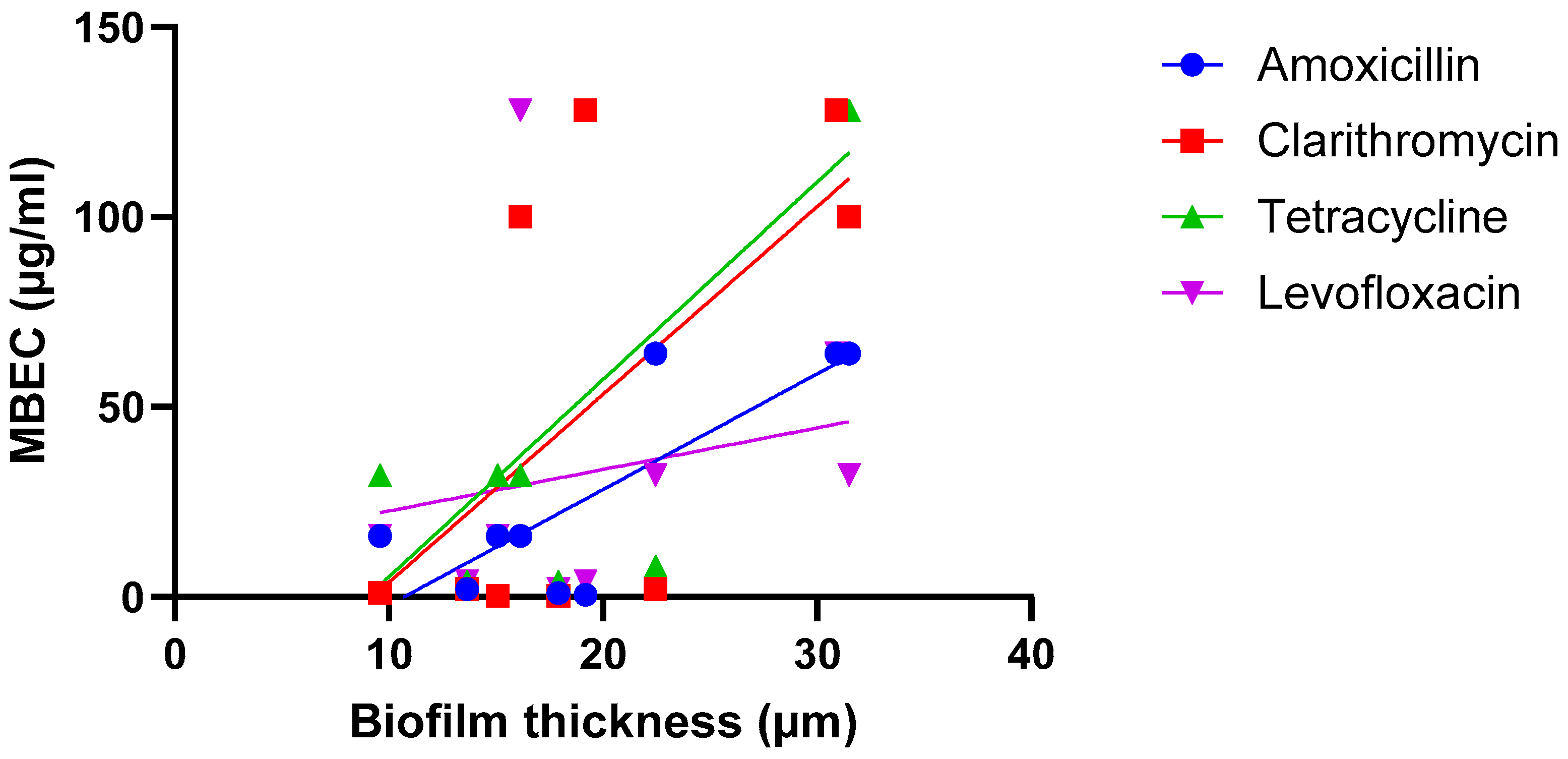
A third hypothesis is that the correlation between biofilm size and susceptibility is antibiotic-dependent (i.e., there is a relationship between size and susceptibility towards antibiotic A, but no—or an inverse—correlation is found for antibiotic B) and several studies point in the direction of this hypothesis being true. In the work conducted by Wu et al. on H. pylori, strong and significant correlations were found when biofilms were exposed to amoxicillin or tetracycline, while weaker and non-significant correlations were found after biofilms were exposed to clarithromycin or levofloxacin [22] (Figure 3; Table 2).
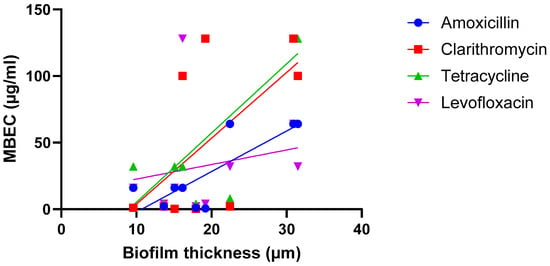
Figure 3.
Exposure of nine H. pylori isolates to four antibiotics suggests the relationship between biofilm formation (quantified using microscopy) and biofilm susceptibility (as quantified by the MBEC) is antibiotic dependent. Data were initially reported by Wu et al. [22]. Statistical analysis of these data is presented in Table 2.
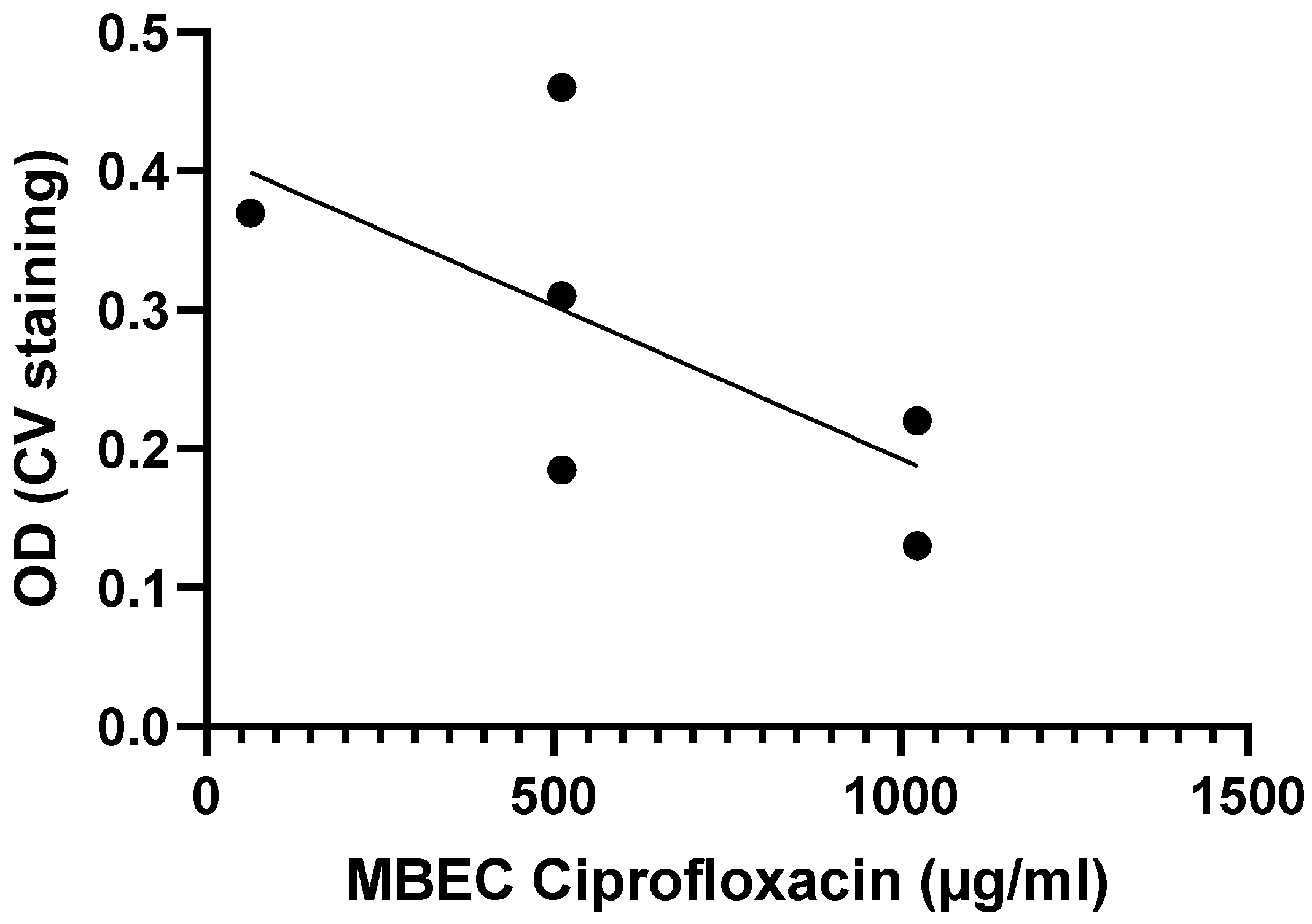
Another example is from the work done by Tomlin et al., who exposed biofilms from six Burkholderia cenocepacia isolates to varying concentrations of ciprofloxacin, ceftazidime, chloramphenicol, and meropenem for 24 h to determine MBEC values [29]. While the MBEC values for ceftazidime, meropenem, and chloramphenicol were above 1024 µg/mL for all six strains, the MBEC values for ciprofloxacin tended to be higher for strains with lower biomass, suggesting a possible inverse correlation between biofilm formation and antibiotic susceptibility for B. cenocepacia (Figure 4), although this correlation is not significant (Table 2).
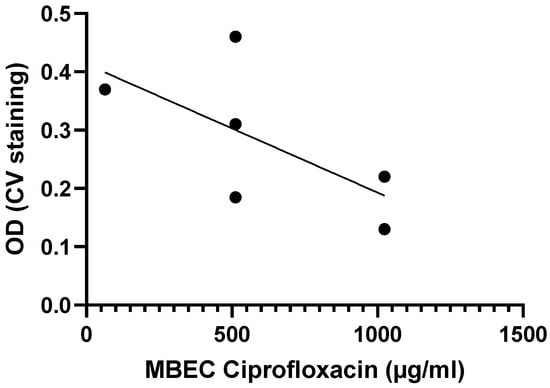
Figure 4.
Exposure of six B. cenocepacia isolates to ciprofloxacin suggests there is a moderate, inverse correlation between biofilm formation (quantified using crystal violet staining) and biofilm susceptibility (as quantified by the MBEC) to ciprofloxacin. Data were initially reported by Tomlin et al. [29]. Statistical analysis of these data is presented in Table 2.
These differences can also be found in the data reported by Alves and colleagues, who collected data on biofilm susceptibility (towards four antifungal drugs) for four different Candida species [28]. For the species C. krusei, high (and significant) r2 values were found for the antifungal drugs fluconazole and amphotericin B, while more moderate (and non-significant) correlations were found for voriconazole and anidulafungin (Table 1) [28]. For C. albicans, on the other hand, low (non-significant) correlations between biofilm formation and fluconazole and amphotericin B susceptibility was found, while this correlation was moderate (albeit still not significant) for voriconazole and anidulafungin (Table 1) [28]. Combined, these data suggest that the relationship between biofilm formation and antimicrobial susceptibility can indeed depend on the antimicrobial agent investigated.
3.5. Impact of the Model System, Quantification Approach, and Other Experimental Parameters
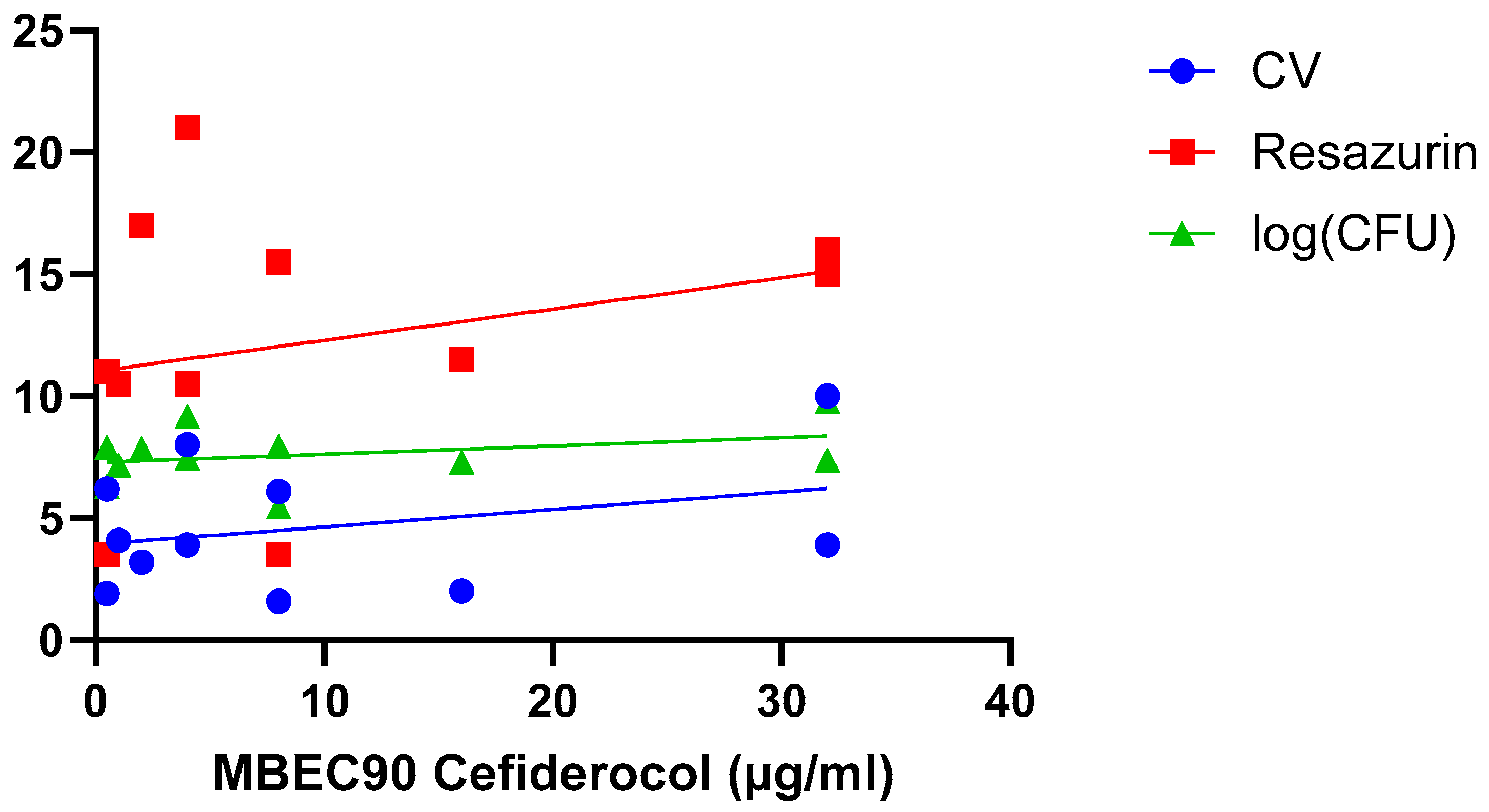
A final hypothesis is that the correlation between biofilm susceptibility and size depends on the experimental parameters used in a particular study, including biofilm model system, biofilm age, treatment time, quantification approach, etc. This has been addressed in two studies. Wu et al. investigated differences in susceptibility towards linezolid between 6 h old and 24 h old S. aureus biofilms, using both crystal violet staining (which allows to quantify ‘total biomass’, as it binds to both dead and living cells, as well as some matrix components) and resazurin-based viability staining (which allows to quantify the number of metabolically active cells) [26]. For both stainings a strong and significant correlation was found for 6 h old biofilm, while for the 24 h old biofilms only a moderate (but non-significant) correlation was observed for crystal violet (Table 2) (i.e., this study suggests both approaches reveal the same trend). Fabrizio et al. compared susceptibility of P. aeruginosa biofilm to cefiderocol using crystal violet and resazurin-based viability staining as well as determination of the number of CFU (which allows to quantify the number of culturable cells) [30] (Figure 5; Table 2). In this particular study, none of the quantification approaches used found a significant correlation between biofilm size and cefiderocol susceptibility (i.e., also data from this study suggest the different approaches yield the same outcome).
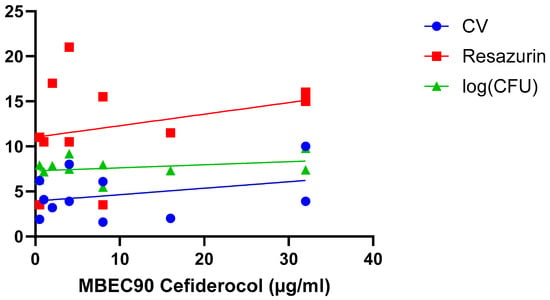
Figure 5.
Exposure of 11 P. aeruginosa isolates to cefiderocol indicates there is no correlation between biofilm formation (quantified using crystal violet staining [blue], resazurin viability staining [red], and CFU counts [green]) and biofilm susceptibility (as quantified by the MBEC90) to cefiderocol. Data were initially reported by Fabrizio et al. [30]. Statistical analysis of these data is presented in Table 2.
4. Discussion
As outlined above, the answer to the question ‘Is increased biofilm formation associated with decreased antimicrobial susceptibility?’ likely depends on the species, the strain collection, the antimicrobial agent investigated, and/or the experimental setup. Moreover, it seems plausible that a combination of several (or even all) of these factors play an important role.
While there are studies that found at least a moderately positive correlation between the amount of biofilm formed and reduced antimicrobial susceptibility, this is not always the case, and statistical support for this correlation is often lacking. The latter can at least partially be attributed to small sample sizes (most studies retrieved contain data for less than 10 isolates, and only very few contain data for more than 20 isolates; Table A1). As a consequence, overemphasizing the relevance of such observations and/or extending these observations to other species/antibiotic combinations than those investigated seems ill advised.
In addition, careful analysis of the existing literature identified a number of important points of attention for future studies.
First of all, the strain-dependent effects observed in several studies indicate that studies investigating the link between biofilm formation and antimicrobial susceptibility should be based on sufficiently large and diverse strain collections. An added advantage of larger strain collections is the ability to carry out properly powered statistical analyses. We illustrate this point by a closer look at the data reported by Alves et al. [28] for three Candida species (C. albicans, C. glabrata, and C. krusei) (Table 1 and Table 2). As mentioned above, when data are analyzed for each species separately, only a minority of the correlations observed are significant. However, when these data are pooled (and data for two C. valida strains are added; this increases the number of datapoints to 24), a different picture emerges, with all correlations being significant (Table 1 and Table 2).
Secondly, it is well known that biofilm susceptibility depends on the test conditions [4,32,33]. While various model systems were used across the studies analyzed for this systematic review (Table A1), we did not identify studies in which this question was addressed in a systematic way in multiple model systems for the same collection of strains. Because of this, we cannot definitively answer the question whether the relation between biofilm formation and biofilm susceptibility is model system-dependent, but it seems reasonable to speculate that it is. Considerable efforts have been made to develop standardized and reproducible biofilm approaches (including several ASTM standard test methods) [34,35] as well as clinically relevant ‘in vivo-like’ models [36,37,38] and the further use of these models might help to address the question about the relationship between biofilm size and susceptibility in a more systematic way.
Thirdly, a large variety of biofilm quantification approaches is used and these measure very different aspects of biofilm biology and chemistry [19,20,39]. This lack of standardization when it comes to quantification makes it difficult to compare studies and to extrapolate data. The dataset compiled did not allow us to address the question whether certain quantification approaches would be better than others, but it is important to emphasize that a thorough validation of the quantification method used (for the organism and antimicrobial agent being investigated) is essential. Crucial aspects of such validation include repeatability (i.e., the within lab variation), reproducibility (i.e., the between lab variation), and responsiveness (i.e., the ability of a method to differentiate between the effect of different concentrations of an antibiotic) [39].
In addition, different studies often use different biofilm susceptibility parameters, further complicating comparisons between studies, and a considerable number of papers were excluded from this systematic review, as they erroneously use MIC values as a proxy for biofilm susceptibility [4]. Biofilm-specific susceptibility parameters, including the biofilm prevention concentration (BPC), the minimum biofilm inhibitory concentration (MBIC), and the minimum biofilm eradication concentration (MBEC) have been defined [4,40] and should be used to quantify biofilm susceptibility.
We also noticed that many of the analyzed publications lacked raw data and/or only contained average or otherwise aggregated data. This precludes the re-use of data and analyses like the one performed in the present systematic review. We urge the biofilm community to make raw data available, by including them as part of the publication and/or by depositing them in general or specialized databases. Attempts to create such databases [41] and guidance on a minimal set of metadata to include [42] were made in the past, but have unfortunately remained rather unsuccessful so far.
Finally, a major limitation of the current systematic review is that it had to rely on data from studies that were mostly set up to address other (biological) questions, i.e., very few studies actually specifically investigated whether the amount of biofilm formed correlates with antibiotic susceptibility. It cannot be ruled out that results from studies specifically designed to answer this question yield a different picture than the one painted here. Obviously, this should not be seen as criticism of the studies we discussed in this systematic review but as a suggestion to help move the field forward.
Author Contributions
Conceptualization, T.C.; methodology, A.M., L.V., and T.C.; formal analysis, A.M. and L.V.; writing—original draft, A.M.; writing—review and editing, L.V. and T.C.; visualization, A.M. and T.C.; supervision, T.C.; project administration, T.C.; funding acquisition, A.M. and T.C. All authors have read and agreed to the published version of the manuscript.
Funding
A.M. gratefully acknowledges financial support for this research by the Fulbright U.S. Student Program, which is sponsored by the U.S. Department of State and the Fulbright Commission for Educational Exchange between the United States, Belgium, and Luxembourg. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Fulbright Program, the Government of the United States, or the Fulbright Commission for Educational Exchange between the United States, Belgium, and Luxembourg.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BPC | Biofilm prevention concentration |
| CFU | Colony forming unit |
| CV | Crystal violet |
| MBEC | Minimum biofilm eradication concentration |
| MIC | Minimum inhibitory concentration |
Appendix A

Table A1.
Complete list and summaries of the publications analyzed for the systematic literature review. Studies are grouped per species/genus. Some studies include data on multiple species.
Table A1.
Complete list and summaries of the publications analyzed for the systematic literature review. Studies are grouped per species/genus. Some studies include data on multiple species.
| Staphylococcus aureus | ||||
|---|---|---|---|---|
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Hon et al. [43] | 5 | Microtiter plate | Crystal violet staining | Mupirocin |
| Liang et al. [44] | 2 | Microtiter plate | Crystal violet staining | Penicillin, ampicillin, meropenem, streptomycin, kanamycin, and gentamicin |
| Silva et al. [23] | 23 | Microtiter plate | Crystal violet staining and XTT viability staining | Amikacin and tetracycline |
| Silva et al. [24] | 18 | Microtiter plate | Crystal violet staining | Ciprofloxacin, erythromycin, and tetracycline |
| Kwiatkowski et al. [25] | 8 | Microtiter plate | Crystal violet staining | Mupirocin |
| Wu et al. [26] | 6 | Microtiter plate | Crystal violet staining and resazurin viability staining | Linezolid |
| de Matos et al. [45] | 6 | Microtiter plate | Crystal violet staining | Gentamicin, linezolid, rifampicin, and vancomycin |
| Abdelhady et al. [46] | 10 | Microtiter plate | Safranin staining | Vancomycin |
| Wells et al. [47] | 2 | Microtiter plate | Crystal violet staining | Oxacillin, vancomycin, and ampicillin |
| Roveta et al. [48] | 3 | Microtiter plate | Crystal violet staining | Moxifloxacin |
| Xu et al. [49] | 6 | Microtiter plate | Crystal violet staining | Daptomycin |
| Lavoie et al. [27] | 11 | Microtiter plate | Crystal violet staining | Daptomycin |
| Coagulase-Negative Staphylococci spp. | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Silva et al. [50] | 19 | Microtiter plate | Crystal violet staining | Tetracycline and amikacin |
| Pseudomonas aeruginosa | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Papa-Ezdra et al. [51] | 2 | Microtiter plate | Crystal violet staining | Rifampicin, meropenem, gentamicin, amikacin, and ciprofloxacin |
| Ruhal et al. [52] | 3 | Glass test tubes | Crystal violet staining | Tobramycin, colistin, and ciprofloxacin |
| Fortes et al. [53] | 2 | Microtiter plate | Crystal violet staining | Polymyxin B |
| Goodyear et al. [54] | 10 | Microtiter plate | Crystal violet staining | Piperacillin, aztreonam, imipenem, colistin, tobramycin, and ciprofloxacin |
| Žiemytė et al. [55] | 3 | Microtiter plate | Crystal violet staining | Ceftazidime, ciprofloxacin, colistin, tobramycin, imipenem, meropenem, and piperacillin-tazobactam |
| Gupta et al. [56] | 4 | Flow tube reactor | Crystal violet staining | Tobramycin and norfloxacin |
| Fabrizio et al. [30] | 11 | Microtiter plate | Crystal violet staining, resazurin viability staining, and determination of number of CFU | Cefiderocol |
| Streptococcus spp. | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Silva et al. [50] | 2 | Microtiter plate | Crystal violet staining | Tetracycline and amikacin |
| Alvim et al. [57] | 4 | Microtiter plate | Crystal violet staining | Penicillin |
| Chen et al. [58] | 2 | Microtiter plate | Crystal violet staining | Chlorhexidine |
| Hall-Stoodley et al. [59] | 6 | Microtiter plate | Crystal violet staining | Azithromycin |
| Roveta et al. [48] | 3 | Microtiter plate | Crystal violet staining | Moxifloxacin |
| Escherichia coli | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Roveta et al. [48] | 3 | Microtiter plate | Crystal violet staining | Moxifloxacin |
| Burkholderia spp. | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Anutrakunchai et al. [60] | 10 | Microtiter plate | Crystal violet staining | Ceftazidime |
| Tomlin et al. [29] | 6 | Microtiter plate | Crystal violet staining | Ceftazidime, chloramphenicol, ciprofloxacin, and meropenem |
| Haemophilus influenzae | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Roveta et al. [48] | 3 | Microtiter plate | Crystal violet staining | Moxifloxacin |
| Acinetobacter baumannii | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Wang et al. [61] | 6 | Microtiter plate | Crystal violet staining | Meropenem, imipenem, sulbactam, colistin, and tigecycline |
| Moraxella catarrhalis | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Roveta et al. [48] | 3 | Microtiter plate | Crystal violet staining | Moxifloxacin |
| Campylobacter jejuni | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Rossi et al. [62] | 2 | Microtiter plate | Crystal violet staining | Ciprofloxacin, colistin, tetracycline, erythromycin, and meropenem |
| Proteus mirabilis | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Li et al. [63] | 2 | Flow cell system | SYTO 62 | Ciprofloxacin |
| Helicobacter pylori | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Yonezawa et al. [64] | 2 | Microtiter plate | Crystal violet staining | Amoxicillin and metronidazole |
| Wu et al. [22] | 9 | Microtiter plate | Microscopy and CFU counts | Amoxicillin, clarithromycin, tetracycline, and levofloxacin |
| Mycobacteroides abscessus | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Oschmann-Kadenbach et al. [31] | 4 | Porous glass beads | CFU counts | Amikacin and tigecycline |
| Bacillus spp. | ||||
| Reference | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| do Canto Canabarro et al. [65] | 4 | Microtiter plate | Crystal violet staining | Penicillin, tetracycline, and gentamicin |
| Candida spp. | ||||
| Authors | Number of Isolates Included | Model System | Biofilm Quantification Method | Antibiotics Tested |
| Alves et al. [28] | 24 | Microtiter plate | Crystal violet staining | Fluconazole, voriconazole, anidulafungin, and amphotericin B |
| Melo et al. [66] | 30 | Microtiter plate | Crystal violet staining | Amphotericin B and fluconazole |
References
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T. Biofilm antimicrobial susceptibility testing: Where are we and where could we be going? Clin. Microbiol. Rev. 2023, 36, e0002423. [Google Scholar] [CrossRef]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Doroshenko, N.; Tseng, B.S.; Howlin, R.P.; Deacon, J.; Wharton, J.A.; Thurner, P.J.; Gilmore, B.F.; Parsek, M.R.; Stoodley, P. Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob. Agents Chemother. 2014, 58, 7273–7282. [Google Scholar] [CrossRef]
- Cao, B.; Christophersen, L.; Kolpen, M.; Jensen, P.O.; Sneppen, K.; Hoiby, N.; Moser, C.; Sams, T. Diffusion Retardation by Binding of Tobramycin in an Alginate Biofilm Model. PLoS ONE 2016, 11, e0153616. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Ciofu, O.; Yang, L.; Wu, H.; Song, Z.; Oliver, A.; Hoiby, N. High beta-lactamase levels change the pharmacodynamics of beta-lactam antibiotics in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Van Acker, H.; Coenye, T. The Role of Efflux and Physiological Adaptation in Biofilm Tolerance and Resistance. J. Biol. Chem. 2016, 291, 12565–12572. [Google Scholar] [CrossRef]
- Van Acker, H.; Gielis, J.; Acke, M.; Cools, F.; Cos, P.; Coenye, T. The Role of Reactive Oxygen Species in Antibiotic-Induced Cell Death in Burkholderia cepacia Complex Bacteria. PLoS ONE 2016, 11, e0159837. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Coenye, T.; Van Acker, H.; Peeters, E.; Sass, A.; Buroni, S.; Riccardi, G.; Mahenthiralingam, E. Molecular mechanisms of chlorhexidine tolerance in Burkholderia cenocepacia biofilms. Antimicrob. Agents Chemother. 2011, 55, 1912–1919. [Google Scholar] [CrossRef]
- Crabbé, A.; Jensen, P.Ø.; Bjarnsholt, T.; Coenye, T. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol. 2019, 27, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Price-Whelan, A.; Dietrich, L.E.P. Gradients and consequences of heterogeneity in biofilms. Nat. Rev. Microbiol. 2022, 20, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Bjarnsholt, T. Microbial Primer: In vivo biofilm. Microbiol. 2023, 169, 001407. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Coenye, T.; Parsek, M.R.; Bjarnsholt, T.; Jakobsen, T.H. What’s in a name? Characteristics of clinical biofilms. FEMS Microbiol. Rev. 2023, 47, fuad050. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Jakobsen, T.H.; Kühl, M.; Kolpen, M.; Jensen, P.Ø.; Bjarnsholt, T. The structure–function relationship of Pseudomonas aeruginosa in infections and its influence on the microenvironment. FEMS Microbiol. Rev. 2022, 46, fuac018. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wu, X.; Wu, D.; Cui, G.; Lee, K.H.; Yang, T.; Zhang, Z.; Liu, Q.; Zhang, J.; Chua, E.G.; Chen, Z. Association Between Biofilm Formation and Structure and Antibiotic Resistance in H. pylori. Infect. Drug Resist. 2024, 17, 2501–2512. [Google Scholar] [CrossRef]
- Silva, V.; Correia, E.; Pereira, J.E.; González-Machado, C.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Biofilm Formation of Staphylococcus aureus from Pets, Livestock, and Wild Animals: Relationship with Clonal Lineages and Antimicrobial Resistance. Antibiotics 2022, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Almeida, L.; Gaio, V.; Cerca, N.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Biofilm Formation of Multidrug-Resistant MRSA Strains Isolated from Different Types of Human Infections. Pathogens 2021, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Grygorcewicz, B.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Sienkiewicz, M.; Wojciechowska-Koszko, I. The Effect of Subinhibitory Concentrations of trans-Anethole on Antibacterial and Antibiofilm Activity of Mupirocin Against Mupirocin-Resistant Staphylococcus aureus Strains. Microb. Drug Resist. 2019, 25, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, T.; Luo, Y.; Li, X.; Zhang, X.; Tang, J.; Ma, X.; Wang, Z. Efficacy of the novel oxazolidinone compound FYL-67 for preventing biofilm formation by Staphylococcus aureus. J. Antimicrob. Chemother. 2014, 69, 3011–3019. [Google Scholar] [CrossRef]
- Lavoie, T.; Daffinee, K.E.; Vicent, M.L.; LaPlante, K.L. Staphylococcus biofilm dynamics and antibiotic resistance: Insights into biofilm stages, zeta potential dynamics, and antibiotic susceptibility. Microbiol. Spectr. 2025, 13, e0291524. [Google Scholar] [CrossRef]
- Alves, A.M.C.V.; Lopes, B.O.; Leite, A.C.R.d.M.; Cruz, G.S.; Brito, É.H.S.d.; Lima, L.F.d.; Černáková, L.; Azevedo, N.F.; Rodrigues, C.F. Characterization of Oral Candida spp. Biofilms in Children and Adults Carriers from Eastern Europe and South America. Antibiotics 2023, 12, 797. [Google Scholar] [CrossRef]
- Tomlin, K.L.; Malott, R.J.; Ramage, G.; Storey, D.G.; Sokol, P.A.; Ceri, H. Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Appl. Environ. Microbiol. 2005, 71, 5208–5218. [Google Scholar] [CrossRef]
- Fabrizio, G.; Truglio, M.; Cavallo, I.; Sivori, F.; Francalancia, M.; Riveros Cabral, R.J.; Comar, M.; Trancassini, M.; Compagnino, D.E.; Diaco, F.; et al. Cefiderocol activity against planktonic and biofilm forms of beta-lactamase-producing pseudomonas aeruginosa from people with cystic fibrosis. J. Glob. Antimicrob. Resist. 2025, 43, 111–119. [Google Scholar] [CrossRef]
- Oschmann-Kadenbach, A.M.; Schaudinn, C.; Borst, L.; Schwarz, C.; Konrat, K.; Arvand, M.; Lewin, A. Impact of Mycobacteroides abscessus colony morphology on biofilm formation and antimicrobial resistance. Int. J. Med. Microbiol. 2024, 314, 151603. [Google Scholar] [CrossRef]
- Subramanian, S.; Huiszoon, R.C.; Chu, S.; Bentley, W.E.; Ghodssi, R. Microsystems for biofilm characterization and sensing—A review. Biofilm 2020, 2, 100015. [Google Scholar] [CrossRef]
- Coenye, T.; Goeres, D.; Van Bambeke, F.; Bjarnsholt, T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin. Microbiol. Infect. 2018, 24, 570–572. [Google Scholar] [CrossRef]
- Gomes, I.B.; Meireles, A.; Goncalves, A.L.; Goeres, D.M.; Sjollema, J.; Simoes, L.C.; Simoes, M. Standardized reactors for the study of medical biofilms: A review of the principles and latest modifications. Crit. Rev. Biotechnol. 2018, 38, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Goeres, D.M.; Gosbell, I.; Vickery, K.; Jensen, S.; Stoodley, P. Approaches to biofilm-associated infections: The need for standardized and relevant biofilm methods for clinical applications. Expert. Rev. Anti Infect. Ther. 2017, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Vyas, H.K.N.; Xia, B.; Mai-Prochnow, A. Clinically relevant in vitro biofilm models: A need to mimic and recapitulate the host environment. Biofilm 2022, 4, 100069. [Google Scholar] [CrossRef] [PubMed]
- De Bleeckere, A.; van Charante, F.; Debord, T.; Vandendriessche, S.; De Cock, M.; Verstraete, M.; Lamret, F.; Lories, B.; Boelens, J.; Reffuveille, F.; et al. A novel synthetic synovial fluid model for investigating biofilm formation and antibiotic susceptibility in prosthetic joint infections. Microbiol. Spectr. 2025, 13, e0198024. [Google Scholar] [CrossRef]
- De Bleeckere, A.; Van den Bossche, S.; De Sutter, P.-J.; Beirens, T.; Crabbé, A.; Coenye, T. High throughput determination of the biofilm prevention concentration for Pseudomonas aeruginosa biofilms using a synthetic cystic fibrosis sputum medium. Biofilm 2023, 5, 100106. [Google Scholar] [CrossRef]
- Allkja, J.; van Charante, F.; Aizawa, J.; Reigada, I.; Guarch-Perez, C.; Vazquez-Rodriguez, J.A.; Cos, P.; Coenye, T.; Fallarero, A.; Zaat, S.A.J.; et al. Interlaboratory study for the evaluation of three microtiter plate-based biofilm quantification methods. Sci. Rep. 2021, 11, 13779. [Google Scholar] [CrossRef]
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef]
- Lourenco, A.; Ferreira, A.; Veiga, N.; Machado, I.; Pereira, M.O.; Azevedo, N.F. BiofOmics: A Web platform for the systematic and standardized collection of high-throughput biofilm data. PLoS ONE 2012, 7, e39960. [Google Scholar] [CrossRef]
- Lourenco, A.; Coenye, T.; Goeres, D.M.; Donelli, G.; Azevedo, A.S.; Ceri, H.; Coelho, F.L.; Flemming, H.C.; Juhna, T.; Lopes, S.P.; et al. Minimum information about a biofilm experiment (MIABiE): Standards for reporting experiments and data on sessile microbial communities living at interfaces. Pathog. Dis. 2014, 70, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.; Liu, S.; Cooksley, C.; Vreugde, S.; Psaltis, A.J. Low pH nasal rinse solution enhances mupirocin antimicrobial efficacy. Rhinology 2022, 60, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, T.Y.; Mao, Y.; Li, X. Biofilm formation of two genetically diverse Staphylococcus aureus isolates under beta-lactam antibiotics. Front. Microbiol. 2023, 14, 1139753. [Google Scholar] [CrossRef] [PubMed]
- de Matos, P.D.M.; Sedaca, S.; Ferreira, D.C.; Iorio, N.L.; Toledo, V.C.S.; Freitas, A.I.C.; Coelho, F.L.; Sousa, C.; Dos Santos, K.R.N.; Pereira, M.O. Antimicrobial synergism against different lineages of methicillin-resistant Staphylococcus aureus carrying SCCmec IV. J. Appl. Microbiol. 2014, 116, 1418–1426. [Google Scholar] [CrossRef][Green Version]
- Abdelhady, W.; Bayer, A.S.; Seidl, K.; Nast, C.C.; Kiedrowski, M.R.; Horswill, A.R.; Yeaman, M.R.; Xiong, Y.Q. Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 1447–1454. [Google Scholar] [CrossRef]
- Wells, C.L.; Henry-Stanley, M.J.; Barnes, A.M.T.; Dunny, G.M.; Hess, D.J. Relation between Antibiotic Susceptibility and Ultrastructure of Staphylococcus aureus Biofilms on Surgical Suture. Surg. Infect. 2011, 12, 297–305. [Google Scholar] [CrossRef]
- Roveta, S.; Schito, A.M.; Marchese, A.; Schito, G.C. Activity of moxifloxacin on biofilms produced in vitro by bacterial pathogens involved in acute exacerbations of chronic bronchitis. Int. J. Antimicrob. Agents 2007, 30, 415–421. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Zhao, H.; Wang, B.; Yu, J.; Shang, Y.; Zhou, Y.; Wu, X. Phenotypic and genetic characterization of daptomycin non-susceptible Staphylococcus aureus strains selected by adaptive laboratory evolution. Front. Cell. Infect. Microbiol. 2024, 14, 1453233. [Google Scholar] [CrossRef]
- Silva, V.; Correia, E.; Pereira, J.E.; González-Machado, C.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Exploring the Biofilm Formation Capacity in S. pseudintermedius and Coagulase-Negative Staphylococci Species. Pathogens 2022, 11, 689. [Google Scholar] [CrossRef]
- Papa-Ezdra, R.; Outeda, M.; Cordeiro, N.F.; Araújo, L.; Gadea, P.; Garcia-Fulgueiras, V.; Seija, V.; Bado, I.; Vignoli, R. Outbreak of Pseudomonas aeruginosa High-Risk Clone ST309 Serotype O11 Featuring blaPER-1 and qnrVC6. Antibiotics 2024, 13, 159. [Google Scholar] [CrossRef]
- Ruhal, R.; Ghosh, M.; Kumar, V.; Jain, D. Mutation of putative glycosyl transferases PslC and PslI confers susceptibility to antibiotics and leads to drastic reduction in biofilm formation in Pseudomonas aeruginosa. Microbiology 2023, 169, 001392. [Google Scholar] [CrossRef] [PubMed]
- Fortes, B.N.; Scheunemann, G.; de Azevedo Melo, A.S.; Ishida, K. Caspofungin alone or combined with polymyxin B are effective against mixed biofilm of Aspergillus fumigatus and carbapenem-resistant Pseudomonas aeruginosa. Res. Microbiol. 2023, 174, 103993. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, M.C.; Garnier, N.E.; Levesque, R.C.; Khursigara, C.M. Liverpool Epidemic Strain Isolates of Pseudomonas aeruginosa Display High Levels of Antimicrobial Resistance during Both Planktonic and Biofilm Growth. Microbiol. Spectr. 2022, 10, e0102422. [Google Scholar] [CrossRef] [PubMed]
- Žiemytė, M.; Carda-Diéguez, M.; Rodríguez-Díaz, J.C.; Ventero, M.P.; Mira, A.; Ferrer, M.D. Real-time monitoring of Pseudomonas aeruginosa biofilm growth dynamics and persister cells’ eradication. Emerg. Microbes Infect. 2021, 10, 2062–2075. [Google Scholar] [CrossRef]
- Gupta, K.; Marques, C.N.H.; Petrova, O.E.; Sauer, K. Antimicrobial Tolerance of Pseudomonas aeruginosa Biofilms Is Activated during an Early Developmental Stage and Requires the Two-Component Hybrid SagS. J. Bacteriol. 2013, 195, 4975–4987. [Google Scholar] [CrossRef]
- Alvim, D.; Oliveira, L.M.A.; Simoes, L.C.; Costa, N.S.; Fracalanzza, S.E.L.; Teixeira, L.M.; Ferreira, R.B.R.; Pinto, T.C.A. Influence of Penicillin on Biofilm Formation by Streptococcus agalactiae Serotype Ia/CC23. Microb. Drug Resist. 2022, 28, 517–524. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Y.; Weir, M.D.; Gao, J.; Imazato, S.; Oates, T.W.; Lei, L.; Wang, S.; Hu, T.; Xu, H.H.K. Effects of S. mutans gene-modification and antibacterial monomer dimethylaminohexadecyl methacrylate on biofilm growth and acid production. Dent. Mater. 2020, 36, 296–309. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Nistico, L.; Sambanthamoorthy, K.; Dice, B.; Nguyen, D.; Mershon, W.J.; Johnson, C.; Hu, F.Z.; Stoodley, P.; Ehrlich, G.D.; et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008, 8, 173. [Google Scholar] [CrossRef]
- Anutrakunchai, C.; Sermswan, R.W.; Wongratanacheewin, S.; Puknun, A.; Taweechaisupapong, S. Drug susceptibility and biofilm formation of Burkholderia pseudomallei in nutrient-limited condition. Trop. Biomed. 2015, 32, 300–309. [Google Scholar]
- Wang, Y.C.; Kuo, S.C.; Yang, Y.S.; Lee, Y.T.; Chiu, C.H.; Chuang, M.F.; Lin, J.C.; Chang, F.Y.; Chen, T.L. Individual or Combined Effects of Meropenem, Imipenem, Sulbactam, Colistin, and Tigecycline on Biofilm-Embedded Acinetobacter baumannii and Biofilm Architecture. Antimicrob. Agents Chemother. 2016, 60, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.A.; Dumont, C.F.; Santos, A.C.S.; Vaz, M.E.L.; Prado, R.R.; Monteiro, G.P.; Melo, C.; Stamoulis, V.J.; Dos Santos, J.P.; de Melo, R.T. Antibiotic Resistance in the Alternative Lifestyles of Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2021, 11, 535757. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, N.; Brady, H.R.; Packman, A.I. Ureolytic Biomineralization Reduces Proteus mirabilis Biofilm Susceptibility to Ciprofloxacin. Antimicrob. Agents Chemother. 2016, 60, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Hojo, F.; Kamiya, S. Effect of Helicobacter pylori biofilm formation on susceptibility to amoxicillin, metronidazole and clarithromycin. Microb. Pathog. 2019, 132, 100–108. [Google Scholar] [CrossRef]
- do Canto Canabarro, M.; Meneghetti, K.L.; Geimba, M.P.; Corcao, G. Biofilm formation and antibiotic susceptibility of Staphylococcus and Bacillus species isolated from human allogeneic skin. Braz. J. Microbiol. 2022, 53, 153–160. [Google Scholar] [CrossRef]
- Melo, A.S.; Bizerra, F.C.; Freymuller, E.; Arthington-Skaggs, B.A.; Colombo, A.L. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med. Mycol. 2011, 49, 253–262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).