Abstract
Polyporus umbellatus is a medicinal fungus primarily used for diuresis, with its sclerotium serving as the medicinal component. The growth and development of sclerotia are reliant on a symbiotic relationship with Armillaria. However, the impact of different Armillaria species on the yield and quality of sclerotia remains unclear. In this study, three Armillaria strains, A35, A541, and A19, were identified through TEF-1α sequence analysis and phylogenetic classification. These strains were classified into three distinct species: A35 as A. ostoyae, A541 as A. gallica, while the taxonomic status of A19 remains unresolved. After four years of co-cultivation with these Armillaria strains, three groups of P. umbellatus sclerotia were harvested and labeled as A35-P, A541-P, and A19-P, respectively. The yields of A35-P, A541-P, and A19-P exhibited significant variations, with A541-P achieving the highest yield (1221 ± 258 g·nest−1), followed by A35-P (979 ± 201 g·nest−1), and A19-P yielding the least (591 ± 54 g·nest−1). HPLC revealed significant differences in the levels of polyporusterone A and polyporusterone B among the groups. The total polysaccharide content, determined via the phenol-sulfuric acid method, also varied significantly, with A541-P recording the highest content (0.897 ± 0.042%), followed by A19-P (0.686 ± 0.058%), and A35-P showing the lowest value (0.511 ± 0.083%). PCA based on these data indicated clear distinctions among A35-P, A541-P, and A19-P, with the three groups forming separate clusters. This study, for the first time, demonstrates the effects of three different Armillaria species on the yield and active compound content of P. umbellatus. These findings provide valuable insights for selecting high-quality Armillaria strains and offer guidance for the artificial cultivation of P. umbellatus.
1. Introduction
Polyporus umbellatus (Pers.) Fries is a fungus belonging to the Polyporaceae family, consisting of two primary parts: the sclerotium and the fruiting body (commonly referred to as mushrooms). The fruiting body, which emerges from the underground sclerotium, is the edible portion. The sclerotium, found beneath the soil, has been recognized as a valuable component of traditional Chinese medicine for over 2000 years, primarily used for its dampness-clearing and diuretic properties [1]. Modern pharmacological research has validated the diuretic [2], renal protective [3], anti-tumor [4], and other beneficial activities of P. umbellatus [5]. The bioactive compounds identified in P. umbellatus are predominantly steroids and polysaccharides [6]. Over 30 steroidal compounds have been isolated and characterized from their sclerotium, fruiting body, and fermenting mycelium [7,8]. For instance, ergosterol and ergosta-4,6,8(14),22-tetraen-3-one, extracted from the sclerotia, have demonstrated diuretic effects [9]. Additional steroids, such as polyporusterone A and polyporusterone B, have exhibited cytotoxicity against leukemia 1210 cells [10], antioxidant activity by inhibiting 2,2-azino-bis(2-aminopropane)dichloride-induced erythrocyte lysis [11], and hair growth-promoting properties [12]. In the Chinese Pharmacopoeia (ChP, 2020), ergosterol is designated as an index compound for evaluating the quality of P. umbellatus sclerotia, with a stipulated minimum content of 0.070%. Polysaccharides, another significant class of active compounds in P. umbellatus, have demonstrated diverse pharmacological activities, including immunomodulatory, anti-tumor, hepatoprotective, and antiradiation effects in in vivo and in vitro studies [13,14].
Given the efficacy of P. umbellatus in treating edema and supporting anti-tumor activities, clinical applications of drugs derived from P. umbellatus, such as the P. umbellatus polysaccharide injection and Wuling-san, have become widespread [15]. Consequently, the artificial cultivation of P. umbellatus is essential to meet market demand and conserve wild P. umbellatus resources. However, the success of artificial cultivation is influenced by factors such as the Armillaria species and environmental conditions, leading to challenges with inconsistent yields and variable quality. The growth and development of P. umbellatus sclerotia are dependent on a special symbiotic relationship with Armillaria, a genus within the family Physalacriaceae [16]. Under natural conditions, there is a special symbiotic relationship between P. umbellatus and Armillaria. Armillaria invades P. umbellatus via its rhizomorphs, triggering the innate immune response of P. umbellatus to resist foreign infection. During this process, the mycelial cells of P. umbellatus undergo lignification, forming isolation cavities resembling the structure of the sclerotia epidermis. These cavities encapsulate the hyphae of both Armillaria and P. umbellatus. Within the isolation cavities, Armillaria digests the separated mycelium of P. umbellatus. Simultaneously, the P. umbellatus mycelium can invade or attach to the Armillaria mycelium and its infection zone’s intercellular spaces to absorb metabolites. This interaction enables the growth of P. umbellatus mycelium and its differentiation into new sclerotia [17]. In artificial cultivation, uncertainties surrounding the source and species of Armillaria can lead to non-growth or the “empty nest” phenomenon, presenting a major limitation for the development of the P. umbellatus industry. Previous studies indicated that infestation with Armillaria increased the ergosterol content in P. umbellatus from 0.0632 to 0.0809%, an improvement of 28%. Additionally, the polysaccharide content rose from 3.69 to 5.45%, representing a 47.7% increase [18]. Further research involving 28 strains of Armillaria isolated from wild P. umbellatus identified A. mellea, A. gallica, A. cepistipes, and A. ostoyae as potential symbiotic partners, indicating that P. umbellatus exhibits low selectivity for symbiotic Armillaria species [19]. Despite this, it remains unclear which specific Armillaria species are optimal for co-cultivation with P. umbellatus to improve yields and quality.
In this study, three Armillaria strains, A19, A35, and A541, were selected for co-cultivation with P. umbellatus. These strains, identified and grouped into distinct Armillaria clades through molecular methods, were used to examine their impact on the yield and contents of ergosterol, polyporusterone A, polyporusterone B, and polysaccharides in P. umbellatus. The findings provide a reference for selecting the most suitable Armillaria strains for the artificial cultivation of P. umbellatus.
2. Materials and Methods
2.1. Strain and Culture of Armillaria
Three Armillaria strains were isolated and purified from wild P. umbellatus sclerotia. These strains were preserved at the Institute of Medicinal Plants, Chinese Academy of Medical Sciences, under preservation numbers A35, A541, and A19. A541 and A35 were isolated from wild P. umbellatus in Liuba and Lueyang, Shaanxi Province, respectively, while A19 was isolated from P. umbellatus in Zhaotong, Yunnan Province. A35, A541, and A19 were cultured on a potato dextrose agar (PDA) medium (1 L of medium consisting of 200 g of potato, 10 g of dextrose, and 12 g of agar) at room temperature in the dark.
For co-cultivation with P. umbellatus, the Armillaria strains on PDA plates were further cultured to spawn due to their rapid growth, which facilitated infection of P. umbellatus and simplified contamination control and transportation. Tree branches from the Fagaceae family were selected as a growth substrate for the Armillaria spawn because of their well-developed sapwood and moderately thick bark [20]. Chestnut tree branches approximately 2 cm in diameter and 5 cm in length were soaked in water for 24 h, bottled, and then submerged in water. The bottles were sterilized, creating a medium suitable for inoculation with Armillaria strains under aseptic conditions. The Armillaria strains were incubated in this medium at room temperature in the dark for 40–50 days until the medium was fully colonized [21]. These cultures were subsequently used for co-cultivation with P. umbellatus.
2.2. DNA Extraction, PCR Amplification, and Sequencing of A35, A541, and A19
Fresh rhizomorphs of A35, A541, and A19, cultured on PDA for approximately 15 days, were collected and ground into powder using liquid nitrogen to facilitate DNA extraction. DNA was extracted following the instructions provided in the DNA extraction kit (Aidlab, Beijing, China). The partial TEF-1α (transcription elongation factor) gene was amplified using the primer pair TEF-F (5′-GGCATCGAGGAGAGAGTCTTG-3′) and TEF-R (5′-TATCTCCAAGGACGGGCAGA-3′) [22]. The PCR amplification system consisted of 12.5 μL of 2× Taq Master Mix (Vazyme, Nanjing, China), 1 μL of each primer (10 μmol·L−1), 2 μL of DNA template, and 8.5 μL of ddH2O, for a total reaction volume of 25 μL. The reaction conditions were as follows: initial denaturation at 95 °C for 3 min, followed by 34 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 15 s, and extension at 72 °C for 60 s, with a final extension step at 72 °C for 5 min. The PCR products were sequenced by RuiBiotech Co., Ltd., Beijing, China.
2.3. Nucleotide Alignment and Phylogenetic Analysis
The sequences obtained from PCR products were analyzed using the BLAST tool at the National Center for Biotechnology Information (NCBI) to identify sequences with high homology. These homologous sequences were downloaded and used to construct a phylogenetic tree with MEGA 7.0 software [23]. Lentinula edodes was designated as the outgroup for the analysis. The evolutionary distance was calculated using the maximum likelihood method, and the confidence levels of the branching points were assessed through bootstrap analysis with 1000 replicates.
2.4. Artificial Co-Cultivation Experiment of P. umbellatus
The artificial co-cultivation experiment was carried out between October 2018 and March 2022 in Liuba County, Shaanxi Province, a major production area of P. umbellatus in China. The experimental site was situated at an altitude of approximately 1450 m, featuring forested, gently sloping terrain with well-drained, humus-rich soil. The co-cultivation methodology followed previously reported protocols [24] with modifications to certain parameters. Nests were dug along the slope, each with dimensions of approximately 30 cm × 30 cm (depth × width). A layer of thin leaves, about 2 cm thick, was spread on the base of each nest. Two segments of tree sticks, each with a diameter of 8–12 cm, were placed side by side with a spacing of 3–5 cm. Each nest was then filled with 250 g of P. umbellatus sclerotia (fresh, tender, and highly viable seeds purchased from Qinzheng Zhuling Development Co., Ltd. in Liuba County, Hanzhong, China), 500 g of small branches, and 1 bottle of Armillaria spawn. The materials were positioned on both sides and on top of the tree sticks. A thin layer of humus soil and leaves was sprinkled over the materials, followed by a 5 cm layer of soil to cover the nest. At least 30 nests of P. umbellatus were co-cultivated with each strain of Armillaria. The nests were spaced 15 cm apart, and after four years of cultivation, the P. umbellatus was harvested. The samples of P. umbellatus co-cultivated with A35, A541, and A19 were designated as A35-P, A541-P, and A19-P, respectively.
2.5. Sample Collection and Yield Comparisons of P. umbellatus Sclerotia
The P. umbellatus sclerotia were harvested in the fourth year of co-cultivation. Surface sediment, residual humus, and other impurities were carefully removed from the sclerotia before weighing. To determine the yield of P. umbellatus after co-cultivation, at least eight nests were dug up from each group. The average yield from each group was calculated, and the differences in yield among the three groups, A35-P, A541-P, and A19-P, were compared.
2.6. High-Performance Liquid Chromatography for Detecting Ergosterol, Polyporusterone A, and Polyporusterone B in P. umbellatus Sclerotia
The reference substance for ergosterol was obtained from Beijing Solarbio Science & Technology Co., Ltd., Beijing, China, while the reference substances for polyporusterone A and polyporusterone B were purchased from Sichuan Scvictory Biotechnology Co., Ltd. (Chengdu, China). The reference substances—ergosterol, polyporusterone A, and polyporusterone B—were accurately weighed at 1.47 mg, 1.57 mg, and 1.57 mg, respectively. Each was diluted in methanol within a volumetric flask to a precise volume of 2 mL, resulting in concentrations of 0.735, 0.785, and 0.785 mg·mL−1, respectively. Different volumes of the single-reference solutions were combined to prepare a mixed-reference solution for qualitative analysis.
Fresh P. umbellatus sclerotia samples from the A19-P, A35-P, and A541-P groups (three samples per group) were collected, washed, and sun-dried. The samples were further dried in an oven at 60 °C until their weight stabilized. The dried samples were then ground into powder and passed through a 40-mesh sieve. From each powdered sample, 3 g was accurately weighed, and 20 volumes of 95% ethanol were added. The samples were soaked in ethanol for 12 h and subsequently subjected to ultrasonication for 1 h. The extracts were filtered and concentrated using rotary evaporation. The resulting concentrate was transferred to an evaporating dish, where the remaining ethanol was allowed to volatilize at room temperature. The residue was redissolved in methanol, and the solution was transferred into a volumetric flask with a final volume of 2 mL [25,26]. Each sample group was processed in triplicate to ensure reproducibility.
High-performance liquid chromatography (HPLC) analysis was conducted using the 1260 Infinity II LC System (Agilent, Santa Clara, CA, USA), which includes a quadruple pump, autosampler, and variable wavelength ultraviolet detector. The experiments utilized a Bridge RP18 column (250 mm × 4.6 mm, 5 μm, Waters, Milford, MA, USA). The flow rate was maintained at 1.0 mL·min−1, and the column temperature was set to room temperature. The detection wavelength was configured as follows: 247 nm for the first 25 min, suitable for detecting polyporusterone A and polyporusterone B, and 283 nm after 25 min for ergosterol detection. Both standard and sample solutions were filtered through a 0.22 μm organic phase filter membrane prior to injection, and 20 μL of each sample solution was injected into the HPLC system. The mobile phase consisted of the following components: A (acetonitrile), B (water), and D (methanol). The elution program was as follows: 0–25 min, A%:B% = 78:22; 25–42 min, 100% D; and 42–45 min, A%:B% = 78:22 [25,26].
2.7. Phenol-Sulfuric Acid Method for Detecting the Polysaccharides in P. umbellatus
The phenol-sulfuric acid method was employed to quantify the polysaccharides in P. umbellatus sclerotia. For the preparation of the reference standard, 10 mg of glucose was accurately weighed and diluted with methanol in a volumetric flask to a final volume of 10 mL. From this solution, volumes of 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0 mL were precisely measured into separate test tubes, and the volume in each tube was adjusted to 2.0 mL with water. Next, 1 mL of freshly prepared 5% phenol solution and 5 mL of concentrated sulfuric acid were added to each test tube. The mixtures were shaken thoroughly, allowed to stand at room temperature for 10 min, and then maintained at 40 °C for 15 min. Afterward, they were cooled in an ice bath, and the absorbance was measured at 490 nm. A standard curve was generated by plotting absorbance (Y-axis) against the glucose reference concentration (X-axis).
To prepare the test solution, powdered P. umbellatus sclerotia from the A19-P, A35-P, and A541-P samples were used. For each sample, 3 g of sclerotia powder was accurately weighed, and 20 mL of ultrapure water was added. The mixture was subjected to ultrasonication for 1 h, and the extract was concentrated under reduced pressure, with the weight recorded. Subsequently, three times the volume of 85% ethanol was added to the concentrate, and the mixture was stored at 4 °C overnight. The mixture was centrifuged for 30 min, and the supernatant was discarded. The precipitate was dissolved in water to produce the test solution, which was appropriately diluted and analyzed using the same procedure described for the standard curve. The total polysaccharide content in P. umbellatus was calculated based on the standard curve [27]. Each sample group was analyzed in triplicate to ensure accuracy.
2.8. Statistical Analysis
The experimental data on the percentage content of the active compounds in P. umbellatus were calculated on a dry weight basis and expressed as mean ± standard deviation. Statistical analyses were conducted using IBM SPSS 25.0 software (IBM Corp., Armonk, NY, USA), with ANOVA, followed by LSD tests to determine the statistical significance, where p < 0.05 was considered significant. Data related to the yield, ergosterol, polyporusterone A, polyporusterone B, and polysaccharide contents of A35-P, A541-P, and A19-P were processed and analyzed using GraphPad Prism 9.5 (GraphPad Software, San Diego, CA, USA) via principal component analysis (PCA). PCA was performed on standardized data, and PCs with eigenvalues greater than 1 were selected for further interpretation.
3. Results
3.1. A35, A541, and A19 Belong to Three Different Species of Armillaria
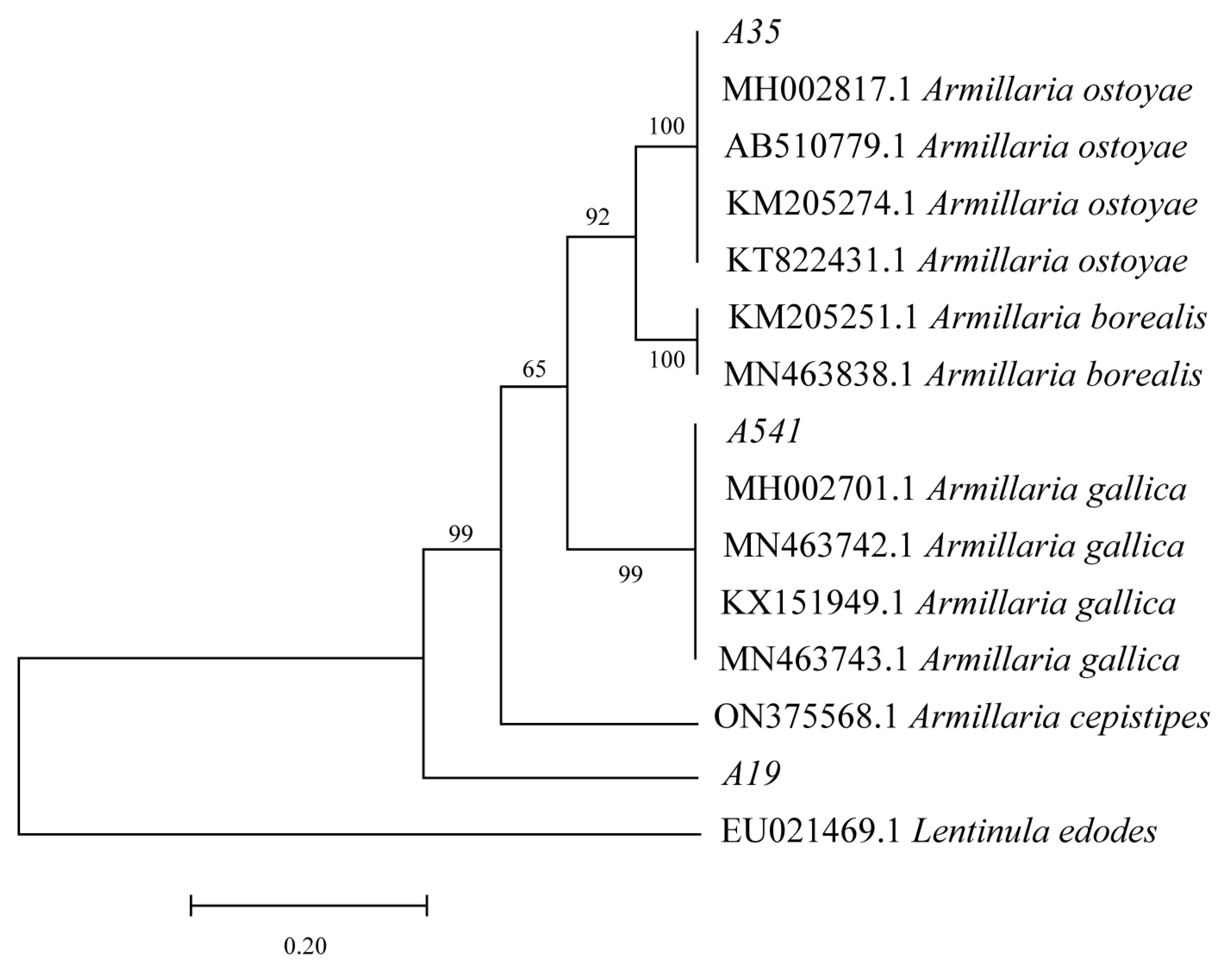
After 15 days of cultivation on the PDA medium, A35, A541, and A19 exhibited distinct differences in growth patterns and rhizomorph characteristics (Figure 1a–c). A541 displayed the longest and most extensively branched fungal rhizomorphs, while the rhizomorphs of A35 were predominantly surrounded by hyphae. The DNA sequences of A35, A541, and A19 were analyzed using BLAST based on the NCBI database. Sequences with over 95% similarity to the queried sequences were downloaded for phylogenetic analysis. Non-comparable regions at both sequence ends were trimmed using MEGA 7.0 software, and the final aligned sequences were employed to construct a phylogenetic tree (Figure 2). The phylogenetic analysis of the TEF-1α sequences revealed that A35, A541, and A19 clustered into separate branches. A35 and A541 were grouped into the A. ostoyae and A. gallica clades, respectively, while A19 could not be clustered with other species and was phylogenetically distinct from the rest.

Figure 1.
Colony morphology of strains A35, A541, and A19 on PDA medium after 15 days, and P. umbellatus sclerotia (A35-P, A541-P, and A19-P) obtained after four years of co-cultivation: (a) A35 grown on PDA medium at room temperature in the dark for 2 weeks; (b) A541 grown on PDA medium under the same conditions; (c) A19 grown on PDA medium under identical conditions; (d) A35-P sclerotia excavated from a nest of P. umbellatus co-cultivated with A35 for four years; (e) A541-P sclerotia excavated from a nest co-cultivated with A541 for four years; (f) A19-P sclerotia excavated from a nest co-cultivated with A19 for four years; (g) Armillaria spawn; (h) P. umbellatus co-cultivated with Armillaria in soil; and (i) Armillaria infecting P. umbellatus.
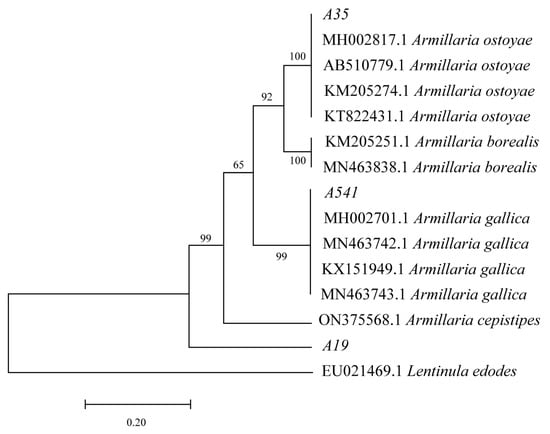
Figure 2.
Molecular phylogenetic analysis was performed using the maximum likelihood method based on TEF-1α sequences. Bootstrap support values (from 1000 replicates) are displayed above the branches, representing the confidence level for each branching node.
3.2. Significant Differences in Yield of P. umbellatus Sclerotia After Co-Cultivation with A35, A541, and A19
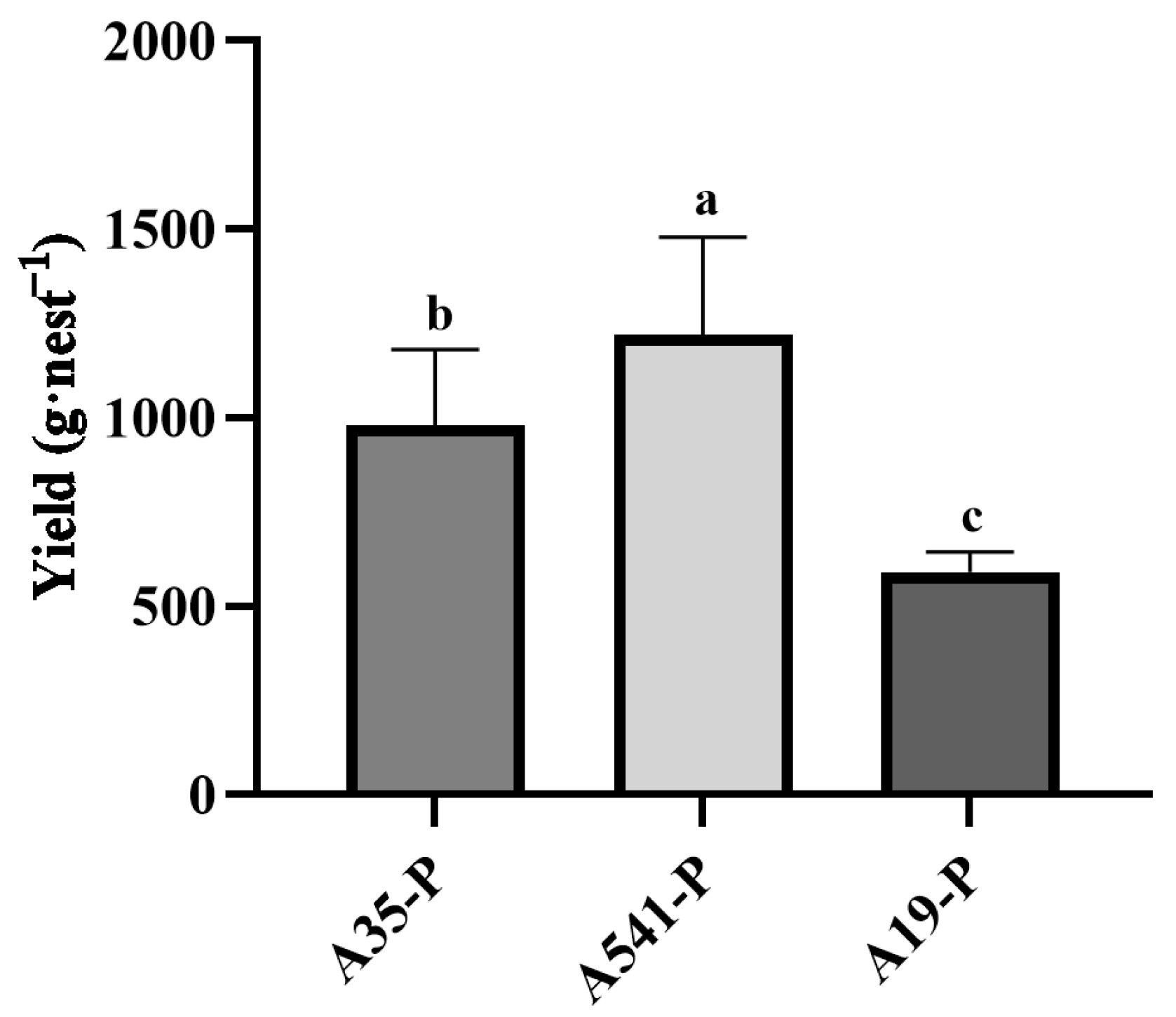
A35, A541, and A19 were all capable of forming a symbiotic relationship with the sclerotia of P. umbellatus. By the fourth year of cultivation, new sclerotia had successfully germinated and developed into mature medicinal materials (Figure 1d–f). Upon co-cultivation with A35, the yield of fresh sclerotia reached (979 ± 201) g·nest−1 (n = 12), starting from an initial seedling weight of 250 g. Co-cultivation with A541 resulted in a yield of (1221 ± 258) g·nest−1 (n = 15), while co-cultivation with A19 produced (591 ± 54) g·nest−1 (n = 8). Variance analysis (ANOVA) revealed significant differences in the yields of P. umbellatus sclerotia among the groups cultivated with A35, A541, and A19 (Figure 3).
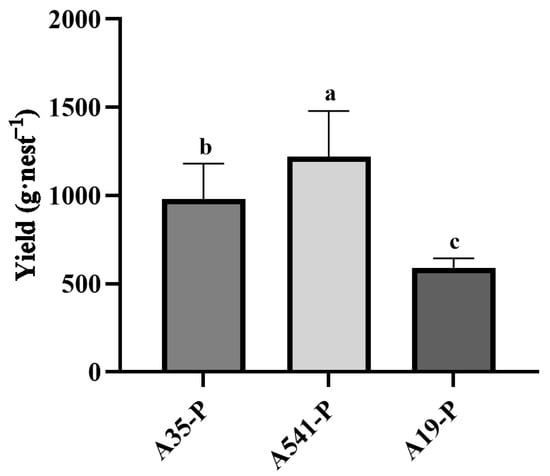
Figure 3.
Statistical analysis differences in yield (g·nest−1) of A35-P, A541-P, and A19-P. Different lowercase letters show significant differences at the p < 0.05 level.
3.3. Validation of Standard Curves and HPLC Quantification Methods
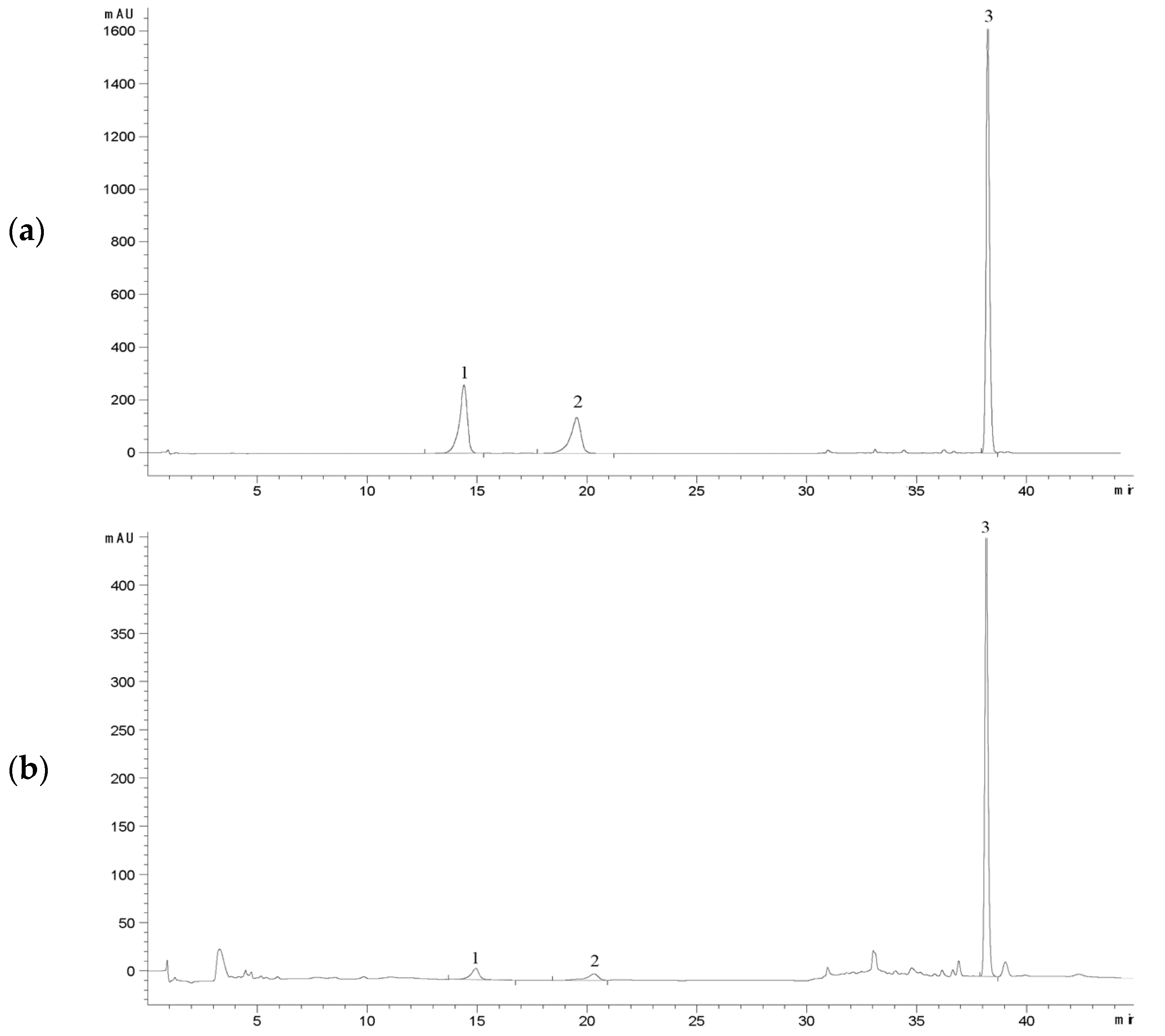
A HPLC method was established for the quantification of ergosterol, polyporusterone A, and polyporusterone B. Validation of the method confirmed that its precision and accuracy met the requirements of the study. The chromatographic peaks for the three target compounds were sharp and symmetrical, with clear separation from one another and no interference from impurities. These attributes were consistent across both the reference standards and all tested samples. The retention times of the components in the test solution closely matched those of the mixed-reference standards (Figure 4).
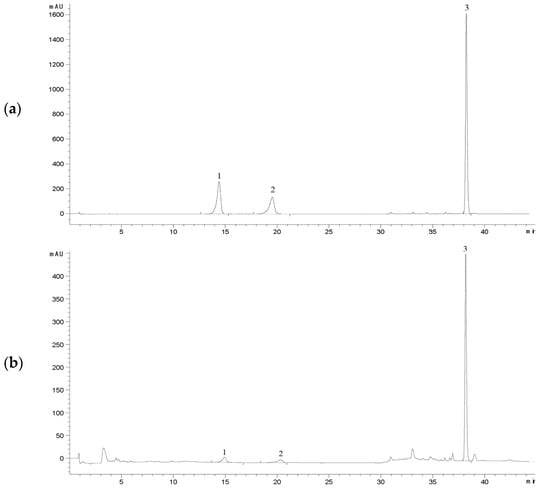
Figure 4.
HPLC chromatograms for the determination of ergosterol, polyporusterone A, and polyporusterone B contents in P. umbellatus samples. (a) Chromatogram of the reference substances: polyporusterone B (1), polyporusterone A (2), and ergosterol (3); (b) chromatogram of substances 1–3 detected in P. umbellatus samples. The detection wavelength was set to 247 nm for the first 25 min and switched to 283 nm after 25 min.
The RSD values for the peak areas of ergosterol, polyporusterone A, and polyporusterone B were 0.62%, 1.10%, and 0.65%, respectively, for six consecutive injections of the sample solution, indicating that the instrument exhibited good precision.
Linear regression analysis was conducted by plotting the concentration of the reference solution on the horizontal axis (X-axis) and the peak area on the vertical axis (Y-axis). The results demonstrated strong linear relationships for all three reference substances within the experimental concentration ranges. The limits of detection (LOD) and limits of quantification (LOQ) for the three components are presented in Table 1.

Table 1.
Linearity, limit of detection, and limit of quantitation of the three types of reference substance.
The RSD values for the peak areas of ergosterol, polyporusterone A, and polyporusterone B were 1.49%, 1.95%, and 0.55%, respectively, indicating that the test solution exhibited good stability within 24 h. Additionally, the RSD values for these compounds, prepared using the same method, were 0.80%, 1.10%, and 1.60%, respectively, demonstrating good reproducibility. The average recoveries of ergosterol, polyporusterone A, and polyporusterone B were 102.74%, 97.90%, and 101.12%, with corresponding RSD values of 1.53%, 4.78%, and 1.59%, confirming the method’s accuracy.
3.4. The Ergosterol, Polyporusterone A, and Polyporusterone B Percentage of Contents of P. umbellatus
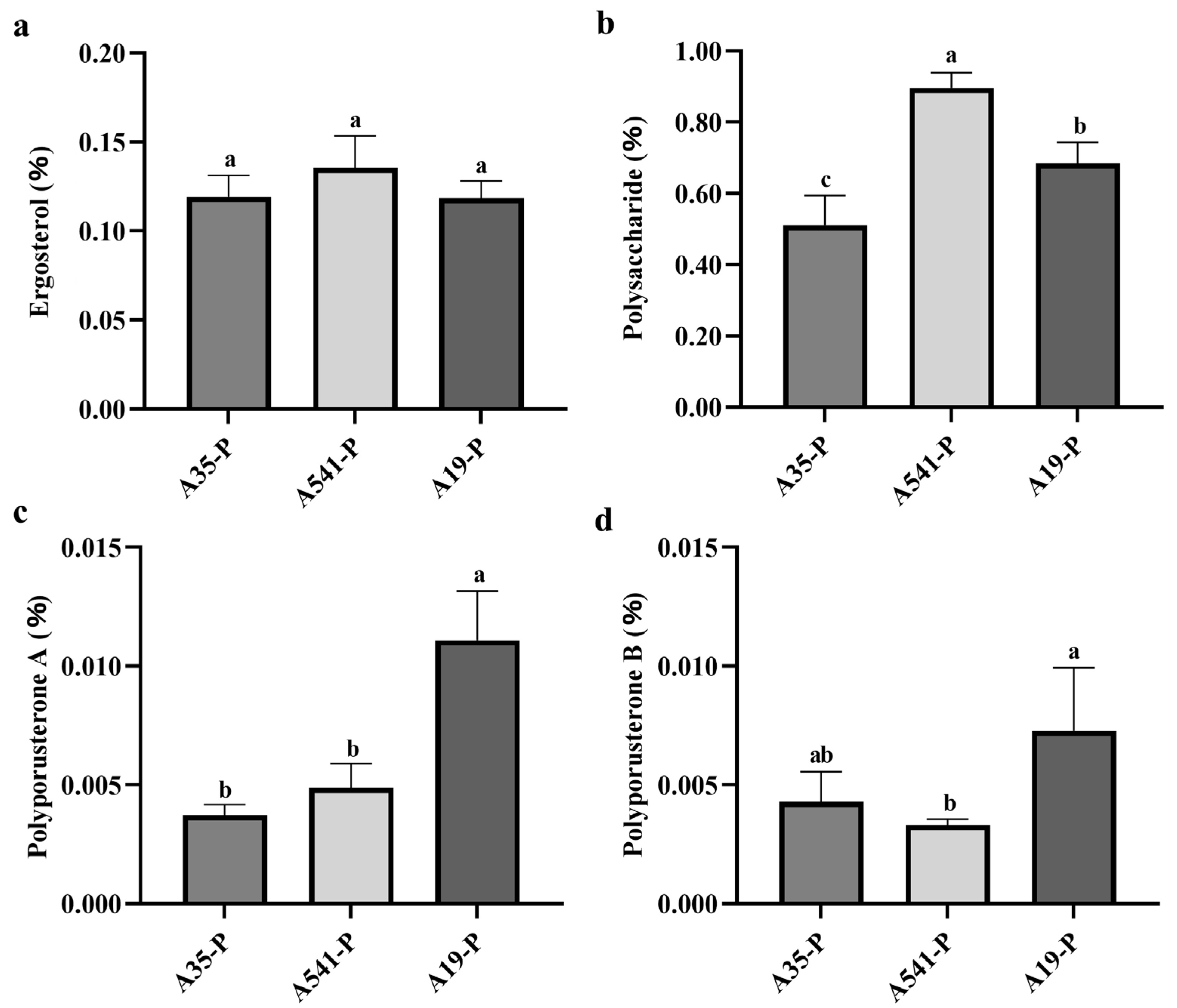
The ergosterol, polyporusterone A, and polyporusterone B contents in the samples were calculated based on the standard curve. The ergosterol content in A35-P, A541-P, and A19-P was 0.119 ± 0.012%, 0.136 ± 0.018%, and 0.118 ± 0.010%, respectively (Figure 5a). These values exceeded the threshold specified in the ChP, indicating that the cultivated sclerotia met the medicinal quality standards. Variance analysis showed differences in the ergosterol content among A35-P, A541-P, and A19-P, but these differences were not statistically significant (Figure 5a). The polyporusterone A content was 0.004 ± 0.0004%, 0.005 ± 0.001%, and 0.011 ± 0.002% in A35-P, A541-P, and A19-P, respectively (Figure 5c). The polyporusterone B content was measured at 0.004 ± 0.001%, 0.003 ± 0.0003%, and 0.007 ± 0.003% in A35-P, A541-P, and A19-P, respectively (Figure 5d). There was no significant difference in polyporusterone A content between A35-P and A541-P, but both were significantly lower than the content in A19-P. Similarly, the polyporusterone B content was highest in A19-P, with a statistically significant difference compared to A541-P, which exhibited the lowest value. The polyporusterone B content in A35-P did not significantly differ from either A19-P or A541-P.
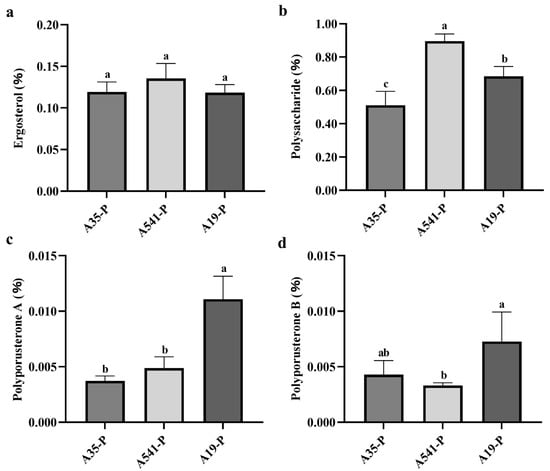
Figure 5.
Statistical analysis of ergosterol, polysaccharide, polyporusterone A, and polyporusterone B contents in A35-P, A541-P, and A19-P. (a) Ergosterol content (%) in A35-P, A541-P, and A19-P; (b) polysaccharide content (%) in A35-P, A541-P, and A19-P; (c) polyporusterone A content (%) in A35-P, A541-P, and A19-P; and (d) polyporusterone B content (%) in A35-P, A541-P, and A19-P. Different lowercase letters indicate significant differences at the p < 0.05 level.
3.5. Total Polysaccharide Contents of P. umbellatus
The total polysaccharide content of P. umbellatus was calculated based on the standard curve y = 4.2455x + 0.0323y = 4.2455x + 0.0323y = 4.2455x + 0.0323 (R2 = 0.9997). The polysaccharide contents were determined to be 0.511 ± 0.083% for A35-P, 0.897 ± 0.042% for A541-P, and 0.686 ± 0.058% for A19-P (Figure 5b). Variance analysis indicated significant differences in the polysaccharide content among the three groups.
3.6. PCA Analysis of P. umbellatus
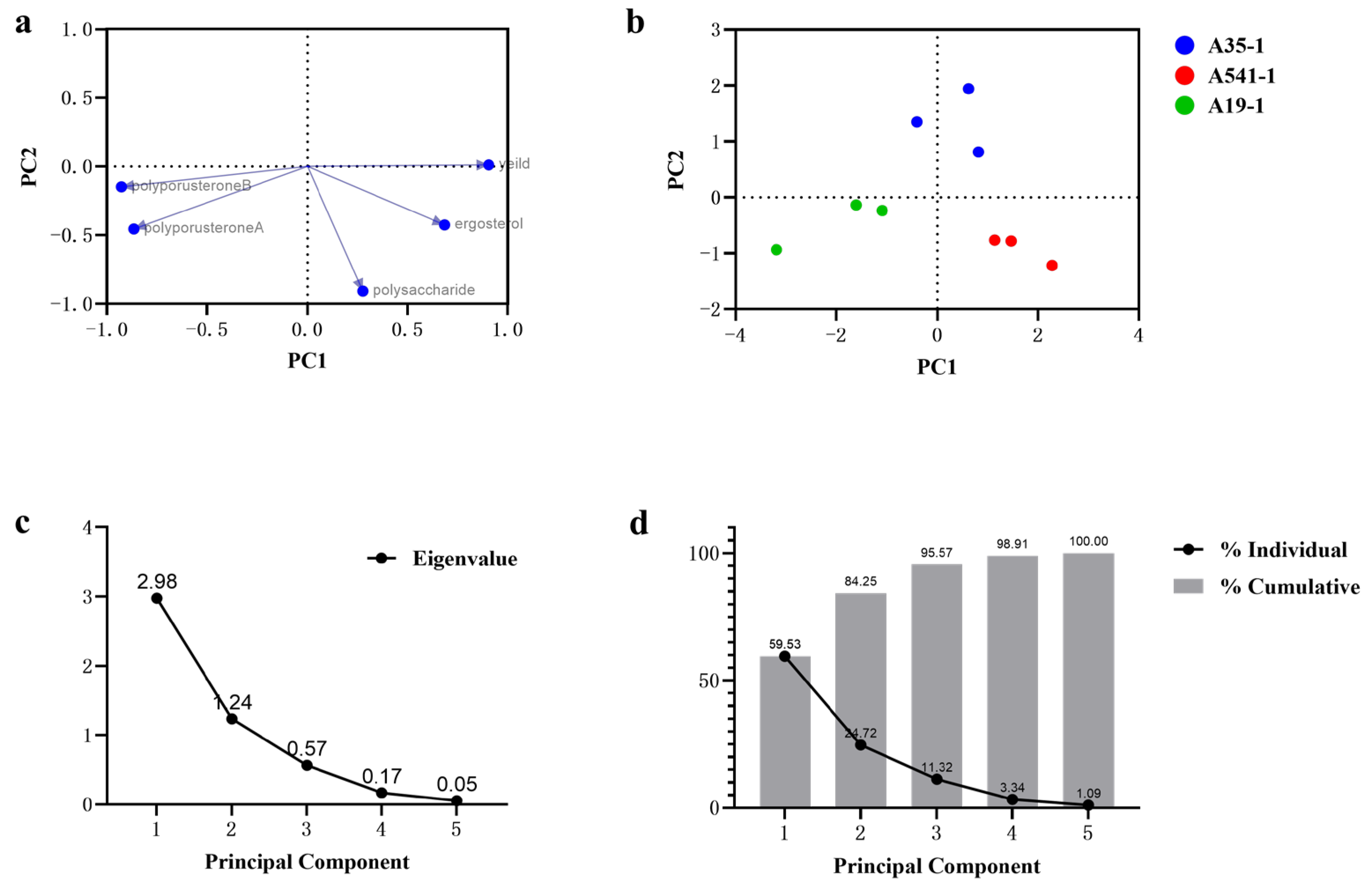
Principal component analysis (PCA) is widely used for distinguishing and discriminating among sample groups or species [28]. PCA was employed to analyze the relationships and differences in yield, ergosterol, polyporusterone A, polyporusterone B, and polysaccharide contents among A35, A541, and A19. The PCA results separated the yield and active compound contents into two primary components, PC1 and PC2. PC1 explained 59.53% of the total variation, while the cumulative variance of PC1 and PC2 accounted for 84.25% of the total variance (Figure 6d). The eigenvectors (principal component vectors) represent specific linear combinations of the variables. From the scree plot (Figure 6c), the eigenvalues for PC1 and PC2 were greater than 1, and the trend stabilized after PC3. Therefore, PC1 and PC2 were selected for the PCA analysis. The loading plots showed that the yield, ergosterol, polyporusterone A, and polyporusterone B contents were strongly correlated with PC1 (values near 1 or −1), while these variables were less correlated with PC2. In contrast, the polysaccharide content showed a stronger correlation with PC2 (Figure 6a). The PC score plots illustrated the clustering of data points into three distinct groups corresponding to A35, A541, and A19, respectively, based on the first two principal components (Figure 6b).
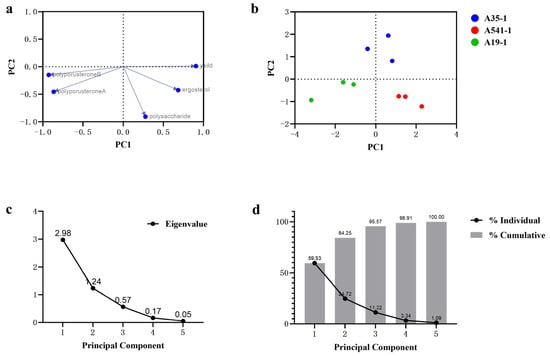
Figure 6.
PCA of A35-P, A541-P, and A19-P samples based on yield, ergosterol, polyporusterone A, polyporusterone B, and polysaccharide contents. (a) The loading plot depicts the grouping of variables using the first two principal components. Yield, ergosterol, polyporusterone A, and polyporusterone B contents showed strong correlations (values close to 1 or −1) with PC1, while polysaccharide content was primarily correlated with PC2. (b) The PC score plot displays the original eigenvalues of each principal component, illustrating clustering patterns for the three sample groups. (c) The scree plot indicates that the eigenvalues of PC1 and PC2 were greater than 1, with the variance trend stabilizing after PC3. (d) The proportion of variance plot reveals that the first (PC1) accounted for 59.53% of the total variation, while the cumulative variance of PC1 and PC2 explained 84.25% of the total variation.
4. Discussion
In recent years, there has been a growing number of reports on Armillaria worldwide, with identification methods advancing from traditional morphological approaches to molecular biological techniques [29,30]. The tef-1α gene encodes a partial protein called translation elongation factor-1α and is noted for its high sequence polymorphism in closely related species, making it a preferred marker for distinguishing fungal species [31]. Our previous research demonstrated that the TEF-1α sequences of Armillaria strains from different sources showed consistent performance, indicating the stability of these sequences across various sources. In contrast, sequence amplification for ITS, IGS, β-tubulin, and LSU exhibited variability among strains from different origins [32]. This stability suggests that TEF-1α sequences are more conserved during the evolution of Armillaria, which may explain their current utility in providing high discrimination among species in molecular systematics research. In this study, TEF-1α sequences were employed to identify and analyze three species of Armillaria isolated from wild P. umbellatus. The results revealed that strain A35 clustered with A. ostoyae, while A541 clustered with A. gallica. However, A19 formed a separate cluster distinct from several Armillaria species, including A. ostoyae and A. gallica, suggesting that A19, A35, and A541 are distinct species. This finding aligns with Liu et al.’s report that P. umbellatus exhibits no selective specialization for symbiotic Armillaria species [19,33]. According to Liu et al., four species—A. cepistipes, A. gallica, A. mellea, and A. ostoyae—have been reported to form symbiotic relationships with P. umbellatus. Consistent with this, the A35 and A541 strains identified in this study clustered with A. ostoyae and A. gallica, respectively. However, the TEF-1α sequence analysis positioned A19 in a distinct taxonomic cluster. This discrepancy may be attributed to sequencing accuracy or limitations in current identification techniques. Further investigation combining advanced methods will be needed to clarify the taxonomic status of A19.
Similar to the symbiotic relationship between Armillaria and P. umbellatus, the growth of the orchid plant Gastrodia elata (“tianma” in Chinese) also depends on Armillaria. Interestingly, the Armillaria species used in the cultivation of G. elata and P. umbellatus sometimes belong to the same species [34]. Previous studies have reported that the yield of G. elata cultivated with Armillaria strain M1 was 2.04 times higher than that achieved with strain M2 [35]. Similarly, another study demonstrated that the gastrodin content in G. elata varied by as much as 1.46 times depending on the Armillaria species used [36]. These findings suggest that the distinct characteristics of different Armillaria species may result in variations in yield and the contents of active compounds in P. umbellatus after co-cultivation. This hypothesis is consistent with our study’s results, which revealed differences in the yield and levels of ergosterol, polyporusterone A, polyporusterone B, and polysaccharides among samples co-cultivated with A35, A541, and A19. All three strains increased the yield of P. umbellatus, with A541-P showing the most pronounced effect. This variation could be attributed to the biological characteristics of the three Armillaria strains, including the rhizomorph thickness and branching, growth rate, and parasitism capacity [37]. Morphologically, under identical medium and culture conditions, A541 exhibited faster growth and more extensive branching compared to the other two strains. The rhizomorphs of A35 were thicker and more inclined to produce mycelium, while A19 displayed slower growth and less branching. These observations suggest that well-branched and thick rhizomorphs may be advantageous for cultivation and production. However, the limited number of Armillaria species studied here raises the question of whether other symbiotic Armillaria species, either from the same or different taxa, exhibit similar culture characteristics and consistent effects on the P. umbellatus yield. Further studies are needed to explore this possibility.
The ergosterol contents of A35-P, A541-P, and A19-P all met the standards specified in the ChP (2020), with no significant differences observed among the three groups. Ergosterol, a crucial component of fungal cell membranes, serves as a marker of fungal biomass [38] and indirectly influences fungal cell wall synthesis [39]. This suggests that differences in yield among A35-P, A541-P, and A19-P may also be related to variations in sclerotial density. In this study, the contents of polyporusterone A and polyporusterone B were significantly higher in A19-P than in A35-P and A541-P. Additionally, the accumulation of ergosterol, polyporusterone A, and polyporusterone B in A35-P, A541-P, and A19-P showed inconsistencies. These variations may be attributed to the conversion mechanism of ergosterol to polyporusterone A in P. umbellatus. Ergosterol can undergo hydroxylation to form a sixth hydroxylated product, which is subsequently oxidized to an α,β-unsaturated bond and transformed into a 2,14-dihydroxylated product via oxidative enzymes. This intermediate product, catalyzed by oxidative enzymes, forms a new unstable transition product with three oxygen bridges. This product is then converted into polyporusterone A with the participation of H+ ions [40]. The polysaccharides of P. umbellatus exhibit various biological functions, including anticancer activities, immune enhancement, and liver protection [14,41,42]. In this study, significant differences in polysaccharide contents were observed among A35-P, A541-P, and A19-P, with A541-P exhibiting the highest content. Although no official content threshold for polysaccharides in P. umbellatus exists, Lu proposed that the total polysaccharide content should not be less than 5% after analyzing 26 P. umbellatus samples [43]. All three groups in this study met the recommended value. The differing levels of polysaccharide accumulation among A35-P, A541-P, and A19-P may be related to variations in the activity of metabolic pathways in P. umbellatus, including polysaccharide synthesis, energy storage, and the expression levels of genes associated with polysaccharide metabolism [44]. Future studies should focus on further purification and characterization of the monosaccharide composition and sugar chain structure in these polysaccharides, as the monosaccharide composition plays a critical role in determining polysaccharide biological activity [45].
Our research demonstrated that the three symbiotic Armillaria strains had distinct effects on the yield and active compound contents of P. umbellatus. Among them, A541-P showed significant advantages in yield, ergosterol content, and polysaccharides, which are key indicators for assessing the yield and quality of P. umbellatus compared to A19-P and A35-P. Phylogenetic analysis revealed that A541 clustered with A. gallica, suggesting that A. gallica may hold significant potential for co-cultivation with P. umbellatus. Another study showed that 22 Armillaria strains were isolated from the sclerotium of P. umbellatus, of which 10 were A. gallica, also indicating that A. gallica might be a dominant species involved in the symbiosis of P. umbellatus [33]. Future studies could explore additional Armillaria species to validate these findings further or investigate whether Armillaria strains of the same species but from different sources affect the yield and quality of P. umbellatus. Similarly, differences in the species, origins, and phylogeny of P. umbellatus may also influence its yield and quality. However, an evolutionary classification of P. umbellatus variants or subspecies is currently lacking. Based on morphological differences, zhushiling and jishiling are generally recognized as the primary types of P. umbellatus [46]. This study focused on zhushiling, the most widely cultivated and medicinally used form of P. umbellatus. Further research is needed to examine how other P. umbellatus phylogenies affect its interaction with Armillaria. These findings have practical implications for selecting suitable Armillaria strains and achieving high-quality and abundant P. umbellatus in production. It is also noteworthy that traditional Chinese medicine (TCM) consists of numerous chemical components, with clinical efficacy resulting from their synergistic effects. A comprehensive evaluation of TCM using multiple indicators is therefore essential. In this study, we assessed the overall quality differences of P. umbellatus co-cultivated with different Armillaria species through PCA. The results highlighted that in addition to ergosterol, polyporusterone A, polyporusterone B, and polysaccharides are critical for the quality control of P. umbellatus.
5. Conclusions
This study is the first to demonstrate significant differences in the effects of three distinct symbiotic Armillaria strains on the yield, steroid content, and polysaccharide content of co-cultivated P. umbellatus sclerotia. These variations are likely attributable to the genetic and morphological differences among the Armillaria strains. Among the strains tested, A541, which is classified as A. gallica, showed the greatest potential for improving both the yield and quality of co-cultivated P. umbellatus sclerotia compared to A19 and A35. These findings provide a valuable reference for selecting symbiotic Armillaria strains in the artificial cultivation of high-quality and high-yield P. umbellatus.
Author Contributions
Conceptualization, B.L. and S.G.; data curation, L.L. and L.Z.; formal analysis, L.L. and S.L.; funding acquisition, B.L. and S.G.; methodology, L.L. and Y.X.; project administration, S.G.; resources, L.L. and Y.X.; writing—original draft, L.L.; writing—review and editing, S.L. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the CAMS Innovation Fund for medical Sciences, grant numbers 2021-I2M-1-031, 2022-I2M-2-001, and 2023-I2M-2-006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript and its additional files. The sequencing original data presented in the study are openly available in SUB2866618.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, W.J.; Ren, H.; Cui, X.; Hu, J.; Qu, T.; Li, N.; Chen, Z. Research progress on effective components, pharmacological mechanisms and clinical use of Polyporus umbellatus in diuresis-promotion and dampness-clearance. China Pharm. 2023, 24, 1399–1403. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Xie, R.M.; Chao, X.; Zhang, Y.; Lin, R.C.; Sun, W.J. Bioactivity-directed isolation, identification of diuretic compounds from Polyporus umbellatus. J. Ethnopharmacol. 2009, 126, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Yan, Z.; Xiong, Q.P.; Chen, X.L.; Lin, Y.T.; Xu, Y.; Bai, L.; Jiang, W.; Zheng, D.H.; Xing, C.Y. Renoprotective effect and mechanism of polysaccharide from Polyporus umbellatus sclerotia on renal fibrosis. Carbohyd. Polym. 2019, 212, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Han, T.; Liu, C.Y.; Ge, C.; Jiang, X.; Liu, Y.P.; Kong, F.; Su, X.Y.; Shi, J.C.; Su, W.T.; et al. Polyporus umbellatus polysaccharide iron-based nanocomposite for synergistic M1 polarization of TAMs and combinational anti-breast cancer therapy. Int. J. Biol. Macromol. 2023, 251, 126323. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, X. Purification, initial characterization and immune activities of polysaccharides from the fungus, Polyporus umbellatus. Food. Sci. Hum. Well. 2014, 3, 73–78. [Google Scholar] [CrossRef]
- He, D.; Ren, Y.; Hua, X.; Zhang, J.; Zhang, B.; Dong, J.; Efferth, T.; Ma, P. Phytochemistry and bioactivities of the main constituents of Polyporus umbellatus (Pers.) Fries. Phytomedicine 2022, 103, 154196. [Google Scholar] [CrossRef]
- Bandara, A.R.; Rapior, S.; Bhat, D.J. Polyporus umbellatus, an edible-medicinal cultivated mushroom with multiple developed health-care products as food, medicine and cosmetics: A review. Cryptogam. Mycol. 2015, 36, 3–42. [Google Scholar] [CrossRef]
- Zhou, W.W.; Guo, S.X. Components of the sclerotia of Polyporus umbellatus. Chem. Nat. Compd. 2009, 45, 124–125. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Qin, X.Y.; Zhang, Y.; Lin, R.C.; Sun, W.J.; Li, X.Y. Quantitative HPLC method and pharmacokinetic studies of ergosta-4,6,8(14),22-tetraen-3-one, a natural product with diuretic activity from Polyporus umbellatus (Pers.) Fries. Biomed. Chromatogr. 2010, 24, 1120–1124. [Google Scholar] [CrossRef]
- Ohsawa, T.; Yukawa, M.; Takao, C.; Murayama, M.; Bando, H. Studies on constituents of fruit body of Polyporus umbellatus and their cytotoxic activity. Chem. Pharm. Bull. 1992, 40, 143–147. [Google Scholar] [CrossRef]
- Sun, Y.; Yasukawa, K. New anti-inflammatory ergostane-type ecdysteroids from the sclerotium of Polyporus umbellatus. Bioorg. Med. Chem. Lett. 2008, 18, 3417–3420. [Google Scholar] [CrossRef]
- Ishida, H.; Inaoka, Y.; Nozawa, A.; Shibatani, J.I.; Fukushima, M.; Tsuji, K. Studies of active substances in herbs used for hair treatment. IV. The structure of the hair regrowth substance, polyporusterone A, from Polyporus umbellatus (Pers.) Fries. Chem. Pharm. Bull. 1999, 47, 1626–1628. [Google Scholar] [CrossRef]
- Guo, Z.; Zang, Y.; Zhang, L. The efficacy of Polyporus umbellatus polysaccharide in treating hepatitis B in China. Prog. Mol. Biol. Transl. 2019, 163, 329–360. [Google Scholar] [CrossRef]
- Liu, C.P.; Li, X.; Lai, G.N.; Li, J.H.; Jia, W.Y.; Cao, Y.Y.; Xu, W.X.; Tan, Q.L.; Zhou, C.Y.; Luo, M.; et al. Mechanisms of macrophage immunomodulatory activity induced by a new polysaccharide isolated from Polyporus umbellatus (Pers.) Fries. Front. Chem. 2020, 8, 581. [Google Scholar] [CrossRef]
- Chen, Z.P.; Zhang, X.; Huang, L.F.; Li, X.; Xu, Z.; Zhu, G.C.; Long, H.Z. Treatment of Idiopathic normal-pressure hydrocephalus by Wuling San combined with conventional western medicine: A randomized, double-blind, placebo-controlled trial. Shanghai J. Tradit. Chin. Med. 2022, 50, 44–47. [Google Scholar] [CrossRef]
- Li, B.; Liu, L.; Zhang, D.; Guo, S. Hallmarks of comparative transcriptome between rhizomorphs and hyphae of Armillaria sp. 541 participating in fungal symbiosis with emphasis on LysM domains. Microorganisms 2023, 11, 1914. [Google Scholar] [CrossRef]
- Guo, S.X.; Xu, J.T. Nutrient source of sclerotia of Grifola umbellata and its relationship to Armillaria mellea. Acta Bot. Sin. 1992, 34, 576–580. [Google Scholar]
- Guo, S.X.; Cao, W.Q.; Wang, Q.Y.; Wang, C.L. Studies on the content of sugar in different parts of Grifola umbellata sclerotium in the courses of symbiosis with Armillaria mellea. Chin. Pharm. J. 2002, 37, 493–495. [Google Scholar] [CrossRef]
- Liu, M.M.; Xing, Y.M.; Zeng, X.; Zhang, D.W.; Guo, S.X. Genetic diversity of Armillaria spp. symbiotic with Polyporus umbellatus in China. Biochem. Syst. Ecol. 2015, 61, 524–530. [Google Scholar] [CrossRef]
- Ren, S.Z.; Gao, Y.P.; Li, H.; Ma, H.H.; Han, X.L.; Yang, Z.T.; Chen, W.J. Research status and application prospects of the medicinal mushroom Armillaria mellea. Appl. Biochem. Biotechnol. 2023, 195, 3491–3507. [Google Scholar] [CrossRef]
- Wang, L.E.; Li, L.J.; Ma, J.; Yin, S.X. The current status of cultivation techniques and industrial development strategies for Polyporus umbellatus. Edible Fungi 2008, 4, 002. [Google Scholar] [CrossRef]
- Liang, J.; Pecoraro, L.; Cai, L.; Yuan, Z.; Zhao, P.; Tsui, C.K.M.; Zhang, Z. Phylogenetic Relationships, Speciation, and Origin of Armillaria in the Northern Hemisphere: A Lesson Based on rRNA and Elongation Factor 1-Alpha. J. Fungi. 2021, 7, 1088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.X.; Wang, Q.Y.; Zhang, J.H.; Xia, H.Y. Studies on the cultivation method of sclerotium forming from hyphae of grifola umbellata. Chin. Pharm. J. 2001, 36, 658–660. [Google Scholar] [CrossRef]
- Zhou, W.W. Study on Chemical Composition and Quality Analysis of Polyporus umbellatus Sclerotinia and Fermented Myce-lium. Ph.D. Thesis, China Union Medical University, Beijing, China, 2008. [Google Scholar]
- Chen, X.M.; Zhou, W.W.; Wang, C.L.; Yang, J.S.; Guo, S.X. Sterone content determination and fingerprint analysis of Polyporus umbellatus sclerotia using high performance liquid chromatography (HPLC). Mycosystema 2017, 36, 83–97. [Google Scholar] [CrossRef]
- Yang, M.S.; Yin, Y.F.; Chen, J.; Li, B.; Hou, M.Y.; Leng, C.Y.; Xing, Y.M.; Guo, S.X. Study on accumulation of polysaccharide and steroid components in Polyporus umbellatus infected by Armillaria spp. Acta Pharm. Sin. 2025, 60, 232–238. [Google Scholar] [CrossRef]
- Karamizadeh, S.; Abdullah, S.M.; Manaf, A.A.; Zamani, M.; Hooman, A. An overview of principal component analysis. J. Signal Inf. Process. 2013, 4, 173–175. [Google Scholar] [CrossRef]
- Kolesnikova, A.I.; Pavlov, I.N.; Litovka, Y.A.; Oreshkova, N.V.; Timofeev, A.A.; Litvinova, E.A.; Petrenko, S.M.; Krutovsky, K.V. Molecular identification of wood-decaying fungi of Armillaria genus widespread in Eastern Siberia and the Far East of Russia using ITS, IGS-1-1 and Tef-1α genetic markers. Mycol. Phytopathol. 2024, 58, 231–245. [Google Scholar] [CrossRef]
- Men, J.X.; Xing, X.K.; Guo, S.X. Biological species and identification methods of the genus Armillaria (Agaricales, Basidiomycota): A review. Mycosystema 2016, 35, 1281–1302. [Google Scholar] [CrossRef]
- Nouripour-Sisakht, S.; Ahmadi, B.; Makimura, K.; Hoog, S.D.; Umeda, Y.; Alshahni, M.M.; Mirhendi, H. Characterization of the translation elongation factor 1-α gene in a wide range of pathogenic Aspergillus species. J. Med. Microbiol. 2017, 66, 419–429. [Google Scholar] [CrossRef]
- Li, S.J.; Liu, Y.Y.; Xu, X.L.; Liu, L.; Li, B.; Guo, S.X. Characteristics and essence of heterozygosity of the DNA sequences amplified from Armillaria species. Mycosystema 2024, 43, 240058. [Google Scholar] [CrossRef]
- Liu, M.M.; Xing, Y.M.; Guo, S.X. Investigation of symbiotic Armillaria species with Chinese traditional medicinal fungus Polyporus umbellatus. J. Chin. Pharm. Sci. 2015, 50, 390–393. [Google Scholar] [CrossRef]
- Men, J.X.; Xing, X.K.; Guo, S.X. A comparison of Armillaria spp. associated with Polyporus umbellatus and Gastrodia elata in China. Mycosystema 2017, 8, 1072–1082. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, F.H.; Bian, Y.B. Effect of different strains of Armillariella mellea on the yield of Gastrodia elata. Acta Edulis Fungi 2004, 11, 46–48. [Google Scholar] [CrossRef]
- Sun, S.Q.; Chen, G.H. Effects on the yield of tall Gastrodia (Gastrodia elata) and the content of Gastrodin by different strains of Armillaria mellea. Shandong Sci. 2003, 2, 10–13. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Yuan, Q.; Guo, L.; Xiao, C.; Yang, C.; Li, L.; Jiang, W.; Zhou, T. Effect of symbiotic fungi-Armillaria gallica on the yield of Gastrodia elata Bl. and insight into the response of soil microbial community. Front. Microbiol. 2023, 14, 1233555. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; von Wachenfeldt, E.; Tranvik, L.J. Ergosterol as a measure of living fungal biomass: Persistence in environmental samples after fungal death. J. Microbiol. Meth. 2004, 59, 253–262. [Google Scholar] [CrossRef]
- Choy, H.L.; Gaylord, E.A.; Doering, T.L. Ergosterol distribution controls surface structure formation and fungal pathogenicity. mBio 2023, 14, e01353-23. [Google Scholar] [CrossRef]
- Xing, Y.M.; Li, B.; Liu, L.; Li, Y.; Yin, S.X.; Yin, S.C. Armillaria mellea symbiosis drives metabolomic and transcriptomic changes in Polyporus umbellatus sclerotia. Front. Microbiol. 2022, 12, 792530. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Mu, J.; Ho, C.T.; Su, J.; Zhang, Y.; Lin, X.; Chen, Z.; Li, B.; Xie, Y. Preparation, physicochemical characterization, and anti-proliferation of selenium nanoparticles stabilized by Polyporus umbellatus polysaccharide. Int. J. Biol. Macromol. 2020, 152, 605–615. [Google Scholar] [CrossRef]
- Peng, K.; Lan, L.S.; Yan, W.X.; Jie, S.L.; Wu, Y.J.; Hua, Z.Y.; Chen, L.S. Polyporus umbellatus polysaccharides ameliorates carbon tetrachloride-induced hepatic injury in mice. Afr. J. Pharm. Pharmacol. 2012, 6, 2686–2691. [Google Scholar]
- Lu, W.J.; Zhou, M.; Liang, Z.S. Quality evaluation standards and factors affecting quality of Polyporus umbellatus. Chin. J. Exp. Tradit. Med. Formulae 2013, 3, 154–196. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, H.; Wu, D.; Zhang, Z.; Zhang, J.; Bao, D.; Yang, Y. Key metabolism pathways and regulatory mechanisms of high polysaccharide yielding in Hericium erinaceus. BMC Genom. 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Q.; Nan, T.G.; Yuan, Y.; Jin, Y.; Yang, Q.; Zhang, M.; Hu, K.Y. Study on polysaccharide content and monosaccharide composition of Polyporus umbellatus from different production areas. China J. Chin. Mater. Medica 2019, 44, 3608–3614. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, S.J.; Li, B.; Guo, S.X. Comparative research progress of Zhushiling and Jishiling. Chin. Pharm. J. 2024, 59, 2199–2204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).