Different Symbiotic Species of Armillaria Affect the Yield and Active Compound Contents of Polyporus umbellatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture of Armillaria
2.2. DNA Extraction, PCR Amplification, and Sequencing of A35, A541, and A19
2.3. Nucleotide Alignment and Phylogenetic Analysis
2.4. Artificial Co-Cultivation Experiment of P. umbellatus
2.5. Sample Collection and Yield Comparisons of P. umbellatus Sclerotia
2.6. High-Performance Liquid Chromatography for Detecting Ergosterol, Polyporusterone A, and Polyporusterone B in P. umbellatus Sclerotia
2.7. Phenol-Sulfuric Acid Method for Detecting the Polysaccharides in P. umbellatus
2.8. Statistical Analysis
3. Results
3.1. A35, A541, and A19 Belong to Three Different Species of Armillaria
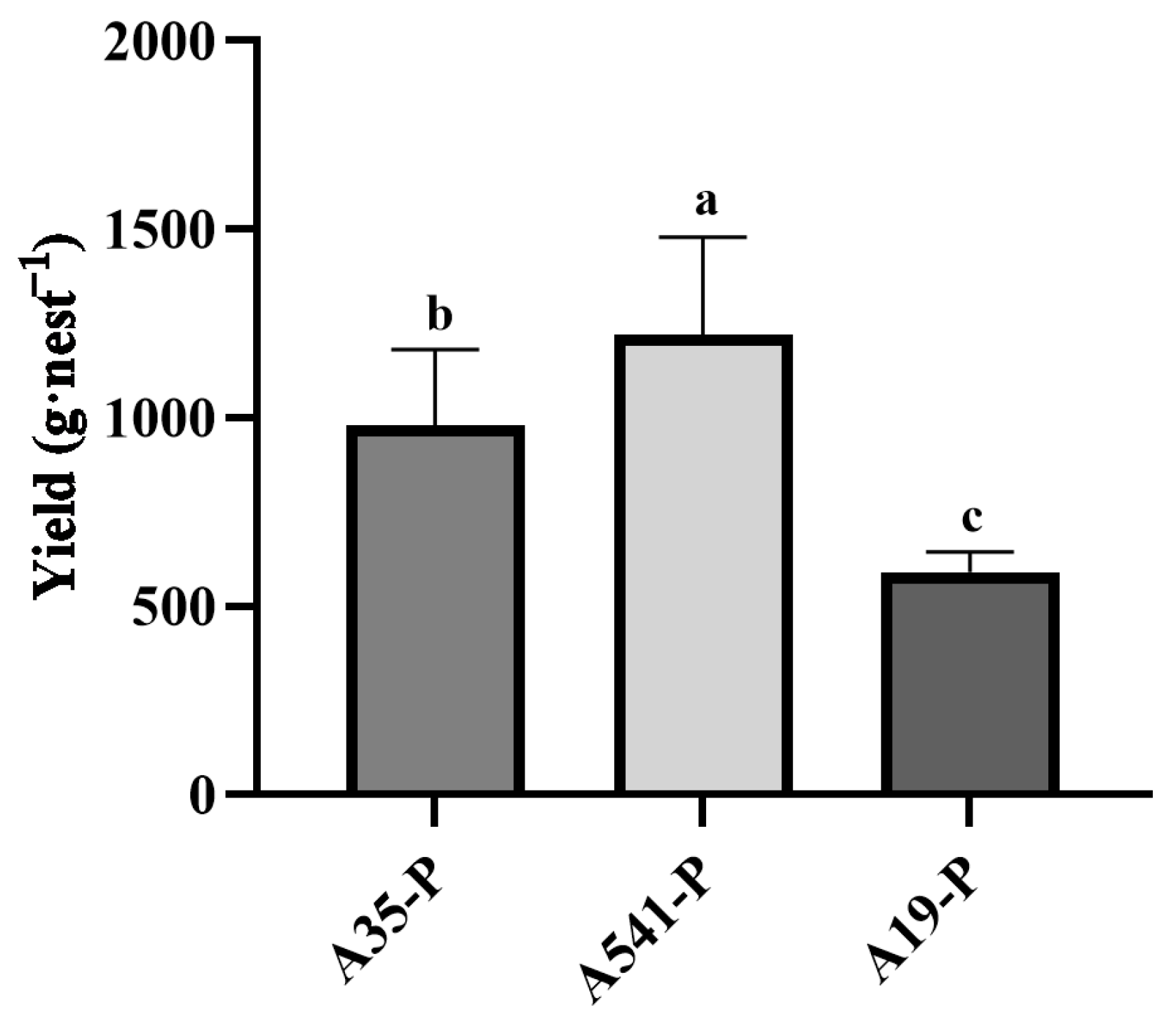
3.2. Significant Differences in Yield of P. umbellatus Sclerotia After Co-Cultivation with A35, A541, and A19
3.3. Validation of Standard Curves and HPLC Quantification Methods
3.4. The Ergosterol, Polyporusterone A, and Polyporusterone B Percentage of Contents of P. umbellatus
3.5. Total Polysaccharide Contents of P. umbellatus
3.6. PCA Analysis of P. umbellatus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, W.J.; Ren, H.; Cui, X.; Hu, J.; Qu, T.; Li, N.; Chen, Z. Research progress on effective components, pharmacological mechanisms and clinical use of Polyporus umbellatus in diuresis-promotion and dampness-clearance. China Pharm. 2023, 24, 1399–1403. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Xie, R.M.; Chao, X.; Zhang, Y.; Lin, R.C.; Sun, W.J. Bioactivity-directed isolation, identification of diuretic compounds from Polyporus umbellatus. J. Ethnopharmacol. 2009, 126, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Yan, Z.; Xiong, Q.P.; Chen, X.L.; Lin, Y.T.; Xu, Y.; Bai, L.; Jiang, W.; Zheng, D.H.; Xing, C.Y. Renoprotective effect and mechanism of polysaccharide from Polyporus umbellatus sclerotia on renal fibrosis. Carbohyd. Polym. 2019, 212, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Han, T.; Liu, C.Y.; Ge, C.; Jiang, X.; Liu, Y.P.; Kong, F.; Su, X.Y.; Shi, J.C.; Su, W.T.; et al. Polyporus umbellatus polysaccharide iron-based nanocomposite for synergistic M1 polarization of TAMs and combinational anti-breast cancer therapy. Int. J. Biol. Macromol. 2023, 251, 126323. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, X. Purification, initial characterization and immune activities of polysaccharides from the fungus, Polyporus umbellatus. Food. Sci. Hum. Well. 2014, 3, 73–78. [Google Scholar] [CrossRef]
- He, D.; Ren, Y.; Hua, X.; Zhang, J.; Zhang, B.; Dong, J.; Efferth, T.; Ma, P. Phytochemistry and bioactivities of the main constituents of Polyporus umbellatus (Pers.) Fries. Phytomedicine 2022, 103, 154196. [Google Scholar] [CrossRef]
- Bandara, A.R.; Rapior, S.; Bhat, D.J. Polyporus umbellatus, an edible-medicinal cultivated mushroom with multiple developed health-care products as food, medicine and cosmetics: A review. Cryptogam. Mycol. 2015, 36, 3–42. [Google Scholar] [CrossRef]
- Zhou, W.W.; Guo, S.X. Components of the sclerotia of Polyporus umbellatus. Chem. Nat. Compd. 2009, 45, 124–125. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Qin, X.Y.; Zhang, Y.; Lin, R.C.; Sun, W.J.; Li, X.Y. Quantitative HPLC method and pharmacokinetic studies of ergosta-4,6,8(14),22-tetraen-3-one, a natural product with diuretic activity from Polyporus umbellatus (Pers.) Fries. Biomed. Chromatogr. 2010, 24, 1120–1124. [Google Scholar] [CrossRef]
- Ohsawa, T.; Yukawa, M.; Takao, C.; Murayama, M.; Bando, H. Studies on constituents of fruit body of Polyporus umbellatus and their cytotoxic activity. Chem. Pharm. Bull. 1992, 40, 143–147. [Google Scholar] [CrossRef]
- Sun, Y.; Yasukawa, K. New anti-inflammatory ergostane-type ecdysteroids from the sclerotium of Polyporus umbellatus. Bioorg. Med. Chem. Lett. 2008, 18, 3417–3420. [Google Scholar] [CrossRef]
- Ishida, H.; Inaoka, Y.; Nozawa, A.; Shibatani, J.I.; Fukushima, M.; Tsuji, K. Studies of active substances in herbs used for hair treatment. IV. The structure of the hair regrowth substance, polyporusterone A, from Polyporus umbellatus (Pers.) Fries. Chem. Pharm. Bull. 1999, 47, 1626–1628. [Google Scholar] [CrossRef]
- Guo, Z.; Zang, Y.; Zhang, L. The efficacy of Polyporus umbellatus polysaccharide in treating hepatitis B in China. Prog. Mol. Biol. Transl. 2019, 163, 329–360. [Google Scholar] [CrossRef]
- Liu, C.P.; Li, X.; Lai, G.N.; Li, J.H.; Jia, W.Y.; Cao, Y.Y.; Xu, W.X.; Tan, Q.L.; Zhou, C.Y.; Luo, M.; et al. Mechanisms of macrophage immunomodulatory activity induced by a new polysaccharide isolated from Polyporus umbellatus (Pers.) Fries. Front. Chem. 2020, 8, 581. [Google Scholar] [CrossRef]
- Chen, Z.P.; Zhang, X.; Huang, L.F.; Li, X.; Xu, Z.; Zhu, G.C.; Long, H.Z. Treatment of Idiopathic normal-pressure hydrocephalus by Wuling San combined with conventional western medicine: A randomized, double-blind, placebo-controlled trial. Shanghai J. Tradit. Chin. Med. 2022, 50, 44–47. [Google Scholar] [CrossRef]
- Li, B.; Liu, L.; Zhang, D.; Guo, S. Hallmarks of comparative transcriptome between rhizomorphs and hyphae of Armillaria sp. 541 participating in fungal symbiosis with emphasis on LysM domains. Microorganisms 2023, 11, 1914. [Google Scholar] [CrossRef]
- Guo, S.X.; Xu, J.T. Nutrient source of sclerotia of Grifola umbellata and its relationship to Armillaria mellea. Acta Bot. Sin. 1992, 34, 576–580. [Google Scholar]
- Guo, S.X.; Cao, W.Q.; Wang, Q.Y.; Wang, C.L. Studies on the content of sugar in different parts of Grifola umbellata sclerotium in the courses of symbiosis with Armillaria mellea. Chin. Pharm. J. 2002, 37, 493–495. [Google Scholar] [CrossRef]
- Liu, M.M.; Xing, Y.M.; Zeng, X.; Zhang, D.W.; Guo, S.X. Genetic diversity of Armillaria spp. symbiotic with Polyporus umbellatus in China. Biochem. Syst. Ecol. 2015, 61, 524–530. [Google Scholar] [CrossRef]
- Ren, S.Z.; Gao, Y.P.; Li, H.; Ma, H.H.; Han, X.L.; Yang, Z.T.; Chen, W.J. Research status and application prospects of the medicinal mushroom Armillaria mellea. Appl. Biochem. Biotechnol. 2023, 195, 3491–3507. [Google Scholar] [CrossRef]
- Wang, L.E.; Li, L.J.; Ma, J.; Yin, S.X. The current status of cultivation techniques and industrial development strategies for Polyporus umbellatus. Edible Fungi 2008, 4, 002. [Google Scholar] [CrossRef]
- Liang, J.; Pecoraro, L.; Cai, L.; Yuan, Z.; Zhao, P.; Tsui, C.K.M.; Zhang, Z. Phylogenetic Relationships, Speciation, and Origin of Armillaria in the Northern Hemisphere: A Lesson Based on rRNA and Elongation Factor 1-Alpha. J. Fungi. 2021, 7, 1088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.X.; Wang, Q.Y.; Zhang, J.H.; Xia, H.Y. Studies on the cultivation method of sclerotium forming from hyphae of grifola umbellata. Chin. Pharm. J. 2001, 36, 658–660. [Google Scholar] [CrossRef]
- Zhou, W.W. Study on Chemical Composition and Quality Analysis of Polyporus umbellatus Sclerotinia and Fermented Myce-lium. Ph.D. Thesis, China Union Medical University, Beijing, China, 2008. [Google Scholar]
- Chen, X.M.; Zhou, W.W.; Wang, C.L.; Yang, J.S.; Guo, S.X. Sterone content determination and fingerprint analysis of Polyporus umbellatus sclerotia using high performance liquid chromatography (HPLC). Mycosystema 2017, 36, 83–97. [Google Scholar] [CrossRef]
- Yang, M.S.; Yin, Y.F.; Chen, J.; Li, B.; Hou, M.Y.; Leng, C.Y.; Xing, Y.M.; Guo, S.X. Study on accumulation of polysaccharide and steroid components in Polyporus umbellatus infected by Armillaria spp. Acta Pharm. Sin. 2025, 60, 232–238. [Google Scholar] [CrossRef]
- Karamizadeh, S.; Abdullah, S.M.; Manaf, A.A.; Zamani, M.; Hooman, A. An overview of principal component analysis. J. Signal Inf. Process. 2013, 4, 173–175. [Google Scholar] [CrossRef]
- Kolesnikova, A.I.; Pavlov, I.N.; Litovka, Y.A.; Oreshkova, N.V.; Timofeev, A.A.; Litvinova, E.A.; Petrenko, S.M.; Krutovsky, K.V. Molecular identification of wood-decaying fungi of Armillaria genus widespread in Eastern Siberia and the Far East of Russia using ITS, IGS-1-1 and Tef-1α genetic markers. Mycol. Phytopathol. 2024, 58, 231–245. [Google Scholar] [CrossRef]
- Men, J.X.; Xing, X.K.; Guo, S.X. Biological species and identification methods of the genus Armillaria (Agaricales, Basidiomycota): A review. Mycosystema 2016, 35, 1281–1302. [Google Scholar] [CrossRef]
- Nouripour-Sisakht, S.; Ahmadi, B.; Makimura, K.; Hoog, S.D.; Umeda, Y.; Alshahni, M.M.; Mirhendi, H. Characterization of the translation elongation factor 1-α gene in a wide range of pathogenic Aspergillus species. J. Med. Microbiol. 2017, 66, 419–429. [Google Scholar] [CrossRef]
- Li, S.J.; Liu, Y.Y.; Xu, X.L.; Liu, L.; Li, B.; Guo, S.X. Characteristics and essence of heterozygosity of the DNA sequences amplified from Armillaria species. Mycosystema 2024, 43, 240058. [Google Scholar] [CrossRef]
- Liu, M.M.; Xing, Y.M.; Guo, S.X. Investigation of symbiotic Armillaria species with Chinese traditional medicinal fungus Polyporus umbellatus. J. Chin. Pharm. Sci. 2015, 50, 390–393. [Google Scholar] [CrossRef]
- Men, J.X.; Xing, X.K.; Guo, S.X. A comparison of Armillaria spp. associated with Polyporus umbellatus and Gastrodia elata in China. Mycosystema 2017, 8, 1072–1082. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, F.H.; Bian, Y.B. Effect of different strains of Armillariella mellea on the yield of Gastrodia elata. Acta Edulis Fungi 2004, 11, 46–48. [Google Scholar] [CrossRef]
- Sun, S.Q.; Chen, G.H. Effects on the yield of tall Gastrodia (Gastrodia elata) and the content of Gastrodin by different strains of Armillaria mellea. Shandong Sci. 2003, 2, 10–13. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Yuan, Q.; Guo, L.; Xiao, C.; Yang, C.; Li, L.; Jiang, W.; Zhou, T. Effect of symbiotic fungi-Armillaria gallica on the yield of Gastrodia elata Bl. and insight into the response of soil microbial community. Front. Microbiol. 2023, 14, 1233555. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; von Wachenfeldt, E.; Tranvik, L.J. Ergosterol as a measure of living fungal biomass: Persistence in environmental samples after fungal death. J. Microbiol. Meth. 2004, 59, 253–262. [Google Scholar] [CrossRef]
- Choy, H.L.; Gaylord, E.A.; Doering, T.L. Ergosterol distribution controls surface structure formation and fungal pathogenicity. mBio 2023, 14, e01353-23. [Google Scholar] [CrossRef]
- Xing, Y.M.; Li, B.; Liu, L.; Li, Y.; Yin, S.X.; Yin, S.C. Armillaria mellea symbiosis drives metabolomic and transcriptomic changes in Polyporus umbellatus sclerotia. Front. Microbiol. 2022, 12, 792530. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Mu, J.; Ho, C.T.; Su, J.; Zhang, Y.; Lin, X.; Chen, Z.; Li, B.; Xie, Y. Preparation, physicochemical characterization, and anti-proliferation of selenium nanoparticles stabilized by Polyporus umbellatus polysaccharide. Int. J. Biol. Macromol. 2020, 152, 605–615. [Google Scholar] [CrossRef]
- Peng, K.; Lan, L.S.; Yan, W.X.; Jie, S.L.; Wu, Y.J.; Hua, Z.Y.; Chen, L.S. Polyporus umbellatus polysaccharides ameliorates carbon tetrachloride-induced hepatic injury in mice. Afr. J. Pharm. Pharmacol. 2012, 6, 2686–2691. [Google Scholar]
- Lu, W.J.; Zhou, M.; Liang, Z.S. Quality evaluation standards and factors affecting quality of Polyporus umbellatus. Chin. J. Exp. Tradit. Med. Formulae 2013, 3, 154–196. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, H.; Wu, D.; Zhang, Z.; Zhang, J.; Bao, D.; Yang, Y. Key metabolism pathways and regulatory mechanisms of high polysaccharide yielding in Hericium erinaceus. BMC Genom. 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Q.; Nan, T.G.; Yuan, Y.; Jin, Y.; Yang, Q.; Zhang, M.; Hu, K.Y. Study on polysaccharide content and monosaccharide composition of Polyporus umbellatus from different production areas. China J. Chin. Mater. Medica 2019, 44, 3608–3614. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, S.J.; Li, B.; Guo, S.X. Comparative research progress of Zhushiling and Jishiling. Chin. Pharm. J. 2024, 59, 2199–2204. [Google Scholar] [CrossRef]


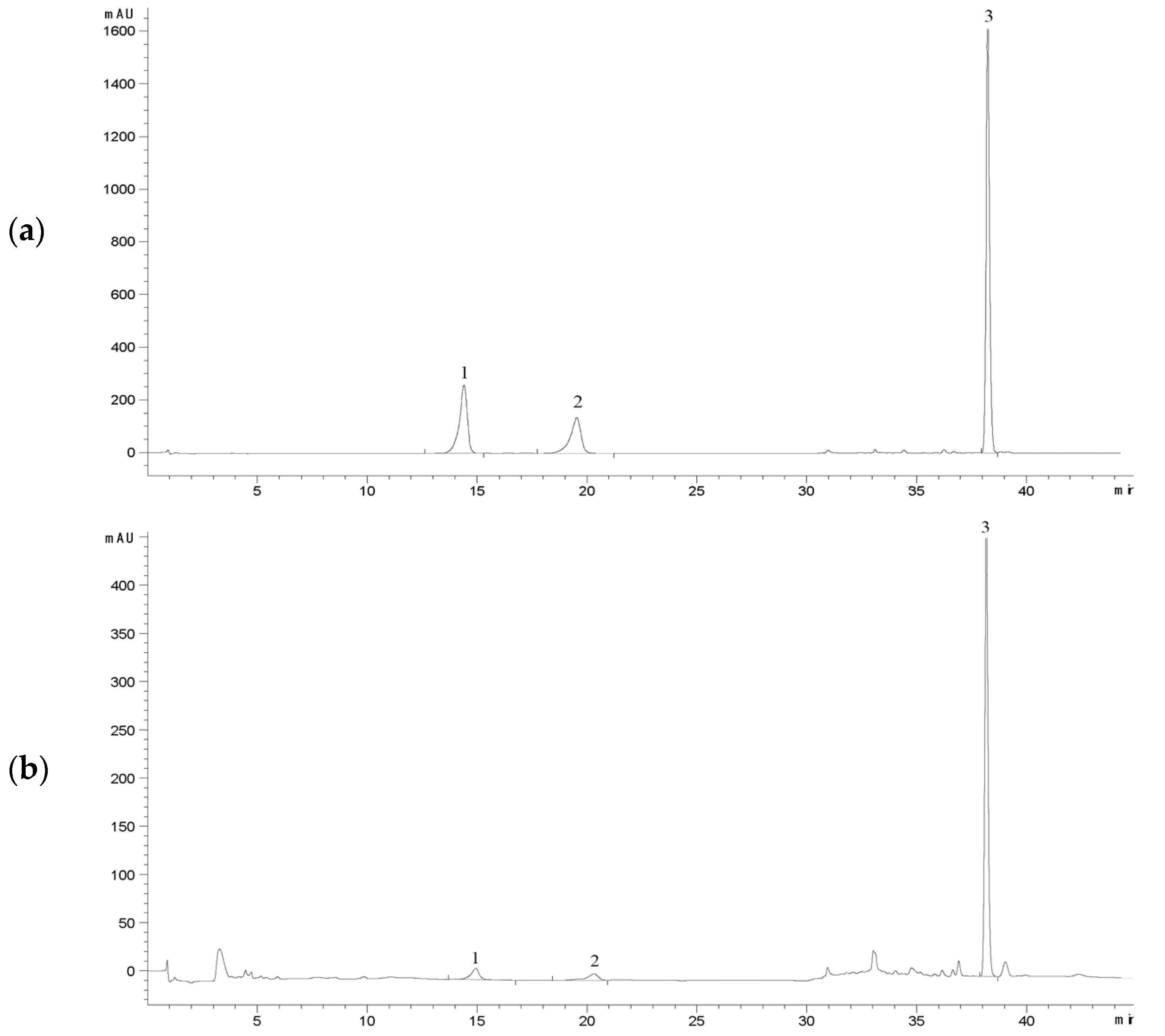


| Substance | Standard Curve | R2 | Linear Range mg·mL−1 | LOD 1 mg·mL−1 | LOQ 2 mg·mL−1 |
|---|---|---|---|---|---|
| ergosterol | y = 24322x − 70.7970 | 0.9990 | 0.0294–0.2058 | 5.948 × 10−4 | 1.487 × 10−3 |
| polyporusterone A | y = 28316x + 15.0590 | 0.9999 | 0.0031–0.0408 | 6.272 × 10−5 | 1.568 × 10−4 |
| polyporusterone B | y = 40549x − 12.3680 | 0.9992 | 0.0031–0.0408 | 8.981 × 10−5 | 2.245 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Xing, Y.; Li, S.; Zhou, L.; Li, B.; Guo, S. Different Symbiotic Species of Armillaria Affect the Yield and Active Compound Contents of Polyporus umbellatus. Microorganisms 2025, 13, 228. https://doi.org/10.3390/microorganisms13020228
Liu L, Xing Y, Li S, Zhou L, Li B, Guo S. Different Symbiotic Species of Armillaria Affect the Yield and Active Compound Contents of Polyporus umbellatus. Microorganisms. 2025; 13(2):228. https://doi.org/10.3390/microorganisms13020228
Chicago/Turabian StyleLiu, Liu, Yongmei Xing, Shoujian Li, Lisi Zhou, Bing Li, and Shunxing Guo. 2025. "Different Symbiotic Species of Armillaria Affect the Yield and Active Compound Contents of Polyporus umbellatus" Microorganisms 13, no. 2: 228. https://doi.org/10.3390/microorganisms13020228
APA StyleLiu, L., Xing, Y., Li, S., Zhou, L., Li, B., & Guo, S. (2025). Different Symbiotic Species of Armillaria Affect the Yield and Active Compound Contents of Polyporus umbellatus. Microorganisms, 13(2), 228. https://doi.org/10.3390/microorganisms13020228







