Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience
Abstract
:1. Introduction
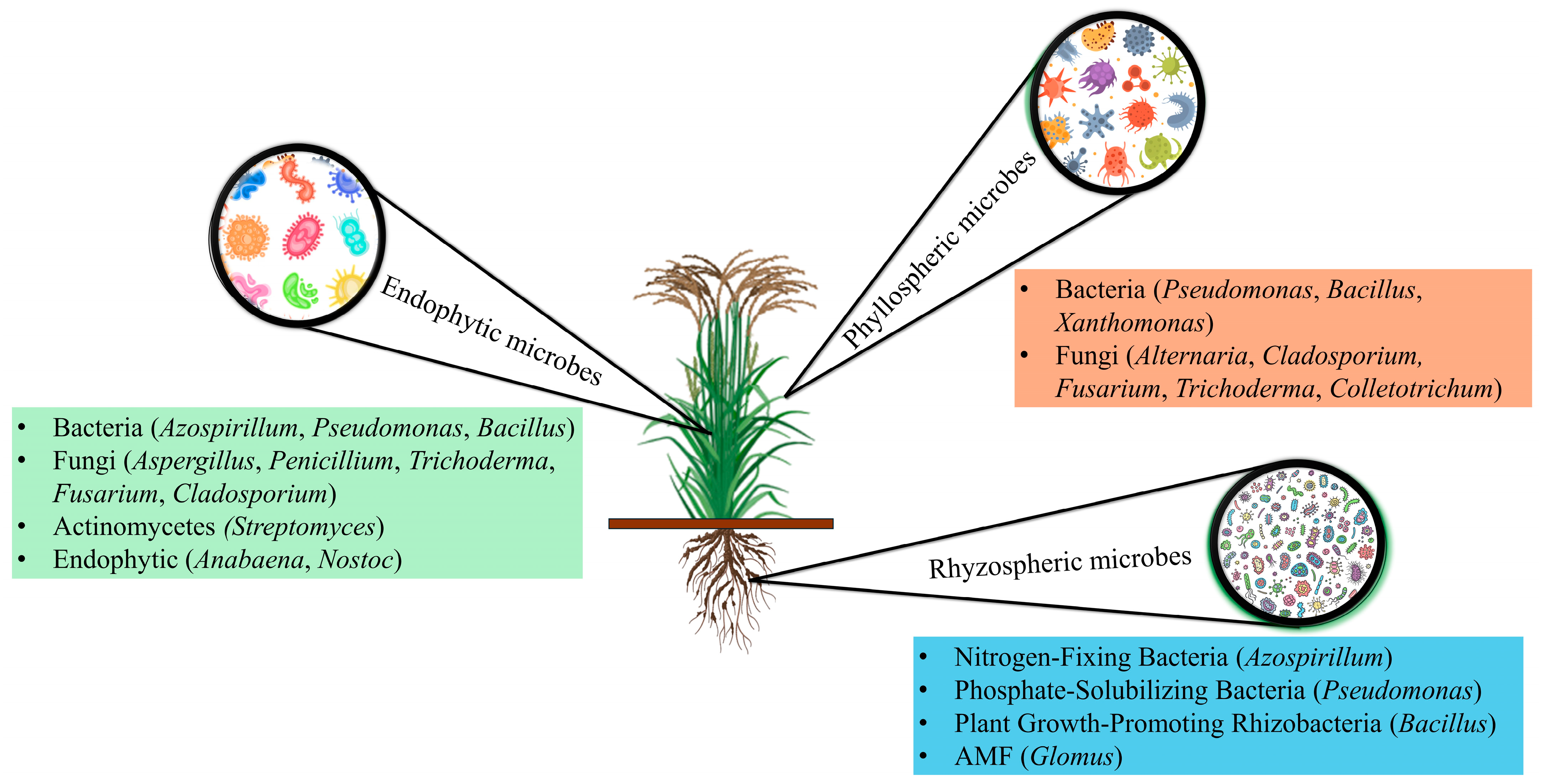
2. Diversity and Functional Dynamics of Rice-Associated Microbiomes
3. The Role of Rhizosphere Microbes in Rice Health and Growth
4. Phyllospheric Microbes and Their Contributions to Rice Growth and Disease Resistance
5. Endophytic Microbes and Their Role in Enhancing Stress Tolerance in Rice
6. Decoding Signalling Pathways in the Rice Rhizosphere
7. How Microbes Enhance Rice Growth and Yield: Mechanisms and Benefits
8. The Role of Microbes in Alleviating Biotic Stresses in Rice
9. Harnessing Microbes to Combat Abiotic Stresses in Rice
10. Metagenomics: Unravelling the Complexities of Rice Microbial Communities
11. Microbiome Engineering: A Pathway to Sustainable Rice Cultivation
12. Microbiome-Shaping (M) Genes: Unlocking New Avenues for Stress-Resilient Traits
13. Overcoming Challenges and Exploring Future Prospects in Rice Microbiome Engineering
14. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hornstein, E.D.; Sederoff, H. Back to the Future: Re-Engineering the Evolutionarily Lost Arbuscular Mycorrhiza Host Trait to Improve Climate Resilience for Agriculture. Crit. Rev. Plant Sci. 2024, 43, 1–33. [Google Scholar] [CrossRef]
- Thakur, N.; Nigam, M.; Mann, N.A.; Gupta, S.; Hussain, C.M.; Shukla, S.K.; Shah, A.A.; Casini, R.; Elansary, H.O.; Khan, S.A. Host-Mediated Gene Engineering and Microbiome-Based Technology Optimization for Sustainable Agriculture and Environment. Funct. Integr. Genom. 2023, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, M.R.; Segrè, D.; Bhatnagar, J.M. Environmental Microbiome Engineering for the Mitigation of Climate Change. Glob. Change Biol. 2023, 29, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Albright, M.B.N.; Louca, S.; E Winkler, D.; Feeser, K.L.; Haig, S.-J.; Whiteson, K.L.; Emerson, J.B.; Dunbar, J. Solutions in Microbiome Engineering: Prioritizing Barriers to Organism Establishment. ISME J. 2022, 16, 331–338. [Google Scholar] [CrossRef]
- Afridi, M.S.; Ali, S.; Salam, A.; Terra, W.C.; Hafeez, A.; Sumaira; Ali, B.; AlTami, M.S.; Ameen, F.; Ercisli, S.; et al. Plant Microbiome Engineering: Hopes or Hypes. Biology 2022, 11, 1782. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, X.; Zhang, C.; Hou, L.; Wu, N.; Zhang, W.; Wang, Y.; Yao, B.; Delaplace, P.; Tian, J. Harnessing Microbial Interactions with Rice: Strategies for Abiotic Stress Alleviation in the Face of Environmental Challenges and Climate Change. Sci. Total Environ. 2023, 912, 168847. [Google Scholar] [CrossRef]
- Beattie, G.A.; Bayliss, K.L.; Jacobson, D.A.; Broglie, R.; Burkett-Cadena, M.; Sessitsch, A.; Kankanala, P.; Stein, J.; Eversole, K.; Lichens-Park, A. From Microbes to Microbiomes: Applications for Plant Health and Sustainable Agriculture. Phytopathology® 2024, 114, 1742–1752. [Google Scholar] [CrossRef]
- Islam, M.T.; Rahman, M.M.; Pandey, P.; Boehme, M.H.; Haesaert, G. Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Islam, S.S.; Hossain, A.; Hasan, M.; Itoh, K.; Tuteja, N. Application of Trichoderma spp. as biostimulants to improve soil fertility for enhancing crop yield in wheat and other crops. In Biostimulants in Alleviation of Metal Toxicity in Plants; Academic Press: Cambridge, MA, USA, 2023; pp. 177–206. [Google Scholar]
- Mia, M.B.; Momotaj, A.; Islam, T. Consortia of Probiotic Bacteria and Their Potentials for Sustainable Rice Production. Sustain. Agrobiol. Des. Dev. Microb. Consortia 2023, 43, 151–176. [Google Scholar] [CrossRef]
- Fatema, K.; Mahmud, N.U.; Gupta, D.R.; Siddiqui, M.N.; Sakif, T.I.; Sarker, A.; Sharpe, A.G.; Islam, T. Enhancing rice growth and yield with weed endophytic bacteria Alcaligenes faecalis and Metabacillus indicus under reduced chemical fertilization. PLoS ONE 2024, 19, e0296547. [Google Scholar] [CrossRef]
- Islam, M.T.; Hashidoko, Y.; Deora, A.; Ito, T.; Tahara, S. Suppression of damping-off disease in hostplants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and anti-biosis against soilborne Peronosporomycetes. Appl. Environ. Microbiol. 2005, 71, 3786–3796. [Google Scholar] [CrossRef]
- Ding, L.J.; Cui, H.L.; Nie, S.A.; Long, X.E.; Duan, G.L.; Zhu, Y.G. Microbiomes inhabiting rice roots and rhizosphere. FEMS Microbiol. Ecol. 2019, 95, fiz040. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Anwar, S.; Nawaz, T.; Fahad, S.; Saud, S.; Ur Rahman, T.; Khan, M.N.; Nawaz, T. Securing a Sustainable Future: The Climate Change Threat to Agriculture, Food Security, and Sustainable Development Goals. J. Umm Al Qura Univ. Appl. Sci. 2024, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Jin, Z.; Smith, P.; Barrett, C.B.; Zhu, Y.G.; Burney, J.; D’Odorico, P.; Fantke, P.; Fargione, J.; et al. Climate Change Exacerbates the Environmental Impacts of Agriculture. Science 2024, 385, eadn3747. [Google Scholar] [CrossRef] [PubMed]
- Uniyal, B.; Kosatica, E.; Koellner, T. Spatial and Temporal Variability of Climate Change Impacts on Ecosystem Services in Small Agricultural Catchments Using the Soil and Water Assessment Tool (SWAT). Sci. Total Environ. 2023, 875, 162520. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Lou, G.; Abbas, W.; Osti, R.; Ahmad, A.; Bista, S.; Ahiakpa, J.K.; He, Y. Improving rice grain quality through ecotype breeding for enhancing food and nutritional security in Asia–Pacific region. Rice 2024, 17, 47. [Google Scholar] [CrossRef]
- Lal, R. Feeding 11 billion on 0.5 billion hectare of area under cereal crops. Food Energy Secur. 2016, 5, 239–251. [Google Scholar] [CrossRef]
- He, X.; Batáry, P.; Zou, Y.; Zhou, W.; Wang, G.; Liu, Z.; Bai, Y.; Gong, S.; Zhu, Z.; Settele, J.; et al. Agricultural Diversification Promotes Sustainable and Resilient Global Rice Production. Nat. Food 2023, 4, 788–796. [Google Scholar] [CrossRef]
- Jamal, M.R.; Kristiansen, P.; Kabir, M.J.; Lobry de Bruyn, L. Challenges and Adaptations for Resilient Rice Production under Changing Environments in Bangladesh. Land 2023, 12, 1217. [Google Scholar] [CrossRef]
- Sarkar, S.; Jaswal, A.; Singh, A. Sources of Inorganic Nonmetallic Contaminants (Synthetic Fertilizers, Pesticides) in Agricultural Soil and Their Impacts on the Adjacent Ecosystems. In Bioremediation of Emerging Contaminants from Soils; Elsevier: Amsterdam, The Netherlands, 2024; pp. 135–161. [Google Scholar] [CrossRef]
- Khan, B.A.; Nadeem, M.A.; Nawaz, H.; Amin, M.M.; Abbasi, G.H.; Nadeem, M.; Ali, M.; Ameen, M.; Javaid, M.M.; Maqbool, R.; et al. Pesticides: Impacts on Agriculture Productivity, Environment, and Management Strategies. In Emerging Contaminants and Plants: Interactions, Adaptations, and Remediation Technologies; Springer: Cham, Switzerland, 2023; pp. 109–134. [Google Scholar] [CrossRef]
- Hossain, M.E.; Shahrukh, S.; Hossain, S.A. Chemical Fertilizers and Pesticides: Impacts on Soil Degradation, Groundwater, and Human Health in Bangladesh. In Environmental Degradation: Challenges and Strategies for Mitigation; Springer International Publishing: Cham, Switzerland, 2022; pp. 63–92. [Google Scholar] [CrossRef]
- Mohd Nizam, S.N.; Haji Baharudin, N.S.; Ahmad, H. Application of Pesticide in Paddy Fields: A Southeast Asia Case Study Review. Environ. Geochem. Health 2023, 45, 5557–5577. [Google Scholar] [CrossRef]
- Krishna, S.; Bhutia, D.D.; Chaubey, R.K.; Sudhir, I. Exploring Plant Microbiome: A Holistic Approach to Sustainable Agriculture. In Microbiome Drivers of Ecosystem Function; Academic Press: Cambridge, MA, USA, 2024; pp. 61–77. [Google Scholar] [CrossRef]
- Rahman, M.; As Sabir, A.; Mukta, J.A.; Khan, M.A.M.; Mohi-Ud-Di, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield, and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S.; Prasad, S.M. Crop Microbiome Dynamics in Stress Management and Green Agriculture. In Microbiome Drivers of Ecosystem Function; Academic Press: Cambridge, MA, USA, 2024; pp. 341–366. [Google Scholar] [CrossRef]
- Mukherjee, A.; Singh, B.N.; Kaur, S.; Sharma, M.; de Araújo, A.S.F.; de Araujo Pereira, A.P.; Morya, R.; Puopolo, G.; Melo, V.M.M.; Verma, J.P. Unearthing the power of microbes as plant microbiome for sustainable agriculture. Microbiol. Res. 2024, 286, 127780. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Chen, W.; Hu, Y.; Wang, Z. Bacillus altitudinis LZP02 improves rice growth by reshaping the rhizosphere microbiome. Plant Soil 2024, 498, 279–294. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Potential of Microalgae and Cyanobacteria to Improve Soil Health and Agricultural Productivity: A Critical View. Environ. Sci. Adv. 2023, 2, 586–611. [Google Scholar] [CrossRef]
- Kumar, U.; Kaviraj, M.; Kundu, S.; Rout, S.; Priya, H.; Nayak, A.K. Microbial Alleviation of Abiotic and Biotic Stresses in Rice. In Sustainable Agriculture Reviews 60: Microbial Processes in Agriculture; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 243–268. [Google Scholar] [CrossRef]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with Rhizosphere Bacteria Can Confer an Adaptive Advantage to Plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial Seed Endophyte Shapes Disease Resistance in Rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant Growth Promoting Rhizobacteria Isolated from Halophytes and Drought-Tolerant Plants: Genomic Characterization and Exploration of Phyto-Beneficial Traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef]
- Etesami, H.; Li, Z.; Maathuis, F.J.; Cooke, J. The Combined Use of Silicon and Arbuscular Mycorrhizas to Mitigate Salinity and Drought Stress in Rice. Environ. Exp. Bot. 2022, 201, 104955. [Google Scholar] [CrossRef]
- Das, D.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-Um, S.; Datta, A. Arbuscular Mycorrhizal Fungi Inoculation and Phosphorus Application Improve Growth, Physiological Traits, and Grain Yield of Rice under Alternate Wetting and Drying Irrigation. J. Plant Physiol. 2022, 278, 153829. [Google Scholar] [CrossRef]
- Compant, S.; Cassan, F.; Kostić, T.; Johnson, L.; Brader, G.; Trognitz, F.; Sessitsch, A. Harnessing the Plant Microbiome for Sustainable Crop Production. Nat. Rev. Microbiol. 2024, 23, 9–23. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus Subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Lu, K.; Chen, R.; Jiang, J. Bacillus subtilis KLBMPGC81 suppresses appressorium-mediated plant infection by altering the cell wall integrity signaling pathway and multiple cell biological processes in Magnaporthe oryzae. Front. Cell. Infect. Microbiol. 2022, 12, 983757. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-Fungal-Interactions: A Detailed Review on Entomopathogenic Fungi Pathogenicity to Combat Insect Pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.A.; Haque, E.; Paul, N.C.; Khaleque, M.A.; Al-Garni, S.M.S.; Islam, M.T. Enhancement of Growth and Grain Yield of Rice in Nutrient Deficient Soils by Rice Probiotic Bacteria. Rice Sci. 2017, 24, 264–273. [Google Scholar] [CrossRef]
- Chi, F.; Shen, S.H.; Cheng, H.P.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol. 2005, 71, 7271–7278. [Google Scholar] [CrossRef]
- Purwani, J.; Pratiwi, E.; Sipahutar, I.A. The Effect of Different Species of Cyanobacteria on the Rice Yield and Nitrogen Use Efficiency Under Different Levels of Nitrogen Fertilizer on Alluvial West Java. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012196. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, P.S. N-Acyl Homoserine Lactone Mediated Quorum Sensing Exhibiting Plant Growth-Promoting and Abiotic Stress Tolerant Bacteria Demonstrates Drought Stress Amelioration. J. Pure Appl. Microbiol. 2022, 16, 669–684. [Google Scholar] [CrossRef]
- Sammauria, R.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial Inoculants: Potential Tool for Sustainability of Agricultural Production Systems. Arch. Microbiol. 2020, 202, 677–693. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Damayani, M.; Herdiyantoro, D.; Suryatmana, P.; Anggraini, D.; Khumairah, F.H. The Application Dosage of Azolla Pinnata in Fresh and Powder Form as Organic Fertilizer on Soil Chemical Properties, Growth and Yield of Rice Plant. AIP Conf. Proc. 2018, 1927, 030017. [Google Scholar]
- Islam, T.; Fatema; Hoque, M.N.; Gupta, D.R.; Mahmud, N.U.; Sakif, T.I.; Sharpe, A.G. Improvement of growth, yield and associated bacteriome of rice by the application of probiotic Paraburkholderia and Delftia. Front. Microbiol. 2023, 14, 1212505. [Google Scholar] [CrossRef] [PubMed]
- Islam, T. CRISPR-Cas technology in modifying food crops. CABI Rev. 2019, 14, 50. [Google Scholar]
- Su, P.; Kang, H.; Peng, Q.; Wicaksono, W.A.; Berg, G.; Liu, Z.; Ma, J.; Zhang, D.; Cernava, T.; Liu, Y. Microbiome Homeostasis on Rice Leaves Is Regulated by a Precursor Molecule of Lignin Biosynthesis. Nat. Commun. 2024, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Nanfack, A.D.; Nguefack, J.; Musonerimana, S.; La China, S.; Giovanardi, D.; Stefani, E. Exploiting the Microbiome Associated with Normal and Abnormal Sprouting Rice (Oryza sativa L.) Seed Phenotypes Through a Metabarcoding Approach. Microbiol. Res. 2024, 279, 127546. [Google Scholar] [CrossRef]
- Rana, R.; Nayak, P.K.; Madhavan, V.N.; Sonti, R.V.; Patel, H.K.; Patil, P.B. Comparative Genomics-Based Insights into Xanthomonas indica, a Non-Pathogenic Species of Healthy Rice Microbiome with Bioprotection Function. Appl. Environ. Microbiol. 2024, 90, e00848-24. [Google Scholar] [CrossRef]
- Eyre, A.W.; Wang, M.; Oh, Y.; Dean, R.A. Identification and Characterization of the Core Rice Seed Microbiome. Phytobiomes J. 2019, 3, 148–157. [Google Scholar] [CrossRef]
- Guo, Y.; Kuzyakov, Y.; Li, N.; Song, B.; Liu, Z.; Adams, J.M.; Yang, L. Rice Rhizosphere Microbiome Is More Diverse but Less Variable Along Environmental Gradients Compared to Bulk Soil. Plant Soil 2024, 1–9. [Google Scholar] [CrossRef]
- Iniesta-Pallarés, M.; Brenes-Álvarez, M.; Lasa, A.V.; Fernández-López, M.; Álvarez, C.; Molina-Heredia, F.P.; Mariscal, V. Changes in Rice Rhizosphere and Bulk Soil Bacterial Communities in the Doñana Wetlands at Different Growth Stages. Appl. Soil Ecol. 2023, 190, 105013. [Google Scholar] [CrossRef]
- Wang, X.; He, S.W.; He, Q.; Ju, Z.C.; Ma, Y.N.; Wang, Z.; Han, J.C.; Zhang, X.X. Early Inoculation of an Endophyte Alters the Assembly of Bacterial Communities across Rice Plant Growth Stages. Microbiol. Spectr. 2023, 11, e04978-22. [Google Scholar] [CrossRef]
- Edwards, J.A.; Santos-Medellín, C.M.; Liechty, Z.S.; Nguyen, B.; Lurie, E.; Eason, S.; Phillips, G.; Sundaresan, V. Compositional Shifts in Root-Associated Bacterial and Archaeal Microbiota Track the Plant Life Cycle in Field-Grown Rice. PLoS Biol. 2018, 16, e2003862. [Google Scholar] [CrossRef]
- Xu, S.; Qiu, Q.; Yan, C.; Xiong, J. Structure, Acquisition, Assembly, and Function of the Root-Associated Microbiomes in Japonica Rice and Hybrid Rice. Agric. Ecosyst. Environ. 2024, 373, 109122. [Google Scholar] [CrossRef]
- Xiong, J.; Lu, J.; Li, X.; Qiu, Q.; Chen, J.; Yan, C. Effect of Rice (Oryza sativa L.) Genotype on Yield: Evidence from Recruiting Spatially Consistent Rhizosphere Microbiome. Soil Biol. Biochem. 2021, 161, 108395. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, Z.; Wei, L.; Wu, J.; Ge, T. Biogeochemical Cycles of Key Elements in the Paddy-Rice Rhizosphere: Microbial Mechanisms and Coupling Processes. Rhizosphere 2019, 10, 100145. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, X.; Shu, D.; Siddique, K.H.; Song, X.; Wu, P.; Li, C.; Zhao, X. Enhancing Soil Health and Nutrient Cycling through Soil Amendments: Improving the Synergy of Bacteria and Fungi. Sci. Total Environ. 2024, 923, 171332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Deng, Y.; Peng, R.; Jiang, H.; Bai, L. Bioremediation of Paddy Soil with Amphitropic Mixture Markedly Attenuates Rice Cadmium: Effect of Soil Cadmium Removal and Fe/S-Cycling Bacteria in Rhizosphere. Sci. Total Environ. 2024, 915, 169876. [Google Scholar] [CrossRef] [PubMed]
- Murase, J.; Asiloglu, R. Protists: The Hidden Ecosystem Players in a Wetland Rice Field Soil. Biol. Fertil. Soils 2024, 60, 773–787. [Google Scholar] [CrossRef]
- Zhang, C.; Mi, W.; Xu, Y.; Zhou, W.; Bi, Y. Long-Term Integrated Rice-Crayfish Culture Disrupts the Microbial Communities in Paddy Soil. Aquac. Rep. 2023, 29, 101515. [Google Scholar] [CrossRef]
- Islam, M.T.; Deora, A.; Hashidoko, Y.; Rahman, A.; Ito, T.; Tahara, S. Isolation and Identification of Potential Phosphate Solubilizing Bacteria from the Rhizoplane of Oryza sativa L. cv. BR29 of Bangladesh. Z. Naturforsch. C 2007, 62, 103–110. [Google Scholar] [CrossRef]
- Cao, X.; Liu, L.; Ma, Q.; Lu, R.; Kong, H.; Kong, Y.; Zhu, L.; Zhu, C.; Tian, W.; Jin, Q.; et al. Optimum Organic Fertilization Enhances Rice Productivity and Ecological Multifunctionality via Regulating Soil Microbial Diversity in a Double Rice Cropping System. Field Crops Res. 2024, 318, 109569. [Google Scholar] [CrossRef]
- Khatibi, S.M.; Dimaano, N.G.; Veliz, E.; Sundaresan, V.; Ali, J. Exploring and Exploiting the Rice Phytobiome to Tackle Climate Change Challenges. Plant Commun. 2024, 3, 101078. [Google Scholar] [CrossRef]
- Nunna, S.A.; Balachandar, D. Rhizobacterial Community Structure Differs Between Landrace and Cultivar of Rice Under Drought Conditions. Curr. Microbiol. 2024, 81, 334. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Dailin, D.J.; Hanapi, S.Z.; Rahman, R.A.; Mehnaz, S.; Shahid, I.; Ho, T.; El Ensahsy, H.A. Role of Microbiome on Healthy Growth and Yield of Rice Plant. In Plant Holobiome Engineering for Climate-Smart Agriculture; Springer Nature: Singapore, 2024; pp. 141–161. [Google Scholar]
- Islam, M.M.; Jana, S.K.; Sengupta, S.; Mandal, S. Impact of Rhizospheric Microbiome on Rice Cultivation. Curr. Microbiol. 2024, 81, 188. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Utami, Y.D.; Dominguez, J.J.; Fuji, M.; Kirita, S.; Imai, S.; Murakami, T.; Hongoh, Y.; Shinjo, R.; Kamiya, T.; et al. Soil nutrition-dependent dynamics of the root-associated microbiome in paddy rice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ramírez-Viga, T.K.; Aguilar, R.; Castillo-Arguero, S.; Chiappa-Carrara, X.; Guadarrama, P.; Ramos-Zapata, J. Wetland plant species improve performance when inoculated with arbuscular mycorrhizal fungi: A meta-analysis of experimental pot studies. Mycorrhiza 2018, 28, 477–493. [Google Scholar] [CrossRef]
- Pivato, B.; Offre, P.; Marchelli, S.; Barbonaglia, B.; Mougel, C.; Lemanceau, P.; Berta, G. Bacterial effects on arbuscular mycorrhizal fungi and mycorrhiza development as influenced by the bacteria, fungi, and host plant. Mycorrhiza 2009, 19, 81–90. [Google Scholar] [CrossRef]
- Ordoñez, Y.M.; Fernandez, B.R.; Lara, L.S.; Rodriguez, A.; Uribe-Vélez, D.; Sanders, I.R. Bacteria with phosphate solubilizing capacity alter mycorrhizal fungal growth both inside and outside the root and in the presence of native microbial communities. PLoS ONE 2016, 11, e0154438. [Google Scholar] [CrossRef]
- Juliyanti, V.; Itakura, R.; Kotani, K.; Lim, S.Y.; Suzuki, G.; Chong, C.W.; Song, B.K.; Rahman, S. Comparative Analysis of Root-Associated Microbes in Tropical Cultivated and Weedy Rice (Oryza spp.) and Temp. Cultiv. Temperate Cultivated Rice. Sci. Rep. 2024, 14, 9656. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.H. The Rice Microbiome: A Model Platform for Crop Holobiome. Phytobiomes J. 2020, 4, 5–18. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant Microbiome—An Account of the Factors that Shape Community Composition and Diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Doni, F.; Mispan, M.S.; Suhaimi, N.S.; Ishak, N.; Uphoff, N. Roles of Microbes in Supporting Sustainable Rice Production Using the System of Rice Intensification. Appl. Microbiol. Biotechnol. 2019, 103, 5131–5142. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Metagenomics Methods for the Study of Plant-Associated Microbial Communities: A Review. J. Microbiol. Methods 2020, 170, 105860. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, N.; Sathishkumar, R.; Selvakumar, G.; Shyamkumar, R.; Arjunekumar, K. Phyllospheric microbiomes: Diversity, ecological significance, and biotechnological applications. Plant Microbiomes Sustain. Agric. 2020, 25, 113–172. [Google Scholar] [CrossRef]
- Schrey, D.; Hartmann, A.; Hampp, R. Rhizosphere interactions. Ecol. Biochem. Environ. Interspecies Interact. 2014, 292–311. [Google Scholar]
- Li, W.; Fang, M.; Shujuan, Z.; Xue, Z. Effect of Glomus Mosseae inoculation on growth and reproduction of rice. In Information Technology and Agricultural Engineering. Advances in Intelligent and Soft Computing; Zhu, E., Sambath, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Maiti, D.; Toppo, N.N.; Variar, M. Integration of crop rotation and arbuscular mycorrhiza (AM) inoculum application for enhancing AM activity to improve phosphorus nutrition and yield of upland rice (Oryza sativa L.). Mycorrhiza 2011, 8, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Luo, J.; Liu, Q.; Ogunyemi, S.O.; Ahmed, T.; Li, B.; Yu, S.; Wang, X.; Yan, C.; Chen, J.; et al. Rice Bacterial Leaf Blight Drives Rhizosphere Microbial Assembly and Function Adaptation. Microbiol. Spectr. 2023, 11, e01059-23. [Google Scholar] [CrossRef]
- Li, P.; Tian, Y.; Yang, K.; Tian, M.; Zhu, Y.; Chen, X.; Hu, R.; Qin, T.; Liu, Y.; Peng, S.; et al. Mechanism of Microbial Action of the Inoculated Nitrogen-Fixing Bacterium for Growth Promotion and Yield Enhancement in Rice (Oryza sativa L.). Adv. Biotechnol. 2024, 2, 32. [Google Scholar] [CrossRef]
- Pang, Z.; Zhao, Y.; Xu, P.; Yu, D. Microbial Diversity of Upland Rice Roots and Their Influence on Rice Growth and Drought Tolerance. Microorganisms 2020, 8, 1329. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Chen, Y.; Zhang, J.; Luo, S.; Tian, C.; Tian, L. Study of Rhizosphere Microbial Community Structures of Asian Wild and Cultivated Rice Showed That Cultivated Rice Had Decreased and Enriched Some Functional Microorganisms in the Process of Domestication. Diversity 2022, 14, 67. [Google Scholar] [CrossRef]
- Bhardwaj, L.; Reddy, B.; Nath, A.J.; Dubey, S.K. Influence of Herbicide on Rhizospheric Microbial Communities and Soil Properties in Irrigated Tropical Rice Field. Ecol. Indic. 2024, 158, 111534. [Google Scholar] [CrossRef]
- Vaid, S.K.; Kumar, B.; Sharma, A.; Shukla, A.; Srivastava, P. Effect of Zn Solubilizing Bacteria on Growth Promotion and Zn Nutrition of Rice. J. Soil Sci. Plant Nutr. 2014, 14, 889–910. [Google Scholar] [CrossRef]
- Hester, E.R.; Vaksmaa, A.; Valè, G.; Monaco, S.; Jetten, M.S.; Lüke, C. Effect of Water Management on Microbial Diversity and Composition in an Italian Rice Field System. FEMS Microbiol. Ecol. 2022, 98, fiac018. [Google Scholar] [CrossRef]
- Wang, P.; Dai, J.; Luo, L.; Liu, Y.; Jin, D.; Zhang, Z.; Li, X.; Fu, W.; Tang, T.; Xiao, Y.; et al. Scale-Dependent Effects of Growth Stage and Elevational Gradient on Rice Phyllosphere Bacterial and Fungal Microbial Patterns in the Terrace Field. Front. Plant Sci. 2022, 12, 766128. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhou, X.; Tian, L.; Zhang, H.; Cai, L.; Tang, F. Temporal and Spatial Variation of Microbial Communities in Stored Rice Grains from Two Major Depots in China. Food Res. Int. 2022, 152, 110876. [Google Scholar] [CrossRef] [PubMed]
- Yeo, F.K.; Cheok, Y.H.; Wan Ismail, W.N.; Kueh-Tai, F.F.; Lam, T.T.; Chong, Y.L. Genotype and Organ Effect on the Occupancy of Phyllosphere Prokaryotes in Different Rice Landraces. Arch. Microbiol. 2022, 204, 600. [Google Scholar] [CrossRef] [PubMed]
- Roman-Reyna, V.; Pinili, D.; Borja, F.N.; Quibod, I.L.; Groen, S.C.; Mulyaningsih, E.S.; Rachmat, A.; Slamet-Loedin, I.H.; Alexandrov, N.; Mauleon, R.; et al. The Rice Leaf Microbiome Has a Conserved Community Structure Controlled by Complex Host-Microbe Interactions. bioRxiv 2019, 615278. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayilara, M.S.; Akinola, S.A.; Babalola, O.O. Biocontrol mechanisms of endophytic fungi. Egypt. J. Biol. Pest Control 2022, 32, 46. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Pecoraro, L.; Wang, X.; Shah, D.; Song, X.; Kumar, V.; Shakoor, A.; Tripathi, K.; Ramteke, P.W.; Rani, R. Biosynthesis pathways, transport mechanisms and biotechnological applications of fungal siderophores. J. Fungi 2021, 8, 21. [Google Scholar] [CrossRef]
- Nwokolo, N.L.; Enebe, M.C.; Chigor, C.B.; Chigor, V.N.; Dada, O.A. The contributions of biotic lines of defence to improving plant disease suppression in soils: A review. Rhizosphere 2021, 19, 100372. [Google Scholar] [CrossRef]
- Mengistu, A.A. Endophytes: Colonization, behaviour, and their role in defence mechanism. Int. J. Microbiol. 2020, 2020, 6927219. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Gong, Y.; Liu, S.; Ji, M.; Tang, R.; Kong, D.; Xue, Z.; Wang, L.; Hu, F.; Huang, L.; et al. Endophytic bacterial communities in wild rice (Oryza officinalis) and their plant growth-promoting effects on perennial rice. Front. Plant Sci. 2023, 14, 1184489. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Liu, Y.; Tang, X.; Li, Y.; Sun, T.; Yan, G.; Yin, C. Drought Stress Increases the Complexity of the Bacterial Network in the Rhizosphere and Endosphere of Rice (Oryza sativa L.). Agronomy 2024, 14, 1662. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Pan, H.; Bai, Y.; Hu, Y.; Jin, S. Effects of Rare Earth Elements on Bacteria in Rhizosphere, Root, Phyllosphere and Leaf of Soil–Rice Ecosystem. Sci. Rep. 2022, 12, 2089. [Google Scholar] [CrossRef]
- Bao, X.; Zou, J.; Zhang, B.; Wu, L.; Yang, T.; Huang, Q. Arbuscular Mycorrhizal Fungi and Microbes Interaction in Rice Mycorrhizosphere. Agronomy 2022, 12, 1277. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased Arbuscular Mycorrhizal Fungal Colonization Reduces Yield Loss of Rice (Oryza sativa L.) Under Drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef]
- Kumar, V.; Nautiyal, C.S. Plant abiotic and biotic stress alleviation: From an endophytic microbial perspective. Curr. Microbiol. 2022, 79, 311. [Google Scholar] [CrossRef]
- Altaf, M.M.; Khan, M.S.; Abulreesh, H.H.; Ahmad, I. Quorum sensing in plant growth-promoting rhizobacteria and its impact on plant-microbe interaction. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Fundamental Mechanisms, Methods and Functions; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1, pp. 311–331. [Google Scholar] [CrossRef]
- Majdura, J.; Jankiewicz, U.; Gałązka, A.; Orzechowski, S. The role of quorum sensing molecules in bacterial–plant interactions. Metabolites 2023, 13, 114. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Baloch, F.B.; Zeng, N.; Gong, H.; Zhang, Z.; Zhang, N.; Baloch, S.B.; Ali, S.; Li, B. Rhizobacterial volatile organic compounds: Implications for agricultural ecosystems’ nutrient cycling and soil health. Heliyon 2024, 10, e40522. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Wu, J.; Yan, W.; Xie, S.; Sun, X.; Ye, B.C.; Chu, X. N-Acylhomoserine Lactonase-Based Hybrid Nanoflowers: A Novel and Practical Strategy to Control Plant Bacterial Diseases. J. Nanobiotechnol. 2022, 20, 347. [Google Scholar] [CrossRef] [PubMed]
- Kelbessa, B.G.; Dubey, M.; Catara, V.; Ghadamgahi, F.; Ortiz, R.; Vetukuri, R.R. Potential of Plant Growth-Promoting Rhizobacteria to Improve Crop Productivity and Adaptation to a Changing Climate. CABI Rev. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Santosh Kumar, V.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao, M.; Chinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU. Physiol. Mol. Biol. Plants 2020, 26, 1099. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhu, Y.; Gao, C.; Zhang, Y.; Gao, Y.; Zhao, Y. Microbial Inoculations Improved Rice Yields by Altering the Presence of Soil Rare Bacteria. Microbiol. Res. 2022, 254, 126910. [Google Scholar] [CrossRef]
- Viswanath, G.; Sekar, J.; Ramalingam, P.V. Detection of Diverse N-Acyl Homoserine Lactone Signaling Molecules Among Bacteria Associated with Rice Rhizosphere. Curr. Microbiol. 2020, 77, 3480–3491. [Google Scholar] [CrossRef]
- Surovy, M.Z.; Rahman, S.; Rostás, M.; Islam, T.; Von Tiedemann, A. Suppressive Effects of Volatile Compounds from Bacillus spp. on Magnaporthe oryzae Triticum (MoT) Pathotype, Causal Agent of Wheat Blast. Microorganisms 2023, 11, 1291. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Arshad, M. Phytohormones in Soils: Microbial Production and Function; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk Amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Jiang, O.; Li, L.; Duan, G.; Gustave, W.; Zhai, W.; Zou, L.; An, X.; Tang, X.; Xu, J. Root Exudates Increased Arsenic Mobility and Altered Microbial Community in Paddy Soils. J. Environ. Sci. 2023, 127, 410–420. [Google Scholar] [CrossRef]
- Cai, G.; Shahbaz, M.; Ge, T.; Hu, Y.; Li, B.; Yuan, H.; Wang, Y.; Liu, Y.; Liu, Q.; Shibistova, O.; et al. Root Exudates with Low C/N Ratios Accelerate CO2 Emissions from Paddy Soil. Land Degrad. Dev. 2022, 33, 1193–1203. [Google Scholar] [CrossRef]
- Wang, L.; Qin, T.; Liu, T.; Guo, L.; Li, C.; Zhai, Z. Inclusion of Microbial Inoculants with Straw Mulch Enhances Grain Yields from Rice Fields in Central China. Food Energy Secur. 2020, 9, e230. [Google Scholar] [CrossRef]
- Zeng, Q.; Man, X.; Huang, Z.; Zhuang, L.; Yang, H.; Sha, Y. Effects of Rice Blast Biocontrol Strain Pseudomonas alcaliphila Ej2 on the Endophytic Microbiome and Proteome of Rice under Salt Stress. Front. Microbiol. 2023, 14, 1129614. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, L.; Lv, M.; Wang, R.; Wang, L.; Yu, S.; Gao, Z.; Li, X. PGPR: Key to enhancing crop productivity and achieving sustainable agriculture. Curr. Microbiol. 2024, 81, 377. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, B.C.; Babalola, O.O.; Hassen, A.I. Rhizosphere competence and applications of plant growth-promoting rhizobacteria in food production—A review. Sci. Afr. 2024, 11, e02081. [Google Scholar]
- Silva, L.I.; Pereira, M.C.; Carvalho, A.M.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-solubilizing microorganisms: A key to sustainable agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Rahimi, S.; Talebi, M.; Baninasab, B.; Gholami, M.; Zarei, M.; Shariatmadari, H. The role of plant growth-promoting rhizobacteria (PGPR) in improving iron acquisition by altering physiological and molecular responses in quince seedlings. Plant Physiol. Biochem. 2020, 155, 406–415. [Google Scholar] [CrossRef]
- Molnár, Z.; Solomon, W.; Mutum, L.; Janda, T. Understanding the mechanisms of Fe deficiency in the rhizosphere to promote plant resilience. Plants 2023, 12, 1945. [Google Scholar] [CrossRef]
- Patil, N.; Raghu, S.; Mohanty, L.; Jeevan, B.; Basana-Gowda, G.; Adak, T.; Annamalai, M.; Rath, P.C.; Sengottayan, S.N.; Govindharaj, G.P. Rhizosphere bacteria isolated from medicinal plants improve rice growth and induce systemic resistance in host against pathogenic fungus. J. Plant Growth Regul. 2024, 43, 770–786. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Du, Z.; Nakagawa, A.; Fang, J.; Ridwan, R.; Astuti, W.D.; Sarwono, K.A.; Sofyan, A.; Widyastuti, Y.; Cai, Y. Cleaner anaerobic fermentation and greenhouse gas reduction of crop straw. Microbiol. Spectr. 2024, 12, e0052024. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.L.; Tang, R.P.; Sun, M.Y.; Chen, T.M.; Duan, X.C.; Lu, X.F.; Liu, D.; Shi, X.C.; Laborda, P.; et al. Pseudomonas putida Represses JA- and SA-Mediated Defence Pathways in Rice and Promotes an Alternative Defence Mechanism Possibly through ABA Signaling. Plants 2020, 9, 1641. [Google Scholar] [CrossRef]
- Kalkhajeh, Y.K.; He, Z.; Yang, X.; Lu, Y.; Zhou, J.; Gao, H.; Ma, C. Co-Application of Nitrogen and Straw-Decomposing Microbial Inoculant Enhanced Wheat Straw Decomposition and Rice Yield in a Paddy Soil. J. Agric. Food Chem. 2021, 4, 100134. [Google Scholar] [CrossRef]
- Huang, K.; Yin, H.; Zheng, Q.; Lv, W.; Shen, X.; Ai, M.; Zhao, Y. Microbial Inoculation Alters Rhizoplane Bacterial Community and Correlates with Increased Rice Yield. Pedobiologia 2024, 104, 150945. [Google Scholar] [CrossRef]
- Hu, M.; Xue, H.; Wade, A.J.; Gao, N.; Qiu, Z.; Long, Y.; Shen, W. Biofertilizer Supplements Allow Nitrogen Fertilizer Reduction, Maintain Yields, and Reduce Nitrogen Losses to Air and Water in China Paddy Fields. Agric. Ecosyst. Environ. 2024, 362, 108850. [Google Scholar] [CrossRef]
- Kumar, S.R.; David, E.M.; Pavithra, G.J.; Sajith, G.K.; Lesharadevi, K.; Akshaya, S.; Bassavaraddi, C.; Navyashree, G.; Arpitha, P.S.; Sreedevi, P.; et al. Enabling Greenhouse Gas Emission Reduction while Improving Rice Yield with a Methane-Derived Microbial Biostimulant. bioRxiv 2024. [Google Scholar] [CrossRef]
- Shahne, A.A.; Shivay, Y.S. Interactions of Microbial Inoculations with Fertilization Options and Crop Establishment Methods on Modulation of Soil Microbial Properties and Productivity of Rice. Int. J. Bio-Resour. Stress Manag. 2024, 15, 1–17. [Google Scholar] [CrossRef]
- Ashouri, R.; Fallah, H.; Niknezhad, Y.; Barari Tari, D. Grain Quality and Yield Response of Rice to Application of Plant Growth-Promoting Bacteria and Amino Acids. J. Plant Nutr. 2023, 46, 4698–4709. [Google Scholar] [CrossRef]
- Zhang, J.; Hussain, S.; Zhao, F.; Zhu, L.; Cao, X.; Yu, S.; Jin, Q. Effects of Azospirillum brasilense and Pseudomonas fluorescens on nitrogen transformation and enzyme activity in the rice rhizosphere. J. Soils Sediments 2018, 18, 1453–1465. [Google Scholar] [CrossRef]
- Danso Ofori, A.; Su, W.; Zheng, T.; Datsomor, O.; Titriku, J.K.; Xiang, X.; Kandhro, A.G.; Ahmed, M.I.; Mawuli, E.W.; Awuah, R.T.; et al. Roles of Phyllosphere Microbes in Rice Health and Productivity. Plants 2024, 13, 3268. [Google Scholar] [CrossRef]
- Cuevas, V.C.; Banaay, C.G. In Situ Bioremediation and Crop Growth Promotion Using Trichoderma Microbial Inoculant (TMI) Ameliorate the Effects of Cu Contamination in Lowland Rice Paddies. Philipp. J. Sci. 2022, 151, 1255–1265. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mahmud, N.U.; Ullah, C.; Rahman, M.; Islam, T. Biological and Biorational Management of Blast Diseases in Cereals Caused by Magnaporthe oryzae. Crit. Rev. Biotechnol. 2021, 41, 994–1022. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.R.; Pan, H.; et al. The Genome Sequence of the Rice Blast Fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhu, M.; Huang, J.; Hsiang, T.; Zheng, L. Biocontrol Potential of a Bacillus subtilis Strain BJ-1 Against the Rice Blast Fungus Magnaporthe oryzae. Can. J. Plant Pathol. 2019, 41, 47–59. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.N.; Wang, X.; Liao, K.; He, S.; Zhao, X.; Guo, H.; Zhao, D.; Wei, H.L. Dynamics of Rice Microbiomes Reveal Core Vertically Transmitted Seed Endophytes. Microbiome 2022, 10, 216. [Google Scholar] [CrossRef]
- Koné, Y.; Alves, E.; da Silveira, P.R.; Cruz-Magalhães, V.; Botelho, F.B.; Ferreira, A.N.; Guimarães, S.D.; de Medeiros, F.H. Microscopic and Molecular Studies in the Biological Control of Rice Blast Caused by Pyricularia oryzae with Bacillus sp. BMH under Greenhouse Conditions. Biol. Control 2022, 172, 104983. [Google Scholar] [CrossRef]
- Lei, L.Y.; Xiong, Z.X.; Li, J.L.; Yang, D.Z.; Li, L.; Chen, L.; Zhong, Q.F.; Yin, F.Y.; Li, R.X.; Cheng, Z.Q.; et al. Biological Control of Magnaporthe oryzae Using Natively Isolated Bacillus subtilis G5 from Oryza officinalis Roots. Front. Microbiol. 2023, 14, 1264000. [Google Scholar] [CrossRef]
- Sarker, A.; Al Masud, M.A.; Deepo, D.M.; Das, K.; Nandi, R.; Ansary, M.W.R.; Islam, A.R.M.T.; Islam, T. Biological and Green Remediation of Heavy Metal Contaminated Water and Soils: A State-of-the-Art Review. Chemosphere 2023, 332, 138861. [Google Scholar] [CrossRef]
- Mirara, F.; Dzidzienyo, D.K.; Mwangi, M. Bacillus amyloliquefaciens D203 Ameliorates Rice Growth and Resistance to Rice Blast Disease. Cogent Food Agric. 2024, 10, 2371943. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Zhang, L.; Zhou, Z.; Zhang, J.; Yang, J.; Gao, X.; Chen, R.; Huang, Z.; Xu, Z.; et al. Isolation of Bacillus siamensis B-612, a Strain That Is Resistant to Rice Blast Disease and an Investigation of the Mechanisms Responsible for Suppressing Rice Blast Fungus. Int. J. Mol. Sci. 2023, 24, 8513. [Google Scholar] [CrossRef]
- Chaowanaprasert, A.; Thanwisai, L.; Siripornadulsil, W.; Siripornadulsil, S. Biocontrol of Blast Disease in KDML105 Rice by Root-Associated Bacteria. Eur. J. Plant Pathol. 2024, 170, 319–336. [Google Scholar] [CrossRef]
- Behera, S.; Behera, S.; Nayak, B.S. Management of Bacterial Leaf Blight of Rice Caused by Xanthomonas oryzae pv. oryzae Through an Integrated Approach in Western Undulating Zones of Odisha. J. Cereal Res. 2024, 16, 37–43. [Google Scholar] [CrossRef]
- Masnilah, R.; Nurmala, F.; Pradana, A.P. In Vitro Study of Phyllosphere Bacteria as Promising Biocontrol Agents Against Bacterial Leaf Blight Disease (Xanthomonas oryzae pv. oryzae) in Rice Plants. J-PEN Borneo J. Ilmu Pertan. 2023, 6, 75–78. [Google Scholar] [CrossRef]
- Rabnawaz, M.; Irshad, G.; Majeed, A.; Yousaf, M.; Javaid, R.A.; Siddique, F.; Rehman, A.; Hanif, A. Trichoderma harzianum as Growth Stimulator and Biological Control Agent Against Bacterial Leaf Blight (BLB) and Blast of Rice. Pak. J. Phytopathol. 2023, 35, 317–326. [Google Scholar] [CrossRef]
- Mahamadou, D.; Adounigna, K.; Amadou, H.B.; Oumarou, H.; Fousseyni, C.; Abdoulaye, H. Isolation and In-Vitro Assessment of Antagonistic Activity of Trichoderma spp. Against Magnaporthe Oryzae oryzae Longorola Strain Causing Rice Blast Dis. Disease in Mali. Afr. J. Microbiol. Res. 2022, 16, 67–75. [Google Scholar] [CrossRef]
- Arellano, A.D.; da Silva, G.M.; Guatimosim, E.; da Rosa Dorneles, K.; Moreira, L.G.; Dallagnol, L.J. Seeds coated with Trichoderma atroviride and soil amended with silicon improve the resistance of Lolium multiflorum against Pyricularia oryzae. Biol. Control 2021, 154, 104499. [Google Scholar] [CrossRef]
- Prismantoro, D.; Akbari, S.I.; Permadi, N.; Dey, U.; Anhar, A.; Miranti, M.; Mispan, M.S.; Doni, F. The Multifaceted Roles of Trichoderma in Managing Rice Diseases for Enhanced Productivity and Sustainability. J. Agric. Food Res. 2024, 18, 101324. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases—A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Priyadarshani, T.D.; Karunarathna, D.D.; Menike, G.D.; Weerasinghe, P.A.; Madushani, M.A.; Madhushan, K.W. Efficiency of Locally Isolated Trichoderma virens for Controlling Rice Brown Leaf Spot Disease Caused by Bipolaris oryzae. Int. Congr. Turk. Sci. Technol. Publ. 2023, 9–13. [Google Scholar]
- Safari Motlagh, M.R.; Jahangiri, B.; Kulus, D.; Tymoszuk, A.; Kaviani, B. Endophytic fungi as potential biocontrol agents against Rhizoctonia solani JG Kühn, the causal agent of rice sheath blight disease. Biology 2022, 11, 1282. [Google Scholar] [CrossRef]
- Bora, B.; Ali, M.S. Evaluation of Microbial Antagonists Against Sarocladium oryzae Causing Sheath Rot Disease of Rice (Oryza sativa L.). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1755–1760. [Google Scholar] [CrossRef]
- Nurhayati, Y.; Suryanti, S.; Wibowo, A. In Vitro Evaluation of Trichoderma asperellum Isolate UGM-LHAF Against Rhizoctonia solani Causing Sheath Blight Disease of Rice. J. Perlind. Tanam. Indones. 2021, 25, 64–73. [Google Scholar] [CrossRef]
- Haque, Z.; Khan, M.R.; Zamir, S.; Pandey, K.; Rajana, R.N.; Gupta, N. Fungal Antagonists and Their Effectiveness to Manage the Rice Root-Knot Nematode, Meloidogyne graminicola. In Sustainable Management of Nematodes in Agriculture: Role of Microbes-Assisted Strategies; Springer International Publishing: Cham, Switzerland, 2024; Volume 2, pp. 237–247. [Google Scholar] [CrossRef]
- Ullah, Q.; Munir, T.; Mumtaz, T.; Chawla, M.; Amir, M.; Ismail, M.; Qasim, M.; Ghaffar, A.; Haidri, I. Harnessing Plant Growth-Promoting Rhizobacteria (PGPRs) for Sustainable Management of Rice Blast Disease Caused by Magnaporthe oryzae: Strategies and Remediation Techniques in Indonesia. Indones. J. Agric. Environ. Anal. 2024, 3, 65–76. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Banerjee, G.; Handique, P.J. Use of an abscisic acid-producing Bradyrhizobium japonicum isolate as biocontrol agent against bacterial wilt disease caused by Ralstonia solanacearum. J. Plant Dis. Prot. 2022, 129, 869–879. [Google Scholar] [CrossRef]
- Jain, A.; Chatterjee, A.; Das, S. Synergistic Consortium of Beneficial Microorganisms in Rice Rhizosphere Promotes Host Defence to Blight-Causing Xanthomonas oryzae pv. oryzae. Planta 2020, 252, 106. [Google Scholar] [CrossRef]
- Sukanya, S.; Thammasittirong, A.; Kittakoop, P.; Prachya, S.; Na-Ranong, S.; Thammasittirong, T. Antagonistic Activity Against Dirty Panicle Rice Fungal Pathogens and Plant Growth-Promoting Activity of Bacillus amyloliquefaciens BAS. J. Microbiol. Biotechnol. 2018, 28, 1527–1535. [Google Scholar] [CrossRef]
- Masum, M.M.I.; Liu, L.; Yang, M.; Hossain, M.M.; Siddiqa, M.M.; Supty, M.E.; Ogunyemi, S.O.; Hossain, A.; An, Q.; Li, B. Halotolerant Bacteria Belonging to Operational Group Bacillus amyloliquefaciens in Biocontrol of the Rice Brown Stripe Pathogen Aidovorax oryzae. J. Appl. Microbiol. 2018, 125, 1852–1867. [Google Scholar] [CrossRef]
- Saikia, K.; Bora, L.C. Exploring Actinomycetes and Endophytes of Rice Ecosystem for Induction of Disease Resistance Against Bacterial Blight of Rice. Eur. J. Plant Pathol. 2021, 159, 67–79. [Google Scholar] [CrossRef]
- Elshakh, A.S.; Anjum, S.I.; Qiu, W.; Almoneafy, A.A.; Li, W.; Yang, Z.; Cui, Z.Q.; Li, B.; Sun, G.C.; Xie, G.L. Controlling and Defence-Related Mechanisms of Bacillus Strains Against Bacterial Leaf Blight of Rice. J. Phytopathol. 2016, 164, 534–546. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.; Zubair, M.; Iqbal, M. Biocontrol of Bacterial Leaf Blight of Rice and Profiling of Secondary Metabolites Produced by Rhizospheric Pseudomonas aeruginosa BRp. Front. Microbiol. 2017, 8, 287562. [Google Scholar] [CrossRef]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-Growth Promotion and Biocontrol Properties of Three Streptomyces spp. Isol. Isolates to Control Bact. Bacterial Rice Pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef]
- de Sousa Oliveira, M.I.; Chaibub, A.A.; Sousa, T.P.; Cortes, M.V.; de Souza, A.C.; da Conceição, E.C.; de Filippi, M.C. Formulations of Pseudomonas fluorescens and Burkholderia pyrrocinia Control Rice Blast of Upland Rice Cultivated Under No-Tillage System. Biol. Control 2020, 144, 104153. [Google Scholar] [CrossRef]
- Campos-Soriano, L.I.; García-Martínez, J.; Segundo, B.S. The Arbuscular Mycorrhizal Symbiosis Promotes the Systemic Induction of Regulatory Defence-Related Genes in Rice Leaves and Confers Resistance to Pathogen Infection. Mol. Plant Pathol. 2012, 13, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Fu, Y.; Qu, Z.; Zhao, H.; Sun, Y.; Lin, Y.; Xie, J.; Cheng, J.; Jiang, D. Isolation and evaluation of the biocontrol potential of Talaromyces spp. against rice sheath blight guided by soil microbiome. Environ. Microbiol. 2021, 23, 5946–5961. [Google Scholar] [CrossRef] [PubMed]
- Someya, N.; Nakajima, M.; Watanabe, K.; Hibi, T.; Akutsu, K. Potential of Serratia marcescens Strain B2 for Biological Control of Rice Sheath Blight. Biocontrol Sci. Technol. 2005, 15, 105–109. [Google Scholar] [CrossRef]
- Vidhyasekaran, P.; Rabindran, R.; Muthamilan, M.; Nayar, K.; Rajappan, K.; Subramanian, N.; Vasumathi, K. Development of Powder Formulation of Pseudomonas fluorescens for Control of Rice Blast. Plant Pathol. 1997, 46, 291–297. [Google Scholar] [CrossRef]
- Yang, D.; Wang, B.; Wang, J.; Chen, Y.; Zhou, M. Activity and Efficacy of Bacillus subtilis Strain NJ-18 Against Rice Sheath Blight and Sclerotinia Stem Rot of Rape. Biol. Control 2009, 51, 61–65. [Google Scholar] [CrossRef]
- Kumar, K.V.K.; Yellareddygari, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Zhou, X.G.; Sudini, H.; Groth, D.E.; Raju, S.K.; Miller, M.E. Efficacy of Bacillus subtilis MBI 600 Against Sheath Blight Caused by Rhizoctonia solani and on Growth and Yield of Rice. Rice Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, J.; Jia, Y.; Tian, C.; Zhao, D.; Wu, X.; Liu, Y.; Wang, D.; Qi, S.; Liu, X.; et al. Bacillus subtilis GB519 promotes rice growth and reduces the damages caused by rice blast fungus Magnaporthe oryzae. PhytoFrontiers™ 2021, 1, 330–338. [Google Scholar] [CrossRef]
- Hossain, M.T.; Khan, A.; Chung, E.J.; Rashid, M.H.; Chung, Y.R. Biological Control of Rice Bakanae by an Endophytic Bacillus oryzicola YC. Plant Pathol. J. 2016, 32, 228. [Google Scholar] [CrossRef]
- Win, K.T.; Oo, A.Z.; Yokoyama, T. Plant Growth and Yield Response Salin. to Salinity Stress of Rice Grown Under Appl. Differ. the Application of Different Nitrogen Levels and Bacillus Pumilus pumilus Strain TUAT—1. Crops 2022, 2, 435–444. [Google Scholar] [CrossRef]
- McNeely, D.; Chanyi, R.M.; Dooley, J.S.; Moore, J.E.; Koval, S.F. Biocontrol of Burkholderia cepacia Complex Bacteria and Bacterial Phytopathogens by Bdellovibrio bacteriovorus. Can. J. Microbiol. 2017, 63, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Hossain, M.T.; Khan, A.; Kim, K.H.; Jeon, C.O.; Chung, Y.R. Bacillus oryzicola sp. nov., an Endophytic Bacterium Isolated from the Roots of Rice with Antimicrobial, Plant Growth Promoting, and Systemic Resistance Inducing Activities in Rice. Plant Pathol. J. 2015, 31, 152. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Li, Z.; Fu, Y.; Wen, Y.; Wei, S. Induction of Defence Responses Against Magnaporthe oryzae in Rice Seedling by a New Potential Biocontrol Agent Streptomyces JD. J. Basic Microbiol. 2018, 58, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Chaibub, A.A.; de Carvalho, J.C.B.; de Sousa Silva, C.; Collevatti, R.G.; Gonçalves, F.J.; de Carvalho Barros Cortes, M.V.; de Filippi, M.C.C.; de Faria, F.P.; Lopes, D.C.B.; de Araújo, L.G. Defence Responses in Rice Plants in Prior and Simultaneous Applications of Cladosporium sp. During Leaf Blast Suppression. Environ. Sci. Pollut. Res. 2016, 23, 21554–21564. [Google Scholar] [CrossRef] [PubMed]
- Chaibub, A.A.; de Sousa, T.P.; de Araújo, L.G.; de Filippi, M.C.C. Molecular and Morphological Characterization of Rice Phylloplane Fungi and Determination of the Antagonistic Activity Against Rice Pathogens. Microbiol. Res. 2020, 231, 126353. [Google Scholar] [CrossRef]
- Subedi, S.; Bohara, A.K.; Thapa, S.; Timilsena, K. Safeguarding Rice Crops in Nepal: Unveiling Strategies Against the Yellow Stem Borer (Scirpophaga incertulas). Discov. Agric. 2024, 2, 64. [Google Scholar] [CrossRef]
- Liu, X.; Matsumoto, H.; Lv, T.; Zhan, C.; Fang, H.; Pan, Q.; Xu, H.; Fan, X.; Chu, T.; Chen, S.; et al. Phyllosphere Microbiome Induces Host Metabolic Defence Against Rice False-Smut Disease. Nat. Microbiol. 2023, 8, 1419–1433. [Google Scholar] [CrossRef]
- Isawa, T.; Yasuda, M.; Awazaki, H.; Minamisawa, K.; Shinozaki, S.; Nakashita, H. Azospirillum sp. Strain B510 Enhances Rice Growth and Yield. Microbes Environ. 2010, 25, 58–61. [Google Scholar] [CrossRef]
- Yasuda, M.; Dastogeer, K.M.; Sarkodee-Addo, E.; Tokiwa, C.; Isawa, T.; Shinozaki, S.; Okazaki, S. Impact of Azospirillum sp. B510 on the Rhizosphere Microbiome of Rice under Field Conditions. Agronomy 2022, 12, 1367. [Google Scholar] [CrossRef]
- Harsonowati, W.; Astuti, R.I.; Wahyudi, A.T. Leaf Blast Disease Reduction by Rice-Phyllosphere Actinomycetes Producing Bioactive Compounds. J. Gen. Plant Pathol. 2017, 83, 98–108. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the Phyllosphere Microbiome to Plant Health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Doni, F.; Suhaimi, N.S.M.; Mispan, M.S.; Fathurrahman, F.; Marzuki, B.M.; Kusmoro, J.; Uphoff, N. Microbial Contributions for Rice Production: From Conventional Crop Management to the Use of ‘Omics’ Technologies. Int. J. Mol. Sci. 2022, 23, 737. [Google Scholar] [CrossRef] [PubMed]
- Onwe, R.O.; Onwosi, C.O.; Ezugworie, F.N.; Ekwealor, C.C.; Okonkwo, C.C. Microbial trehalose boosts the ecological fitness of biocontrol agents, the viability of probiotics during long-term storage and plants tolerance to environmental-driven abiotic stress. Sci. Total Environ. 2022, 806, 150432. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.R.; Gill, S.P.; Kaundal, A.; Sandhu, D. Strategies for combating plant salinity stress: The potential of plant growth-promoting microorganisms. Front. Plant Sci. 2024, 15, 1406913. [Google Scholar] [CrossRef]
- Adeniji, A.; Huang, J.; Li, S.; Lu, X.; Guo, R. Hot viewpoint on how soil texture, soil nutrient availability, and root exudates interact to shape microbial dynamics and plant health. Plant Soil 2024, 28, 1–22. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Seed biopriming with salinity-tolerant isolates of Trichoderma harzianum alleviates salt stress in rice: Growth, physiological and biochemical characteristics. J. Plant Pathol. 2012, 354, 353–365. [Google Scholar]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and Physiological Responses of Rice (Oryza sativa L.) as Influ. By Influenced by Trichoderma Harzianum Under harzianum under Drought Stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A.K.; et al. Dose-Dependent Response of Trichoderma harzianum in Improving Drought Tolerance in Rice Genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef]
- Bashyal, B.; Parmar, P.; Zaidi, N.; Aggarwal, R. Molecular Programming of Drought-Challenged Trichoderma harzianum-Bioprimed Rice (Oryza sativa L.). Front. Microbiol. 2021, 12, 655165. [Google Scholar] [CrossRef]
- Zaidi, N.; Singh, M.; Kumar, S.; Sangle, U.N.; Singh, R.S.; Prasad, R.; Singh, S.; Singh, S.; Yadav, A.; Singh, A.; et al. Trichoderma harzianum Improves the Performance of Stress-Tolerant Rice Varieties in Rainfed Ecologies of Bihar, India. Field Crops Res. 2017, 220, 97–104. [Google Scholar] [CrossRef]
- Morán-Diez, E.; Rubio, B.; Dominguez, S.; Hermosa, R.; Monte, E.; Nicolás, C. Transcriptomic Response of Arabidopsis thaliana After 24 h Incubation with the Biocontrol Fungus Trichoderma harzianum. J. Plant Physiol. 2012, 169, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Subramanian, R.B. Characterization of Root-Associated Bacteria from Paddy and Its Growth-Promotion Efficacy. 3 Biotech 2014, 4, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.; Trivedi, P.; Asif, M.; Chauhan, P.; Nautiyal, C. Unraveling Aspects of Bacillus amyloliquefaciens Mediated Enhanced Production of Rice under Biotic Stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zhao, S.; Javed, M.; Khan, K.; Bano, A.; Shen, R.; Masood, S. Bacillus pumilus Enhances Tolerance in Rice (Oryza sativa L.) to Comb. Stress. Combined Stresses of NaCl and High Boron Due Ltd. to Limited Uptake of Na. Environ. Exp. Bot. 2016, 124, 120–129. [Google Scholar] [CrossRef]
- Rajkumar, M.; Bruno, L.; Jeyakumar, R. Alleviation of Environmental Stress in Plants: The Role of Beneficial Pseudomonas spp. Crit. Rev. Environ. Sci. Technol. 2017, 47, 372–407. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.; Patel, S. Combination of Endophytic and Rhizospheric Plant Growth Promoting Rhizobacteria in Oryza sativa Shows Higher Accumulation of Osmoprotectant Against Saline Stress. Acta Physiol. Plant. 2011, 33, 797–802. [Google Scholar] [CrossRef]
- Ashwitha, K.; Rangeshwaran, R.; Vajid, N.; Sivakumar, G.; Jalali, S.; Rajalaksmi, K.; Manjunath, H. Characterization of Abiotic Stress Tolerant Pseudomonas spp. Occur. Occurring in Indian Soils. J. Biol. Control 2013, 27, 319–328. [Google Scholar]
- Tiwari, S.; Prasad, V.; Chauhan, P.; Lata, C. Bacillus amyloliquefaciens Confers Tolerance to Various Abiotic Stresses and Modulates Plant Response to Phytohormones through Osmoprotection and Gene Expression Regulation in Rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, B.; Lee, B.; Jo, K.; Lee, N.; Chung, C.; Lee, Y.; Lee, J. Purification and Characterization of Cellulase Produced by Bacillus amyoliquefaciens DL-3 Utilizing Rice Hull. Bioresour. Technol. 2008, 99, 378–386. [Google Scholar] [CrossRef]
- Kakar, K.; Ren, X.; Nawaz, Z.; Cui, Z.; Li, B.; Xie, G.; Hassan, M.; Ali, E.; Sun, G. A Consortium of Rhizobacterial Strains and Biochemical Growth Elicitors Improve Cold and Drought Stress Tolerance in Rice (Oryza sativa L.). Plant Biol. 2016, 18, 471–483. [Google Scholar] [CrossRef]
- Bisht, N.; Mishra, S.; Chauhan, P. Bacillus amyloliquefaciens Inoculation Alters Physiology of Rice (Oryza sativa L. var. IR-36) through Modulating Carbohydrate Metabolism to Mitigate Stress Induced by Nutrient Starvation. Int. J. Biol. Macromol. 2019, 143, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Naser, I.B.; Mahmud, N.U.; Sarker, A.; Hoque, M.N.; Islam, T. A Highly Salt-Tolerant Bacterium Brevibacterium sediminis Promotes the Growth of Rice (Oryza sativa L.) Seedlings. Stresses 2022, 2, 275–289. [Google Scholar] [CrossRef]
- Choi, J.; Choudhury, A.; Walitang, D.; Lee, Y.; Sa, T. ACC Deaminase-Producing Brevibacterium linens RS16 Enhances Heat-Stress Tolerance of Rice (Oryza sativa L.). Physiol. Plant. 2021, 174, e13584. [Google Scholar] [CrossRef]
- Singh, N.; Marwa, N.; Mishra, J.; Verma, P.C.; Rathaur, S.; Singh, N. Brevundimonas diminuta Mediated Alleviation of Arsenic Toxicity and Plant Growth Promotion in Oryza sativa L. Ecotoxicol. Environ. Saf. 2016, 125, 25–34. [Google Scholar] [CrossRef]
- Udayashankar, A.C.; Nayaka, S.C.; Reddy, M.S.; Srinivas, C. Plant Growth-Promoting Rhizobacteria Mediate Induced Systemic Resistance in Rice against Bacterial Leaf Blight Caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2011, 59, 114–122. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Thangappan, S.; Uthandi, S. Plant Growth-Promoting Bacillus sp. Cahoots Moisture Stress Alleviation in Rice Genotypes By by Triggering Antioxid. Def. Antioxidant Defence System. Microbiol. Res. 2020, 239, 126518. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Martín-Cardoso, H.; Olivé, M.; Pla, E.; Catala-Forner, M.; Martínez-Eixarch, M.; San Segundo, B. Effect of root colonization by arbuscular mycorrhizal fungi on growth, productivity and blast resistance in rice. Rice 2020, 13, 42. [Google Scholar] [CrossRef]
- Xu, Y.; Lambers, H.; Feng, J.; Tu, Y.; Peng, Z.; Huang, J. The role of arbuscular mycorrhizal fungi in micronutrient homeostasis and cadmium uptake and transfer in rice under different flooding intensities. Ecotoxicol. Environ. Saf. 2024, 284, 116978. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.N.; Tiwari, R.K.; Sahu, P.K.; Yadav, J.; Srivastava, A.K.; Kumar, S. Salinity Alleviation and Reduction in Oxidative Stress by Endophytic and Rhizospheric Microbes in Two Rice Cultivars. Plants 2023, 12, 976. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Zhang, N.; Shen, Q.; Zhang, R. Beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 induces plant salt tolerance through spermidine production. Mol. Plant-Microbe Interact. 2017, 30, 423–432. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of Abscisic Acid-Producing Endophytic Bacteria Enhances Salinity Stress Tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Li, P.; Ye, S.; Chen, J.; Wang, L.; Li, Y.; Ge, L.; Wu, G.; Song, L.; Wang, C.; Sun, Y.; et al. Combined Metagenomic and Metabolomic Analyses Reveal That Bt Rice Planting Alters Soil CN Metabolism. ISME Commun. 2023, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chandrasekhar, C.N. Effect of PGPR on Growth Promotion of Rice (Oryza sativa L.) under Salt Stress. Asian J. Plant Sci. Res. 2014, 4, 62–67. [Google Scholar]
- Singh, D.P.; Singh, V.; Gupta, V.K.; Shukla, R.; Prabha, R.; Sarma, B.K.; Patel, J.S. Microbial Inoculation in Rice Regulates Antioxidative Reactions and Defence-Related Genes to Mitigate Drought Stress. Sci. Rep. 2020, 10, 4818. [Google Scholar] [CrossRef]
- Devarajan, A.K.; Muthukrishanan, G.; Truu, J.; Truu, M.; Ostonen, I.; Kizhaeral S., S.; Panneerselvam, P.; Kuttalingam Gopalasubramanian, S. The Foliar Application of Rice Phyllosphere Bacteria Induces Drought-Stress Tolerance in Oryza sativa (L). Plants 2021, 10, 387. [Google Scholar] [CrossRef]
- Lei, Z.; Ding, Y.; Xu, W.; Zhang, Y. Microbial community structure in rice rhizosheaths under drought stress. J. Plant Ecol. 2023, 16, rtad012. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The Role of Plant Growth Promoting Rhizobacteria in Plant Drought Stress Responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Saha, C.; Mukherjee, G.; Agarwal-Banka, P.; Seal, A. A Consortium of Non-Rhizobial Endophytic Microbes from Typha angustifolia Functions as Probiotic in Rice and Improves Nitrogen Metabolism. Plant Biol. 2016, 18, 938–946. [Google Scholar] [CrossRef]
- Cassán, F.; Maiale, S.; Masciarelli, O.; Vidal, A.; Luna, V.; Ruiz, O. Cadaverine Production by Azospirillum brasilense and Its Possible Role in Plant Growth Promotion and Osmotic Stress Mitigation. Eur. J. Soil Biol. 2009, 45, 12–19. [Google Scholar] [CrossRef]
- de Almeida Leite, R.; Martins da Costa, E.; Cabral Michel, D.; do Amaral Leite, A.; de Oliveira-Longatti, S.M.; de Lima, W.; Konstantinidis, K.T.; de Souza Moreira, F.M. Genomic Insights into Organic Acid Production and Plant Growth Promotion by Different Species of Phosphate-Solubilizing Bacteria. World J. Microbiol. Biotechnol. 2024, 40, 311. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A.; Vadassery, J.; Verma, N.; Oelmüller, R.; Dua, M.; Nevo, E.; Johri, A.K. PiHOG1, a Stress Regulator MAP Kinase from the Root Endophyte Fungus Piriformospora indica, Confers Salinity Stress Tolerance in Rice Plants. Sci. Rep. 2016, 6, 36765. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lei, P.; Wang, Q.; Ma, J.; Zhan, Y.; Jiang, K.; Xu, Z.; Xu, H. The Endophyte Pantoea alhagi NX-11 Alleviates Salt Stress Damage to Rice Seedlings by Secreting Exopolysaccharides. Front. Microbiol. 2020, 10, 3112. [Google Scholar] [CrossRef]
- Adeleke, R.A.; Obieze, C.C.; Mukoro, C.; Chikere, C.B.; Tsipinana, S.; Nciizah, A. Phosphorus fertilizer application and tillage practices influence bacterial community composition: Implication for soil health. Arch. Agron. Soil Sci. 2023, 69, 803–820. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of Composition of Microbiomes: A Novel Method for Studying Microbial Composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Xu, Y.; Zhu, W. Metagenomic Analysis Reveals Significant Differences in Microbiome and Metabolic Profiles in the Rumen of Sheep Fed Low N Diet with Increased Urea Supplementation. FEMS Microbiol. Ecol. 2020, 96, fiaa117. [Google Scholar] [CrossRef]
- Forbes, J.D.; Knox, N.C.; Ronholm, J.; Pagotto, F.; Reimer, A. Metagenomics: The Next Culture-Independent Game Changer. Front. Microbiol. 2017, 8, 1069. [Google Scholar] [CrossRef]
- Islam, T. Genomic Surveillance for Tackling Emerging Plant Diseases, with Special Reference to Wheat Blast. CABI Rev. 2024, 19, 1. [Google Scholar] [CrossRef]
- Lv, L.; Huang, H.; Lv, J.; Xu, X.; Cao, D.; Rao, Z.; Geng, F.; Kang, Y. Unique Dissolved Organic Matter Molecules and Microbial Communities in Rhizosphere of Three Typical Crop Soils and Their Significant Associations Based on FT-ICR-MS and High-Throughput Sequencing Analysis. Sci. Total Environ. 2024, 919, 170904. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Z.; Li, X.; Wang, W.; Gu, A.; Liu, Y. Analysis of Endophytic Bacterial Diversity in Rice Seeds with Regional Characteristics in Yunnan Province, China, Based on High-Throughput Sequencing Technology. Curr. Microbiol. 2023, 80, 287. [Google Scholar] [CrossRef]
- Cheng, Z.; Zheng, Q.; Shi, J.; He, Y.; Yang, X.; Huang, X.; Wu, L.; Xu, J. Metagenomic and Machine Learning-Aided Identification of Biomarkers Driving Distinctive Cd Accumulation Features in the Root-Associated Microbiome of Two Rice Cultivars. ISME Commun. 2023, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Gan, P.; Kiguchi, Y.; Anda, M.; Sasaki, K.; Shibata, A.; Iwasaki, W.; Suda, W.; Shirasu, K. Uncovering Microbiomes of the Rice Phyllosphere Using Long-Read Metagenomic Sequencing. Commun. Biol. 2024, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Guo, J.; Noman, M.; Lv, L.; Manzoor, N.; Qi, X.; Li, B. Metagenomic and biochemical analyses reveal the potential of silicon to alleviate arsenic toxicity in rice (Oryza sativa L.). Environ. Pollut. 2024, 345, 123537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhao, W.; Sun, S.; Yang, X.; Mao, H.; Sheng, L.; Chen, Z. Community metagenomics reveals the processes of cadmium resistance regulated by microbial functions in soils with Oryza sativa root exudate input. Sci. Total Environ. 2024, 949, 175015. [Google Scholar] [CrossRef]
- Wang, C.; Chang, J.; Tian, L.; Sun, Y.; Wang, E.; Yao, Z.; Ye, L.; Zhang, H.; Pang, Y.; Tian, C. A Synthetic Microbiome Based on Dominant Microbes in Wild Rice Rhizosphere to Promote Sulfur Utilization. Rice 2024, 17, 18. [Google Scholar] [CrossRef]
- Ge, L.; Mao, C.; Wu, Y.; Wang, L.; Chao, S.; Lv, B.; Ye, S.; Wang, X.; Zhao, K.; Chen, J.; et al. Soil Nutrient Cycling and Microbiome Responses to Bt Rice Cultivation. Plant Soil 2024, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Pu, Q.; Zhang, C.; Chen, Y.; Lin, Z.; Hu, X.; Li, O. Complete Genome of Sphingomonas paucimobilis ZJSH1, an Endophytic Bacterium from Dendrobium officinale with Stress Resistance and Growth Promotion Potential. Arch. Microbiol. 2023, 205, 132. [Google Scholar] [CrossRef]
- Patel, A.; Sahu, K.P.; Mehta, S.; Javed, M.; Balamurugan, A.; Ashajyothi, M.; Sheoran, N.; Ganesan, P.; Kundu, A.; Gopalakrishnan, S.; et al. New Insights on Endophytic Microbacterium-Assisted Blast Disease Suppression and Growth Promotion in Rice: Revelation by Polyphasic Functional Characterization and Transcriptomics. Microorganisms 2023, 11, 362. [Google Scholar] [CrossRef]
- Sahu, K.P.; Patel, A.; Kumar, M.; Sheoran, N.; Mehta, S.; Reddy, B.; Eke, P.; Prabhakaran, N.; Kumar, A. Integrated Metabarcoding and Culturomic-Based Microbiome Profiling of Rice Phyllosphere Reveal Diverse and Functional Bacterial Communities for Blast Disease Suppression. Front. Microbiol. 2021, 12, 780458. [Google Scholar] [CrossRef]
- Krishnappa, C.; Mushineni, A.; Reddy, B.; Kumar, M.; Sahu, K.P.; Patel, A.; Sheoran, N.; Rajashekara, H.; V., G.; Kumar, A. Fine-Scale Mapping of the Microbiome on Phylloplane and Spermoplane of Aromatic and Non-Aromatic Rice Genotypes. Folia Microbiol. 2023, 68, 889–910. [Google Scholar] [CrossRef]
- Sondo, M.; Wonni, I.; Koïta, K.; Rimbault, I.; Barro, M.; Tollenaere, C.; Moulin, L.; Klonowska, A. Diversity and Plant Growth Promoting Ability of Rice Root-Associated Bacteria in Burkina Faso and Cross-Comparison with Metabarcoding Data. PLoS ONE 2023, 18, e0287084. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Liu, Y.; Zhu, W.; Yuan, Z.; Su, X.; Ding, C. Differences in Phyllosphere Microbiomes Among Different Populus spp. in the Same Habitat. Front. Plant Sci. 2023, 14, 1143878. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-Efficiency TALEN-Based Gene Editing Produces Disease-Resistant Rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Zafar, K.; Khan, M.Z.; Amin, I.; Mukhtar, Z.; Zafar, M.; Mansoor, S. Employing Template-Directed CRISPR-Based Editing of the OsALS Gene to Create Herbicide Tolerance in Basmati Rice. AoB Plants 2023, 15, plac059. [Google Scholar] [CrossRef]
- Sam, V.H.; Van, P.T.; Ha, N.T.; Ha, N.T.T.; Huong, P.T.T.; Hoi, P.X.; Phuong, N.D.; Le Quyen, C. Design and Transfer of OsSWEET14-Editing T-DNA construct to bac thom 7 rice cultivar. J. Biol. TẠp Chí Sinh HỌc 2021, 43, 99–108. [Google Scholar] [CrossRef]
- Mathsyaraja, S.; Lavudi, S.; Vutukuri, P.R. Enhancing Resistance to Blast Disease Through CRISPR/Cas9 Gene Editing Technology in OsHDT701 Gene in RPBio-226 Rice Cv. (Oryza sativa L.). J. Appl. Biol. Biotechnol. 2024, 12, 289–294. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H. Harnessing CRISPR/Cas9 for Enhanced Disease Resistance in Hot Peppers: A Comparative Study on CAMLO2-Gene-Editing Efficiency Across Six Cultivars. Int. J. Mol. Sci. 2023, 24, 16775. [Google Scholar] [CrossRef]
- Zafar, K.; Khan, M.Z.; Amin, I.; Mukhtar, Z.; Yasmin, S.; Arif, M.; Ejaz, K.; Mansoor, S. Precise CRISPR-Cas9 Mediated Genome Editing in Super Basmati Rice for Resistance Against Bacterial Blight by Targeting the Major Susceptibility Gene. Front. Plant Sci. 2020, 11, 575. [Google Scholar] [CrossRef]
- Ji, Z.; Sun, H.; Wei, Y.; Li, M.; Wang, H.; Xu, J.; Lei, C.; Wang, C.; Zhao, K. Ectopic Expression of Executor Gene Xa23 Enhances Resistance to Both Bacterial and Fungal Diseases in Rice. Int. J. Mol. Sci. 2022, 23, 6545. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere Microbiome: Engineering Bacterial Competitiveness for Enhancing Crop Production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, X.; Wang, Y.; Liu, L.; Li, Y.; Yang, Y.; Liu, L.; Zou, L.; Chen, G. A Varied AvrXa23-like TALE Enables the Bacterial Blight Pathogen to Avoid Being Trapped by Xa23 Resistance Gene in Rice. J. Adv. Res. 2022, 42, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.R.; Chadha-Mohanty, P. Novel Alleles of Rice eIF4G Generated by CRISPR/Cas9-Targeted Mutagenesis Confer Resistance to Rice Tungro Spherical Virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Que, Z.; Xia, Y.; Tang, N.; Li, D.; He, R.; Cao, M. Knock Out of the Annexin Gene OsAnn3 via CRISPR/Cas9-Mediated Genome Editing Decreased Cold Tolerance in Rice. J. Plant Biol. 2017, 60, 539–547. [Google Scholar] [CrossRef]
- Shen, L.; Wang, C.; Fu, Y.; Wang, J.; Liu, Q.; Zhang, X.; Yan, C.; Qian, Q.; Wang, K. QTL Editing Confers Opposing Yield Performance in Different Rice Varieties. J. Integr. Plant Biol. 2018, 60, 89–93. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Y.; Li, R.; He, M.; Wang, F.; Xu, Y.; Liu, X.; Pan, T.; Tian, X.; Bu, Q.; et al. Improvement of Herbicide Resistance in Rice by Using CRISPR/Cas9 System. Chin. J. Rice Sci. 2022, 36, 459. [Google Scholar]
- Ganie, S.A.; Wani, S.H.; Henry, R.; Hensel, G. Improving Rice Salt Tolerance by Precision Breeding in a New Era. Curr. Opin. Plant Biol. 2021, 60, 101996. [Google Scholar] [CrossRef]
- Ashokkumar, S.; Jaganathan, D.; Ramanathan, V.; Rahman, H.; Palaniswamy, R.; Kambale, R.; Muthurajan, R. Creation of Novel Alleles of Fragrance Gene OsBADH2 in Rice through CRISPR/Cas9 Mediated Gene Editing. PLoS ONE 2020, 15, e0237018. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y.; et al. Knockout of OsNramp5 Using the CRISPR/Cas9 System Produces Low Cd-Accumulating Indica Rice without Compromising Yield. Sci. Rep. 2017, 7, 14438. [Google Scholar] [CrossRef]
- Duan, Y.B.; Li, J.; Qin, R.Y.; Xu, R.F.; Li, H.; Yang, Y.C.; Ma, H.; Li, L.; Wei, P.C.; Yang, J.B. Identification of a Regulatory Element Responsible for Salt Induction of Rice OsRAV2 through Ex Situ and In Situ Promoter Analysis. Plant Mol. Biol. 2016, 90, 49–62. [Google Scholar] [CrossRef]
- Zhang, X.; Long, Y.; Huang, J.; Xia, J. OsNAC45 is involved in ABA response and salt tolerance in rice. Rice 2020, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xiao, Y.; Niu, M.; Meng, W.; Li, L.; Zhang, X.; Liu, D.; Zhang, G.; Qian, Y.; Sun, Z.; et al. ARGONAUTE2 enhances grain length and salt tolerance by activating BIG GRAIN3 to modulate cytokinin distribution in rice. Plant Cell 2020, 32, 2292–2306. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Ma, W.; Haq, F.; Li, Y.; Shah, S.M.A.; Zhu, B.; Zhu, C.; Zou, L.; Chen, G. Tale-Triggered and ITale-Suppressed Xa1 Resistance to Bacterial Blight Is Independent of OsTfiiaγ1 or OsTfiiaγ5 in Rice. J. Exp. Bot. 2021, 72, 3249–3262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, N.; Liu, Y.X.; Zhang, X.; Hu, B.; Qin, Y.; Xu, H.; Wang, H.; Guo, X.; Qian, J.; et al. Root Microbiota Shift in Rice Correlates with Resident Time in the Field and Developmental Stage. Sci. China Life Sci. 2018, 61, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cui, Y.; Zhang, Y.; Zhang, Y.; Liang, M.; Chen, H.; Lan, J.; Song, G.; Lou, J. The Initiation, Propagation and Dynamics of CRISPR-SpyCas9 R-Loop Complex. Nucleic Acids Res. 2018, 46, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.R.; Salas-González, I.; Conway, J.M.; Finkel, O.M.; Gilbert, S.; Russ, D.; Teixeira, P.J.; Dangl, J.L. The Plant Microbiome: From Ecology to Reductionism and Beyond. Annu. Rev. Microbiol. 2020, 74, 81–100. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, L.; Nian, H.; Jin, J.; Lian, T. Linking Plant Functional Genes to Rhizosphere Microbes: A Review. Plant Biotechnol. J. 2023, 21, 902–917. [Google Scholar] [CrossRef]
- Ma, L.; Rocha, F.I.; Lee, J.; Choi, J.; Tejera, M.; Sooksa-Nguan, T.; Boersma, N.; Van Loocke, A.; Heaton, E.; Howe, A. The impact of stand age and fertilization on the soil microbiome of Miscanthus× giganteus. Phytobiomes J. 2021, 5, 51–59. [Google Scholar] [CrossRef]
- Goossens, P.; Spooren, J.; Baremans, K.C.; Andel, A.; Lapin, D.; Echobardo, N.; Pieterse, C.M.; Van Den Ackerveken, G.; Berendsen, R.L. Congruent Downy Mildew-Associated Microbiomes Reduce Plant Disease and Function as Transferable Resistobiomes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing Functional and Translational Microbiome Research Using Meta-Omics Approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef]
- Song, Y.; Wilson, A.J.; Zhang, X.-C.; Thoms, D.; Sohrabi, R.; Song, S.; Geissmann, Q.; Liu, Y.; Walgren, L.; He, S.Y.; et al. FERONIA Restricts Pseudomonas in the Rhizosphere Microbiome via Regulation of Reactive Oxygen Species. Nat. Plants 2021, 7, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Morales Moreira, Z.; Briggs, A.L.; Zhang, X.C.; Diener, A.C.; Haney, C.H. PSKR1 Balances the Plant Growth–Defence Trade-off in the Rhizosphere Microbiome. Nat. Plants 2023, 9, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Y.; Wang, N.R.; Haney, C.H. Mechanisms in Plant–Microbiome Interactions: Lessons from Model Systems. Curr. Opin. Plant Biol. 2021, 62, 102003. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Bidirectional Interaction Between Phyllospheric Microbiotas and Plant Volatile Emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef]
- Zhan, C.; Wang, M. Disease Resistance Through M Genes. Nat. Plants 2024, 10, 352–353. [Google Scholar] [CrossRef]
- Zhan, C.; Matsumoto, H.; Liu, Y.; Wang, M. Pathways to Engineering the Phyllosphere Microbiome for Sustainable Crop Production. Nat. Food 2022, 3, 997–1004. [Google Scholar] [CrossRef]
- Huang, S.; Zha, X.; Fu, G. Affecting Factors of Plant Phyllosphere Microbial Community and Their Responses to Climatic Warming—A Review. Plants 2023, 12, 2891. [Google Scholar] [CrossRef]
- Shankar, N.; Shetty, P.; Melo, T.C.; Kesseli, R. Multi-Generation Ecosystem Selection of Rhizosphere Microbial Communities Associated with Plant Genotype and Biomass in Arabidopsis thaliana. Microorganisms 2023, 11, 2932. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, M.; Chakdar, H.; Pandiyan, K.; Kumar, S.C.; Zeyad, M.T.; Singh, B.N.; Ravikiran, K.T.; Mahto, A.; Srivastava, A.K.; et al. Influence of Host Genotype in Establishing Root Associated Microbiome of Indica Rice Cultivars for Plant Growth Promotion. Front. Microbiol. 2022, 13, 1033158. [Google Scholar] [CrossRef]
- Haldar, S.; Mondal, S.; Kumari, A.; Ghosh, A.; Chattopadhyay, D.; Ghosh, A. Rhizosphere Microbiome Engineering. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 377–396. [Google Scholar] [CrossRef]
- Huang, Y.; Sheth, R.U.; Zhao, S.; Cohen, L.A.; Dabaghi, K.; Moody, T.; Sun, Y.; Ricaurte, D.; Richardson, M.; Velez-Cortes, F.; et al. High-Throughput Microbial Culturomics Using Automation and Machine Learning. Nat. Biotechnol. 2023, 41, 1424–1433. [Google Scholar] [CrossRef]
- McArdle, A.J.; Kaforou, M. Sensitivity of Shotgun Metagenomics to Host DNA: Abundance Estimates Depend on Bioinformatic Tools and Contamination Is the Main Issue. Access Microbiol. 2020, 2, acmi000104. [Google Scholar] [CrossRef]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil Microbiome Engineering for Sustainability in a Changing Environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Levin, S.; Rodriguez-Iturbe, I. Linking plant disease risk and precipitation drivers: A dynamical systems framework. Am. Nat. 2013, 181, E1–E16. [Google Scholar] [CrossRef] [PubMed]
- Loiko, N.; Islam, M.N. Plant–soil microbial interaction: Differential adaptations of beneficial vs. pathogenic bacterial and fungal communities to climate-induced drought. Agronomy 2024, 14, 1949. [Google Scholar] [CrossRef]


| Beneficial Antagonistic Microbes | Phytopathogens | Observed Effects | References |
|---|---|---|---|
| Bacillus amyloliquefaciens and Aspergillus spinulosporus | Xanthomonas oryzae pv. oryzae | Increase the expression of defence-related enzymes, and proteins, and elevate levels of total phenols | [167] |
| Curvularia lunata, Fusarium semitectum, and Helminthosporium oryzae | Suppress the germ tube elongation and mycelial development in fungal infections | [168] | |
| Acidovorax oryzae | Cell membrane damage results in decreased cell count, biofilm development, and impaired swimming capability | [169] | |
| Consortium of S. fimicarius, S. laurentii, P. putida, and Metarhizium anisopliae | X. oryzae pv. oryzae | Decrease the occurrence of leaf blight | [170] |
| B. subtilis, B. amyloliquefaciens, and B. methyltrophicus | X. oryzae pv. oryzae | Activate the defence-related enzymes | [171] |
| P. aeruginosa | X. oryzae pv. oryzae | Activates the defence enzymes | [172] |
| Streptomyces spp. | B. glumae | Inhibits the growth of B. glumae and promotes plant growth | [173] |
| P. fluorescens | M. oryzae | Reduces the physical damage caused by M. oryzae | [174] |
| Glomus intraradices | M. oryzae | Enhances the expression of defence-related genes (OsNPR1, OsAP2, OsEREBP, and OsJAmyb) | [175] |
| Talaromyces spp. | R. solani | Increases the expression of defence-related genes and defence enzymes synthesis | [176] |
| Serratia marcescens | R. solani | Decreases the occurrence of sheath blight | [177] |
| P. fluorescens | P. oryzae | Induces integrated stress response (ISR) in rice against P. oryzae | [178] |
| Bacillus subtilis | R. solani | Reduces the frequency of sheath blight | [179] |
| R. solani | Decreases the occurrence of sheath blight and enhanced plant growth | [180,181] | |
| Bacillus subtilis | M. oryzae | Reduces (by over 50%) blast disease, enhances systemic resistance, improves plant resilience | [88,143,145,182,183] |
| B. oryzicola | Gibberella fujikuroi | Decrease Bakanae disease severity by 46–78%. | [184] |
| B. glumae | Stimulates the resistance and enhancement of plant development | [185] | |
| Streptomyces spp. | M. oryzae | Enhances the defensive enzyme activity | [186] |
| Cladosporium cladosporioides | M. oryzae | Increase the enzyme activity and expression of defence-related genes like JIOsPR10, LOX-RLL, and PR1b | [187,188] |
| B. subtilis, B. amyloliquefaciens, and B. methyltrophicus | X. oryzae pv. oryzae | Activate ISR leads to increase the activity of defence-related enzymes | [172] |
| P. aeruginosa | X. oryzae pv. oryzae | Increases the functions of defence-associated enzymes | [173] |
| Streptomyces spp. | B. glumae | Suppresses Burkholderia glumae development | [174] |
| Consortium of S. fimicarius, S. laurentii, P. putida, and Metarhizium anisopliae | X. oryzae pv. oryzae | Decrease the occurrence of leaf blight | [171] |
| Bacillus thuringiensis | Scirpophaga incertulas | Reduces pest damage, enhances plant growth and yield | [189] |
| Pseudomonas fluorescens | X. oryzae pv. oryzae | Reduces bacterial blight, promotes plant growth, increases disease resistance | [153,154] |
| Azospirillum brasilense | M. oryzae X. oryzae | Enhances growth, induces resistance against multiple pathogens, improves disease tolerance | [165] |
| Bacillus velezensis | P. oryzae Bipolaris oryzae | Reduces fungal disease incidence, enhances plant growth, improves grain yield | [166] |
| B. megaterium | R. solani | Suppresses sheath blight, promotes plant growth, increases disease resistance | [166] |
| B. toyonensis | B. oryzae | Suppresses sheath blight, promotes plant growth, increases disease resistance | [166] |
| Bradyrhizobium japonicum | Ralstonia solanacearum | Controls bacterial wilt, enhances plant health and disease resistance | [166] |
| Trichoderma spp. | R. solani P. oryzae Fusarium spp. B. oryzae | Suppresses fungal pathogens, promotes growth, reduces disease incidence | [155,156,157] |
| T. asperellum | R. solani | Suppresses fungal growth, reduces disease severity | [163] |
| Lactobacillus spp. and Aspergillus spp. | Ustilaginoidea virens | Reduces pathogen infection and disease severity in rice panicle | [190] |
| Azospirillum spp. | Various soilborne pathogens | Increases rice growth and yield, enhanced stress resistance | [191,192] |
| Saccharothrix spp. | P. oryzae | Produces host plant beneficial bioactive compounds | [193] |
| Beneficial Microbes | Mechanism | References |
|---|---|---|
| Trichoderma harzianum | Improves root development in water scarcity like salinity stress | [200] |
| Increases the expression of aquaporin, dehydrin, and malondialdehyde genes, as well as other physiological factors | [201] | |
| Improves seed germination and seedling growth at different stress conditions decreasing oxidative damage and lipid peroxidation | [202] | |
| Enhances the phenol levels, peroxidase activity, lignin content, and cell membrane integrity | [203] | |
| Increases the levels of antioxidant enzymes and secondary metabolites in plants | [204] | |
| Improves gene expression associated with stress response | [199] | |
| Enhances the efficiency of photosynthetic, antioxidant enzymes, and physiological adaptation in saline environments | [205] | |
| Pseudomonas pseudoalcaligenes and Bacillus pumilus | Decrease the toxicity of reactive oxygen species (ROS) | [206] |
| Increase the amount of osmoprotectants in rice, like glycine betaine-like quaternary compounds, to help shoots grow more when they are under saline stress | [207] | |
| Inhibit the absorption of Na+ ions Synthesize growth related metabolites and enzymes | [123,208] | |
| Reduce sodium uptake in roots under saline stress conditions | [209] | |
| Protect cells from saline stress | [210] | |
| Enhance the synergistic interaction among several PGPR strains | [210] | |
| Reduce abiotic stress by increasing plant hormone, osmolytes, antioxidants, and growth-regulated genes | [211] | |
| B. amyloliquefaciens | Improves photosynthesis, hormone signalling, stress response, and carbohydrate metabolism | [212] |
| Enhances the synthesis of secondary metabolites, hormones, and enzymes | [213,214] | |
| Increases the production of indole-3-acetic acid, siderophores, and cellulase under stress condition | [214] | |
| Increases biomass, relative water, and proline content under stress conditions | [212,215] | |
| Brevibacterium sp. | Increases tolerance to salinity stress | [216] |
| Increases the expression of stress linked genes | [217,218] | |
| Reduces ethylene release and reactive oxygen species levels in rice | [217] | |
| Decreases arsenic absorption in rice plants and lower stress-related enzyme activity | [218] | |
| Bacillus sp. | Enhances levels of phenylalanine ammonia lyase, peroxidase, and polyphenol oxidase to combat bacterial leaf blight | [218] |
| Enhances tolerance to water stress | [217] | |
| Inhibits sodium ion absorption and enhances antioxidant enzyme | [219] | |
| Enhances resistance to cold and drought stress | [214] | |
| Glomus intraradices | Increases resistance to rice blast disease and improves phosphorus nutrition | [220] |
| AMF | Reduces Cd uptake in rice and improves micronutrient (Zn and Fe) under flooding conditions | [221] |
| Funneliformis mosseae + Piriformospora indica | Increase salinity tolerance | [220] |
| Approach | Method | Outcome | References |
|---|---|---|---|
| CRISPR-Cas9 Gene Editing | Gene editing to enhance disease resistance | CRISPR/Cas9 protocol for genome editing of Pyricularia oryzae (rice blast fungus). Enables gene disruption, base editing, and functional genomics | [256] |
| Bacterial blight resistance | Developed bacterial blight-resistant rice by silencing OsSWEET11, OsSWEET13, and OsSWEET14 genes, which regulate sugar transport in the plant | [276] | |
| Resistance enhancement via CRISPR | Improved rice resistance to Magnaporthe oryzae by editing the OsHDT701 gene | [260] | |
| Blast disease resistance | Amino acid substitution in ALS gene of basmati rice significantly improves resistance to bacterial blight | [258] | |
| CRISPR editing for enhanced stress tolerance | Improved drought and salt stress tolerance in rice by editing the OsDST gene | [270] | |
| Salt and abiotic stress tolerance | Improved salt stress tolerance in rice via CRISPR editing of OsRAV2 and OsDST genes | [114,273] | |
| Reduced heavy metal accumulation | Reduced arsenic, cadmium, and calcium accumulation in rice by editing OsHAK1, OsNramp5, and OsARM1 genes | [271] | |
| Microbial Inoculation | Beneficial microbial strains | Introduction of beneficial microbes (e.g., Pseudomonas, Bacillus) to enhance nitrogen fixation and suppress pathogens in rice | [5] |
| Endophytic microbial engineering | Modification of endophytic bacteria to promote plant health and stress tolerance by enhancing the plant microbiome | [255] | |
| Rhizosphere microbial community manipulation | Modulation of the plant rhizosphere microbiome to improve disease resistance and nutrient uptake in rice | [260] | |
| Synthetic Biology | Engineering synthetic microbial consortia | Design and application of synthetic microbial communities to optimize plant–microbe interactions and improve plant resilience | [256] |
| Microbe-engineered growth-promoting substances | Engineering microbes to produce beneficial compounds (e.g., antimicrobial peptides, growth regulators) to enhance plant health | [5] | |
| Traditional Breeding | Selection for microbiome-supportive traits | Traditional breeding to select rice varieties that support beneficial microbial communities through exudate production or root architecture | [268] |
| Breeding for enhanced plant–microbe interactions | Breeding rice varieties with traits that favour beneficial plant–microbe interactions, such as improved exudate profiles that attract beneficial microbes | [265] | |
| Metagenomics/Microbiome Profiling | High-throughput sequencing of microbiomes | Profiling rice microbiomes to identify beneficial microbes and determine how plant varieties impact microbial communities. | [268] |
| Microbial community analysis and optimization | Metagenomic analysis to identify microbial communities that enhance plant resilience to stresses like drought and disease | [5] | |
| Environmental Modification | Soil amendments to enhance microbial diversity | Use of biochar, organic fertilizers, and other soil amendments to promote beneficial microbiomes in the rhizosphere of rice | [274] |
| Fertilizer application to modulate microbiome | Modulation of the plant microbiome through strategic fertilizer application, enhancing nutrient availability and plant health | [277] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misu, I.J.; Kayess, M.O.; Siddiqui, M.N.; Gupta, D.R.; Islam, M.N.; Islam, T. Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience. Microorganisms 2025, 13, 233. https://doi.org/10.3390/microorganisms13020233
Misu IJ, Kayess MO, Siddiqui MN, Gupta DR, Islam MN, Islam T. Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience. Microorganisms. 2025; 13(2):233. https://doi.org/10.3390/microorganisms13020233
Chicago/Turabian StyleMisu, Israt Jahan, Md. Omar Kayess, Md. Nurealam Siddiqui, Dipali Rani Gupta, M. Nazrul Islam, and Tofazzal Islam. 2025. "Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience" Microorganisms 13, no. 2: 233. https://doi.org/10.3390/microorganisms13020233
APA StyleMisu, I. J., Kayess, M. O., Siddiqui, M. N., Gupta, D. R., Islam, M. N., & Islam, T. (2025). Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience. Microorganisms, 13(2), 233. https://doi.org/10.3390/microorganisms13020233










