Microbiota Dysbiosis: A Key Modulator in Preeclampsia Pathogenesis and Its Therapeutic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Selection
2.3. Data Extraction and Analysis
3. Results
3.1. Gut Microbiota and Preeclampsia
3.2. Gut–Placenta Microbiota Axis
3.3. Vaginal Microbiota and Preeclampsia Risk
3.4. Placental Microbiota and Preeclampsia
3.5. Impact of the Microbiota on Maternal Immune System Function
3.6. External Factors Modulating Microbiota and Preeclampsia Risk
3.7. Interventions Targeting Microbiota to Prevent Preeclampsia
4. Discussion
4.1. Principal Findings
4.2. Clinical Implication
4.3. Research Implication
4.4. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Torres, J.; Villafan-Bernal, J.R.; Martinez-Portilla, R.J.; Hidalgo-Carrera, J.A.; Estrada-Gutierrez, G.; Adalid-Martinez-Cisneros, R.; Rojas-Zepeda, L.; Acevedo-Gallegos, S.; Camarena-Cabrera, D.M.; Cruz-Martínez, M.Y.; et al. Performance of machine-learning approach for prediction of pre-eclampsia in a middle-income country. Ultrasound Obstet. Gynecol. 2024, 63, 350–357. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torres, J.; Espino, Y.S.S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef] [PubMed]
- Colonetti, T.; Limas Carmo Teixeira, D.; Grande, A.J.; Rodrigues Uggioni, M.L.; Generoso, J.; Harding, S.; Rodriguez-Mateos, A.; Rech, P.; Rosa Silva, F.; Toreti, I.; et al. The role of intestinal microbiota on pre-eclampsia: Systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 291, 49–58. [Google Scholar] [CrossRef]
- Huang, L.; Cai, M.; Li, L.; Zhang, X.; Xu, Y.; Xiao, J.; Huang, Q.; Luo, G.; Zeng, Z.; Jin, C.; et al. Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 2021, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, X.; Ying, C. Advances in Research on the Relationship Between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms 2023, 11, 991. [Google Scholar] [CrossRef]
- Lv, L.J.; Li, S.H.; Li, S.C.; Zhong, Z.C.; Duan, H.L.; Tian, C.; Li, H.; He, W.; Chen, M.C.; He, T.W.; et al. Early-Onset Preeclampsia Is Associated With Gut Microbial Alterations in Antepartum and Postpartum Women. Front. Cell. Infect. Microbiol. 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bian, G.; Zheng, M.; Lu, G.; Chan, W.Y.; Li, W.; Yang, K.; Chen, Z.J.; Du, Y. Fertility factors affect the vaginal microbiome in women of reproductive age. Am. J. Reprod. Immunol. 2020, 83, e13220. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lin, C.Y.; Yeh, Y.M.; Yang, L.Y.; Lee, Y.S.; Chao, A.; Chin, C.Y.; Chao, A.S.; Yang, C.Y. Severe preeclampsia is associated with a higher relative abundance of Prevotella bivia in the vaginal microbiota. Sci. Rep. 2020, 10, 18249. [Google Scholar] [CrossRef] [PubMed]
- Beckers, K.F.; Sones, J.L. Maternal microbiome and the hypertensive disorder of pregnancy, preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1–H10. [Google Scholar] [CrossRef] [PubMed]
- Giannella, L.; Grelloni, C.; Quintili, D.; Fiorelli, A.; Montironi, R.; Alia, S.; Delli Carpini, G.; Di Giuseppe, J.; Vignini, A.; Ciavattini, A. Microbiome Changes in Pregnancy Disorders. Antioxidants 2023, 12, 463. [Google Scholar] [CrossRef]
- Jin, J.; Gao, L.; Zou, X.; Zhang, Y.; Zheng, Z.; Zhang, X.; Li, J.; Tian, Z.; Wang, X.; Gu, J.; et al. Gut Dysbiosis Promotes Preeclampsia by Regulating Macrophages and Trophoblasts. Circ. Res. 2022, 131, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, X.; Li, X.; Xin, S.; Zhang, F.; Liu, F.; Zeng, Y.; Wu, J.; Zou, Y.; Xiong, X. Landscapes of gut bacterial and fecal metabolic signatures and their relationship in severe preeclampsia. J. Transl. Med. 2024, 22, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association between gut microbiota and preeclampsia-eclampsia: A two-sample Mendelian randomization study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Meijer, S.; Pasquinelli, E.; Renzi, S.; Lavasani, S.; Nouri, M.; Erlandsson, L.; Cavalieri, D.; Hansson, S.R. Gut Micro-and Mycobiota in Preeclampsia: Bacterial Composition Differences Suggest Role in Pathophysiology. Biomolecules 2023, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Gewirtz, A.T. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol. Pathol. 2014, 42, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Záhumenský, J.; Hederlingová, J.; Pšenková, P. The importance of maternal microbiome in pregnancy. Ces. Gynekol. 2017, 82, 211–217. [Google Scholar]
- Ishimwe, J.A. Maternal microbiome in preeclampsia pathophysiology and implications on offspring health. Physiol. Rep. 2021, 9, e14875. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237–265. [Google Scholar] [CrossRef]
- Yoffe, L.; Kuperman, A.A.; Isakov, O.; Haguel, D.; Polsky, A.L.; Farberov, L.; Pillar, N.; Gurevich, V.; Haviv, I.; Shomron, N. Assessing the involvement of the placental microbiome and virome in preeclampsia using non coding RNA sequencing. J. Perinat. Med. 2021, 49, 1071–1083. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef]
- VandeVusse, L.; Hanson, L.; Safdar, N. Perinatal outcomes of prenatal probiotic and prebiotic administration: An integrative review. J. Perinat. Neonatal Nurs. 2013, 27, 288–301; quiz E281–E282. [Google Scholar] [CrossRef]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: A randomised controlled trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Coughlin, K.B.; Frederick, I.O.; Sorensen, T.K.; Williams, M.A. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am. J. Hypertens. 2008, 21, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Cheng, D.C.; Cao, Y.N.; Su, Y.; Chen, L.; Liu, W.Y.; Yu, Y.X.; Xu, X.M. The effect of dietary fiber supplement on prevention of gestational diabetes mellitus in women with pre-pregnancy overweight/obesity: A randomized controlled trial. Front. Pharmacol. 2022, 13, 922015. [Google Scholar] [CrossRef]
- Perry, A.; Stephanou, A.; Rayman, M.P. Dietary factors that affect the risk of pre-eclampsia. BMJ Nutr. Prev. Health 2022, 5, 118–133. [Google Scholar] [CrossRef]
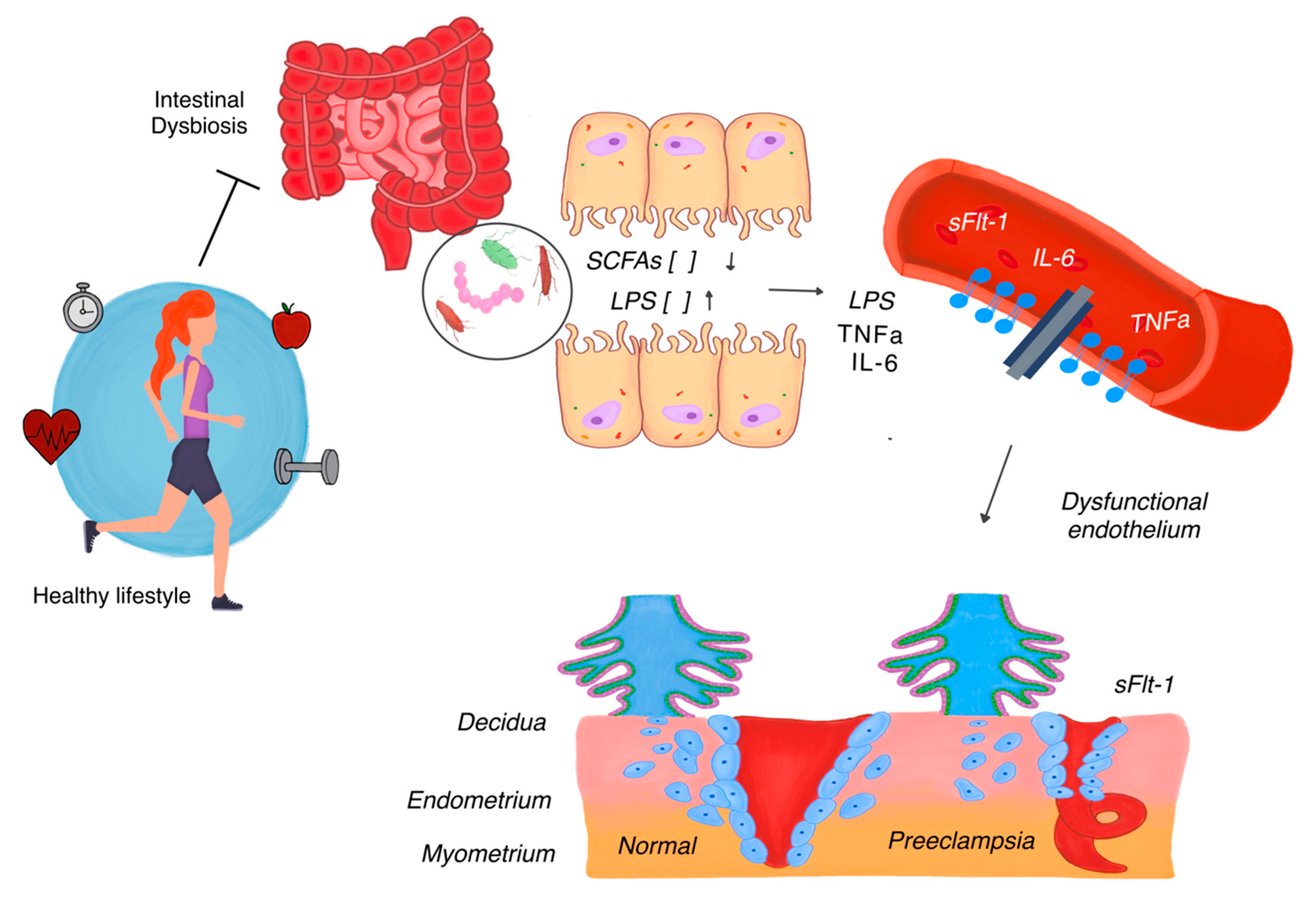
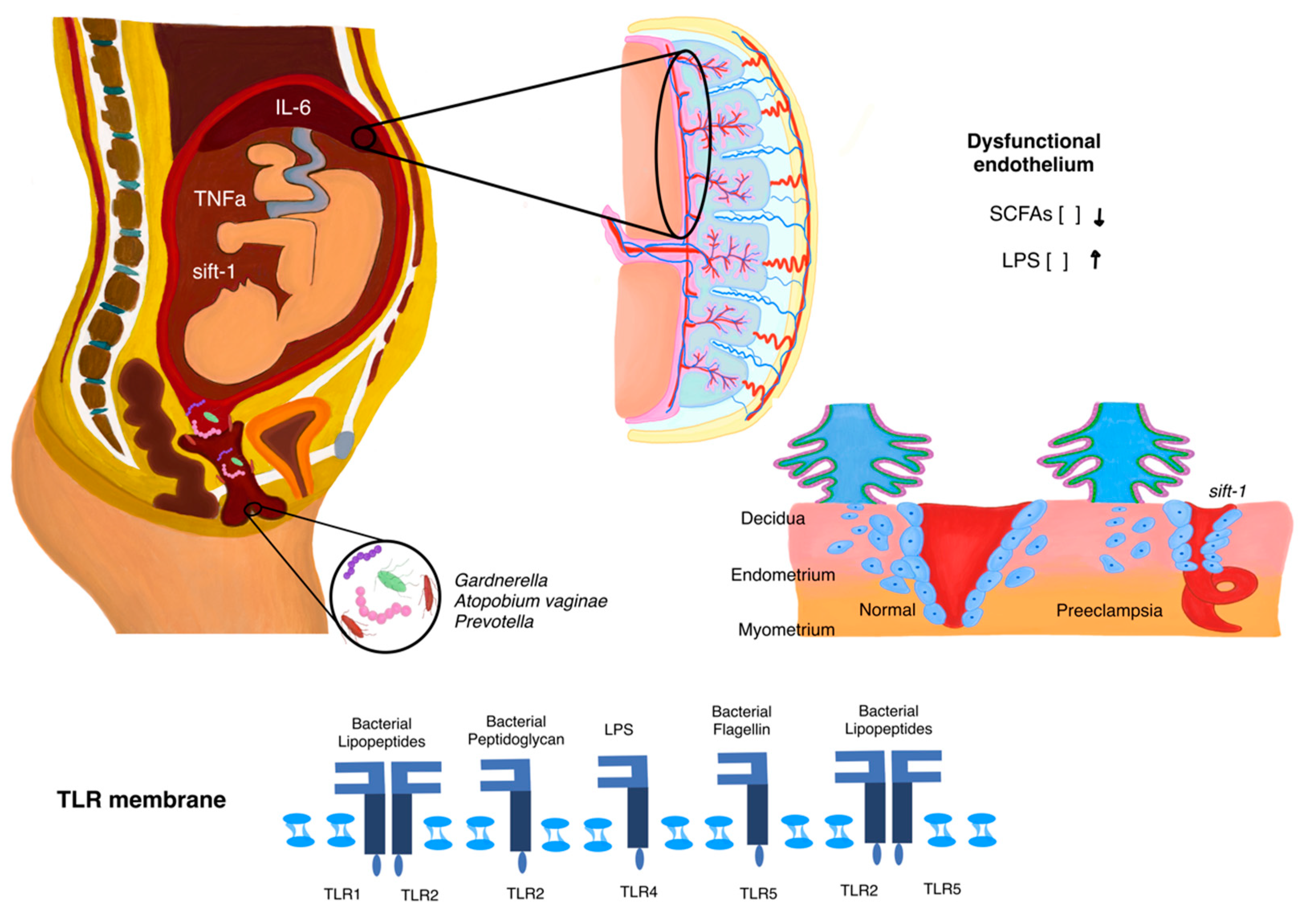
| Aspect | Description | Observed Alterations | Effect | Key Factor |
|---|---|---|---|---|
| Gut Microbiota | Refers to the collection of microorganisms residing in the intestine, which are critical for immune regulation, metabolic processes, and inflammation during pregnancy. | Decrease in beneficial bacteria (Lactobacillus, Bifidobacterium) responsible for producing SCFAs. Increase in pro-inflammatory bacteria such as Escherichia coli and Enterococcus faecalis. | Reduced SCFA production weakens the gut barrier, leading to increased intestinal permeability. Bacterial endotoxins (e.g., LPS) enter the bloodstream, triggering systemic inflammation and immune system activation. Systemic inflammation exacerbates endothelial dysfunction, a hallmark of preeclampsia. | SCFAs (butyrate, propionate), gut permeability, LPS. |
| Vaginal Microbiota | Refers to microorganisms in the vaginal tract, which maintain local homeostasis, prevent infections, and support immune regulation. | Reduction of Lactobacillus spp., which maintains an acidic pH. Overgrowth of anaerobic bacteria such as Gardnerella vaginalis and Prevotella. | Loss of vaginal acidity allows pathogenic bacteria to thrive, causing local inflammation. Inflammation increases the risk of ascending infections, which can reach the uterus and placenta. Ascending infections impair placental function and disrupt spiral artery remodeling, leading to placental insufficiency and fetal hypoxia. | Lactobacillus spp., Gardnerella vaginalis, Prevotella, local pH balance, ascending infections. |
| Placental Microbiota | Refers to emerging evidence suggesting the placenta may host its own microbiota, although its existence and role are debated. | Detection of microbial DNA from genera such as Firmicutes, Proteobacteria, and Bacteroidetes. Evidence remains inconclusive about whether these microbes are viable or contaminants. | Microbial DNA may interfere with critical placental functions such as trophoblast invasion and angiogenesis. Disruption in placental immune signaling contributes to placental insufficiency, reduced nutrient exchange, and exacerbated maternal inflammation. | Microbial DNA, inflammatory signals, trophoblast invasion, angiogenesis disruption. |
| Interventions Targeting Microbiota | Strategies aimed at restoring microbial balance using dietary or pharmacological approaches to improve maternal and fetal health outcomes. | Use of probiotics to replenish beneficial bacteria such as Lactobacillus and Bifidobacterium. Use of prebiotics such as dietary fibers to stimulate the growth of beneficial bacteria. | Probiotics and prebiotics enhance SCFA production, reduce systemic inflammation, and strengthen the intestinal and vaginal barriers. These interventions may mitigate risk factors for preeclampsia by restoring microbial homeostasis. | Probiotics [Lactobacillus, Bifidobacterium], prebiotics [dietary fiber], SCFAs production. |
| External Factors Modulating Microbiota | Exogenous factors that influence microbiota composition during pregnancy and may contribute to dysbiosis. | Diet: high-fiber diets support beneficial bacteria, while high-fat or processed food diets promote dysbiosis. Antibiotics: alter the composition and diversity of protective microbiota. Obesity: associated with a pro-inflammatory microbiota profile and reduced microbial diversity. | High-fiber diets promote microbial diversity and SCFA production, reducing inflammation. Antibiotics can disrupt microbial communities, increasing susceptibility to inflammatory complications. Obesity fosters a dysbiotic, pro-inflammatory microbiota that exacerbates systemic inflammation and endothelial dysfunction. | Fiber, processed foods, antibiotics, obesity, metabolic inflammation. |
| Aspect | Description | Implications in Preeclampsia |
|---|---|---|
| Microbiota Composition | Detection of bacterial DNA from Firmicutes, Proteobacteria, and Bacteroidetes in placental tissue. | May represent viable microbial communities or contamination; functional activity and relevance remain uncertain. Advances in sequencing, such as metagenomics and transcriptomics, aim to distinguish true colonization from contamination. |
| Potential Mechanisms | Activation of TLRs by microbial components such as LPS or bacterial DNA. | Triggers pro-inflammatory cascades, shifting the balance toward inflammation within the placenta. Impairs trophoblast invasion and angiogenesis, contributing to vascular dysfunction and placental insufficiency. |
| Dysregulated release of pro-inflammatory cytokines, including TNF-α and IL-6. | Increases endothelial dysfunction and systemic inflammation, leading to impaired spiral artery remodeling and reduced placental perfusion. | |
| Exacerbation of anti-angiogenic factors such as sFlt-1. | Disrupts trophoblast-endothelial interactions, impairing nutrient and oxygen exchange between mother and fetus. Amplifies placental insufficiency and hypoxia. | |
| Effects on the Placenta | Disruption of immune interactions within the placenta. | Alters local immune tolerance and inflammatory signaling, further compromising vascular remodeling and placental function. |
| Reduction in trophoblast invasion and spiral artery remodeling. | Leads to placental hypoxia, fetal nutrient restriction, and exacerbation of systemic inflammation. | |
| Current Knowledge Status | Evidence suggests microbial signals may reflect systemic maternal changes rather than a distinct placental microbiome. Small bacterial loads complicate differentiation between true colonization and contamination. | Limited data on functional microbial activity; unclear whether alterations are causative in preeclampsia or secondary to systemic changes. Further studies are essential to clarify microbial viability and functional relevance. |
| Future Research Directions | Use of metagenomics, transcriptomics, and advanced functional assays to characterize microbial communities and differentiate contamination from true colonization. | Identification of functional microbial components and their role in inflammation, angiogenesis, and vascular remodeling. Experimental models to explore microbiota–host interactions and their impact on placental health and preeclampsia. |
| External Factor | Description | Effect on Microbiota | Implications for Preeclampsia |
|---|---|---|---|
| Diet | High-fat or ultra-processed foods promote dysbiosis. | Reduces microbial diversity and short-chain fatty acid production. | Imbalanced diets increase systemic inflammation and the risk of preeclampsia. |
| Antibiotic Use | Disrupts protective bacterial communities; sometimes necessary but with potential long-term effects. | Reduces microbial diversity. | Heightens susceptibility to inflammatory complications. |
| Obesity | Associated with reduced microbial diversity and a pro-inflammatory microbiota profile. | Creates a pro-inflammatory microbiota with decreased diversity. | Increases the risk of metabolic and hypertensive disorders, including preeclampsia. |
| Stress | Alters gut microbiota, increasing intestinal permeability and systemic inflammation. | Increases intestinal permeability and systemic inflammation. | Contributes to endothelial dysfunction and other preeclampsia-related complications. |
| Sedentary Lifestyle | Aggravates the negative effects of other factors. | Reduces microbiota resilience to harmful factors. | Potentially increases inflammation and metabolic imbalance, predisposing to preeclampsia. |
| Intervention | Mechanism of Action | Potential Benefits in Preeclampsia |
|---|---|---|
| Probiotics | Contain beneficial strains like Lactobacillus and Bifidobacterium. Enhance microbial diversity and modulate gut and vaginal flora. | Reduce systemic inflammation. Strengthen intestinal and vaginal mucosal barriers. Lower pro-inflammatory cytokines (e.g., TNF-α, IL-6). |
| Prebiotics | Dietary fibers that stimulate the growth of beneficial bacteria and enhance SCFA production. | Support gut barrier integrity. Promote anti-inflammatory pathways. Decrease intestinal permeability and endotoxin translocation. |
| Mediterranean Diet | Emphasizes high intake of fruits, vegetables, whole grains, legumes, and unsaturated fats (e.g., olive oil). | Increase microbial diversity. Boost SCFAs production. Mitigate systemic inflammation and endothelial dysfunction. |
| Fiber-Rich Diets | Diets with elevated fiber intake to support the growth of beneficial bacterial species. | Promote Lactobacillus and Bifidobacterium proliferation. Improve vascular function. Reduce inflammation and oxidative stress. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Torres, J.; Basurto-Serrano, J.A.; Camacho-Martinez, Z.A.; Guadarrama-Sanchez, F.R.; Monroy-Muñoz, I.E.; Perez-Duran, J.; Solis-Paredes, J.M.; Martinez-Portilla, R.; Espino-y-Sosa, S.; Ramirez-Gonzalez, A.; et al. Microbiota Dysbiosis: A Key Modulator in Preeclampsia Pathogenesis and Its Therapeutic Potential. Microorganisms 2025, 13, 245. https://doi.org/10.3390/microorganisms13020245
Torres-Torres J, Basurto-Serrano JA, Camacho-Martinez ZA, Guadarrama-Sanchez FR, Monroy-Muñoz IE, Perez-Duran J, Solis-Paredes JM, Martinez-Portilla R, Espino-y-Sosa S, Ramirez-Gonzalez A, et al. Microbiota Dysbiosis: A Key Modulator in Preeclampsia Pathogenesis and Its Therapeutic Potential. Microorganisms. 2025; 13(2):245. https://doi.org/10.3390/microorganisms13020245
Chicago/Turabian StyleTorres-Torres, Johnatan, Jorge Alberto Basurto-Serrano, Zaira Alexi Camacho-Martinez, Francisco Rafael Guadarrama-Sanchez, Irma Eloisa Monroy-Muñoz, Javier Perez-Duran, Juan Mario Solis-Paredes, Raigam Martinez-Portilla, Salvador Espino-y-Sosa, Andrea Ramirez-Gonzalez, and et al. 2025. "Microbiota Dysbiosis: A Key Modulator in Preeclampsia Pathogenesis and Its Therapeutic Potential" Microorganisms 13, no. 2: 245. https://doi.org/10.3390/microorganisms13020245
APA StyleTorres-Torres, J., Basurto-Serrano, J. A., Camacho-Martinez, Z. A., Guadarrama-Sanchez, F. R., Monroy-Muñoz, I. E., Perez-Duran, J., Solis-Paredes, J. M., Martinez-Portilla, R., Espino-y-Sosa, S., Ramirez-Gonzalez, A., Guadarrama-Mora, R., & Rojas-Zepeda, L. (2025). Microbiota Dysbiosis: A Key Modulator in Preeclampsia Pathogenesis and Its Therapeutic Potential. Microorganisms, 13(2), 245. https://doi.org/10.3390/microorganisms13020245


_Di_Marco.png)







