The Role of Skunks in the Epidemiology of Rabies in the State of Yucatan from 2000 to 2022: Current Perspectives and Future Research Directions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Selection
2.2. Diagnosis and Antigenic Characterization of the Rabies Virus
2.3. Genetic Characterization of the Rabies Virus
2.4. Genetic Characterization of Skunk Species
2.4.1. Design of Specific Oligonucleotides
2.4.2. Standardization of Polymerase Chain Reaction (PCR) for Genetic Characterization of Skunks’ Species
2.5. Sequencing and Phylogenetic Reconstruction of the Rabies Virus
2.6. Sequencing and Phylogenetic Reconstruction of Skunks Species
2.7. Genetic Distance Analysis of the Cyt b Sequences
3. Results
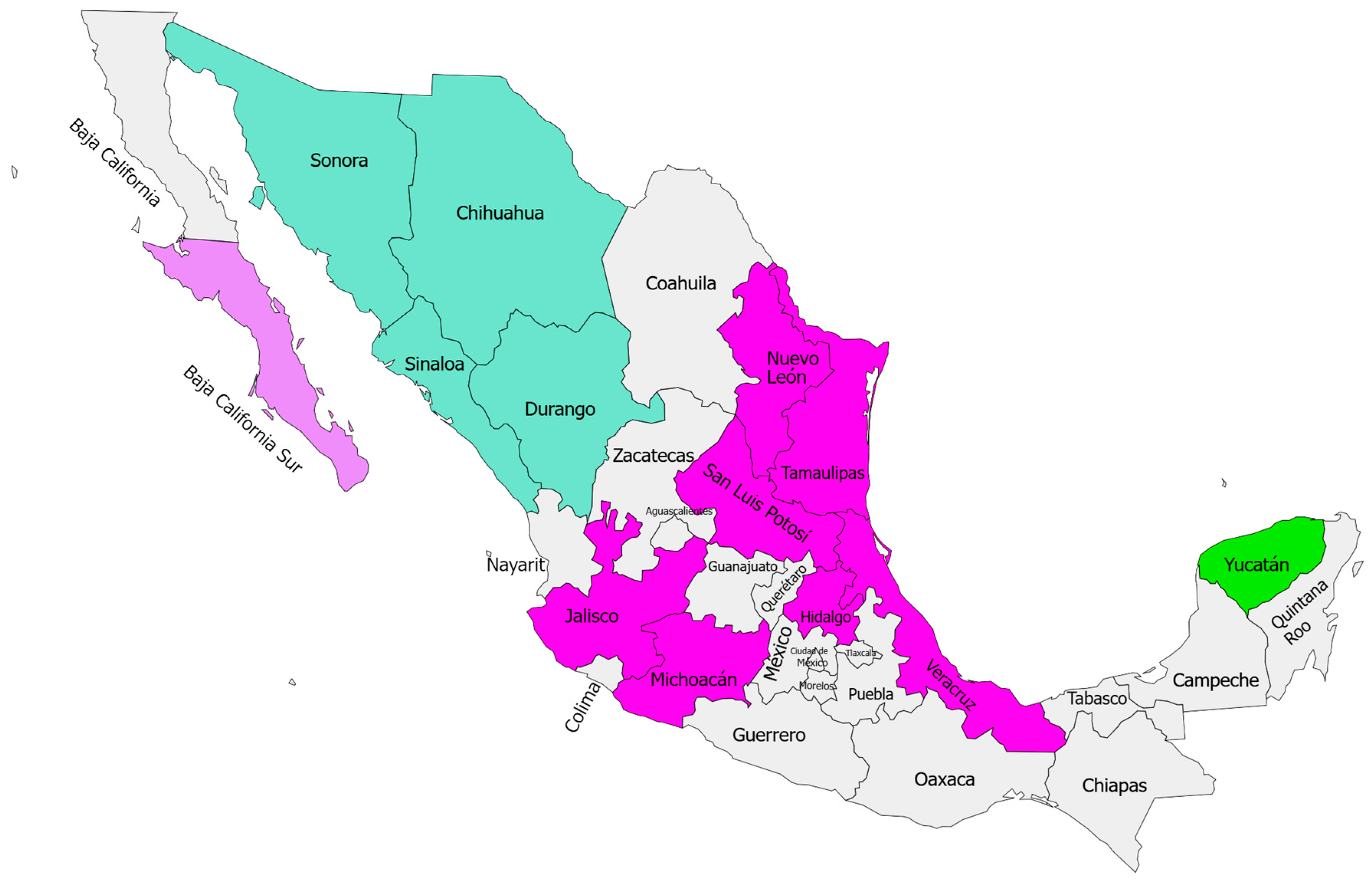
3.1. Rabies Virus Diagnosis and Antigenic Characterization
Rabies Virus Genetic Characterization
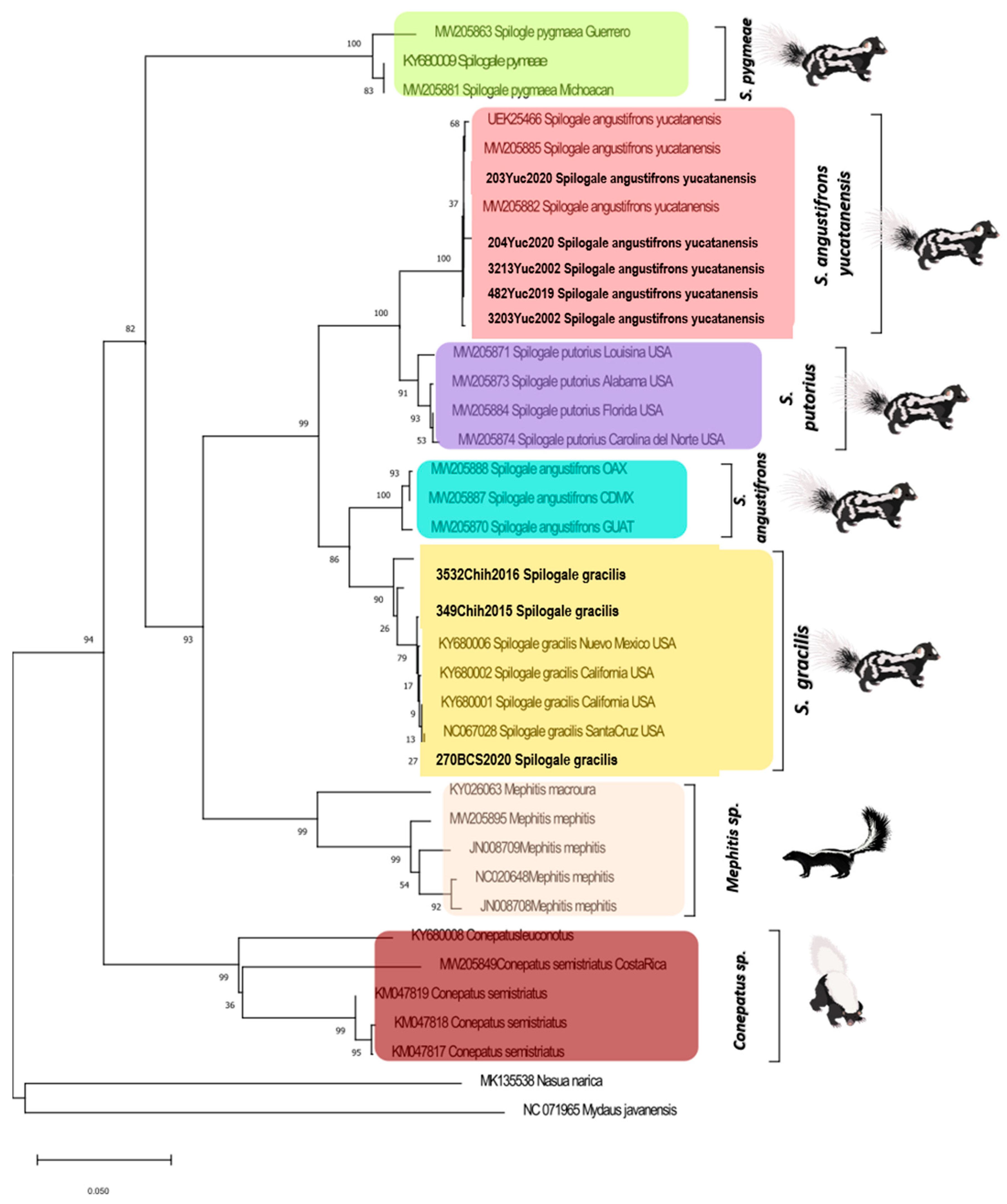
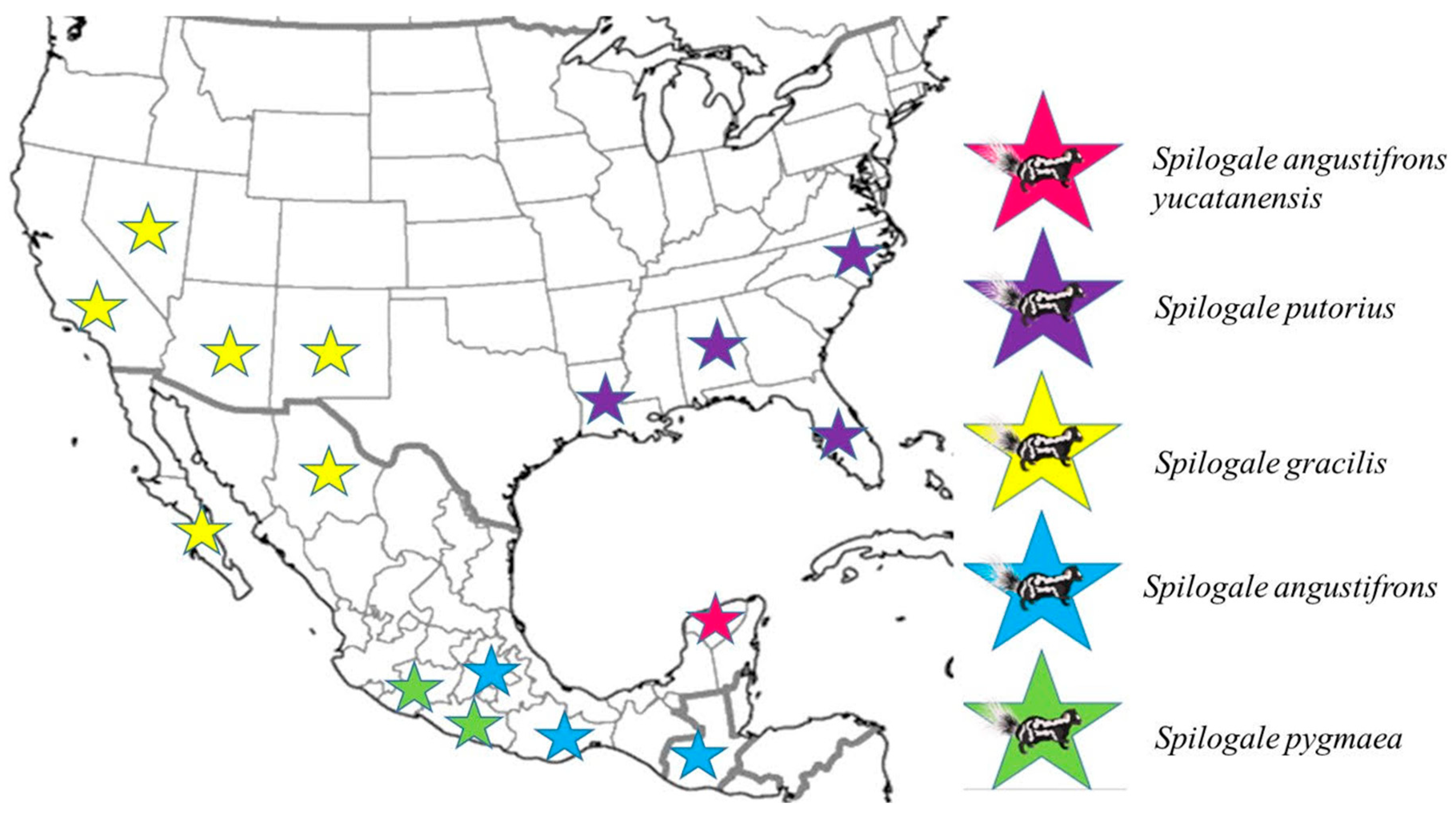
3.2. Skunk Genetic Characterization
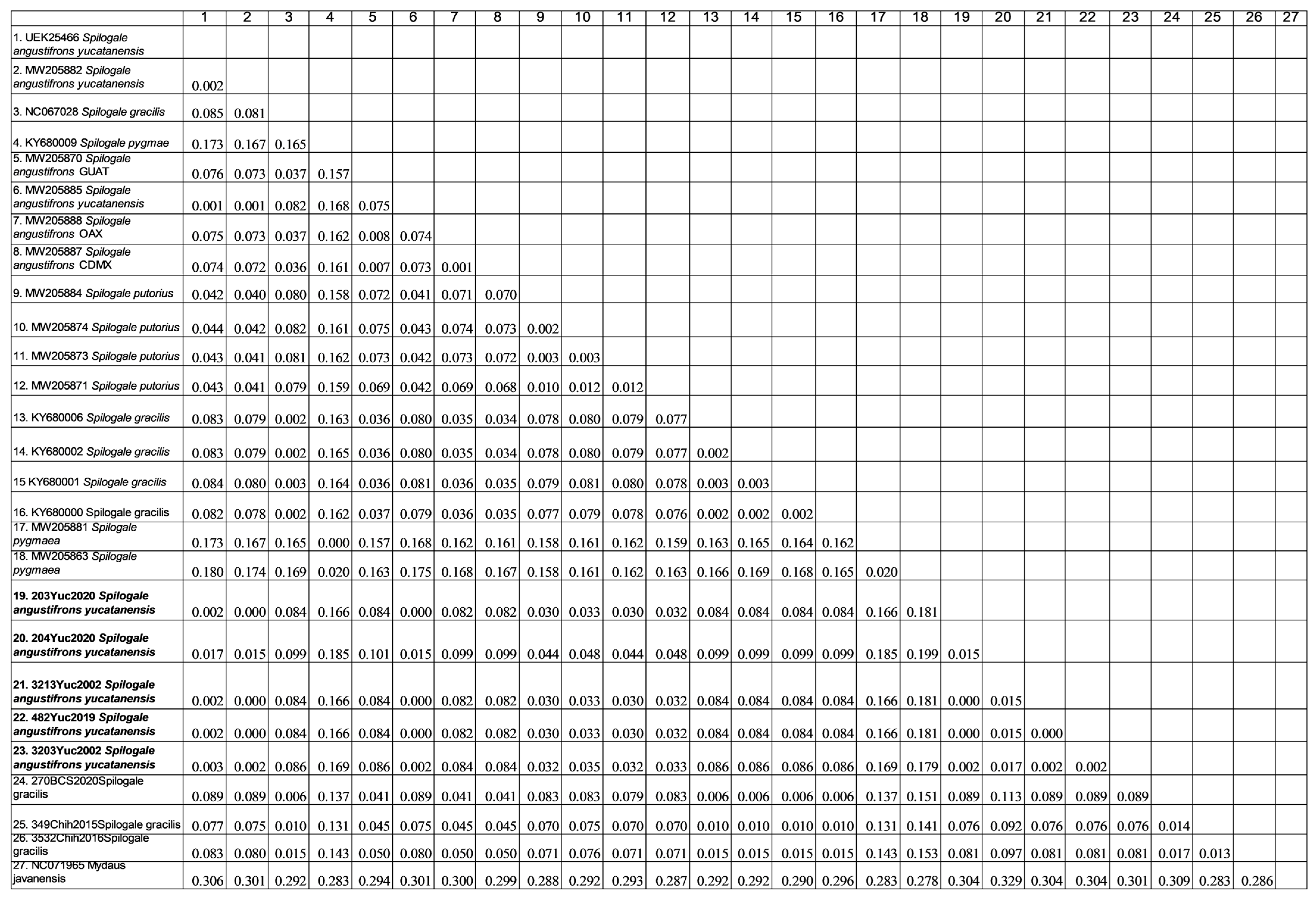
Genetic Distance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rupprecht, C.E.; Hanlon, C.A.; Hemachudha, T. Rabies re-examined. Lancet Infect. Dis. 2002, 2, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Frantchez, V.; Medina, J. Rabia: 99.9% mortal, 100% prevenible. Rev. Medica Del. Urug. 2018, 34, 164–171. [Google Scholar] [CrossRef]
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The spread and evolution of rabies virus: Conquering new frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Rabies Fact Sheet; WHO: Geneva, Switzerland, 2019; Available online: http://www.who.int/mediacentre/factsheets/fs099/es/ (accessed on 15 May 2024).
- Krebs, J.W.; Wilson, M.L.; Childs, J.E. Rabies: Epidemiology, Prevention, and Future Research. J. Mammal. 1995, 76, 681–694. [Google Scholar] [CrossRef]
- Garcés-Ayala, F.; Aréchiga-Ceballos, N.; Ortiz-Alcántara, J.M.; González-Durán, E.; Pérez-Agüeros, S.I.; Méndez-Tenorio, A.; Torres-Longoria, B.; López-Martínez, I.; Hernández-Rivas, L.; Díaz-Quiñonez, J.A.; et al. Molecular characterization of atypical antigenic variants of canine rabies virus reveals its reintroduction by wildlife vectors in southeastern Mexico. Arch. Virol. 2017, 162, 3629–3637. [Google Scholar] [CrossRef]
- Aréchiga Ceballos, N.; Puebla Rodríguez, P.; Aguilar Setién, Á. The New Face of Human Rabies in Mexico, What’s Next After Eradicating Rabies in Dogs. Vector-Borne Zoonotic Dis. 2022, 22, 69–75. [Google Scholar] [CrossRef]
- Ramírez-Pulido, J.; Castro-Campillo, A. Lista Taxonómica de los Mamíferos Terrestres de México; Museum of Texas Tech University: Lubbock, TX, USA, 1996. [Google Scholar] [CrossRef]
- Juárez Agis, N.; García Sánchez, S.; Olivier Salomé, B.; Zeferino Torres, J.Y.; Rivas González, M. Los Zorrillos en México un grupo vulnerable poco conocido. Biodiversitas 2020, 153, 12–16. [Google Scholar]
- Fehlner-Gardiner, C. Rabies control in North America-past, present and future. Rev. Sci. Et Tech. 2018, 37, 421–437. [Google Scholar] [CrossRef]
- Jaramillo-Reyna, E.; Almazán-Marín, C.; de la O-Cavazos, M.E.; Valdéz-Leal, R.; Bañuelos-Álvarez, A.H.; Zúñiga-Ramos, M.A.; Melo-Munguía, M.; Gómez-Sierra, M.; Sandoval-Borja, A.; Chávez-López, S.; et al. Public veterinary medicine: Public health rabies virus variants identified in Nuevo Leon State, Mexico, from 2008 to 2015. J. Am. Vet. Med. Assoc. 2020, 256, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Aranda, M.; López-de Buen, L. Rabies in skunks from Mexico. J. Wildl. Dis. 1999, 35, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pulido, J.; Arroyo-Cabrales, J.; Castro-Campillo, A. Estado actual y relación nomenclatural de los mamíferos terrestres de México. Acta Zoológica Mex. 2005, 21, 21–82. [Google Scholar] [CrossRef]
- Gilbert, A.T. Rabies virus vectors and reservoir species. Rev. Sci. Et Tech. 2018, 37, 371–384. [Google Scholar] [CrossRef]
- Ewer, R.F. The Carnivores; Cornell University Press: Ithaca, NY, USA, 1998. [Google Scholar]
- Dragoo, J.W.; Sheffield, S.R. Conepatus leuconotus (Carnivora: Mephitidae). Mamm. Species 2009, 827, 1–8. [Google Scholar] [CrossRef]
- Koepfli, K.P.; Dragoo, J.W.; Wang, X. The evolutionary history and molecular systematics of the Musteloidea. In Biology and Conservation of Musteloids; Oxford University Press: Oxford, UK, 2017; pp. 75–91. [Google Scholar]
- McDonough, M.M.; Ferguson, A.W.; Dowler, R.C.; Gompper, M.E.; Maldonado, J.E. Phylogenomic systematics of the spotted skunks (Carnivora, Mephitidae, Spilogale): Additional species diversity and Pleistocene climate change as a major driver of diversification. Mol. Phylogenetics Evol. 2022, 167, 107266. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.; Harrington, L.; Newman, C. Dramatis Personae: An Introduction to the Wild Musteloids; Macdonald, D., Newman, C., Harrington, l., Eds.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Hernández-Sánchez, A.; Santos-Moreno, A.; Pérez-Irineo, G. The Mephitidae in the Americas: A review of the current state of knowledge and future research priorities. Mamm. Biol. 2022, 102, 307–320. [Google Scholar] [CrossRef]
- Hass, C.C.; Dragoo, J.W. Rabies in hooded and striped skunks in Arizona. J. Wildl. Dis. 2006, 42, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Loza-Rubio, E.; Nadin-Davis, S.A.; Morales Salinas, E. Caracterización molecular y biológica del virus de la Rabia que circula en zorrillos de México enfocado a la variante del gen de la fosfoproteína (P). Rev. Mex. De Cienc. Pecu. 2012, 3, 155–170. [Google Scholar]
- Velasco-Villa, A.; Reeder, S.A.; Orciari, L.A.; Yager, P.A.; Franka, R.; Blanton, J.D.; Zuckero, L.; Hunt, P.; Oertli, E.H.; Robinson, L.E.; et al. Enzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United States. Emerg. Infect. Dis. 2008, 14, 1849. [Google Scholar] [CrossRef]
- Ceballos, G.; Miranda, A. Los Mamíferos de Chamela, Jalisco: Manual de Campo; Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 1986. [Google Scholar]
- Puebla-Rodríguez, P.; Almazán-Marín, C.; Garcés-Ayala, F.; Rendón-Franco, E.; Chávez-López, S.; Gómez-Sierra, M.; Sandoval-Borja, A.; Martínez-Solís, D.; Escamilla-Ríos, B.; Sauri-González, I.; et al. Rabies virus in white-nosed coatis (Nasua narica) in Mexico: What do we know so far? Front. Vet. Sci. 2023, 10, 1090222. [Google Scholar] [CrossRef]
- Salgado-Cardoso, A.M.; Olave-Leyva, J.I.; Morales, I.; Aguilar-Setién, A.; López-Martínez, I.; Aréchiga-Ceballos, N. Cats: The New Challenge for Rabies Control in the State of Yucatan, Mexico. Pathogens 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- CONABIO. National Commission for the Knowledge and Use of Biodiversity. 2024. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 21 June 2024).
- Leopold, S.A. Fauna Silvestre de México: Aves y Mamíferos de Caza; Primera Edición en Español; Instituto Mexicano de Recursos Naturales Renovables: Mexico City, Mexico, 1965. [Google Scholar]
- Marmi, J.; López-Giráldez, J.F.; Domingo-Roura, X. Phylogeny, evolutionary history and taxonomy of the Mustelidae based on sequences of the cytochrome b gene and a complex repetitive flanking region. Zool. Scr. 2004, 33, 481–499. [Google Scholar] [CrossRef]
- Dragoo, J.W.; Bradley, R.D.; Honeycutt, R.L.; Templeton, J.W. Phylogenetic relationships among the skunks: A molecular perspective. J. Mamm. Evol. 1993, 1, 255–267. [Google Scholar] [CrossRef]
- Floyd, C.H.; Van Vuren, D.H.; Crooks, K.R.; Jones, K.L.; Garcelon, D.K.; Belfiore, N.M.; Dragoo, J.W.; May, B. Genetic differentiation of island spotted skunks, Spilogale gracilis amphiala. J. Mammal. 2011, 92, 148–158. [Google Scholar] [CrossRef]
- Ferguson, A.W.; McDonough, M.M.; Guerra, G.I.; Rheude, M.; Dragoo, J.W.; Ammerman, L.K.; Dowler, R.C. Phylogeography of a widespread small carnivore, the western spotted skunk (Spilogale gracilis) reveals temporally variable signatures of isolation across western North America. Ecol. Evol. 2017, 7, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.A.; Dowler, R.C.; Perkins, J.C.; Ferguson, A.W.; McDonough, M.M.; Ammerman, L.K. Genetic variation in the eastern spotted skunk (Spilogale putorius) with emphasis on the plains spotted skunk (S. p. interrupta). J. Mammal. 2018, 99, 1237–1248. [Google Scholar] [CrossRef]
- Howell, A.H. Revision of the Skunks of the Genus Spilogale (No. 26); US Government Printing Office: Washington, DC, USA, 1906.
- Van Gelder, R.G. A Taxonomic Revision of the Spotted Skunks (Genus Spilogale); University of Illinois at Urbana-Champaign: Champaign, IL, USA, 1958; Bulletin of the American Museum of Natural History. 117 (5). Manhattan, New York, USA 1959. [Google Scholar]
- Howell, A.H. Three new skunks of the genus Spilogale. Proc. Biol. Soc. Wash. 1902, 15, 242. [Google Scholar]
- Owen, J.G.; Baker, R.J.; Williams, S.L. Karyotypic Variation in Spotted Skunks (Carnivora: Mustelidae: Spilogale) from Texas, Mexico and El Salvador. Tex. J. Sci. 1996, 48, 119–122. [Google Scholar]
- Wozencraft, W.C. Order carnivora. In Mammal Species of the World: A Taxonomic and Geographic Reference; Johns Hopkins University Press: Baltimore, MD, USA, 2005; Volume 1, pp. 532–628. [Google Scholar]
- Diaz, A.M.; Papo, S.; Rodriguez, A.; Smith, J.S. Antigenic analysis of rabies-virus isolates from Latin America and the Caribbean. J. Vet. Med. Ser. B 1994, 41, 153–160. [Google Scholar] [CrossRef]
- Markotter, W.; Kuzmin, I.; Rupprecht, C.E.; Randles, J.; Sabeta, C.T.; Wandeler, A.I.; Nel, L.H. Isolation of Lagos bat virus from water mongoose. Emerg. Infect. Dis. 2006, 12, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.R.; Baron, E.J.; Pfaller, M.A.; Tenover, F.C.; Yolken, R.H.; Morgan, D.R. Manual of Clinical Microbiology, 6th ed.; 1995; Volume 3, p. 449. [Google Scholar]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; The UGENE team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ayala, F.; Aguilar-Setién, Á.; Almazán-Marín, C.; Cuautle-Zavala, C.; Chávez-López, S.; Martínez-Solís, D.; Gómez-Sierra, M.; Sandoval-Borja, A.; Escamilla-Ríos, B.; López-Martínez, I.; et al. Rabies Virus Variants Detected from Cougar (Puma concolor) in Mexico 2000–2021. Pathogens 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Greenwood, R.J.; Newton, W.E.; Pearson, G.L.; Schamber, G.J. Population and movement characteristics of radio-collared striped skunks in North Dakota during an epizootic of rabies. J. Wildl. Dis. 1997, 33, 226–241. [Google Scholar] [CrossRef]
- Krebs, J.W.; Mandel, E.J.; Swerdlow, D.L.; Rupprecht, C.E. Rabies surveillance in the United States during 2004. J. Am. Vet. Med. Assoc. 2005, 227, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.J.; Messenger, S.; Rohde, R.E.; Smith, J.; Cheshier, R.; Hanlon, C.; Rupprecht, C.E. Bat-associated rabies virus in skunks. Emerg. Infect. Dis. 2006, 12, 1274. [Google Scholar] [CrossRef] [PubMed]
- Oertli, E.H.; Wilson, P.J.; Hunt, P.R.; Sidwa, T.J.; Rohde, R.E. Epidemiology of rabies in skunks in Texas. J. Am. Vet. Med. Assoc. 2009, 234, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.K.; Hanlon, C.A.; Goodin, D.G.; Davis, R.; Moore, M.; Moore, S.; Anderson, G.A. Bayesian spatiotemporal pattern and eco-climatological drivers of striped skunk rabies in the North Central Plains. PLoS Neglected Trop. Dis. 2016, 10, e0004632. [Google Scholar] [CrossRef]
- Wohlers, A.; Lankau, E.W.; Oertli, E.H.; Maki, J. Challenges to controlling rabies in skunk populations using oral rabies vaccination: A review. Zoonoses Public Health 2018, 65, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.W.; Honeycutt, R.L.; Schmidly, D.J. Taxonomic status of white-backed hog-nosed skunks, genus Conepatus (Carnivora: Mephitidae). J. Mammal. 2003, 84, 159–176. [Google Scholar] [CrossRef]
- Parker, R.L. Rabies in skunks. In The Natural History of Rabies; Baer, G.M., Ed.; Academic Press: New York, NY, USA, 1975; pp. 41–51. [Google Scholar]
- Tinline, R.R.; MacInnes, C. D Ecogeographic patterns of rabies in southern Ontario based on time series analysis. J. Wildl. Dis. 2004, 40, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Nadin-Davis, S.A.; Moore, M.; Hanlon, C. Genetic characterization and phylogenetic analysis of skunk-associated rabies viruses in North America with special emphasis on the central plains. Virus Res. 2013, 174, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mejenes-López, S.d.M.A.; Gálvez-Aguilera, X.; Escalona-Segura, G.; Vargas-Contreras, J.A.; Retana-Guiascón, O.G.; Cab-Paat, G.d.L. First record of the coexistence of two mesocarnivores in the Yucatán Peninsula, México. Therya Notes 2021, 2, 79–84. [Google Scholar] [CrossRef]
- Contreras-Moreno, F.; E Simá-Pantí, D.; Cruz-Romo, L.; Petrone, S.; Méndez-Saint, G.; Méndez-Tun, J.; Jesús-Espinosa, D.; Cruz-Molina, I.; Mayor, C.C.-C.Y.; Duque, V. Registros destacados de Spilogale angustifrons en la Reserva de la Biosfera Calakmul. Rev. Colomb. De Cienc. Anim.—RECIA 2022, 14, e913. [Google Scholar] [CrossRef]
- Riddle, B.R.; Hafner, D.J. A step-wise approach to integrating phylogeographic and phylogenetic biogeographic perspectives on the history of a core North American warm deserts biota. J. Arid. Environ. 2006, 66, 435–461. [Google Scholar] [CrossRef]
- Hillman, S.S.; Drewes, R.C.; Hedrick, M.S.; Hancock, T.V. Physiological vagility and its relationship to dispersal and neutral genetic heterogeneity in vertebrates. J. Exp. Biol. 2014, 217, 3356–3364. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Van Vuren, D.H.; McEachern, M.B.; Crooks, K.R.; Dragoo, J.W.; May, B. Spatial and genetic organization of the island spotted skunk, Spilogale gracilis amphiala. Southwest. Nat. 2013, 58, 481–486. [Google Scholar] [CrossRef]
- Lesmeister, D.B.; Millspaugh, J.J.; Gompper, M.E.; Mong, T.W. Eastern spotted skunk (Spilogale putorius) survival and cause-specific mortality in the Ouachita Mountains, Arkansas. Am. Midl. Nat. 2010, 164, 52–60. [Google Scholar] [CrossRef]
- Weyandt, S.E.; Van Den Bussche, R.A. Phylogeographic structuring and volant mammals: The case of the pallid bat (Antrozous pallidus). J. Biogeogr. 2007, 34, 1233–1245. [Google Scholar] [CrossRef]
- Vázquez-Domínguez, E.; Arita, H.T. The Yucatan peninsula: Biogeographical history 65 million years in the making. Ecography 2010, 33, 212–219. [Google Scholar] [CrossRef]
- Loza, E.; Aguilar-Setien, A.; Tordo, N. Evidencias de una nueva variante de virus de rabia en México, que circula en zorrillos. In Folleto de Investigación; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP): Ciudad de México, México, 1998. [Google Scholar]
- Velasco-Villa, A.; Orciari, L.A.; Souza, V.; Juárez-Islas, V.; Gomez-Sierra, M.; Castillo, A.; Flisser, A.; Rupprecht, C.E. Molecular epizootiology of rabies associated with terrestrial carnivores in Mexico. Virus Res. 2005, 111, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Villa, A.; Mauldin, M.R.; Shi, M.; Escobar, L.E.; Gallardo-Romero, N.F.; Damon, I.; Olson, V.A.; Streicker, D.G.; Emerson, G. The history of rabies in the Western Hemisphere. Antivir. Res. 2017, 146, 221–232. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Smith, J.S.; Fekadu, M.; Childs, J.E. The ascension of wildlife rabies: A cause for public health concern or intervention? Emerg. Infect. Dis. 1995, 1, 107. [Google Scholar] [CrossRef] [PubMed]

| No. InDRE | Year | Institution | Municipality | State | Origin Diagnosis | Antigenic Variant | GenBank Access Rabies Virus | GenBank Access Cyt b |
|---|---|---|---|---|---|---|---|---|
| 3203MxskYuc02 | 2002 | State Public Health Laboratory of Yucatan | ND | Yucatan | Positive | Atypical | NP | PV012461 |
| 3213MxskYuc02 | 2002 | State Public Health Laboratory of Yucatan | ND | Yucatan | Positive | RABVV1 | NP | PV012462 |
| 482MxskYuc19 | 2019 | State Public Health Laboratory of Yucatan | ND | Yucatan | Positive | Atypical | PQ963930 | PV012463 |
| 203MxskYuc20 | 2020 | State Public Health Laboratory of Yucatan | Kinchil | Yucatan | Positive | Atypical | PQ963931 | PV012464 |
| 204MxskYuc20 | 2020 | State Public Health Laboratory of Yucatan | Kinchil | Yucatan | Positive | Atypical | PQ963932 | PV012465 |
| 270MxskBCS20 | 2020 | State Public Health Laboratory of BCS | ND | Baja California Sur | Positive | RABVV10 | NP | PV012466 |
| 349MxskChih15 | 2015 | State Public Health Laboratory of Chihuahua | ND | Chihuahua | Positive | RABVV1-“ Atypical” | NP | PV012467 |
| 3124MxskChih16 | 2016 | State Public Health Laboratory of Chihuahua | ND | Chihuahua | Positive | RABVV1-“ Atypical” | NP | PV012468 |
| Genus | Oligo | Sequence | Expected Molecular Weight | |
|---|---|---|---|---|
| Spilogale | Sa FW | 5′ TCAWCATGATGAAACTTCGGTTCC 3′ | Fw | 678 bp |
| Spilogale | Sa RV | 5′ CCTGTTTCATGAAGGAACAGTAAATG 3′ | Rv | |
| Conepatus | Cs FW | 5′ GCTCTCTACTCGGAAyCTGC 3′ | Fw | 679 bp |
| Conepatus | Cs RV | 5′ TCGTGTAGGAATAATAGGTGGAC 3′ | Rv | |
| Mephitis | Mm FW | 5′ TCAwCATGATGAAACTTCGGTTCC 3′ | Fw | 678 bp |
| Mephitis | Mm RV | 5′ CCTGTTTCATGTAGGAAATAGTAAGTG3′ | Rv |
| Reagent | Volume |
|---|---|
| Buffer Mix | 5 µL |
| Taq DNA Polymerasa | 1 µL |
| Primer Fw (20 pmol/µL) | 2 µL |
| Primer Rv (20 pmol/µL) | 2 µL |
| Water PCR | 9.5 µL |
| dNTPs (10 nM) | 2.5 µL |
| MgCl2 | 3 µL |
| Thermocycler | Program |
|---|---|
| Step | |
| 1 cycle | 3 min 94 °C |
| 39 cycles | 30 seg 94 °C |
| 39 cycles | 15 seg 60 °C |
| 39 cycles | 30 seg 72 °C |
| 1 cycle | 7 min 72 °C |
| ∞ | 4 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puebla-Rodríguez, P.; García-González, O.P.; Sánchez-Sánchez, R.; Díaz-Sánchez, M.; Del Mazo, J.C.; Sauri-González, I.; Alonzo-Góngora, A.; García-Rodríguez, G.; López-Martínez, I.; Aréchiga-Ceballos, N. The Role of Skunks in the Epidemiology of Rabies in the State of Yucatan from 2000 to 2022: Current Perspectives and Future Research Directions. Microorganisms 2025, 13, 262. https://doi.org/10.3390/microorganisms13020262
Puebla-Rodríguez P, García-González OP, Sánchez-Sánchez R, Díaz-Sánchez M, Del Mazo JC, Sauri-González I, Alonzo-Góngora A, García-Rodríguez G, López-Martínez I, Aréchiga-Ceballos N. The Role of Skunks in the Epidemiology of Rabies in the State of Yucatan from 2000 to 2022: Current Perspectives and Future Research Directions. Microorganisms. 2025; 13(2):262. https://doi.org/10.3390/microorganisms13020262
Chicago/Turabian StylePuebla-Rodríguez, Paola, Octavio Patricio García-González, Rocío Sánchez-Sánchez, Mauricio Díaz-Sánchez, Juan Carlos Del Mazo, Isaías Sauri-González, Adriana Alonzo-Góngora, Gabriel García-Rodríguez, Irma López-Martínez, and Nidia Aréchiga-Ceballos. 2025. "The Role of Skunks in the Epidemiology of Rabies in the State of Yucatan from 2000 to 2022: Current Perspectives and Future Research Directions" Microorganisms 13, no. 2: 262. https://doi.org/10.3390/microorganisms13020262
APA StylePuebla-Rodríguez, P., García-González, O. P., Sánchez-Sánchez, R., Díaz-Sánchez, M., Del Mazo, J. C., Sauri-González, I., Alonzo-Góngora, A., García-Rodríguez, G., López-Martínez, I., & Aréchiga-Ceballos, N. (2025). The Role of Skunks in the Epidemiology of Rabies in the State of Yucatan from 2000 to 2022: Current Perspectives and Future Research Directions. Microorganisms, 13(2), 262. https://doi.org/10.3390/microorganisms13020262






