Characterization of Microbial Carbon Metabolism in Karst Soils from Citrus Orchards and Analysis of Its Environmental Drivers
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Soil Sampling and Pre-Treatment
2.3. Soil Physicochemical Properties Analysis
2.4. Determination of Soil Microbial Biomass Indicators
2.4.1. Soil Microbial Number
2.4.2. Soil Microbial Biomass Carbon and Nitrogen
2.4.3. Soil Microbial Quotient
2.5. Soil Microbial Utilization of Carbon Sources
2.6. Data Analysis
2.6.1. Statistical Analysis
2.6.2. The Calculation Formula of Soil Microbial Carbon Source Metabolism and Community Functional Diversity
3. Results
3.1. Comparison of Physicochemical Properties and Nutrients of Soils in Karst and Non-Karst Regions
3.2. Comparison of Soil Microbial Number and Biomass Between Karst and Non-Karst Regions
3.2.1. Soil Microbial Number
3.2.2. Soil Microbial Biomass
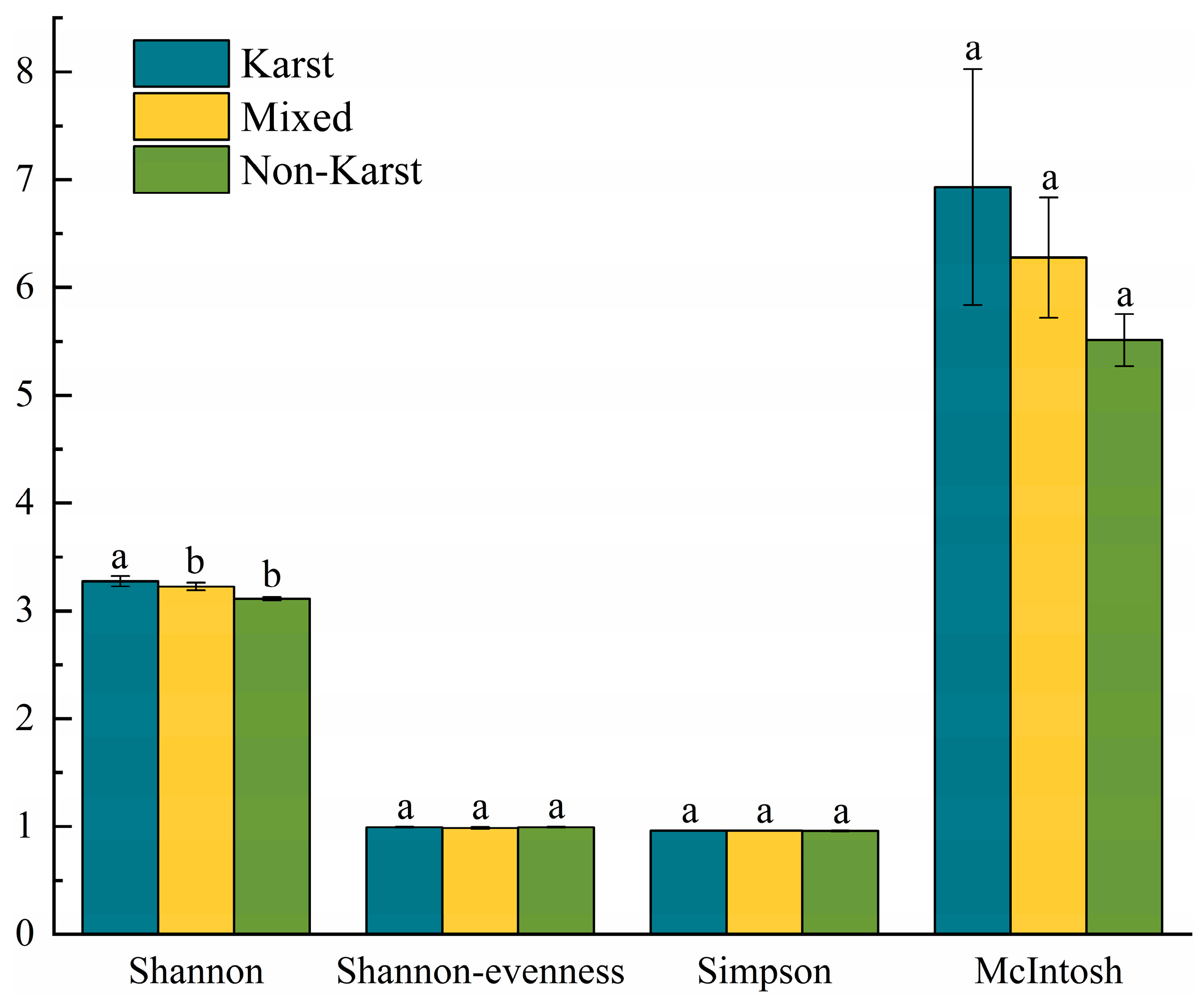
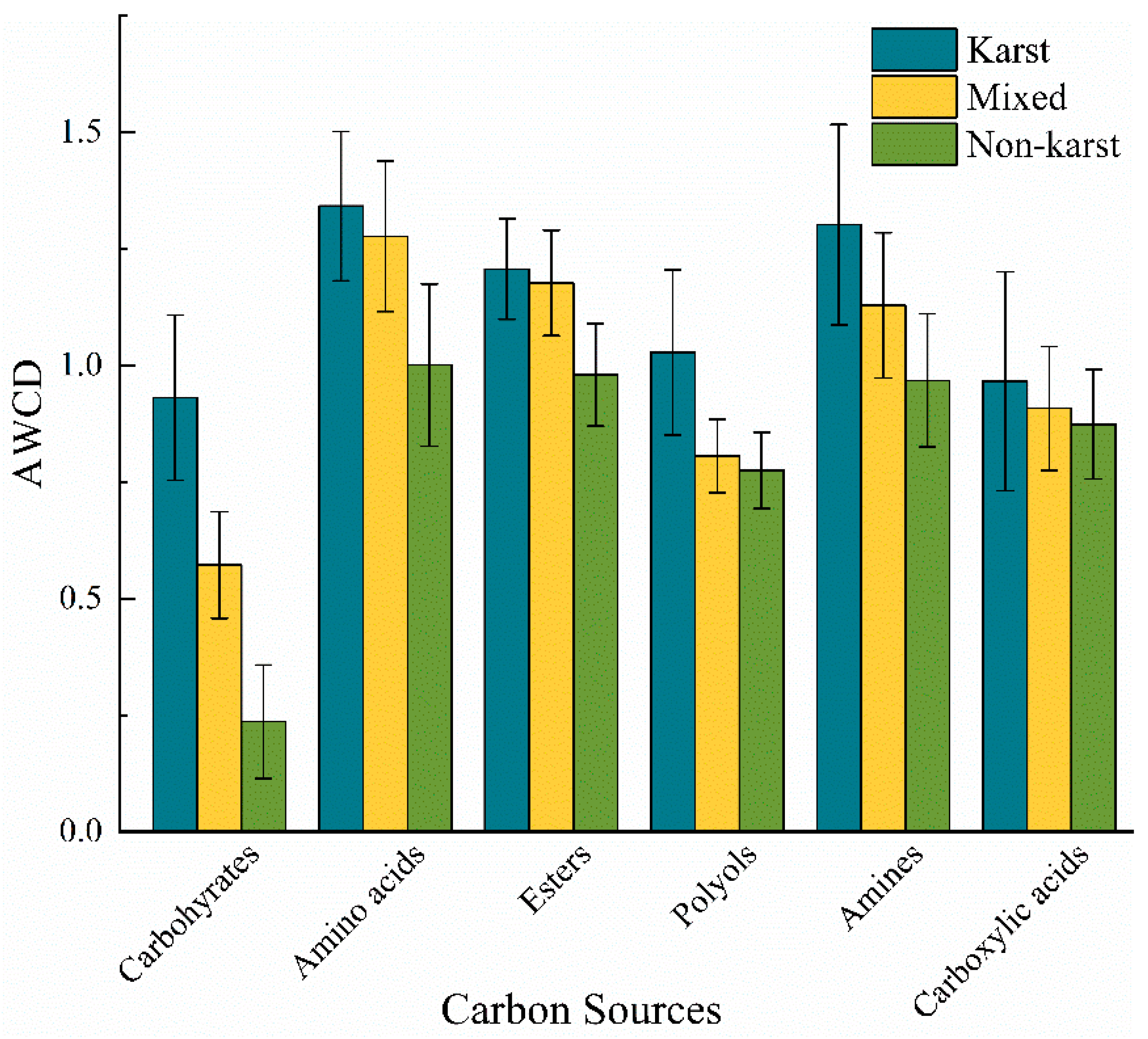
3.3. Soil Microbial Carbon Source Metabolic Diversity
3.3.1. Soil Microbial Carbon Source Metabolic Activity
3.3.2. Soil Microbial Community Functional Diversity
3.3.3. Soil Microbial Carbon Source Utilization Intensity
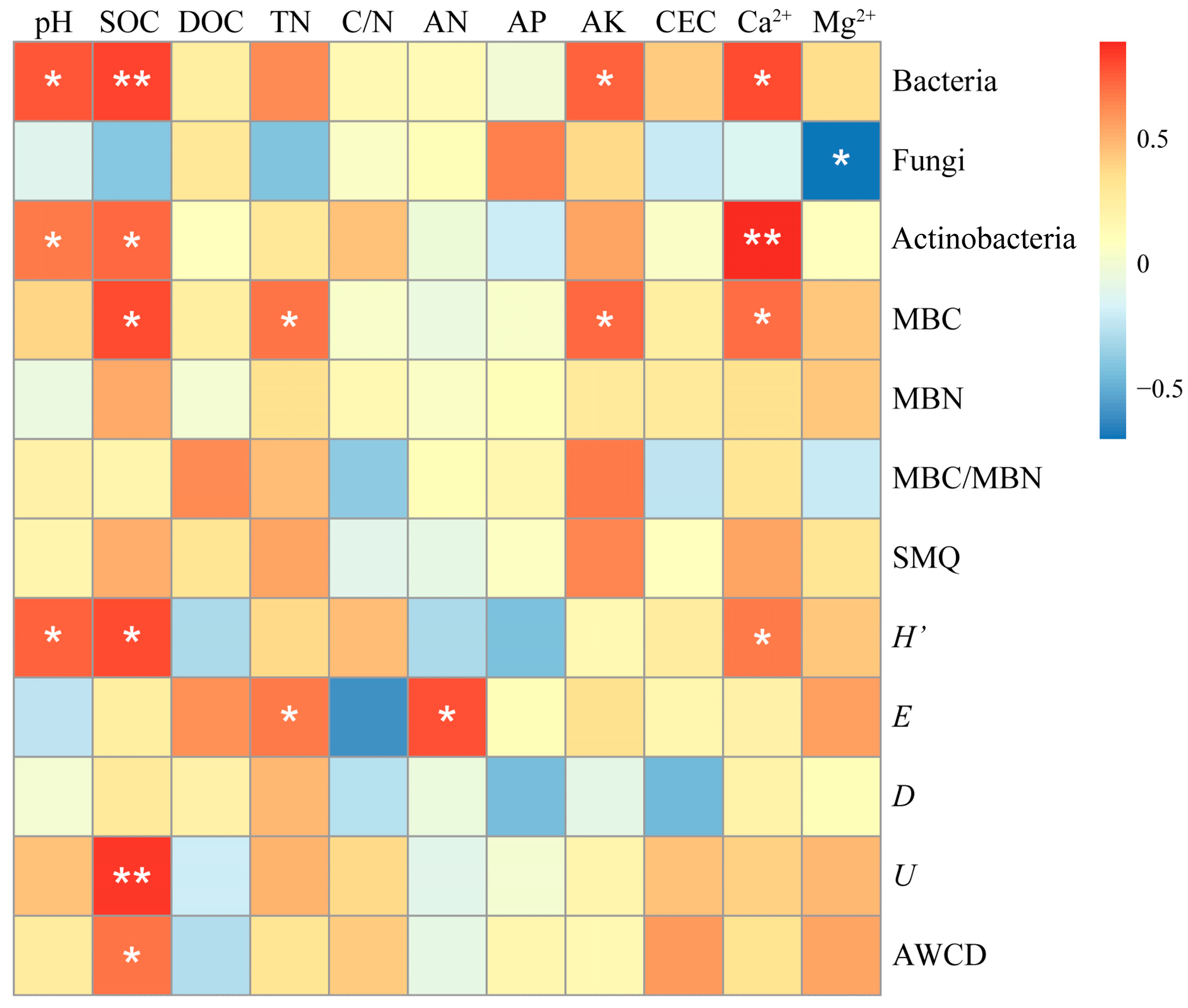
3.3.4. Relationship Between Soil Microbial Number, Community Diversity Index, and Metabolic Activity with Soil Physicochemical Properties
4. Discussion
4.1. Influence Factors of Soil Microbial Quantity and Biomass in Karst Regions
4.2. Influence Factors of Carbon Source Metabolism Diversity of Soil Microorganisms in Karst Regions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the Crucial Role of Soil Microorganisms in Carbon Cycling: A Review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bai, B.; Cao, J.; Wu, Z. Experimental Insights into the Stability of Karst Carbon Sink by Submerged Macrophytes. Environ. Earth Sci. 2024, 83, 422. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Bai, X.; Luo, W.; Tang, H.; Cao, Y.; Wu, L.; Chen, F.; Li, Q.; Zeng, C.; et al. Spatiotemporal Distribution and National Measurement of the Global Carbonate Carbon Sink. Sci. Total Environ. 2018, 643, 157–170. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. Available online: https://data.stats.gov.cn/ (accessed on 6 August 2024).
- Ghorbani, M.; Amirahmadi, E.; Konvalina, P.; Moudrý, J.; Kopecký, M.; Hoang, T.N. Carbon Pool Dynamic and Soil Microbial Respiration Affected by Land Use Alteration: A Case Study in Humid Subtropical Area. Land 2023, 12, 459. [Google Scholar] [CrossRef]
- Bauhus, J.; Khanna, P. The Significance of Microbial Biomass in Forest Soils. In Going Underground-Ecological Studies in Forest Soils; Research Signpost: Trivandrum, India, 1999; pp. 77–110. ISBN 81-86481-88-5. [Google Scholar]
- Huang, Z.; Qin, Y.; He, X.; Zhang, M.; Ren, X.; Yu, W.; Ji, K. Analysis on Metabolic Functions of Rhizosphere Microbial Communities of Pinus massoniana Provenances with Different Carbon Storage by Biolog Eco Microplates. Front. Microbiol. 2024, 15, 1365111. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, X.; Wang, X.; Shu, Q.; Liu, X.; Wu, H.; Gao, S. Functional Diversity of Soil Microorganisms and Influencing Factors in Three Typical Water-Conservation Forests in Danjiangkou Reservoir Area. Forests 2023, 14, 67. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Karanja, N.N.; Gachene, C.K.K.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M.L. Short-Term Dynamics of Soil Organic Matter Fractions and Microbial Activity in Smallholder Potato-Legume Intercropping Systems. Appl. Soil Ecol. 2019, 142, 123–135. [Google Scholar] [CrossRef]
- Luo, R.; Kuzyakov, Y.; Liu, D.; Fan, J.; Luo, J.; Lindsey, S.; He, J.-S.; Ding, W. Nutrient Addition Reduces Carbon Sequestration in a Tibetan Grassland Soil: Disentangling Microbial and Physical Controls. Soil Biol. Biochem. 2020, 144, 107764. [Google Scholar] [CrossRef]
- Dongdong, C.; Qi, L.; Lili, H.; Qian, X.; Xin, C.; Fuquan, H.; Liang, Z. Soil Nutrients Directly Drive Soil Microbial Biomass and Carbon Metabolism in the Sanjiangyuan Alpine Grassland. J. Soil Sci. Plant Nutr. 2023, 23, 3548–3560. [Google Scholar] [CrossRef]
- Iqbal, A.; Ali, I.; Yuan, P.; Khan, R.; Liang, H.; Wei, S.; Jiang, L. Combined Application of Manure and Chemical Fertilizers Alters Soil Environmental Variables and Improves Soil Fungal Community Composition and Rice Grain Yield. Front. Microbiol. 2022, 13, 856355. [Google Scholar] [CrossRef]
- Du, Y.; Wei, Y.; Zhou, Y.; Wang, Y.; Zhang, A.; Wang, T.; Li, Z. Temporal Variation of Microbial Nutrient Limitation in Citrus Plantations: Insights from Soil Enzyme Stoichiometry. Environ. Res. 2024, 258, 119275. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095. [Google Scholar] [CrossRef]
- Deng, R.; Chen, X.; Qiu, L.-P.; Chen, J.-Z.; Meng, S.-L. Bacterial Community Structure and Diversity in the Aqueous Environment of Shihou Lake and Its Relationship with Environmental Factors. Indian J. Microbiol. 2021, 61, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Shi, F.; Liu, Y.; Wang, F.; Dong, S.; Li, M. Composition and Diversity of Soil Microbial Community Associated with Land Use Types in the Agro–Pastoral Area in the Upper Yellow River Basin. Front. Plant Sci. 2022, 13, 819661. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, M.; Cong, J.; Qi, Q.; Xiao, Y.; Cong, W.; Deng, Y.; Zhou, J.; Zhang, Y. Soil pH Exerts Stronger Impacts than Vegetation Type and Plant Diversity on Soil Bacterial Community Composition in Subtropical Broad-Leaved Forests. Plant Soil 2020, 450, 273–286. [Google Scholar] [CrossRef]
- Espinoza-Corral, R.; Schwenkert, S.; Schneider, A. Characterization of the Preferred Cation Cofactors of Chloroplast Protein Kinases in Arabidopsis thaliana. FEBS Open Bio 2023, 13, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-Y. Calcium Ion Channels in Saccharomyces cerevisiae. J. Fungi 2023, 9, 524. [Google Scholar] [CrossRef]
- Zhan, Y.; Yan, N.; Miao, X.; Li, Q.; Chen, C. Different Responses of Soil Environmental Factors, Soil Bacterial Community, and Root Performance to Reductive Soil Disinfestation and Soil Fumigant Chloropicrin. Front. Microbiol. 2021, 12, 796191. [Google Scholar] [CrossRef] [PubMed]
- Groffman, P.M.; Fisk, M.C.; Driscoll, C.T.; Likens, G.E.; Fahey, T.J.; Eagar, C.; Pardo, L.H. Calcium Additions and Microbial Nitrogen Cycle Processes in a Northern Hardwood Forest. Ecosystems 2006, 9, 1289–1305. [Google Scholar] [CrossRef]
- Denardin, L.G.d.O.; Martins, A.P.; Flores, J.P.M.; Alves, L.A.; Pires, C.B.; Machado, D.R.; Anghinoni, I.; Carvalho, P.C.F.; Kuzyakov, Y.; Rice, C.W.; et al. Fertilization Effects on Soil Microbial Composition and Nutrient Availability in Integrated Rice-Livestock Production Systems. Appl. Soil Ecol. 2022, 174, 104420. [Google Scholar] [CrossRef]
- Stautz, J.; Hellmich, Y.; Fuss, M.F.; Silberberg, J.M.; Devlin, J.R.; Stockbridge, R.B.; Hänelt, I. Molecular Mechanisms for Bacterial Potassium Homeostasis. J. Mol. Biol. 2021, 433, 166968. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, W.; Chen, R.; Wang, H.; Duan, Y.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Quicklime and Superphosphate Alleviating Apple Replant Disease by Improving Acidified Soil. ACS Omega 2022, 7, 7920–7930. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, H.; Dai, X.; Chen, M.; Luo, J.; Yang, R.; Ding, F. Effect of Karst Microhabitats on the Structure and Function of the Rhizosphere Soil Microbial Community of Rhododendron pudingense. Sustainability 2023, 15, 7104. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wang, W.; Cheng, X.; Wang, Y.; Li, Q.; Li, L.; Ma, L.; Lu, X.; Tuovinen, O.H. Nitrate Determines the Bacterial Habitat Specialization and Impacts Microbial Functions in a Subsurface Karst Cave. Front. Microbiol. 2023, 14, 1115449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky Desertification in Southwest China: Impacts, Causes, and Restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Beng, K.C.; Corlett, R.T. Identifying the Mechanisms That Shape Fungal Community and Metacommunity Patterns in Yunnan, China. Fungal Ecol. 2019, 42, 100862. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, Z.; Yuan, W.; Chen, W.; Li, X.; Xiong, L.; Cheng, G. Microbial Communities and Soil Respiration during Rice Growth in Paddy Fields from Karst and Non-Karst Areas. Agronomy 2023, 13, 2001. [Google Scholar] [CrossRef]
- Zhu, G.; Zhou, L.; He, X.; Wei, P.; Lin, D.; Qian, S.; Zhao, L.; Luo, M.; Yin, X.; Zeng, L.; et al. Effects of Elevation Gradient on Soil Carbon and Nitrogen in a Typical Karst Region of Chongqing, Southwest China. J. Geophys. Res. Biogeosci. 2022, 127, e2021JG006742. [Google Scholar] [CrossRef]
- Santillán, J.; López-Martínez, R.; Aguilar-Rangel, E.J.; Hernández-García, K.; Vásquez-Murrieta, M.S.; Cram, S.; Alcántara-Hernández, R.J. Microbial Diversity and Physicochemical Characteristics of Tropical Karst Soils in the Northeastern Yucatan Peninsula, Mexico. Appl. Soil Ecol. 2021, 165, 103969. [Google Scholar] [CrossRef]
- Wang, W.; Peng, P.; Li, J.; Liao, X.; Zhang, W.; Wang, K.; Zhao, J. Effects of Vegetation Succession on Soil Microbial Communities on Karst Mountain Peaks. Forests 2024, 15, 586. [Google Scholar] [CrossRef]
- Lu, R. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Li, X.; Jin, Z.; Xiong, L.; Tong, L.; Zhu, H.; Zhang, X.; Qin, G. Effects of Land Reclamation on Soil Bacterial Community and Potential Functions in Bauxite Mining Area. Int. J. Environ. Res. Public Health 2022, 19, 16921. [Google Scholar] [CrossRef]
- Wang, Z.; Hasi, E.; Han, X.; Qingda, M. Fractal Characterization of Soil Particle Size Distribution under Different Land Use Patterns on the North Slope of Wula Mountain in China. J. Soils Sediments 2024, 24, 1148–1164. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Liu, H.; Zhang, F. Long-Term Cultivation Drives Soil Carbon, Nitrogen, and Bacterial Community Changes in the Black Soil Region of Northeastern China. Land Degrad. Dev. 2024, 35, 428–441. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Xin, Y. Changes in and Evaluation of Surface Soil Quality in Populus × xiaohei Shelterbelts in Midwestern Heilongjiang Province, China. J. For. Res. 2021, 32, 1221–1233. [Google Scholar] [CrossRef]
- Bi, X.; Chu, H.; Fu, M.; Xu, D.; Zhao, W.; Zhong, Y.; Wang, M.; Li, K.; Zhang, Y. Distribution Characteristics of Organic Carbon (Nitrogen) Content, Cation Exchange Capacity, and Specific Surface Area in Different Soil Particle Sizes. Sci. Rep. 2023, 13, 12242. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.K.; Mani, C.; Singh, J.; Kim, J.-G.; Mudgett, M.B. A New Leaf Blight of Rice Caused by Pantoea ananatis in India. Plant Dis. 2011, 95, 1582. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, H.; Liu, J.; Luo, H.; Zou, W. Systematic Review of Actinomycetes in the Baijiu Fermentation Microbiome. Foods 2022, 11, 3551. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Lin, X.; Dai, Y.; Lin, G.; Liu, X.; Yang, X. First Report of Sanqi (Panax notoginseng) Root Rot Caused by Pythium Vexans in China. Plant Dis. 2022, 107, 235. [Google Scholar] [CrossRef] [PubMed]
- Bailey, V.L.; Peacock, A.D.; Smith, J.L.; Bolton, H. Relationships between Soil Microbial Biomass Determined by Chloroform Fumigation–Extraction, Substrate-Induced Respiration, and Phospholipid Fatty Acid Analysis. Soil Biol. Biochem. 2002, 34, 1385–1389. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Długosz, J.; Frąc, M.; Gryta, A.; Breza-Boruta, B. Enzymatic Activity and Functional Diversity of Soil Microorganisms along the Soil Profile—A Matter of Soil Depth and Soil-Forming Processes. Geoderma 2022, 416, 115779. [Google Scholar] [CrossRef]
- Ge, Z.; Du, H.; Gao, Y.; Qiu, W. Analysis on Metabolic Functions of Stored Rice Microbial Communities by BIOLOG ECO Microplates. Front. Microbiol. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Gu, J.; Sun, W.; Li, Y.-D.; Fu, Q.-X.; Wang, X.-J.; Gao, H. Changes in the Soil Nutrient Levels, Enzyme Activities, Microbial Community Function, and Structure during Apple Orchard Maturation. Appl. Soil Ecol. 2014, 77, 18–25. [Google Scholar] [CrossRef]
- Chavan, S.; Nadanathangam, V. Shifts in Metabolic Patterns of Soil Bacterial Communities on Exposure to Metal Engineered Nanomaterials. Ecotoxicol. Environ. Saf. 2020, 189, 110012. [Google Scholar] [CrossRef] [PubMed]
- Németh, I.; Molnár, S.; Vaszita, E.; Molnár, M. The Biolog EcoPlateTM Technique for Assessing the Effect of Metal Oxide Nanoparticles on Freshwater Microbial Communities. Nanomaterials 2021, 11, 1777. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; He, X.; Liang, H.; Wu, Q.; Sun, X.; Liu, M.; Shen, P. Multi-Year Crop Rotation and Quicklime Application Promote Stable Peanut Yield and High Nutrient-Use Efficiency by Regulating Soil Nutrient Availability and Bacterial/Fungal Community. Front. Microbiol. 2024, 15, 1367184. [Google Scholar] [CrossRef]
- Hong, L.; Yao, Y.; Lei, C.; Hong, C.; Zhu, W.; Zhu, F.; Wang, W.; Lu, T.; Qi, X. Declined Symptoms in Myrica rubra: The Influence of Soil Acidification and Rhizosphere Microbial Communities. Sci. Hortic. 2023, 313, 111892. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Hartemink, A.E.; Barrow, N.J. Soil pH—Nutrient Relationships: The Diagram. Plant Soil 2023, 486, 209–215. [Google Scholar] [CrossRef]
- Mendoza, B.; Béjar, J.; Luna, D.; Osorio, M.; Jimenez, M.; Melendez, J.R. Differences in the Ratio of Soil Microbial Biomass Carbon (MBC) and Soil Organic Carbon (SOC) at Various Altitudes of Hyperalic Alisol in the Amazon Region of Ecuador. F1000Research 2020, 9, 443. [Google Scholar] [CrossRef]
- Marinari, S.; Vittori Antisari, L. Effect of Lithological Substrate on Microbial Biomass and Enzyme Activity in Brown Soil Profiles in the Northern Apennines (Italy). Pedobiologia 2010, 53, 313–320. [Google Scholar] [CrossRef]
- Babur, E. Effects of Parent Material on Soil Microbial Biomass Carbon and Basal Respiration within Young Afforested Areas. Scand. J. For. Res. 2019, 34, 94–101. [Google Scholar] [CrossRef]
- Orlova, N.; Orlova, E.; Abakumov, E.; Smirnova, K.; Chukov, S. Humic Acids Formation during Compositing of Plant Remnants in Presence of Calcium Carbonate and Biochar. Agronomy 2022, 12, 2275. [Google Scholar] [CrossRef]
- Akberdin, I.R.; Collins, D.A.; Hamilton, R.; Oshchepkov, D.Y.; Shukla, A.K.; Nicora, C.D.; Nakayasu, E.S.; Adkins, J.N.; Kalyuzhnaya, M.G. Rare Earth Elements Alter Redox Balance in Methylomicrobium alcaliphilum 20ZR. Front. Microbiol. 2018, 9, 2735. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Xiao, Z.; Liu, J.; Zhu, Y.; Shuai, K.; Chen, X.; Liu, Y.; Hu, R.; Peng, G.; Li, J.; et al. Vertical Differences in Carbon Metabolic Diversity and Dominant Flora of Soil Bacterial Communities in Farmlands. Sci. Rep. 2024, 14, 9445. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Guo, H.; Shafiqul Islam, M.; Zaki, H.E.M.; Wang, Z.; Wang, H.; Qi, X.; Guo, J.; Sun, L.; Wang, Q.; et al. Improvement Effect of Biochar on Soil Microbial Community Structure and Metabolites of Decline Disease Bayberry. Front. Microbiol. 2023, 14, 1154886. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Zhao, C.; Hu, X.; Yin, C. Nitrogen Fertilization Increases Soil Microbial Biomass and Alters Microbial Composition Especially Under Low Soil Water Availability. Microb. Ecol. 2023, 86, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in Root Growth and Development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sindhu, S.S.; Glick, B.R. Potassium Solubilizing Microorganisms as Potential Biofertilizer: A Sustainable Climate-Resilient Approach to Improve Soil Fertility and Crop Production in Agriculture. J. Plant Growth Regul. 2024, 43, 2503–2535. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Pirdashti, H.; Lendeh, K.S. Phosphate and Potassium-Solubilizing Bacteria Effect on the Growth of Rice. Ecol. Eng. 2017, 103, 164–169. [Google Scholar] [CrossRef]
- Bot, P.J.; Abbasi, G.H.; Akhtar, J.; Anwar-Ul-Haq, M.; Malik, W. Exogenous Potassium Differentially Mitigates Salt Stress in Tolerant and Sensitive Maize Hybrids. Pak. J. Bot. 2015, 46, 135–146. [Google Scholar]
- Minick, K.J.; Fisk, M.C.; Groffman, P.M. Soil Ca Alters Processes Contributing to C and N Retention in the Oa/A Horizon of a Northern Hardwood Forest. Biogeochemistry 2017, 132, 343–357. [Google Scholar] [CrossRef]
- Morales, J.; Martínez-Alcántara, B.; Bermejo, A.; Millos, J.; Legaz, F.; Quiñones, A. Effect of Calcium Fertilization on Calcium Uptake and Its Partitioning in Citrus Trees. Agronomy 2023, 13, 2971. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Gao, J.; Ma, H.; Li, Z.; Hao, Y.; Qiu, X.; Ru, J.; Song, J.; Wan, S. Changes in Plant Litter and Root Carbon Inputs Alter Soil Respiration in Three Different Forests of a Climate Transitional Region. Agric. For. Meteorol. 2024, 358, 110212. [Google Scholar] [CrossRef]
- Weng, X.; Li, J.; Sui, X.; Li, M.; Yin, W.; Ma, W.; Yang, L.; Mu, L. Soil Microbial Functional Diversity Responses to Different Vegetation Types in the Heilongjiang Zhongyangzhan Black-Billed Capercaillie Nature Reserve. Ann. Microbiol. 2021, 71, 26. [Google Scholar] [CrossRef]
- Sofo, A.; Ricciuti, P. A Standardized Method for Estimating the Functional Diversity of Soil Bacterial Community by Biolog® EcoPlatesTM Assay—The Case Study of a Sustainable Olive Orchard. Appl. Sci. 2019, 9, 4035. [Google Scholar] [CrossRef]
- He, W.; Ye, W.; Sun, M.; Li, Y.; Chen, M.; Wei, M.; Hu, G.; Yang, Q.; Pan, H.; Lou, Y.; et al. Soil Phosphorus Availability and Stoichiometry Determine Microbial Activity and Functional Diversity of Fluvo-Aquic Soils under Long-Term Fertilization Regimes. J. Soils Sediments 2022, 22, 1214–1227. [Google Scholar] [CrossRef]
- Xu, H.; Yu, M.; Cheng, X. Abundant Fungal and Rare Bacterial Taxa Jointly Reveal Soil Nutrient Cycling and Multifunctionality in Uneven-Aged Mixed Plantations. Ecol. Indic. 2021, 129, 107932. [Google Scholar] [CrossRef]
- Wagg, C.; Hautier, Y.; Pellkofer, S.; Banerjee, S.; Schmid, B.; van der Heijden, M.G. Diversity and Asynchrony in Soil Microbial Communities Stabilizes Ecosystem Functioning. eLife 2021, 10, e62813. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, R.; Jie, X.; Lian, T.; Zang, H.; Zeng, Z.; Yang, Y. Organic Management Improved the Multifunctionality in Recolonization Soil by Increasing Microbial Diversity and Function. Funct. Ecol. 2024, 38, 2207–2219. [Google Scholar] [CrossRef]
- Hamidović, S.; Cvijović, G.G.; Waisi, H.; Životić, L.; Šoja, S.J.; Raičević, V.; Lalević, B. Response of Microbial Community Composition in Soils Affected by Coal Mine Exploitation. Environ. Monit. Assess. 2020, 192, 364. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhang, S.; Niu, B.; Pei, Y.; Song, S.; Lei, T.; Yun, H. Soil Texture Influences Soil Bacterial Biomass in the Permafrost-Affected Alpine Desert of the Tibetan Plateau. Front. Microbiol. 2022, 13, 1007194. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of Organic Substitution Rates on Soil Quality and Fungal Community Composition in a Tea Plantation with Long-Term Fertilization. Biol. Fertil. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Wang, C.; Hou, X.; Islam, Z.U.; Zhang, Z.; Zhu, B.; Yang, T. Driving Factors of Microbial Community Abundance and Structure in Typical Forest Soils of Sanjiang Plain, Northeast China. Sustainability 2022, 14, 8040. [Google Scholar] [CrossRef]
- Wang, D.; Yi, W.; Zhou, Y.; He, S.; Tang, L.; Yin, X.; Zhao, P.; Long, G. Intercropping and N Application Enhance Soil Dissolved Organic Carbon Concentration with Complicated Chemical Composition. Soil Tillage Res. 2021, 210, 104979. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Xie, B.; Hu, S.; Zheng, Y.; Jin, P. Effects of Exogenous Calcium and Calcium Chelant on Cold Tolerance of Postharvest Loquat Fruit. Sci. Hortic. 2020, 269, 109391. [Google Scholar] [CrossRef]
- Tang, Y.; Lou, W.; Yan, X.; Li, S.; Wang, P.; Zhou, Y.; Zhan, T.; Zhang, S.; Hu, C.; Wang, X.; et al. The Pivotal Role of Secondary Nutrients and Micronutrients in Regulating Fruit Quality and Root Exudates Metabolism Profile of Citrus. Plant Soil 2024, 500, 461–479. [Google Scholar] [CrossRef]
- Fang, M.; Wang, X.; Jia, Z.; Qiu, Q.; Li, P.; Chen, L.; Yang, H. A Simple and Efficient Method for the Substrate Identification of Amino Acid Decarboxylases. Int. J. Mol. Sci. 2022, 23, 14551. [Google Scholar] [CrossRef]
- Rijk, I.J.C.; Ekblad, A. Carbon and Nitrogen Cycling in a Lead Polluted Grassland Evaluated Using Stable Isotopes (δ13C and δ15N) and Microbial, Plant and Soil Parameters. Plant Soil 2020, 449, 249–266. [Google Scholar] [CrossRef]

| Regions | pH (H2O) | SOC (g/kg) | DOC (mg/kg) | TN (g/kg) | C/N | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | CEC (cmol/kg) | Exchangable Ca2+ (cmol/kg) | Exchangable Mg2+ (cmol/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KR | 7.60 ± 0.30 a | 17.69 ± 2.36 a | 546.44 ± 24.20 a | 0.77 ± 0.08 a | 22.85 ± 2.07 a | 62.99 ± 13.63 a | 14.35 ± 6.60 a | 1021.62 ± 116.34 a | 11.08 ± 2.13 a | 48.13 ± 22.80 a | 5.04 ± 1.58 a |
| MR | 6.90 ± 0.19 ab | 13.81 ± 0.48 b | 476.7 ± 59.49 a | 0.60 ± 0.10 a | 23.40 ± 4.16 a | 60.79 ± 21.41 a | 11.53 ± 4.39 a | 506.79 ± 39.85 b | 10.63 ± 0.54 a | 7.58 ± 2.16 b | 4.17 ± 1.51 a |
| NK | 6.18 ± 0.69 b | 13.11 ± 0.07 b | 571.4 ± 77.46 a | 0.64 ± 0.10 a | 20.76 ± 3.29 a | 74.95 ± 8.54 a | 22.13 ± 4.95 a | 833.56 ± 180.08 a | 10.19 ± 2.00 a | 4.08 ± 3.19 b | 3.38 ± 1.35 a |
| Regions | Bacteria/×106 CFU/g | Fungi/×103 CFU/g | Actinobacteria/×105 CFU/g |
|---|---|---|---|
| KR | 5.69 ± 0.39 a | 5.42 ± 1.56 a | 3.02 ± 1.04 a |
| MR | 4.41 ± 0.37 b | 4.82 ± 2.18 a | 2.19 ± 0.31 a |
| NKR | 4.25 ± 0.71 b | 8.41 ± 2.36 a | 1.85 ± 0.19 a |
| Regions | MBC (mg/kg) | MBN (mg/kg) | MBC/MBN | SMQ (%) |
|---|---|---|---|---|
| KR | 608.24 ± 63.80 a | 136.1 ± 50.63 a | 4.74 ± 1.13 a | 3.45 ± 0.18 a |
| MR | 213.04 ± 80.01 b | 73.89 ± 20.40 a | 2.86 ± 0.45 a | 2.75 ± 0.62 b |
| NKR | 359.57 ± 79.75 b | 117.21 ± 70.82 a | 3.93 ± 2.29 a | 2.58 ± 0.94 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Jin, Z.; Li, X.; Zhu, H.; Fang, F.; Luo, T.; Li, J. Characterization of Microbial Carbon Metabolism in Karst Soils from Citrus Orchards and Analysis of Its Environmental Drivers. Microorganisms 2025, 13, 267. https://doi.org/10.3390/microorganisms13020267
Wang S, Jin Z, Li X, Zhu H, Fang F, Luo T, Li J. Characterization of Microbial Carbon Metabolism in Karst Soils from Citrus Orchards and Analysis of Its Environmental Drivers. Microorganisms. 2025; 13(2):267. https://doi.org/10.3390/microorganisms13020267
Chicago/Turabian StyleWang, Shixuan, Zhenjiang Jin, Xuesong Li, Hongying Zhu, Fang Fang, Ting Luo, and Jia Li. 2025. "Characterization of Microbial Carbon Metabolism in Karst Soils from Citrus Orchards and Analysis of Its Environmental Drivers" Microorganisms 13, no. 2: 267. https://doi.org/10.3390/microorganisms13020267
APA StyleWang, S., Jin, Z., Li, X., Zhu, H., Fang, F., Luo, T., & Li, J. (2025). Characterization of Microbial Carbon Metabolism in Karst Soils from Citrus Orchards and Analysis of Its Environmental Drivers. Microorganisms, 13(2), 267. https://doi.org/10.3390/microorganisms13020267





