Internalization of Lactobacillus crispatus Through Caveolin-1-Mediated Endocytosis Boosts Cellular Uptake but Blocks the Transcellular Passage of Neisseria meningitidis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Cell Lines and Growth Conditions
2.3. Adherence Assay
2.4. Gentamicin Invasion Assay
2.5. Flow Cytometry Invasion Assay
2.6. Fluorescence Microscopy to Detect Intracellular Bacteria
2.7. CEACAM Detection
2.8. Expression of Virulence Factors
2.9. Internalization Blocking Assay
2.10. Fluorescence Microscopy to Detect Host Cell Components
2.11. Transcytosis
2.12. Long-Term Survival Inside Cells
2.13. Statistics
3. Results
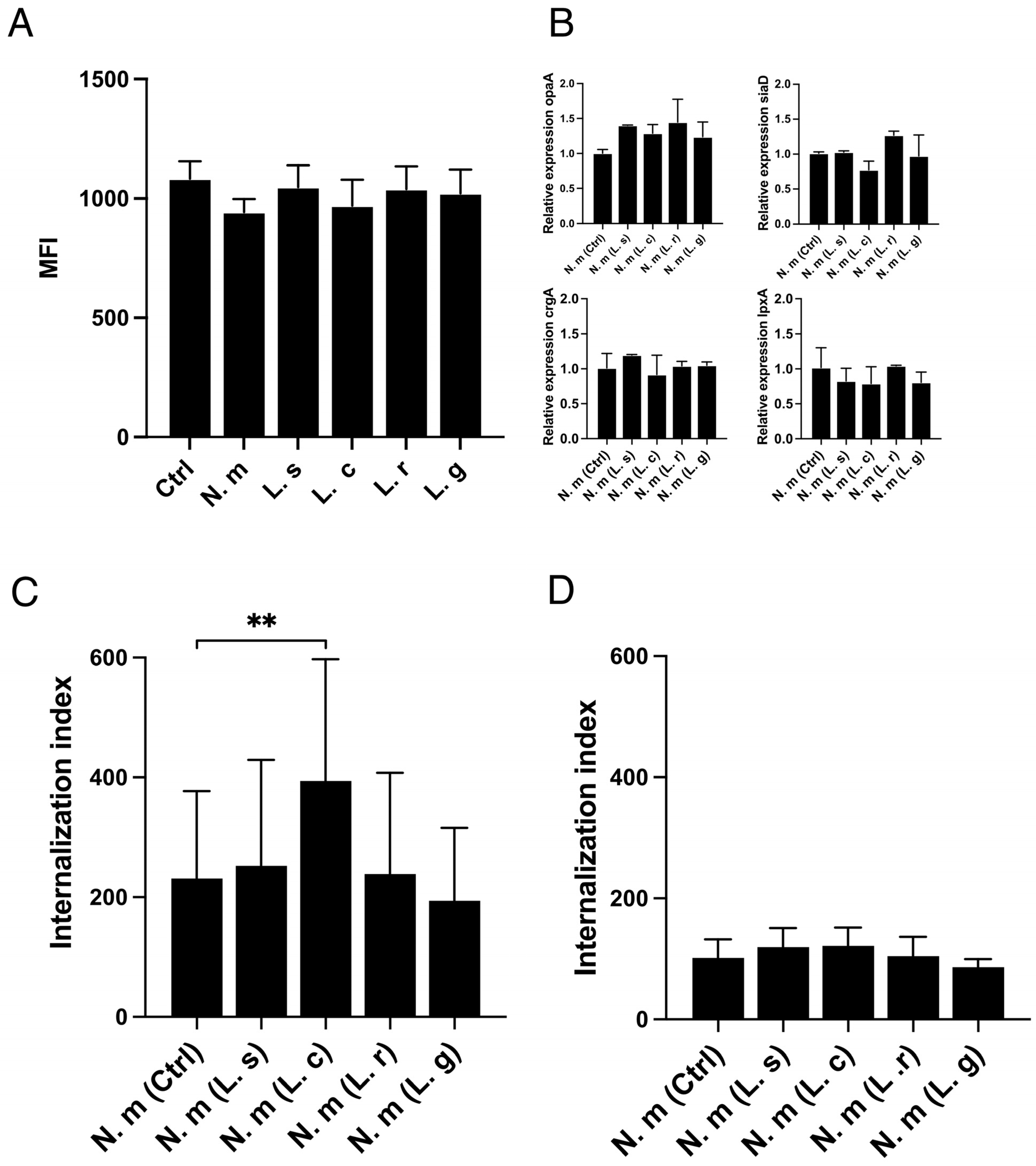
3.1. Increased Internalization of Neisseria Meningitidis into Human Epithelial Cells in the Presence of Lactobacillus crispatus
3.2. L. crispatus Must Be in Contact with Host Cells to Increase the Uptake of Both Live and Fixed N. meningitidis
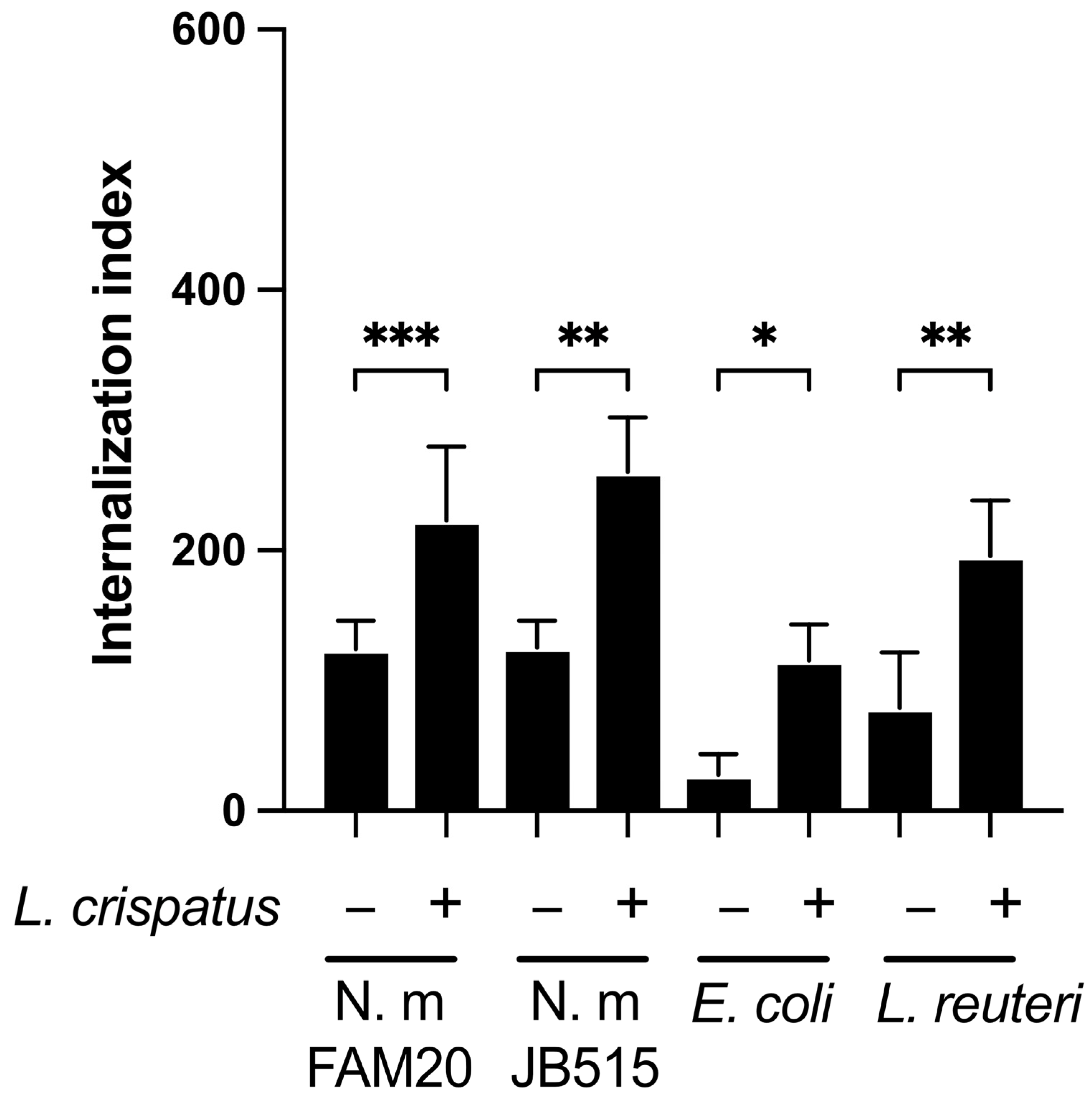
3.3. L. crispatus Enhances the Epithelial Uptake of Both Pathogenic and Commensal Bacteria
3.4. L. crispatus Can Internalize Itself in Both Immortalized and Primary Epithelial Cells
3.5. Internalization of L. crispatus via the Caveolin-Mediated Endocytic Pathway
3.6. Caveolin and Cholesterol but Not Flotillin Were Recruited Around L. crispatus
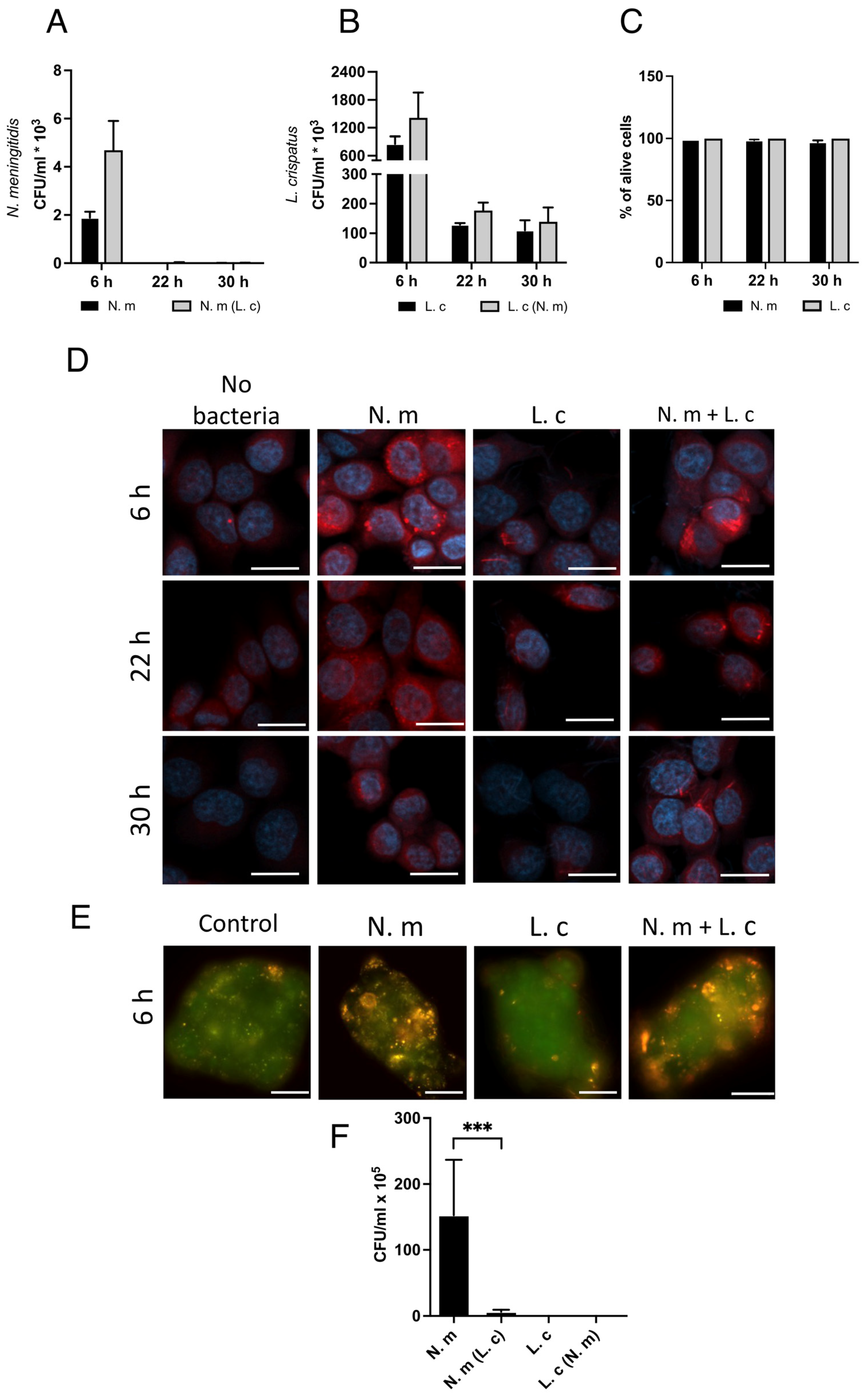
3.7. L. crispatus Survives Inside Cells, Whereas N. meningitidis Is Killed over Time
3.8. L. crispatus Prevents N. meningitidis from Exiting Epithelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caugant, D.A.; Maiden, M.C. Meningococcal carriage and disease--population biology and evolution. Vaccine 2009, 27 (Suppl. 2), B64–B70. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.S. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine 2009, 27 (Suppl. 2), B71–B77. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Griffiths, N.J.; Borodina, E.; Virji, M. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin. Sci. 2010, 118, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Rudel, T.; Scheurerpflug, I.; Meyer, T.F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature 1995, 373, 357–359. [Google Scholar] [CrossRef]
- Tchoupa, A.K.; Schuhmacher, T.; Hauck, C.R. Signaling by epithelial members of the CEACAM family—Mucosal docking sites for pathogenic bacteria. Cell Commun. Signal. 2014, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Deghmane, A.E.; Giorgini, D.; Larribe, M.; Alonso, J.M.; Taha, M.K. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol. Microbiol. 2002, 43, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Albiger, B.; Johansson, L.; Jonsson, A.B. Lipooligosaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect. Immun. 2003, 71, 155–162. [Google Scholar] [CrossRef]
- Barrile, R.; Kasendra, M.; Rossi-Paccani, S.; Merola, M.; Pizza, M.; Baldari, C.; Soriani, M.; Aricò, B. Neisseria meningitidis subverts the polarized organization and intracellular trafficking of host cells to cross the epithelial barrier. Cell. Microbiol. 2015, 17, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Abrahamsson, T.R.; Artacho, A.; Collado, M.C.; Mira, A.; Jenmalm, M.C. Oral microbiota maturation during the first 7 years of life in relation to allergy development. Allergy 2018, 73, 2000–2011. [Google Scholar] [CrossRef]
- Sillanpää, J.; Martínez, B.; Antikainen, J.; Toba, T.; Kalkkinen, N.; Tankka, S.; Lounatmaa, K.; Keränen, J.; Höök, M.; Westerlund-Wikström, B.; et al. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 2000, 182, 6440–6450. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Kong, J.; Hu, S.; Kong, W.; Lu, W.; Liu, W. Characterization of a S-layer protein from Lactobacillus crispatus K313 and the domains responsible for binding to cell wall and adherence to collagen. Appl. Microbiol. Biotechnol. 2013, 97, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Mathipa-Mdakane, M.G.; Thantsha, M.S. Lacticaseibacillus rhamnosus: A Suitable Candidate for the Construction of Novel Bioengineered Probiotic Strains for Targeted Pathogen Control. Foods 2022, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Faas, M.M.; de Vos, P.L. plantarum, L. salivarius, and L. lactis attenuate Th2 responses and increase Treg frequencies in healthy mice in a strain dependent manner. PLoS ONE 2012, 7, e47244. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediators Inflamm. 2013, 2013, 237921. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I. Pharmacological inhibition of endocytic pathways: Is it specific enough to be useful? Methods Mol. Biol. 2008, 440, 15–33. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Büttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlötzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, P.; Nabi, I.R. Regulation of raft-dependent endocytosis. J. Cell. Mol. Med. 2007, 11, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.S.; Corrêa, G.; Einicker-Lamas, M.; Coutinho-Silva, R. Host-cell lipid rafts: A safe door for micro-organisms? Biol. Cell 2010, 102, 391–407. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, D.A.; Navarro-Lleo, N.; Bauerl, C.; Campista-Leon, S.; Coll-Marques, J.M.; Perez-Martinez, G. Factors Affecting Spontaneous Endocytosis and Survival of Probiotic Lactobacilli in Human Intestinal Epithelial Cells. Microorganisms 2022, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Kallstrom, H.; Normark, S.; Jonsson, A.B. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol. Microbiol. 1997, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.S.; Cohen, I.R.; Norins, L.C.; Schroeter, A.L.; Reising, G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J. Bacteriol. 1968, 96, 596–605. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Engstrand, L.; Jonsson, H. Lactobacillus gastricus sp. nov., Lactobacillus antri sp. nov., Lactobacillus kalixensis sp. nov. and Lactobacillus ultunensis sp. nov., isolated from human stomach mucosa. Int. J. Syst. Evol. Microbiol. 2005, 55, 77–82. [Google Scholar] [CrossRef]
- de Klerk, N.; Maudsdotter, L.; Gebreegziabher, H.; Saroj, S.D.; Eriksson, B.; Eriksson, O.S.; Roos, S.; Linden, S.; Sjolinder, H.; Jonsson, A.B. Lactobacilli Reduce Helicobacter pylori Attachment to Host Gastric Epithelial Cells by Inhibiting Adhesion Gene Expression. Infect. Immun. 2016, 84, 1526–1535. [Google Scholar] [CrossRef]
- Coleman, W.G.; Goebel, P.J.; Leive, L. Genetic analysis of Escherichia coli O111:B4, a strain of medical and biochemical interest. J. Bacteriol. 1977, 130, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Rangan, S.R. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer 1972, 29, 117–121. [Google Scholar] [CrossRef]
- Pils, S.; Schmitter, T.; Neske, F.; Hauck, C.R. Quantification of bacterial invasion into adherent cells by flow cytometry. J. Microbiol. Methods 2006, 65, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.D.; Obradovic, M.; Dillon, J.R.; Ng, S.H.; Wilson, H.L. Development of flow cytometry based adherence assay for Neisseria gonorrhoeae using 5′-carboxyfluorosceinsuccidyl ester. BMC Microbiol. 2019, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Mohan Nair, M.K.; Venkitanarayanan, K. Role of bacterial OmpA and host cytoskeleton in the invasion of human intestinal epithelial cells by Enterobacter sakazakii. Pediatr. Res. 2007, 62, 664–669. [Google Scholar] [CrossRef]
- Spaniol, V.; Heiniger, N.; Troller, R.; Aebi, C. Outer membrane protein UspA1 and lipooligosaccharide are involved in invasion of human epithelial cells by Moraxella catarrhalis. Microbes Infect. 2008, 10, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Virji, M.; Makepeace, K.; Ferguson, D.J.; Achtman, M.; Sarkari, J.; Moxon, E.R. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol. Microbiol. 1992, 6, 2785–2795. [Google Scholar] [CrossRef]
- Goldmann, O.; Lang, J.C.; Rohde, M.; May, T.; Molinari, G.; Medina, E. Alpha-hemolysin promotes internalization of Staphylococcus aureus into human lung epithelial cells via caveolin-1- and cholesterol-rich lipid rafts. Cell. Mol. Life Sci. 2024, 81, 435. [Google Scholar] [CrossRef] [PubMed]
- Spörl, F.; Wunderskirchner, M.; Ullrich, O.; Bömke, G.; Breitenbach, U.; Blatt, T.; Wenck, H.; Wittern, K.P.; Schrader, A. Real-time monitoring of membrane cholesterol reveals new insights into epidermal differentiation. J. Investig. Dermatol. 2010, 130, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Sadeghloo, Z.; Saffarian, P.; Hakemi-Vala, M.; Sadeghi, A.; Yadegar, A. The modulatory effect of Lactobacillus gasseri ATCC 33323 on autophagy induced by extracellular vesicles of Helicobacter pylori in gastric epithelial cells in vitro. Microb. Pathog. 2024, 188, 106559. [Google Scholar] [CrossRef] [PubMed]
- Ilver, D.; Kallstrom, H.; Normark, S.; Jonsson, A.B. Transcellular passage of Neisseria gonorrhoeae involves pilus phase variation. Infect. Immun. 1998, 66, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Vielfort, K.; Sjölinder, H.; Roos, S.; Jonsson, H.; Aro, H. Adherence of clinically isolated lactobacilli to human cervical cells in competition with Neisseria gonorrhoeae. Microbes Infect. 2008, 10, 1325–1334. [Google Scholar] [CrossRef]
- Gebremariam, H.G.; Qazi, K.R.; Somiah, T.; Pathak, S.K.; Sjölinder, H.; Sverremark Ekström, E.; Jonsson, A.B. Lactobacillus gasseri Suppresses the Production of Proinflammatory Cytokines in Helicobacter pylori-Infected Macrophages by Inhibiting the Expression of ADAM17. Front. Immunol. 2019, 10, 2326. [Google Scholar] [CrossRef]
- van der Veer, C.; Hertzberger, R.Y.; Bruisten, S.M.; Tytgat, H.L.P.; Swanenburg, J.; de Kat Angelino-Bart, A.; Schuren, F.; Molenaar, D.; Reid, G.; de Vries, H.; et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota. Microbiome 2019, 7, 49. [Google Scholar] [CrossRef]
- Johswich, K. Innate immune recognition and inflammation in Neisseria meningitidis infection. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Wosen, J.E.; Mukhopadhyay, D.; Macaubas, C.; Mellins, E.D. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front. Immunol. 2018, 9, 2144. [Google Scholar] [CrossRef]
- Mikucki, A.; McCluskey, N.R.; Kahler, C.M. The Host-Pathogen Interactions and Epicellular Lifestyle of Neisseria meningitidis. Front. Cell. Infect. Microbiol. 2022, 12, 862935. [Google Scholar] [CrossRef]
- Schmitter, T.; Pils, S.; Weibel, S.; Agerer, F.; Peterson, L.; Buntru, A.; Kopp, K.; Hauck, C.R. Opa proteins of pathogenic neisseriae initiate Src kinase-dependent or lipid raft-mediated uptake via distinct human carcinoembryonic antigen-related cell adhesion molecule isoforms. Infect. Immun. 2007, 75, 4116–4126. [Google Scholar] [CrossRef] [PubMed]
- Virji, M.; Makepeace, K.; Ferguson, D.J.; Watt, S.M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol. 1996, 22, 941–950. [Google Scholar] [CrossRef]
- Sutherland, T.C.; Quattroni, P.; Exley, R.M.; Tang, C.M. Transcellular passage of Neisseria meningitidis across a polarized respiratory epithelium. Infect. Immun. 2010, 78, 3832–3847. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.L.; Datta, A.; Ambrose, K.; Lo, M.; Davies, J.K.; Carlson, R.W.; Stephens, D.S.; Kahler, C.M. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis. J. Biol. Chem. 2004, 279, 35053–35062. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and molecular biology of airway mucins. Int. Rev. Cell Mol. Biol. 2013, 303, 139–202. [Google Scholar] [CrossRef]
- Audry, M.; Robbe-Masselot, C.; Barnier, J.P.; Gachet, B.; Saubaméa, B.; Schmitt, A.; Schönherr-Hellec, S.; Léonard, R.; Nassif, X.; Coureuil, M. Airway Mucus Restricts Neisseria meningitidis Away from Nasopharyngeal Epithelial Cells and Protects the Mucosa from Inflammation. mSphere 2019, 4, e00494-19. [Google Scholar] [CrossRef] [PubMed]
- Brunworth, J.D.; Garg, R.; Mahboubi, H.; Johnson, B.; Djalilian, H.R. Detecting nasopharyngeal reflux: A novel pH probe technique. Ann. Otol. Rhinol. Laryngol. 2012, 121, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Flieger, A.; Frischknecht, F.; Hacker, G.; Hornef, M.W.; Pradel, G. Pathways of host cell exit by intracellular pathogens. Microb. Cell 2018, 5, 525–544. [Google Scholar] [CrossRef]
- Peters, S.; Mohort, K.; Claus, H.; Stigloher, C.; Schubert-Unkmeir, A. Interaction of Neisseria meningitidis carrier and disease isolates of MenB cc32 and MenW cc22 with epithelial cells of the nasopharyngeal barrier. Front. Cell. Infect. Microbiol. 2024, 14, 1389527. [Google Scholar] [CrossRef] [PubMed]
- Filippakis, D.; Gkentzi, D.; Dimitriou, G.; Karatza, A. Neonatal meningococcal disease: An update. J. Matern. Fetal Neonatal Med. 2022, 35, 4190–4195. [Google Scholar] [CrossRef]
- Argentini, C.; Fontana, F.; Alessandri, G.; Lugli, G.A.; Mancabelli, L.; Ossiprandi, M.C.; van Sinderen, D.; Ventura, M.; Milani, C.; Turroni, F. Evaluation of Modulatory Activities of Lactobacillus crispatus Strains in the Context of the Vaginal Microbiota. Microbiol. Spectr. 2022, 10, e0273321. [Google Scholar] [CrossRef] [PubMed]
- Foschi, C.; Salvo, M.; Cevenini, R.; Parolin, C.; Vitali, B.; Marangoni, A. Vaginal Lactobacilli Reduce Neisseria gonorrhoeae Viability through Multiple Strategies: An in Vitro Study. Front. Cell Infect. Microbiol. 2017, 7, 502. [Google Scholar] [CrossRef]
- Corbett, G.A.; Corcoran, S.; Feehily, C.; Soldati, B.; Rafferty, A.; MacIntyre, D.A.; Cotter, P.D.; McAuliffe, F.M. Preterm-birth-prevention with Lactobacillus crispatus oral probiotics: Protocol for a double blinded randomised placebo-controlled trial (the PrePOP study). Contemp. Clin. Trials 2024, 149, 107776. [Google Scholar] [CrossRef] [PubMed]
- Mändar, R.; Sõerunurk, G.; Štšepetova, J.; Smidt, I.; Rööp, T.; Kõljalg, S.; Saare, M.; Ausmees, K.; Le, D.D.; Jaagura, M.; et al. Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: A randomised double-blind placebo controlled clinical trial. Benef. Microbes 2023, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sanders, H.; Brehony, C.; Maiden, M.C.J.; Vipond, C.; Feavers, I.M. The effect of iron availability on transcription of the Neisseria meningitidis fHbp gene varies among clonal complexes. Microbiology (Reading) 2012, 158, 869–876. [Google Scholar] [CrossRef]
- Sigurlásdóttir, S.; Wassing, G.M.; Zuo, F.; Arts, M.; Jonsson, A.B. Deletion of D-Lactate Dehydrogenase A in. Front. Microbiol. 2019, 10, 422. [Google Scholar] [CrossRef]
- Sigurlásdóttir, S.; Engman, J.; Eriksson, O.S.; Saroj, S.D.; Zguna, N.; Lloris-Garcerá, P.; Ilag, L.L.; Jonsson, A.B. Host cell-derived lactate functions as an effector molecule in Neisseria meningitidis microcolony dispersal. PLoS Pathog. 2017, 13, e1006251. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lidberg, K.; Pilheden, S.; Relloso Ortiz de Uriarte, M.; Jonsson, A.-B. Internalization of Lactobacillus crispatus Through Caveolin-1-Mediated Endocytosis Boosts Cellular Uptake but Blocks the Transcellular Passage of Neisseria meningitidis. Microorganisms 2025, 13, 479. https://doi.org/10.3390/microorganisms13030479
Lidberg K, Pilheden S, Relloso Ortiz de Uriarte M, Jonsson A-B. Internalization of Lactobacillus crispatus Through Caveolin-1-Mediated Endocytosis Boosts Cellular Uptake but Blocks the Transcellular Passage of Neisseria meningitidis. Microorganisms. 2025; 13(3):479. https://doi.org/10.3390/microorganisms13030479
Chicago/Turabian StyleLidberg, Kenny, Sarah Pilheden, Mikel Relloso Ortiz de Uriarte, and Ann-Beth Jonsson. 2025. "Internalization of Lactobacillus crispatus Through Caveolin-1-Mediated Endocytosis Boosts Cellular Uptake but Blocks the Transcellular Passage of Neisseria meningitidis" Microorganisms 13, no. 3: 479. https://doi.org/10.3390/microorganisms13030479
APA StyleLidberg, K., Pilheden, S., Relloso Ortiz de Uriarte, M., & Jonsson, A.-B. (2025). Internalization of Lactobacillus crispatus Through Caveolin-1-Mediated Endocytosis Boosts Cellular Uptake but Blocks the Transcellular Passage of Neisseria meningitidis. Microorganisms, 13(3), 479. https://doi.org/10.3390/microorganisms13030479





