Effects of Microorganisms in Fish Aquaculture from a Sustainable Approach: A Review
Abstract
:1. Introduction
2. Methodology Applied for the Literature Review
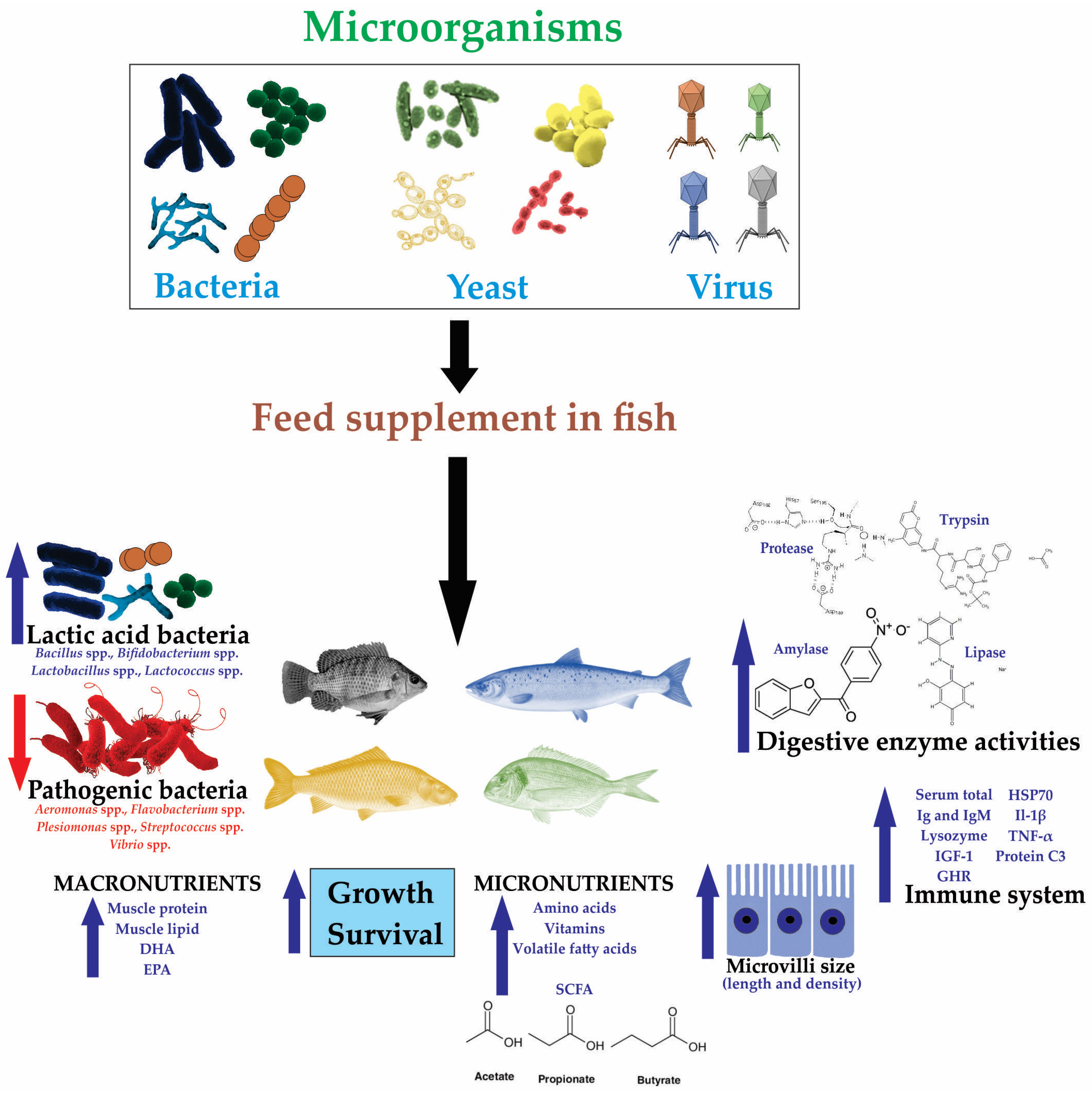
3. Effects of Microorganisms on Fish Aquaculture
3.1. Effects of Bacteria in Fish Aquaculture
3.2. Effects of Yeasts in Fish Aquaculture
3.3. Effects of Virus in Fish Aquaculture
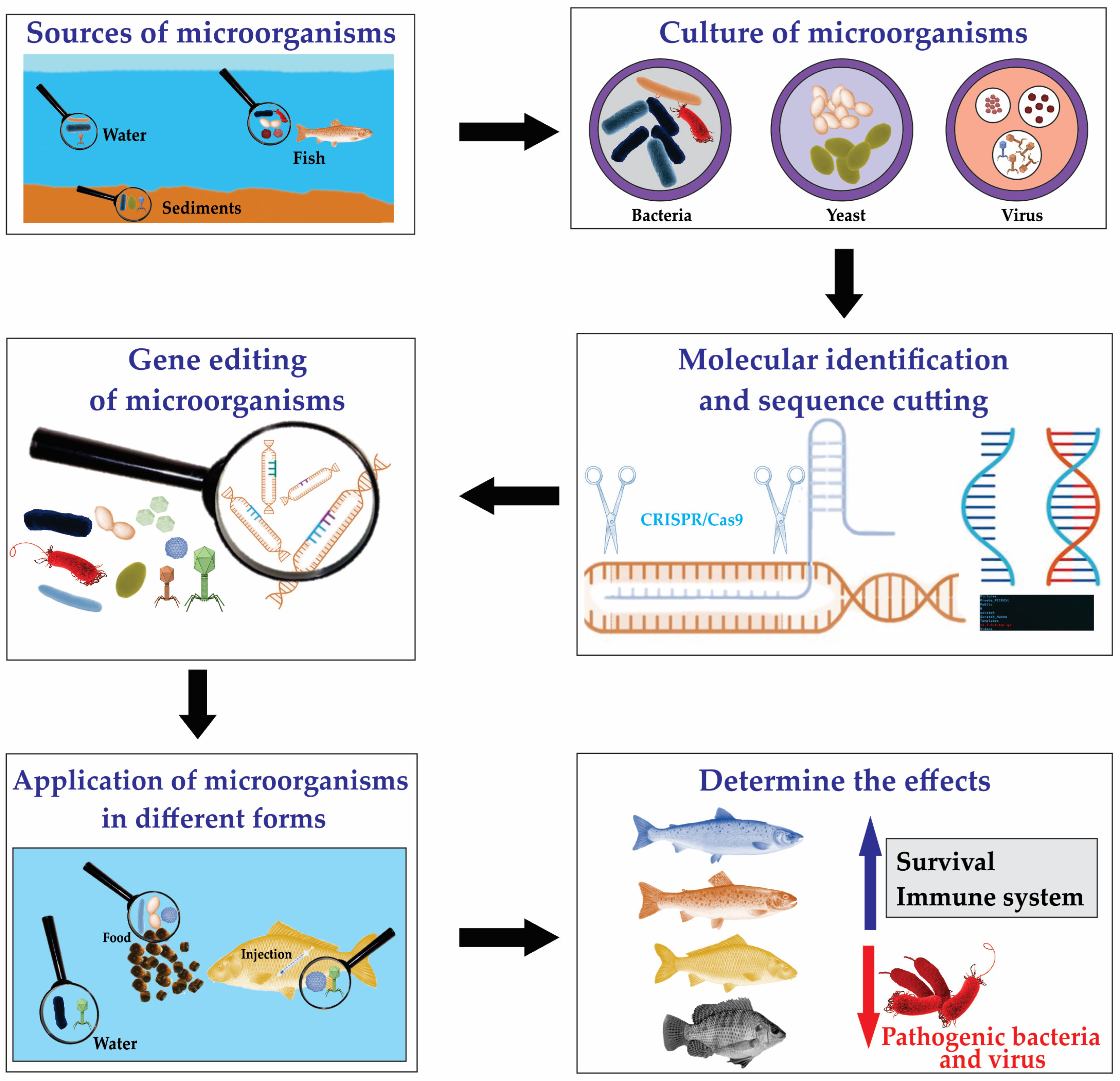
4. Use of Genetically Modified Microorganisms in Fish Aquaculture
5. Challenges and Future Perspectives
5.1. Challenges in the Use of Microorganisms in Aquaculture
5.2. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action. 2024. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/66538eba-9c85-4504-8438-c1cf0a0a3903/content/cd0683en.html (accessed on 9 December 2024).
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. 2022. Available online: https://www.fao.org/3/cc0461en/cc0461en.pdf (accessed on 6 December 2024).
- Tacon, A.G.J.; Metian, M. Fish Matters: Importance of Aquatic Foods in Human Nutrition and Global Food Supply. Rev. Fish. Sci. Res. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All. 2016. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/20e618b3-93a1-488a-9697-798f6b6c6b35/content (accessed on 9 December 2024).
- Singh, A.; Deb, R.; Rajendran, N. Benefits and Risks of Genetically Modified Organisms in Aquaculture. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 79–84. [Google Scholar]
- ONU. DESAFÍOS GLOBALES Población: Una Población en Crecimiento. 2023. Available online: https://www.un.org/es/global-issues/population (accessed on 18 December 2024).
- World Bank. Reducing Disease Risks in Aquaculture. World Bank Report #88257-GLB.2014. Available online: https://documents1.worldbank.org/curated/es/110681468054563438/pdf/882570REPLACEM00NAME0Reantaso0Melba.pdf (accessed on 15 December 2024).
- Cain, K. The many challenges of disease management in aquaculture. J. World Aquac. Soc. 2022, 53, 1080–1083. [Google Scholar] [CrossRef]
- Zahran, E.; Hafez, E.E.; Mohd Altaf Hossain, F.; Elhadidy, M.; Shaheen, A.A. Saprolegniosis in Nile Tilapia: Identification, Molecular Characterization, and Phylogenetic Analysis of Two Novel Pathogenic Saprolegnia Strains. J. Aquat. Anim. Health 2017, 29, 43–49. [Google Scholar] [CrossRef]
- Ali, S.E.; Gamil, A.A.A.; Skaar, I.; Evensen, O.; Charo-Karisa, H. Efficacy and safety of boric acid as a preventive treatment against Saprolegnia infection in Nile tilapia (Oreochromis niloticus). Sci. Rep. 2019, 9, 18013. [Google Scholar] [CrossRef]
- Priya, J.T.A.; Kappalli, S. Modern biotechnological strategies for vaccine development in aquaculture—Prospects and challenges. Vaccine 2022, 40, 5873–5881. [Google Scholar] [CrossRef]
- Sarkar, P.; Raju, V.S.; Kuppusamy, G.; Rahman, M.A.; Elumalai, P.; Harikrishnan, R.; Arshad, A.; Arockiaraj, J. Pathogenic fungi affecting fishes through their virulence molecules. Aquaculture 2022, 548, 737553. [Google Scholar] [CrossRef]
- Senthamarai, D.M.; Rajan, M.R.; Bharathi, P.V. Current risks of microbial infections in fish and their prevention methods: A review. Microb. Pathog. 2023, 185, 106400. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Panche, A.N. Studies on exploring the potentials of gut microbiomes to mitigate the bacterial and viral diseases of fish and shellfish in aquaculture farming. Microbe 2024, 2, 100031. [Google Scholar] [CrossRef]
- Jørgensen, L.v.G. The fish parasite Ichthyophthirius multifiliis—Host immunology, vaccines and novel treatments. Fish Shellfish Immunol. 2017, 67, 586–595. [Google Scholar] [CrossRef]
- Paladini, G.; Longshaw, M.; Gustineiii, A.; Shinn, A.P. Parasitic diseases in aquaculture: Their biology, diagnosis and control. In Diagnosis and Control of Diseases of Fish and Shellfish; Austin, B., Newaj-Fyzul, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 37–107. [Google Scholar]
- Sudhagar, A.; Kumar, G.; El-Matbouli, M. The Malacosporean Myxozoan Parasite Tetracapsuloides bryosalmonae: A Threat to Wild Salmonids. Pathogens 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, P.; Fischer, S.M.; Alexander, J.; James, C.T.; Paul, A.J.; Greiner, R.; Lewis, M.A. Myxobolus cerebralis establishment and spread: A graphical synthesis. Can. J. Fish. Aquat. Sci. 2022, 79, 677–691. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Attia, M.M.; Marzouk, M.S.; Korany, R.M.S.; Elgendy, M.Y.; Soliman, A.W.; Prince, A.; Hamada, A.H. Investigating dynamics, etiology, pathology, and therapeutic interventions of Caligus clemensi and Vibrio alginolyticus co-infection in farmed marine fish. Sci. Rep. 2024, 14, 20704. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; John, K.R.; Rajan, P.; Krishnan, R.; Safeena, M.P. Co-infection of Lactococcus garvieae and Aeromonas hydrophila in cultured Nile Tilapia in Kerala, India. Braz. J. Microbiol. 2024, 55, 2071–2083. [Google Scholar] [CrossRef]
- Yang, Q.; Tu, Y.Y.; Zhang, N.; Miao, B.; Zhang, Y.Z.; Deng, X.T.; He, T.; Zhu, S. Co-infections of Aeromonas dhakensis and Chryseobacterium indologenes in largemouth bass (Micropterus salmoides). Aquaculture 2024, 579, 740259. [Google Scholar] [CrossRef]
- Guan, L.; Li, X.; Chen, J.; Wang, L.; Zhang, X.; Sun, H.; Li, Y.; Yang, M.; Qin, Q.; Wang, S. Co-infection of nervous necrosis virus and Vibrio harveyi increased mortality and worsened the disease severity in the orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 2025, 158, 110117. [Google Scholar] [CrossRef]
- Islam, S.I.; Rodkhum, C.; Taweethavonsawat, P. An overview of parasitic co-infections in tilapia culture. Aquacult. Int. 2024, 32, 899–927. [Google Scholar] [CrossRef]
- Oidtmann, B.; Peeler, E.; Lyngstad, T.; Brun, E.; Bang Jensen, B.; Stärk, K.D.C. Risk-based methods for fish and terrestrial animal disease surveillance. Prev. Vet. Med. 2013, 112, 13–26. [Google Scholar] [CrossRef]
- Peeler, E.J.; Oidtmann, B.C.; Midtlyng, P.J.; Miossec, L.; Gozlan, R.E. Non-native aquatic animals introductions have driven disease emergence in Europe. Biol. Invasions 2011, 13, 1291–1303. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and managing microbes in aquaculture—Towards a sustainable industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Chen, j.; Jin, H.; Wang, T.; Zhu, W.; Li, L. Underestimated nutrient from aquaculture ponds to Lake Eutrophication: A case study on Taihu Lake Basin. J. Hydrol. 2024, 630, 130749. [Google Scholar] [CrossRef]
- Do, H.-L.; Thuy, T.D. Productivity response and production risk: A study of mangrove forest effects in aquaculture in the Mekong River Delta. Ecol. Econ. 2022, 194, 107326. [Google Scholar] [CrossRef]
- McSherry, M.; Davis, R.P.; Andradi-Brown, D.A.; Ahmadia, G.N.; Van Kempen, M.; Wingard Brian, S. Integrated mangrove aquaculture: The sustainable choice for mangroves and aquaculture? Front. For. Glob. Chang. 2023, 6, 1094306. [Google Scholar] [CrossRef]
- Fan, B.; Li, Y.; Zhang, Z.; Yang, Y.; Li, Y. Exploring Cumulative Vulnerability of Mangrove Forests to Intensive Coastal Anthropogenic Threats. Ecosyst. Health Sustain. 2024, 10, e0153. [Google Scholar] [CrossRef]
- Alfred, O.; Shaahu, A.; Orban, D.A.; Egwenomhe, M. An overview on understanding the basic concept of fish diseases in aquaculture. Iconic. Res. Eng. J. 2020, 4, 83–91. [Google Scholar]
- Admasu, F.; Wakjira, M. Pathology of Epizootic-Infectious Diseases of Fishes in Aquaculture. Biomed. J. Sci. Tech. Res. 2021, 40, 31984–31995. [Google Scholar] [CrossRef]
- Subasinghe, R.; Alday-Sanz, V.; Bondad-Reantaso, M.G.; Jie, H.; Shinn, A.P.; Sorgeloos, P. Biosecurity: Reducing the burden of disease. J. World Aquac. Soc. 2023, 54, 397–426. [Google Scholar] [CrossRef]
- Mo, W.Y.; Chen, Z.; Leung, H.M.; Leung, A.O.W. Application of veterinary antibiotics in China’s aquaculture industry and their potential human health risks. Environ. Sci. Pollut. Res. 2015, 24, 8978–8989. [Google Scholar] [CrossRef]
- Amillano-Cisneros, J.M.; Fuentes-Valencia, M.A.; Leyva-Morales, J.B.; Davizón, Y.A.; Marquéz-Pacheco, H.; Valencia-Castañeda, G.; Maldonado-Coyac, J.A.; Ontiveros-García, L.A.; Badilla-Medina, C.N. Prebiotics in Global and Mexican Fish Aquaculture: A Review. Animals 2023, 13, 3607. [Google Scholar] [CrossRef]
- Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A Review of Antibiotics, Antibiotic Resistant Bacteria, and Resistance Genes in Aquaculture: Occurrence, Contamination, and Transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef]
- Imtiaz, N.; Anwar, Z.; Waiho, K.; Shi, C.; Mu, C.; Wang, C.; Qingyang, W. A review on aquaculture adaptation for fish treatment from antibiotic to vaccine prophylaxis. Aquac. Int. 2024, 32, 2643–2668. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Approved Aquaculture Drugs. 2024. Available online: https://www.fda.gov/animal-veterinary/aquaculture/approved-aquaculture-drugs (accessed on 10 December 2024).
- Ajayi, A.O.; Odeyemi, A.T.; Akinjogunla, O.J.; Adeyeye, A.B.; Ayo-ajayi, I. Review of antibiotic-resistant bacteria and antibiotic resistance genes within the one health framework. Infect. Ecol. Epidemiol. 2024, 14, 2312953. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Parra, M.; Maisey, K.; Vargas, R.A.; Cabezas-Cruz, A.; Gonzalez, A.; Tello, M.; Bermúdez-Humarán, L.G. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms 2024, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- WCED (World Commission on Environment and Development). Report of the World Commission on Environment and Development. 1987. Available online: https://digitallibrary.un.org/record/139811?v=pdf#files (accessed on 6 December 2024).
- Idowu, S.O.; Capaldi, N.; Zu, L.; Das Gupta, A. Encyclopedia of Corporate Social Responsibility. 2013. Available online: https://link.springer.com/referencework/10.1007/978-3-642-28036-8 (accessed on 28 November 2024).
- Kenis, M.; Hurley, B.P.; Colombari, F.; Lawson, S.; Sun, J.; Wilcken, C.; Weeks, R.; Sathyapala, S. Guide to the Classical Biological Control of Insect Pests in Planted and Natural Forests. 2019. Available online: http://www.fao.org/3/ca3677en/CA3677EN.pdf (accessed on 12 December 2024).
- Shams, A.; Fischer, A.; Bodnar, A.; Kliegman, M. Perspectives on Genetically Engineered Microorganisms and Their Regulation in the United States. ACS Synth. Biol. 2024, 13, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Dehler, C.E.; Secombes, C.J.; Martin, S.A.M. Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L.). Aquaculture 2017, 467, 149–157. [Google Scholar] [CrossRef]
- Guangxin, G.; Li, K.; Zhu, Q.; Zhao, C.; Li, C.; He, Z.; Hu, S.; Ren, Y. Improvements of immune genes and intestinal microbiota composition of turbot (Scophthalmus maximus) with dietary oregano oil and probiotics. Aquaculture 2022, 547, 737442. [Google Scholar] [CrossRef]
- Luan, Y.; Li, M.; Zhou, W.; Yao, Y.; Yang, Y.; Zhang, Z.; Ringø, E.; Olsen, R.E.; Clarke, J.L.; Xie, S.; et al. The Fish Microbiota: Research Progress and Potential Applications. Engineering 2023, 29, 137–146. [Google Scholar] [CrossRef]
- McInnes, M.D.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; PRISMA-DTA Group. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, R.E.; Lagisz, M.; Jennions, M.D.; Koricheva, J.; Noble, D.W.; Parker, T.H.; Gurevitch, J.; Page, M.J.; Stewart, G.; Moher, D.; et al. Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: A PRISMA extension. Biol. Rev. 2021, 96, 1695–1722. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseerf, L.; Tetzlaffg, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Food and Agriculture Organization/World Health Organization. Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation. FAO Food and Nutrition Paper, Rome. 2006. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 17 December 2024).
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, S.J.; Baker, R.T.; Bogwald, J.; Castex, M.; Ringo, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Jitendrasinh, R.R.; Kotiya, A.S.; Dipakbhai, J.M. Bioactive Feed Ingredients used in Aquaculture: A Review. J. Sci. Res. Rep. 2024, 30, 399–414. [Google Scholar] [CrossRef]
- Rousta, N.; Aslan, M.; Yesilcimen Akbas, M.; Ozcan, F.; Sar, T.; Taherzadeh, M.J. Effects of fungal based bioactive compounds on human health. Crit. Rev. Food Sci. Nutr. 2024, 64, 7004–7027. [Google Scholar] [CrossRef]
- Irianto, A.; Austin, B. Probiotics in aquaculture. J. Fish Dis. 2002, 25, 633–642. [Google Scholar] [CrossRef]
- Banerjee, G.; Ray, A.K. The advancement of probiotics research and its application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef]
- Linh, N.T.H.; Nagai, S.; Nagasaka, N.; Okane, S.; Taoka, Y. Effect of Lactococcus lactis K-C2 on the growth performance, amino acid content and gut microflora of amberjack Seriola dumerili. Fish. Sci. 2018, 84, 1051–1062. [Google Scholar] [CrossRef]
- Ringø, E.; Van Doan, H.; Lee, S.H.; Soltani, M.; Hoseinifar, S.H.; Harikrishnan, R.; Song, S.K. Probiotics, lactic acid bacteria and bacilli: Interesting supplementation for aquaculture. J. Appl. Microbiol. 2020, 129, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, Y.; Gao, C.; Zhang, F.; Xia, R.; Li, D.; Hu, J.; Ran, C.; Zhang, Z.; Liu-Clarke, J.; et al. Surface display system for probiotics and its application in aquaculture. Rev. Aquac. 2020, 12, 2333–2350. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.; Dhama, K.; Abdel-Latif, H.M. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Jinendiran, S.; Archana, R.; Sathishkumar, R.; Kannan, R.; Selvakumar, G.; Sivakumar, N. Dietary Administration of Probiotic Aeromonas veronii V03 on the Modulation of Innate Immunity, Expression of Immune-Related Genes and Disease Resistance Against Aeromonas hydrophila Infection in Common Carp (Cyprinus carpio). Probiotics Antimicrob. Proteins 2021, 13, 1709–1722. [Google Scholar] [CrossRef]
- Medina, A.; García-Márquez, J.; Morinigo, M.Á.; Arijo, S. Effect of the Potential Probiotic Vibrio proteolyticus DCF12.2 on the Immune System of Solea senegalensis and Protection against Photobacterium damselae subsp. piscicida and Vibrio harveyi. Fishes 2023, 8, 344. [Google Scholar] [CrossRef]
- Sáenz de Rodrigáñez, M.A.; Díaz-Rosales, P.; Chabrillón, M.; Smidt, H.; Arijo, S.; León-Rubio, J.M.; Alarcón, F.J.; Balebona, M.C.; Moriñigo, M.A.; Cara, J.B.; et al. Effect of dietary administration of probiotics on growth and intestine functionality of juvenile Senegalese sole (Solea senegalensis, Kaup 1858). Aquac. Nut. 2009, 15, 177–185. [Google Scholar] [CrossRef]
- Wu, Z.X.; Feng, X.; Xie, L.L.; Peng, X.Y.; Yuan, J.; Chen, X.X. Effect of probiotic Bacillus subtilis Ch9 for grass carp, Ctenopharyngodon idella (Valenciennes, 1844), on growth performance, digestive enzyme activities and intestinal microflora. J. Appl. Ichthyol. 2012, 28, 721–727. [Google Scholar] [CrossRef]
- Xu, Y.J.; Wang, Y.B.; Lin, J.D. Use of Bacillus coagulans as a Dietary Probiotic for the Common Carp, Cyprinus carpio. J. World Aquac. Soc. 2014, 45, 403–411. [Google Scholar] [CrossRef]
- Beck, B.R.; Kim, D.; Jeon, J.; Lee, S.M.; Kim, H.K.; Kim, O.J.; Lee, J., II.; Suh, B.S.; Do, H.K.; Lee, K.H.; et al. The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2015, 42, 177–183. [Google Scholar] [CrossRef]
- Pourgholam, M.A.; Khara, H.; Safari, R.; Yazdani-Sadati, M.A.; Sadegh Aramli, M. Dietary Administration of Lactobacillus plantarum Enhanced Growth Performance and Innate Immune Response of Siberian Sturgeon, Acipenser baerii. Probiotics Antimicrob. Proteins 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.M.; El-Sayed, A.F.M.; Mahmoud, M.M. Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). J. Appl. Microbiol. 2016, 120, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Mokashe, N.U.; Chaudhari, B.L. Effect of Dietary Probiotic Lactobacillus helveticus on Growth Performance, Antioxidant Levels, and Absorption of Essential Trace Elements in Goldfish (Carassius auratus). Probiotics Antimicrob. Proteins 2018, 11, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.V.; Onoda, S.; Khanh, T.V.; Hai, P.D.; Trung, N.T.; Hoang, L.; Koshio, S. Evaluation of dietary Heat-killed Lactobacillus plantarum strain L-137 supplementation on growth performance, immunity and stress resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 498, 371–379. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Fan, W.; Huang, M.; Liu, M. Effects of dietary Lactobacillus delbrueckii on growth performance, body composition, digestive and absorptive capacity, and gene expression of common carp (Cyprinus carpio Huanghe var). Aquac. Nut. 2019, 25, 166–175. [Google Scholar] [CrossRef]
- Munni, M.J.; Akther, K.R.; Ahmed, S.; Hossain, M.A.; Roy, N.C. Effects of Probiotics, Prebiotics, and Synbiotics as an Alternative to Antibiotics on Growth and Blood Profile of Nile Tilapia (Oreochromis niloticus). Aquac. Res. 2023, 2798279. [Google Scholar] [CrossRef]
- Liaqat, R.; Fatima, S.; Komal, W.; Minahal, Q.; Kanwal, Z.; Suleman, M.; Carter, C.G. Effects of Bacillus subtilis as a single strain probiotic on growth, disease resistance and immune response of striped catfish (Pangasius hypophthalmus). PLoS ONE 2024, 19, e0294949. [Google Scholar] [CrossRef]
- Pérez-Jiménez, G.M.; Alvarez-Villagomez, C.S.; Martínez-Porchas, M.; Garibay-Valdez, E.; Sepúlveda-Quiroz, C.A.; Méndez-Marín, O.; De la Rosa-García, S.D.C. The indigenous probiotic Lactococcus lactis PH3-05 enhances the growth, digestive physiology, and gut microbiota of the tropical gar (Atractosteus tropicus) larvae. Animals 2024, 14, 2663. [Google Scholar] [CrossRef]
- Heo, W.; Kim, Y.; Kim, E.Y.; Bai, S.C.; Kong, I. Effects of dietary probiotic, Lactococcus lactis subsp. lactis I2, supplementation on the growth and immune response of olive flounder (Paralichthys olivaceus). Aquaculture 2013, 376–379, 20–24. [Google Scholar] [CrossRef]
- Aini, N.; Putri, D.S.Y.R.; Achhlam, D.H.; Fatimah, F.; Andriyono, S.; Hariani, D.; Wahyuningsih, S.P.A. Supplementation of Bacillus subtilis and Lactobacillus casei to increase growth performance and immune system of catfish (Clarias gariepinus) due to Aeromonas hydrophila infection. Vet. World 2024, 17, 602. [Google Scholar] [CrossRef]
- Meidong, R.; Buatong, A.; Nakao, M.; Sakai, K.; Tongpim, S. Mixed culture of Bacillus aerius B81e and Lactiplantibacillus paraplantarum L34b-2 derived from in vivo screening using hybrid catfish exhibits high probiotic effects on Pangasius bocourti. J. Biosci. Bioeng. 2021, 132, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Amenyogbe, E.; Yang, E.J.; Xie, R.T.; Huang, J.S.; Chen, G. Influences of indigenous isolates Pantoea agglomerans RCS2 on growth, proximate analysis, hematological parameters, digestive enzyme activities, serum biochemical parameters, antioxidants activities, intestinal morphology, disease resistance, and molecular immune response in juvenile cobia fish (Rachycentron canadum). Aquaculture 2022, 551, 737942. [Google Scholar] [CrossRef]
- Arghideh, M.; Hoseinifar, S.H.; Nasrabadi, R.G.; Mazandarani, M.; El-Haroun, E.; Van Doan, H. Evaluation of soil-derived Streptomyces chartreusis KU324443 effects as probiotic on growth performance, antioxidant enzyme activity, mucosal and serum immune parameters, and related gene expression in common carp (Cyprinus carpio) fingerlings. Aquac. Nutr. 2022, 2022, 2278130. [Google Scholar] [CrossRef]
- Omar, A.A.; Gado, M.S.; Kandel, H.E.; Farrag, F.A.; Shukry, M. Probiotic efficacy in aquaculture: The role of Technospore® (Bacillus coagulans) in improving Nile tilapia (Oreochromis niloticus) performance and disease resistance: A study on gut health, immunological response, and gene expression. Probiotics Antimicrob. Proteins 2024, 1–18. [Google Scholar] [CrossRef]
- Adeshina, I.; Abubakar, M.I.O.; Ajala, B.E. Dietary supplementation with Lactobacillus acidophilus enhanced the growth, gut morphometry, antioxidant capacity, and the immune response in juveniles of the common carp, Cyprinus carpio. Fish Physiol. Biochem. 2020, 46, 1375–1385. [Google Scholar] [CrossRef]
- Akbari, H.; Shekrabi, S.P.H.; Soltani, M.; Mehrgan, M.S. Effects of potential probiotic Enterococcus casseliflavus (EC-001) on growth performance, immunity, and resistance to Aeromonas hydrophila infection in common carp (Cyprinus carpio). Probiotics Antimicrob. Proteins 2021, 13, 1316–1325. [Google Scholar] [CrossRef]
- Hooshyar, Y.; Abedian Kenari, A.; Paknejad, H.; Gandomi, H. Effects of Lactobacillus rhamnosus ATCC 7469 on different parameters related to health status of rainbow trout (Oncorhynchus mykiss) and the protection against Yersinia ruckeri. Probiotics Antimicrob. Proteins 2020, 12, 1370–1384. [Google Scholar] [CrossRef]
- Silva, V.V.; Salomão, R.A.S.; Mareco, E.A.; Dal Pai, M.; Santos, V.B. Probiotic additive affects muscle growth of Nile tilapia (Oreochromis niloticus). Aquac. Res. 2021, 52, 2061–2069. [Google Scholar] [CrossRef]
- Shadyeva, L.A.; Romanova, E.M.; Romanov, V.V.; Spirina, E.V. Vitamin content in meat when growing African catfish with probiotics. In IOP Conf. Ser. Earth Environ. Sci. 2022, 954, 012069. [Google Scholar] [CrossRef]
- Allameh, S.K.; Ringø, E.; Yusoff, F.M.; Daud, H.M.; Ideris, A. Dietary supplement of Enterococcus faecalis on digestive enzyme activities, short-chain fatty acid production, immune system response and disease resistance of Javanese carp (Puntius gonionotus, Bleeker 1850). Aquac. Nutr. 2015, 23, 331–338. [Google Scholar] [CrossRef]
- Soltani, M.; Badzohreh, G.; Mirzargar, S.; Farhangi, M.; Shekarabi, P.H.; Lymbery, A. Growth Behavior and Fatty Acid Production of Probiotics, Pediococcus acidilactici and Lactococcus lactis, at Different Concentrations of Fructooligosaccharide: Studies Validating Clinical Efficacy of Selected Synbiotics on Growth Performance of Caspian Roach (Rutilus frisii kutum) Fry. Probiotics Antimicrob. Proteins. 2019, 11, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K. Production of eicosapentaenoic acid from marine bacteria. Lipids 1996, 31, S297–S300. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.; Abedian Kenari, A.; Yazdani Sadati, M. Influence of dietary acetic acid, protexin (probiotic), and their combination on growth performance, intestinal microbiota, digestive enzymes, immunological parameters, and fatty acids composition in Siberian sturgeon (Acipenser baerii, Brandt, 1869). Aquac. Int. 2021, 29, 891–910. [Google Scholar] [CrossRef]
- Schubiger, C.B.; Orfe, L.H.; Sudheesh, P.S.; Cain, K.D.; Shah, D.H.; Call, D.R. Entericidin is required for a probiotic treatment (Enterobacter sp. strain C6-6) to protect trout from cold-water disease challenge. Appl. Environ. Microbiol. 2015, 81, 658–665. [Google Scholar] [CrossRef]
- Maas, R.M.; Deng, Y.; Dersjant-Li, Y.; Petit, J.; Verdegem, M.C.; Schrama, J.W.; Kokou, F. Exogenous enzymes and probiotics alter digestion kinetics, volatile fatty acid content and microbial interactions in the gut of Nile tilapia. Sci. Rep. 2021, 11, 8221. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, E.; Lu, M.; Xie, J. Effects of probiotic supplementation on gut microbiota as well as metabolite profiles within Nile tilapia, Oreochromis niloticus. Aquaculture 2020, 527, 735428. [Google Scholar] [CrossRef]
- Santos, H.O.; May, T.L.; Bueno, A.A. Eating more sardines instead of fish oil supplementation: Beyond omega-3 polyunsaturated fatty acids, a matrix of nutrients with cardiovascular benefits. Front. Nutr. 2023, 10, 1107475. [Google Scholar] [CrossRef]
- Tacon, A.G.; Coelho, R.T.; Levy, J.; Machado, T.M.; Neiva, C.R.; Lemos, D. Annotated bibliography of selected papers dealing with the health benefits and risks of fish and seafood consumption. Rev. Fish. Sci. Aquac. 2024, 32, 211–305. [Google Scholar] [CrossRef]
- Izquierdo, M.; Fernández-Palacios, H. Importancia de la nutrición en la reproducción de peces. ITEA 2004, 100, 289–296. [Google Scholar]
- Chen, X.-Q.; Zhao, W.; Xie, S.-W.; Xie, J.-J.; Zhang, Z.-H.; Tian, L.-X.; Liu, Y.-J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef]
- Sanahuja, I.; Ruiz, A.; Firmino, J.P.; Reyes-López, F.E.; Ortiz-Delgado, J.B.; Vallejos-Vidal, E.; Tort, L.; Tovar-Ramírez, D.; Cerezo, I.M.; Moriñigo, M.A.; et al. Debaryomyces hansenii supplementation in low fish meal diets promotes growth, modulates microbiota and enhances intestinal condition in juvenile marine fish. J. Anim. Sci. Biotechnol. 2023, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, O.A.; Akintayo, I.A.; Adesina, S.A.; Akintunde, E.I.; Oluwole, T.O. The Dietary Effects of Saccharomyces cerevisiae on Nile Tilapia (Oreochromis niloticus) Juveniles Challenged with Aeromonas hydrophila. Ethiop. J. Sci. Sustain. Dev. 2024, 11, 17–26. [Google Scholar] [CrossRef]
- Singh, A.; Vidakovic, A.; Singh, A.; Dicksved, J.; Schnürer, A.; Lundh, T. Yarrowia lipolytica yeast as a dietary supplement for rainbow trout (Oncorhynchus mykiss): Effects on gut microbiota, health and immunity. Aquaculture 2024, 590, 741065. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, S.; Zou, T.; Han, D.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S.; Zhou, W. Effects of dietary yeast culture on growth performance, immune response and disease resistance of gibel carp (Carassius auratus gibelio CAS III). Fish Shellfish Immunol. 2018, 82, 400–407. [Google Scholar] [CrossRef]
- Rhema, Z.A.; Al-Noor, J.M. Health and nutritional performance of young common carp Cyprinus carpio L. feeding diets with added bakery yeast Saccharomyces cerevisiae. Int. J. Health Sci. 2022, 6, 2424–2437. [Google Scholar] [CrossRef]
- Feng, Z.; Zhong, Y.; He, G.; Sun, H.; Chen, Y.; Zhou, W.; Lin, S. Yeast culture improved the growth performance, liver function, intestinal barrier and microbiota of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 2022, 120, 706–715. [Google Scholar] [CrossRef]
- Sutthi, N.; Thaimuangphol, W. Effects of yeast (Saccharomyces cerevisiae) on growth performances, body composition and blood chemistry of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) under different salinity conditions. Iran. J. Fish. Sci. 2020, 19, 1428–1446. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Rohani, M.F.; Shahjahan, M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquac. Rep. 2021, 21, 100800. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; El Asely, A.M.; Hussein, M.N.; Khattaby, A.E.R.A.; Sabry, E.A.; Abdelsalam, M.; Samir, F. Effects of dietary fermented Saccharomyces cerevisiae extract (Hilyses) supplementation on growth, hematology, immunity, antioxidants, and intestinal health in Nile tilapia. Sci. Rep. 2024, 14, 12583. [Google Scholar] [CrossRef]
- Mashhadizadeh, N.; Khezri, S.; Esfahani, D.E.; Mohammadzadeh, S.; Ahmadifar, E.; Ahmadifar, M.; El-Haroun, E. Enhancing growth performance, antioxidant defense, immunity response, and resistance against heat stress in Nile tilapia (Oreochromis niloticus) fed Saccharomyces boulardii and/or Bifidobacterium bifidum. Aquac. Rep. 2024, 39, 102462. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Tovar-Ramírez, D.; Ascencio-Valle, F.; Civera-Cerecedo, R.; Gracia-López, V.; Barbosa-Solomieu, V. Effects of dietary live yeast Debaryomyces hansenii on the immune and antioxidant system in juvenile leopard grouper Mycteroperca rosacea exposed to stress. Aquaculture 2008, 280, 39–44. [Google Scholar] [CrossRef]
- Tovar-Ramirez, D.; Zambonino-Infante, J.; Cahu, C.; Gatesoupe, F.J.; Vazquez-Juarez, R. Influence of dietary live yeast on European sea bass (Dicentrarchus labrax) larval development. Aquaculture 2004, 234, 415–427. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Alamillo, E.; Angulo, C. Probiotic and Immunomodulatory Activity of Marine Yeast Yarrowia lipolytica Strains and Response Against Vibrio parahaemolyticus in Fish. Probiotics Antimicrob. Proteins 2021, 13, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Retcheski, M.C.; Maximowski, L.V.; Escorsin, K.J.S.; de Almeida Rosa Kurosaki, J.K.; Romão, S.; Bitencourt, T.B.; Garcia Parra, J.E.; Helena, L. Yarrowia lipolytica biomass—A potential additive to boost metabolic and physiological responses of Nile tilapia. Fish Physiol. Biochem. 2023, 49, 655–670. [Google Scholar] [CrossRef]
- Neuls, L.; Souza, V.J.; Romão, S.; Bitencourt, T.B.; Ramos, C.J.R.; Parra, J.E.G.; Cazarolli, L.H. Immunomodulatory effects of Yarrowia lipolytica as a food additive in the diet of Nile tilapia. Fish Shellfish Immunol. 2021, 119, 272–279. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Xia, D.; Yang, K.; Yang, Y.; Huang, Z.; Yu, W. Effect of Dietary Marine Red Yeast Rhodotorula mucilaginosa on the Growth Performance, and also Non-Specific Immune Responses of Juvenile Golden Pompano trachinotus Ovatus when Challenged with Vibrio Harveyi. Isr. J. Aquac. 2016, 68. [Google Scholar] [CrossRef]
- Van Doan, H.; Tapingkae, W.; Chaiyaso, T.; Wangkahart, E.; Panchan, R.; Sutthi, N. Effects of Red Yeast (Sporidiobolus pararoseus) on Growth, Innate Immunity, Expression of Immune-related Genes and Disease Resistance of Nile Tilapia (Oreochromis niloticus). Probiotics Antimicrob. Proteins 2022, 15, 1312–1326. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Guluarte, C.; Ceballos-Francisco, D.; Angulo, C.; Esteban, M.Á. Dietary yeast Sterigmatomyces halophilus enhances mucosal immunity of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 64, 165–175. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Madsen, L.; Bertelsen, S.K.; Dalsgaard, I.; Middelboe, M. Dispersal and Survival of Flavobacterium psychrophilum Phages In Vivo in Rainbow Trout and In Vitro under Laboratory Conditions: Implications for Their Use in Phage Therapy. Appl. Environ. Microbiol. 2013, 79, 4853–4861. [Google Scholar] [CrossRef]
- Mateus, L.; Costa, L.; Silva, Y.J.; Pereira, C.; Cunha, A.; Almeida, A. Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture 2014, 424–425, 167–173. [Google Scholar] [CrossRef]
- Salmond, G.P.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. La OMS Publica un Informe Sobre el Estado de Desarrollo de Antibacterianos. Available online: https://www.who.int/es/news/item/14-06-2024-who-releases-report-on-state-of-development-of-antibacterials (accessed on 19 January 2025).
- Preenanka, R.; Safeena, M.P. Morphological, biological and genomic characterization of lytic phages against Streptococcus agalactiae causing streptococcosis in tilapia. Microb. Pathog. 2023, 174, 105919. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, S.; Han, S.; Wang, D.; Zhao, J.; Xu, L.; Liu, H.; Lu, T. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 2020, 523, 735193. [Google Scholar] [CrossRef]
- Mohan Raj, J.R.; Karunasagar, I. Phages amid antimicrobial resistance. Crit. Rev. 2019, 45, 701–711. [Google Scholar] [CrossRef]
- Fuentes-Valencia, M.A.; Gil, C.A.C.; Martínez, P.C.A.; Baizabal, A.V.M.; Valdez, A.J.J. El enemigo de mi amigo es… Un virus que ataca a las bacterias: Los bacteriófagos. Rev. Digit. Univ. 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Z.; Gu, Y.; Huang, A.; Wang, J.; Hao, H. Phage Products for Fighting Antimicrobial Resistance. Microorganisms 2022, 10, 1324. [Google Scholar] [CrossRef]
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Baloch, Z. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Mahony, J.; McAuliffe, O.; Ross, R.P.; Van Sinderen, D. Bacteriophages as biocontrol agents of food pathogens. Curr. Opin. Biotechnol. 2011, 22, 157–163. [Google Scholar] [CrossRef]
- Jun, J.W.; Han, J.E.; Tang, K.F.; Lightner, D.V.; Kim, J.; Seo, S.W.; Park, S.C. Potential application of bacteriophage pVp-1: Agent combating Vibrio parahaemolyticus strains associated with acute hepatopancreatic necrosis disease (AHPND) in shrimp. Aquaculture 2016, 457, 100–103. [Google Scholar] [CrossRef]
- Makarov, R.; Lomelí, O.C.O.; Zermeño, C.L.A.; García, Á.E.; Gutiérrez, R.J.N.; Cardona, F.C.S.; Martínez, D.S.F. Evaluation of a cocktail of phages for the control of presumptive Vibrio parahaemolyticus strains associated to acute hepatopancreatic necrosis disease. Aquac. Res. 2019, 50, 3107–3116. [Google Scholar] [CrossRef]
- Hossain, M.M.M.; Tanni, L.N.; Rahman, M.A.; Farjana, N.; Moon, R.S.; Tonni, N.Z.; Saha, P.K. Bacteriophage and non-pathogenic Vibrio to control diseases in shrimp aquaculture. Comp. Immunol. Rep. 2024, 6, 200126. [Google Scholar] [CrossRef]
- Muliya Sankappa, N.; Shivani Kallappa, G.; Kallihosuru Boregowda, K.; Mandrira Ramakrishna, N.; Kattapuni Suresh, P.; Shriraje Balakrishna, D.; Abernathy, J.W. Novel lytic bacteriophage AhFM11 as an effective therapy against hypervirulent Aeromonas hydrophila. Sci. Rep. 2024, 14, 16882. [Google Scholar] [CrossRef] [PubMed]
- Deshotel, M.B.; Dave, U.M.; Farmer, B.; Kemboi, D.; Nelson, D.C. Bacteriophage endolysin treatment for systemic infection of Streptococcus iniae in hybrid striped bass. Fish Shellfish Immunol. 2024, 145, 109296. [Google Scholar] [CrossRef]
- De Zoysa, M. Marine Bacteriophages for the Biocontrol of Fish and Shellfish Diseases. In Marine Microbiology; Kim, S.-W., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 161–172. [Google Scholar]
- Donati, V.L.; Dalsgaard, I.; Sundell, K.; Castillo, D.; Er-Rafik, M.; Clark, J.; Madsen, L. Phage-mediated control of Flavobacterium psychrophilum in aquaculture: In vivo experiments to compare delivery methods. Front. Microbiol. 2021, 12, 628309. [Google Scholar] [CrossRef]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Kurtböke, D.İ. Protective effects of bacteriophages against Aeromonas hydrophila causing motile Aeromonas septicemia (MAS) in striped catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef]
- Silva, Y.J.C.; Moreirinha, C.; Pereira, L.; Costa, R.J.; Cunha, A.; Gomes, N.C.; Calado, R.; Almeida, A. Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with Phage AS-A. Aquaculture 2016, 450, 225–233. [Google Scholar] [CrossRef]
- Jia, K.; Yang, N.; Zhang, X.; Cai, R.; Zhang, Y.; Tian, J.; Raza, S.H.A.; Kang, Y.; Qian, A.; Li, Y.; et al. Genomic, Morphological and Functional Characterization of Virulent Bacteriophage IME-JL8 Targeting Citrobacter freundii. Front. Microbiol. 2020, 11, 585261. [Google Scholar] [CrossRef]
- Han, G.; Huang, T.; Liu, X.; Liu, R. Bacteriophage EPP-1, a potential antibiotic alternative for controlling edwardsiellosis caused by Edwardsiella piscicida while mitigating drug-resistant gene dissemination. Sci. Rep. 2024, 14, 9399. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Chandrarathna, H.P.S.U.; Dananjaya, S.H.S.; De Zoysa, M.; Lee, J. Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 2020, 63, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.I.; Nakai, T. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microbiol. 2000, 66, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cong, C.; Wang, L.; Li, X.; Li, J.; Yang, H.; Xu, Y. Protective effectiveness of feeding phage cocktails in controlling Vibrio harveyi infection of turbot Scophthalmus maximus. Aquaculture 2021, 535, 736390. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Yang, H.; Li, J.; Xiao, S.; Hu, S.; Yan, F.; Xia, L.; Zhang, Y. Screening of a Plesiomonas shigelloides phage and study of the activity of its lysis system. Virus Res. 2021, 306, 198581. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Sugimoto, R.; Park, K.H.; Matsuoka, S.; Mori, K.I.; Nishioka, T.; Maruyama, K. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Organ. 1999, 37, 33–41. [Google Scholar] [CrossRef]
- Gordillo-Altamirano, F.L.; Barr, J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef]
- Rehman, S.; Ali, Z.; Khan, M.; Bostan, N.; Naseem, S. The dawn of phage therapy. Rev. Med. Virol. 2019, 29, e2041. [Google Scholar] [CrossRef]
- Harper, R.D.; Abedon, T.S.; Burrowes, H.B.; McConville, L.M. Bacteriophages: Biology, Technology, Therapy, 2nd ed.; Springer Nature: Cham, Switzerland, 2021; pp. 1–1206. [Google Scholar] [CrossRef]
- Khan, A.; Rao, T.S.; Joshi, H.M. Phage therapy in the Covid-19 era: Advantages over antibiotics. Curr. Res. Microb. Sci. 2022, 3, 100115. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Price, D.E.R.E.K.; Chalmers, W.H.; McClure, J. Understanding antibiotic treatment failures in salmon aquaculture. Asian Fish Sci. 2020, 33, 33–38. [Google Scholar] [CrossRef]
- Jaenisch, R.; Mintz, B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc. Natl. Acad. Sci. USA 1974, 71, 1250–1254. [Google Scholar] [CrossRef]
- Chourrout, D.; Guyomard, R.; Houdebine, L.M. High efficiency gene transfer in rainbow trout (Salmo gairdneri rich.) by microinjection into egg cytoplasm. Aquaculture 1986, 51, 143–150. [Google Scholar] [CrossRef]
- Dunham, R.A.; Eash, J.; Askins, J.; Townes, T.M. Transfer of the Metallothionein-Human Growth Hormone Fusion Gene into Channel Catfish. Trans. Am. Fish. Soc. 1987, 116, 87–91. [Google Scholar] [CrossRef]
- Rahman, M.A.; Maclean, N. Production of transgenic tilapia Oreochromis niloticus by onecell-stage microinjection. Aquaculture 1992, 105, 219–232. [Google Scholar] [CrossRef]
- Devlin, R.H.; Leggatt, R.A.; Benfey, T.J. Genetic modification of growth in fish species used in aquaculture: Phenotypic and physiological responses. Fish Physiol. 2020, 38, 237–272. [Google Scholar] [CrossRef]
- Hallerman, E.M.; Dunham, R.; Houston, R.D.; Walton, M.; Wargelius, A.; Wray-Cahen, D. Towards production of genome-edited aquaculture species. Rev. Aquac. 2022, 15, 404–408. [Google Scholar] [CrossRef]
- Moran, M.N.; Jones, D.B.; Jensen, S.A.; Marcoli, R.; Jerry, D.R. Optimising commercial traits through gene editing in aquaculture: Strategies for accelerating genetic improvement. Rev. Aquac. 2023, 16, 1127–1159. [Google Scholar] [CrossRef]
- Yadav, D.K.; Yadav, N.; Khurana, S.M.P. Chapter 26—Vaccines: Present status and applications. In Animal Biotechnology, 2nd ed.; Ashish, S.V., Anchal, S., Eds.; Academic Press: Boca Raton, FL, USA, 2020; pp. 523–542. [Google Scholar] [CrossRef]
- Kamionka, M. Engineering of Therapeutic Proteins Production in Escherichia coli. Curr. Pharm. Biotechnol. 2011, 12, 268–274. [Google Scholar] [CrossRef]
- Lerner, A.; Benzvi, C.; Vojdani, A. The Potential Harmful Effects of Genetically Engineered Microorganisms (GEMs) on the Intestinal Microbiome and Public Health. Microorganisms 2024, 12, 238. [Google Scholar] [CrossRef]
- Goeddel, D.V.; Kleid, D.G.; Bolivar, F.; Heyneker, H.L.; Yansura, D.G.; Crea, R.; Hirose, T.; Kraszewski, A.; Itakura, K.; Riggs, A.D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc. Natl. Acad. Sci. USA 1979, 76, 106–110. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef]
- Wesseler, J.; Kleter, G.; Meulenbroek, M.; Purnhagen, K.P. EU regulation of genetically modified microorganisms in light of new policy developments: Possible implications for EU bioeconomy investments. Appl. Econ. Perspect. Policy 2023, 45, 839–859. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Ramesh, B.; Srinivasan, S. Removal of toxic heavy metals using genetically engineered microbes: Molecular tools, risk assessment and management strategies. Chemosphere 2022, 298, 134341. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M.N. Genetically engineered microorganisms for environmental remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Torres, O.; Abad-Sojos, S.; Pardo, S.; Bucio, E. Application of Genetically Modified Microorganisms for Bioremediation of Polluted Environments. In Genetically Engineered Organisms in Bioremediation, 1st ed.; Inamuddin, D.R., Oluwaseun Adetunji, C., Imran Ahamed, M., Altalhi, T., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 18–51. [Google Scholar]
- Thammasorn, T.; Jitrakorn, S.; Charoonnart, P.; Sirimanakul, S.; Rattanarojpong, T.; Chaturongakul, S.; Saksmerprome, V. Probiotic Bacteria (Lactobacillus Plantarum) Expressing Specific Double-Stranded RNA and Its Potential for Controlling Shrimp Viral and Bacterial Diseases. Aquac. Int. 2017, 25, 1679–1692. [Google Scholar] [CrossRef]
- Riet, J.; Costa-Filho, J.; Dall’Agno, L.; Medeiros, L.; Azevedo, R.; Nogueira, L.F.; Maggioni, R.; Pedrosa, V.F.; Romano, L.A.; Altenbuchner, J.; et al. Bacillus Subtilis Expressing Double-Strand RNAs (DsRNAs) Induces RNA Interference Mechanism (RNAi) and Increases Survival of WSSV-Challenged Litopenaeus vannamei. Aquaculture 2021, 541, 736834. [Google Scholar] [CrossRef]
- Fajardo, C.; De Donato, M.; Macedo, M.; Charoonnart, P.; Saksmerprome, V.; Yang, L.; Purton, S.; Mancera, J.M.; Costas, B. RNA Interference Applied to Crustacean Aquaculture. Biomolecules 2024, 14, 1358. [Google Scholar] [CrossRef]
- Costa Filho, J.; Riet, J.; Santos, K.; de Sousa, O.V.; Maggioni, R.; Feijó, R.G.; Wasielesky, W.; Marins, L.F. Genetic manipulation of native Bacillus cereus: A biotechnological tool for aquaculture. J. Appl. Aquac. 2020, 34, 197–207. [Google Scholar] [CrossRef]
- Bandari, N.M.; Abootaleb, M.; Nikokar, I.; Karimli, M. Biologically engineered probiotic supplement production containing phytase enzyme for livestock, poultry, and aquaculture consumption. J. Basic Appl. Zool. 2024, 85, 41. [Google Scholar] [CrossRef]
- Nakharuthai, C.; Boonanuntanasarn, S.; Kaewda, J.; Manassila, P. Isolation of Potential Probiotic Bacillus spp. from the Intestine of Nile Tilapia to Construct Recombinant Probiotic Expressing CC Chemokine and Its Effectiveness on Innate Immune Responses in Nile Tilapia. Animals 2023, 13, 986. [Google Scholar] [CrossRef]
- Nematollahi, A.; Decostere, A.; Pasmans, F.; Haesebrouck, F. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 2003, 26, 563–574. [Google Scholar] [CrossRef]
- Sloboda, S. Development of Genetic Manipulation Techniques for the Fish Pathogen Flavobacterium psychrophilum. Cornerstone: A Collection of Scholarly and Creative Works for Minnesota State University. Master’s Thesis, Minnesota State University, Mankato, Mankato, MN, USA, 2022. Available online: https://cornerstone.lib.mnsu.edu/etds/1261 (accessed on 11 December 2024).
- Poobalane, S.; Thompson, K.D.; Ardo, L.; Verjan, N.; Han, H.-J.; Jeney, G.; Hirono, I.; Aoki, T.; Adams, A. Production and efficacy of an Aeromonas hydrophila recombinant S-layer protein vaccine for fish. Vaccine 2010, 28, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Hua, X.; Wang, Y.; Wang, Y.; Chen, Y.; Shi, W.; Tang, L.; Li, Y.; Liu, M. Oral immunization with a recombinant Lactobacillus expressing CK6 fused with VP2 protein against IPNV in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hua, X.; Ren, X.; Duan, K.; Gao, S.; Sun, J.; Feng, Y.; Zhou, Y.; Guan, X.; Li, D.; et al. Oral immunization with recombinant Lactobacillus casei displayed AHA1-CK6 and VP2 induces protection against infectious pancreatic necrosis in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2020, 100, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, S.; Zhao, Z.; Guo, Z.-R.; Ma, R.; Wang, G.-X.; Zhu, B. Surface display of spring viremia of carp virus glycoprotein on Lactococcus lactis and its protection efficacy in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2020, 104, 262–268. [Google Scholar] [CrossRef]
- Kong, Y.D.; Kang, Y.H.; Tian, J.X.; Zhang, D.; Zhang, L.; Tao, L.T.; Wu, T.L.; Li, Y.; Wang, G.Q.; Shan, X.F. Oral immunization with recombinant Lactobacillus casei expressing flaB confers protection against Aeromonas veronii challenge in common carp, Cyprinus carpio. Fish Shellfish Immunol. 2019, 87, 627–637. [Google Scholar] [CrossRef]
- Chen, C.; Zu, S.; Zhang, D.; Zhao, Z.; Ji, Y.; Xi, H.; Shan, X.; Qian, A.; Han, W.; Gu, J. Oral vaccination with recombinant Lactobacillus casei expressing Aha1 fused with CTB as an adjuvant against Aeromonas veronii in common carp (Cyprinus carpio). Microb. Cell Fact. 2022, 21, 114. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Zhang, D.; Guan, Y.; Siddiqui, S.A.; Feng-Shan, X.; Cong, B. Oral vaccination with recombinant Lactobacillus casei expressing Aeromonas hydrophila Aha1 against A. hydrophila infections in common carps. Virulence 2022, 13, 794–807. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, D.-X.; Chen, C.; Kong, L.-C.; Hu, X.-Y.; Shan, X.-F.; Qian, A.-D. Immunization effect of recombinant Lactobacillus casei displaying Aeromonas veronii Aha1 with an LTB adjuvant in carp. Fish Shellfish Immunol. 2023, 135, 108660. [Google Scholar] [CrossRef]
- Li, H.-J.; Yang, B.-T.; Sun, Y.-F.; Zhao, T.; Hao, Z.-P.; Gu, W.; Sun, M.-X.; Cong, W.; Kang, Y.-H. Oral vaccination with recombinant Lactobacillus casei with surface displayed OmpK fused to CTB as an adjuvant against Vibrio mimicus infection in Carassius auratus. Fish Shellfish Immunol. 2023, 135, 108659. [Google Scholar] [CrossRef]
- Cui, L.-C.; Guan, X.-T.; Liu, Z.-M.; Tian, C.-Y.; Xu, Y.-G. Recombinant lactobacillus expressing G protein of spring viremia of carp virus (SVCV) combined with ORF81 protein of koi herpesvirus (KHV): A promising way to induce protective immunity against SVCV and KHV infection in cyprinid fish via oral vaccination. Vaccine 2015, 33, 3092–3099. [Google Scholar] [CrossRef]
- Jia, S.; Zhou, K.; Pan, R.; Wei, J.; Liu, Z.; Xu, Y. Oral immunization of carps with chitosan–alginate microcapsule containing probiotic expressing spring viremia of carp virus (SVCV) G protein provides effective protection against SVCV infection. Fish Shellfish Immunol. 2020, 105, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, B.-T.; Kang, Y.-H.; Cong, W. Efficacy of a recombinant Lactobacillus plantarum Lp-pPG-Malt as an oral vaccine candidate against Aeromonas hydrophila infection in crucian carp. Fish Shellfish Immunol. 2023, 136, 108737. [Google Scholar] [CrossRef]
- Aonullah, A.A.; Nuryati, S.; Alimuddin; Murtini, S. Efficacy of koi herpesvirus DNA vaccine administration by immersion method on Cyprinus carpio field scale culture. Aquac. Res. 2016, 48, 2655–2662. [Google Scholar] [CrossRef]
- Luo, S.X.; Yan, L.M.; Zhang, X.H.; Yuan, L.; Fang, Q.; Zhang, Y.A.; Dai, H. Yeast surface display of capsid protein VP7 of Grass carp reovirus: Fundamental investigation for the development of vaccine against hemorrhagic disease. J. Microbiol. Biotechnol. 2015, 25, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Xu, L.M.; Liu, M.; Cao, Y.S.; LaPatra, S.E.; Yin, J.S.; Liu, H.B.; Lu, T.Y. Preliminary study of an oral vaccine against infectious hematopoietic necrosis virus using improved yeast Surface display technology. Mol. Immunol. 2017, 85, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J.Ø. Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Intamaso, U.; Chutoam, P.; Poomipak, W.; Pirarat, N. Cell surface display of red-grouper nervous necrosis virus capsid protein on Pichia pastoris. Adv. Microbiol. 2018, 8, 830–845. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Esteban, M.A.; Angulo, C. Yarrowia lipolytica, health benefits for animals. Appl. Microbiol. Biotechnol. 2021, 105, 7577–7592. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Tan, P.; Xu, D.; Wang, L.; Ding, Z.; Shao, Q. Yarrowia lipolytica as a promising protein source for Pacific white shrimp (Litopenaeus vannamei) diet: Impact on growth performance, metabolism, antioxidant capacity, and apparent digestibility. Front. Mar. Sci. 2024, 11, 1370371. [Google Scholar] [CrossRef]
- Osmond, A.T.Y.; Colombo, S.M. The future of genetic engineering to provide essential dietary nutrients and improve growth performance in aquaculture: Advantages and challenges. J. World Aquac. Soc. 2019, 50, 490–509. [Google Scholar] [CrossRef]
- Aksnes, I.; Braaen, S.; Markussen, T.; Åkesson, C.P.; Villoing, S.; Rimstad, E. Genetically modified attenuated salmonid alphavirus: A potential strategy for immunization of Atlantic salmon. J. Fish Dis. 2021, 44, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Elaswad, A.; Dunham, R. Disease reduction in aquaculture with genetic and genomic technology: Current and future approaches. Rev. Aquac. 2017, 10, 876–898. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, K.H. Genetically engineered viral hemorrhagic septicemia virus (VHSV) vaccines. Fish Shellfish Immunol. 2019, 95, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Moriette, C.; Leberre, M.; Lamoureux, A.; Lai, T.L.; Bremont, M. Recovery of a recombinant salmonid alphavirus fully attenuated and protective for rainbow trout. J. Virol. 2006, 80, 4088–4098. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Pan, W.; Lin, Y.; He, J.; Luo, Z.; Li, Z.; Weng, S.; He, J.; Guo, C. Development of a gene-deleted live attenuated candidate vaccine against fish virus (ISKNV) with low pathogenicity and high protection. Iscience 2021, 24, 102750. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Mondal, H.; Thomas, J.A. review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Ramos-Vivas, J.; Superio, J.; Galindo-Villegas, J.; Acosta, F. Phage therapy as a focused management strategy in aquaculture. Int. J. Mol. Sci. 2021, 22, 10436. [Google Scholar] [CrossRef]
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture 2019, 513, 734423. [Google Scholar] [CrossRef]
- Liu, L.; Helal, S.E.; Peng, N. CRISPR-Cas-based engineering of probiotics. BioDesign Res. 2023, 5, 0017. [Google Scholar] [CrossRef]
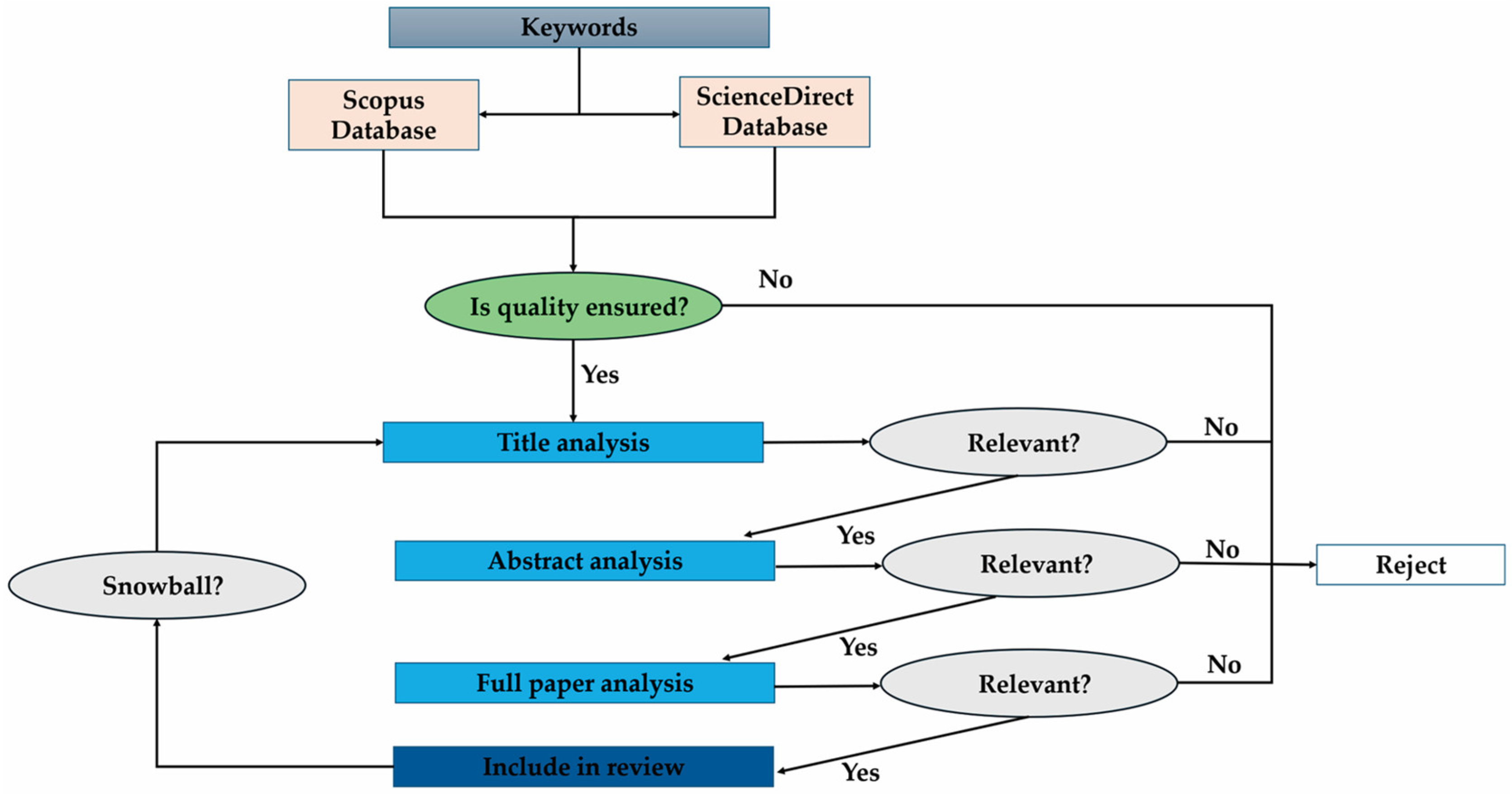
| Keyword Strings | Science Direct | Scopus | Used for | Documents Selected |
|---|---|---|---|---|
| EM + FA | 12,971 | 946 | Introduction | 35 |
| FA + P + SA | 4647 | 1502 | Introduction | 14 |
| B + FA | 20,529 | 2211 | Effects of bacteria on fish aquaculture | 44 |
| Y + FA | 6971 | 234 | Effects of yeasts on fish aquaculture | 22 |
| V + FA | 10,756 | 581 | Effects of bacteriophages on fish aquaculture | 34 |
| GMM + FA | 8425 | 8 | Use of genetically modified microorganisms in fish aquaculture | 43 |
| Fish Species | Microorganism | Concentration or Dose | Origin of Microorganism | Effect | Reference |
|---|---|---|---|---|---|
| Senegalese sole Solea senegalensis | Shewanella spp. | 1 × 109 CFU/g | Isolated from skin of Sparus aurata | ↑ Growth | [68] |
| Grass carp Ctenopharyngodon idella | Bacillus subtilis Ch9 | 3 and 5 × 109 CFU/kg | Isolated from intestine of Ctenopharyngodon idella | ↑ Growth ↑ Bacteria Bifidobacterium and Lactobacillus ↑ Enzyme activity (protease, amylase and lipase) | [69] |
| Common carp Cyprinus carpio | Bacillus coagulans | 1, 2 and 4 × 107 CFU/g | Isolated from C. carpio | ↑ Growth | [70] |
| Olive flounder Paralichthys olivaceus | Lactobacillus plantarum FGL0001 | 1 × 107 CFU/g | Isolated from hindgut of P. olivaceus | ↑ Growth | [71] |
| Siberian sturgeon Acipenser baerii | Lactobacillus plantarum | 1 × 108 CFU/g | Isolated from the digestive tracts of Oncorhynchus mykiss | ↑ Growth and innate immune response | [72] |
| Nile tilapia Oreochromis niloticus | Lactobacillus plantarum AH 78 | 1% | Isolated from corals along the Egyptian coasts of the Mediterranean Sea | ↑ Growth, immune response and survival ↑ Total protein in muscle | [73] |
| Goldfish Carassius auratus | Lactobacillus helveticus | 3 × 107 CFU/g | Isolated from Indian traditional fermented food | ↑ Growth | [74] |
| Nile tilapia Oreochromis niloticus | Lactobacillus plantarum L-137 | 50 ppm | Isolated from a fermented fish and rice dish | ↑ Growth | [75] |
| Common carp Cyprinus carpio | Lactobacillus delbrueckii | 1 × 106 CFU/g | From Angel Company, Wuhan, China | ↑ Growth | [76] |
| Nile tilapia Oreochromis niloticus | Bacillus spp. | 1 × 109 CFU/g | From Pond Care, SKF BioJoc Fish Probiotic, Bangladesh | ↑ Growth | [77] |
| Striped catfish Pangasius hypophthalmus | Bacillus subtilis | 1 × 108 and 1 × 1010 CFU/g | From ECOSH, Estonian | ↑ Growth, body protein and digestive enzymes (amylase and protease) | [78] |
| Tropical gar Atractosteus tropicus | Lactococcus lactis PH3-05 | 1 × 104, 1 × 106 and 1 × 108 CFU/g | Isolated from the intestine of an adult male of A. tropicus | ↑ Growth, survival and digestive enzymes | [79] |
| Olive flounder Paralichthys olivaceus | Lactococcus lactis | 1 × 108 CFU/mL | Isolated from the intestine of P. olivaceus | ↑ Growth performance parameters | [80] |
| Catfish Clarias gariepinus | Bacillus subtilis and Lactobacillus casei | 1 × 108 CFU/mL | Not specified | ↑ Growth and immune system | [81] |
| Asian catfish Pangasius bocourti | Bacillus aerius B81e and Lactiplantibacillus paraplantarum L34b-2 | 1 × 107 CFU/g | Strain B81e isolated from the intestine of P. bocourti Strain L34b-2 isolated from fermented food samples | ↑ Growth performance parameters and immune system (lysozymes) | [82] |
| Cobia Rachycentron canadum | Pantoea agglomerans RCS2 | 1 × 1010 and 1 × 1012 CFU/mL | Isolates from R. canadum | ↑ Growth and activity of digestive enzymes | [83] |
| Common carp Cyprinus carpio | Streptomyces chartreusis | 1 × 106 and 1 × 107 CFU/g | Isolated from soil ecosystem | ↑ Growth performance parameters ↑ Serum total Ig and lysozyme activity | [84] |
| Nile tilapia Oreochromis niloticus | Bacillus coagulans DSM 32016 | 0.02, 0.04, and 0.08% | Isolated from canned tomatoes | ↑ Immune-related genes, including liver IGF-1, GHR, HSP70, IL-1β, and TNF-α and IL-1β and intestinal C-lysozyme and TNF-α | [85] |
| Common carp Cyprinus carpio | Lactobacillus acidophilus ATCC 4356 | 1 × 106 CFU/kg | Isolated from human intestine | ↑ Growth performance parameters | [86] |
| Common carp Cyprinus carpio | Enterococcus casseliflavus | 1 × 1012 CFU/kg | Isolated from the intestine of C. carpio | ↑ Growth performance parameters | [87] |
| Rainbow trout Oncorhynchus mykiss | Lactobacillus rhamnosus ATCC 7469 | 1 × 109 CFU/kg | Purchased from Persian Type Culture Collection | ↑ Growth performance parameters | [88] |
| Nile tilapia Oreochromis niloticus | Mixture of Bacillus, Bifidobacterium, Enterococcus, Lactobacillus, Pediococcus sp. and B. subtilis | 7 × 1010 CFU/kg | Provided by Biomart Nutrição Animal Importação e Exportação LTDA | ↑ Growth performance parameters | [89] |
| African catfish Clarias gariepinu | Probiotic sporothermine (Bacillus subtilis and B. licheniformis) | 0.2% | Provided by Ulyanovsk State University, Russia | ↑ Vitamins B3, B5, B6, C, and E in the muscle | [90] |
| Amberjack Seriola dumerili | Lactococcus lactis K-C2 | 2 × 1010 CFU/g | Isolated from fermented vegetables | ↑ Amino acids in the gut content | [61] |
| Javanese carp Puntius gonionotus | Enterococcus faecalis | 2 × 107 CFU/g | Isolated from the intestine of Channa striatus | ↑ SCFA (propionic and butyric acids) | [91] |
| Caspian Roach Rutilus frisii kutum | Pediococcus acidilactici and Lactococcus lactis | 1 × 107 and 1 × 1010 CFU/g | P. acidilactici comercial Bactocell® (Lallemand animal nutrition, Blagnac, France). L. lactis isolated from juvenile sturgeon Acipenser persicus gut | ↑ SCFA (acetic and butyric acids) | [92] |
| Pacific mackerel Pneurnatophorus japonicus | Shewanella putrefaciens | 2 × 1010 viable cells/mL | Isolated from the intestine of contents of P. japonicus | ↑ EPA | [93] |
| Siberian sturgeon Acipenser baerii | Lactobacillus plantarum, L. delbrueckii subsp. Bulgaricus, L. acidophilus, L. rhamnosus, Bifidobacterium bifidum Streptococcus salivarius subsp. Thermophilus and Enterococcus faecium | 0.01% | Commercial probiotic name of Protexin® (ADM Protexin Limited, Somerset, UK) | ↑ DHA and EPA | [94] |
| Rainbow trout Oncorhynchus mykiss | Enterobacter sp. (strain C6-6) | 1.03 × 107 CFU/g | Isolated from the intestine of O. mykiss | ↑ Entericidin protein ↑ Protection against Flavobacterium psychrophilum | [95] |
| Nile tilapia Oreochromis niloticus | Bacillus amyloliquefaciens | 3.0 × 103 and 7.9 × 104CFU/g | Commercial probiotic mix (Enviva® PRO 202 GT, Danisco Animal Nutrition, Wiltshire, UK) | ↑ Volatile fatty acids | [96] |
| Nile tilapia Oreochromis niloticus | Lactococcus lactis subsp. lactis JCM5805 | 1 × 108 CFU/g | Provided by China General Microbiological Culture Collection Center (CGMCC) | ↑ Volatile fatty acids | [97] |
| Fish Species | Microorganism | Concentration or Dose | Origin of Microorganism | Effect | Reference |
|---|---|---|---|---|---|
| Gibel carp Carassius auratus gibelio | Saccharomyces cerevisiae | 4 and 6% | Purchased from Enhalor Biotechnology Company (Beijing, China) | ↑ Immune system (IL-1β) and survival rate in presence of pathogen Aeromonas hydrophila | [105] |
| Common carp Cyprinus carpio | Saccharomyces cerevisiae | 1.5% | Obtained from the local markets of Basrah, Turkey | ↑ Growth performance parameters (total weight gain, relative growth rate and feed conversion efficiency) | [106] |
| Largemouth bass Micropterus salmoides | Saccharomyces cerevisiae | 3% | Culture (from Beijing Enhalor International Tech Co., Ltd., Beijing, China) | ↑ Growth ↑ Abundance of beneficial bacteria (Lactobacillus, Bacillus and Bifidobacterium) ↓ Abundance of potential pathogenic bacteria Plesiomonas | [107] |
| Nile tilapia Oreochromis niloticus | Saccharomyces cerevisiae | 0.5% | Obtained as a commercial preparation (Perfect®, Dejo Co., Ltd., Bangkok, Thailand) | ↑ Growth performance | [108] |
| Nile tilapia Oreochromis niloticus | Saccharomyces cerevisiae | 0.4% | Culture (from Angel Yeast Co., Ltd., Yichang, China) | ↑ Growth ↑ Length, width and area of villus in gut | [109] |
| Nile tilapia Oreochromis niloticus | Saccharomyces cerevisiae | 0.1, 0.2, and 0.3% | Hilyses® commercial products (ICC Industrial Comércio Exportaçãoe Importação SA, São Paulo, Brazil) | ↑ Growth and weight gain | [110] |
| Nile tilapia Oreochromis niloticus | Saccharomyces cerevisiae | 2, 2.5 and 3% | From palm wine | ↑ Growth ↑ Resistance to pathogen Aeromonas hydrophila | [103] |
| Nile tilapia Oreochromis niloticus | Saccharomyces boulardii and Bifidobacterium bifidum | Saccharomyces boulardii (1 × 1010 CFU/g), Bifidobacterium bifidum (1.5 × 108 CFU/mL) and mixture of both | Acquired from the Iranian Biological Resource Center (Tehran, Iran) | ↑ Growth and immune responses | [111] |
| Leopard grouper Mycteroperca rosacea | Debaryomyces hansenii | 1 × 106 CFU/g | Isolated from the intestine of O. mykiss | ↑ Immune system and resistance against pathogen Amyloodinium ocellatum | [112] |
| Gilthead seabream Sparus aurata | Debaryomyces hansenii | 1.1% | Isolated from the intestine of O. mykiss | ↑ Growth ↓ Abundance of opportunistic bacteria Pseudomonas spp. and Acinetobacter spp. | [102] |
| Sea bass Dicentrarchus labrax | Debaryomyces hansenii | 1.1% | Isolated from the intestine of O. mykiss | ↑ Survival and digestive enzymes (trypsin and lipase) | [113] |
| Pacific red snapper Lutjanus peru | Yarrowia lipolytica | 1 × 108 CFU/mL | Isolated from the world’s largest open-air saltern known in Baja California Sur, Mexico | ↑ Innate immune and antioxidant enzyme activities in presence of pathogen Vibrio parahaemolyticus | [114] |
| Nile tilapia Oreochromis niloticus | Yarrowia lipolytica | 3, 5 and 7% | Provided from Federal University of Rio Grande do Sul (UFRGS, Porto Alegre, Brazil) | ↑ Digestive enzymes (chymotrypsin, trypsin and sucrose) ↑ Protein and lipid contents in fish muscle | [115] |
| Rainbow trout Oncorhynchus mykiss | Yarrowia lipolytica | 2 and 5% | Isolated from sewage from a wastewater treatment plant in Uppsala, Sweden | ↑ Expression of immune genes | [104] |
| Nile tilapia Oreochromis niloticus | Yarrowia lipolytica | 3, 5 and 7% | Provided from Federal University of Rio Grande do Sul (UFRGS, Porto Alegre, Brazil) | ↑ Growth promoter and immunostimulant | [116] |
| Golden Pompano Trachinotus ovatus | Rhodotorula mucilaginosa | 1, 2, 3, 4, 5 and 8% | Provided by Xinhailisheng technology company (Guangzhou, China) | ↑ Growth, lysozyme activity and resistance 100% survival rate against the pathogen Vibrio harveyi | [117] |
| Nile tilapia Oreochromis niloticus | Rhodotorula mucilaginosa | 1% | Supplied by South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences | ↑ Growth and protein content in the whole-body ↑ Immune system (lysozyme) and villi height of mid-intestine ↑ Survival rate in presence of pathogen Streptococcus iniae | [101] |
| Nile tilapia Oreochromis niloticus | Sporidiobolus pararoseus | 1 and 2% | By product of the biodiesel production process | ↑ Growth ↑ Immune response against pathogen Streptococcus agalactiae | [118] |
| Gilthead seabream Sparus aurata | Sterigmatomyces halophilus | 0.55 and 1.1% | Isolated from the world’s largest open-air saltern in Baja California Sur, Mexico | ↑ Trypsin and immune related gene expression (IL-1β, TNF-α, IgM, C3 and lysozyme), in presence of pathogen Vibrio parahaemolyticus | [119] |
| Fish Species | Phage Strain Name/Virus Taxonomic Family | Concentration or Dose | Origin of Microorganism | Effect | Reference |
|---|---|---|---|---|---|
| Gram-negative bacteria Aeromonas hydrophila | |||||
| Rohu Labeo rohita | AhFM11/Straboviridae | I: 1.5 × 105 PFU/fish B: 1.5 × 107 PFU/mL F: 1.5 × 107 PFU/g of feed pellets. MOI = 1000 | From river | I: showed 100% survival, B: 95% survival, F: 93% feeding of phage top-coated feed | [136] |
| Aeromonas salmonicida | |||||
| Senegalese sole Solea senegalensis | AS-A/Myoviridae | W: 1 × 1010 PFU/mL (MOI 100) | From sewage | No mortality, in the control group mortality 36% | [141] |
| Cytrobacter freundii | |||||
| Common carp Cyprinus carpio | IME-JL8/Siphoviridae | I: 1 × 108 PFU/mL | From sewage | Decrement pro-inflammatory cytokines | [142] |
| Edwardsiella piscicida | |||||
| Zebrafish Danio rerio | EPP-1/Heunggongvirae | I: MOI of 0.1, 1, 5, and 10) | From aquaculture wastewater | Treatment with MOI 1 significantly improved survival, similar in effectiveness to the florfenicol therapy group | [143] |
| Edwardsiella tarda | |||||
| Zebrafish Danio rerio | ETP-1/Podoviridae | B: 9.85 × 108 PFU/mL | From fish farm water | The survival rate was higher in phage-exposed fish (68%) compared to that of the control (18%) until 4 days post-challenge | [144] |
| Pseudomonas plecoglossicida | |||||
| Ayu fish Plecoglossus altivelis | PTH-9802/Myoviridae PPpW-3 and PPpW/Podoviridae | F: 1 × 107 PFU/g feed | From farm water | Survival rate of 78% | [145] |
| Vibrio harveyi | |||||
| Turbot Scophthalmus maximus | PVHp5/Au-tographiviridae PVHp8/Myoviridae | F: phage cocktail (MOI 1,10, 100) | From water | 80% survival at MOI 10–100, normal fish growth | [146] |
| Flavobacterium psychrophilum | |||||
| Rainbow trout Oncorhynchus mykiss | FpV4/Podoviridae FPSV-D22/Siphoviridae | F: bacteriophage cocktails by spraying (1.6 × 108 PFU/g) or by irreversible immobilization (8.3 × 107 PFU/g). I: 1.7 × 107 PFU/fish). W: 1 × 105−1 × 108 PFU/mL (MOI = 1). | From fecal water samples From rainbow trout organs | I: 80% survival compared to the control group of 57% | [139] |
| Plesiomonas shigelloides | |||||
| Grass carp Ctenopharyngodon idella | PSP01/Siphoviridae | I | From intestine of C. idella | Strong protective effect, increased survival by 33% | [147] |
| Gram-positive bacteria Streptococcus agalactiae | |||||
| Nile tilapia Oreochromis niloticus | 1A/Myoviri-dae | I: 100 μL of phage) F: 3 mL/10 g feed B: 200 μL phage/L water (MOI 1) | From fish farm water | B: highest protection with 80% survival compared to applications I and F with 70% and 50% animal survival | [125] |
| Lactococcus garvieae | |||||
| Yellowtail Seriola quinqueradiata | PLG-Y16/Siphoviridae | I: 1 × 107.5 PFU/fish F: 2% fish body feeding rate | From municipal wastewater | Both administrations with potential for use of phage therapy to control the disease | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amillano-Cisneros, J.M.; Fuentes-Valencia, M.A.; Leyva-Morales, J.B.; Savín-Amador, M.; Márquez-Pacheco, H.; Bastidas-Bastidas, P.d.J.; Leyva-Camacho, L.; De la Torre-Espinosa, Z.Y.; Badilla-Medina, C.N. Effects of Microorganisms in Fish Aquaculture from a Sustainable Approach: A Review. Microorganisms 2025, 13, 485. https://doi.org/10.3390/microorganisms13030485
Amillano-Cisneros JM, Fuentes-Valencia MA, Leyva-Morales JB, Savín-Amador M, Márquez-Pacheco H, Bastidas-Bastidas PdJ, Leyva-Camacho L, De la Torre-Espinosa ZY, Badilla-Medina CN. Effects of Microorganisms in Fish Aquaculture from a Sustainable Approach: A Review. Microorganisms. 2025; 13(3):485. https://doi.org/10.3390/microorganisms13030485
Chicago/Turabian StyleAmillano-Cisneros, Jesús Mateo, María Anel Fuentes-Valencia, José Belisario Leyva-Morales, Macario Savín-Amador, Henri Márquez-Pacheco, Pedro de Jesús Bastidas-Bastidas, Lucía Leyva-Camacho, Zamaria Yoselin De la Torre-Espinosa, and César Noé Badilla-Medina. 2025. "Effects of Microorganisms in Fish Aquaculture from a Sustainable Approach: A Review" Microorganisms 13, no. 3: 485. https://doi.org/10.3390/microorganisms13030485
APA StyleAmillano-Cisneros, J. M., Fuentes-Valencia, M. A., Leyva-Morales, J. B., Savín-Amador, M., Márquez-Pacheco, H., Bastidas-Bastidas, P. d. J., Leyva-Camacho, L., De la Torre-Espinosa, Z. Y., & Badilla-Medina, C. N. (2025). Effects of Microorganisms in Fish Aquaculture from a Sustainable Approach: A Review. Microorganisms, 13(3), 485. https://doi.org/10.3390/microorganisms13030485






