Non-Rhizobial Endophytes (NREs) of the Nodule Microbiome Have Synergistic Roles in Beneficial Tripartite Plant–Microbe Interactions
Abstract
1. Introduction
Review Methodology
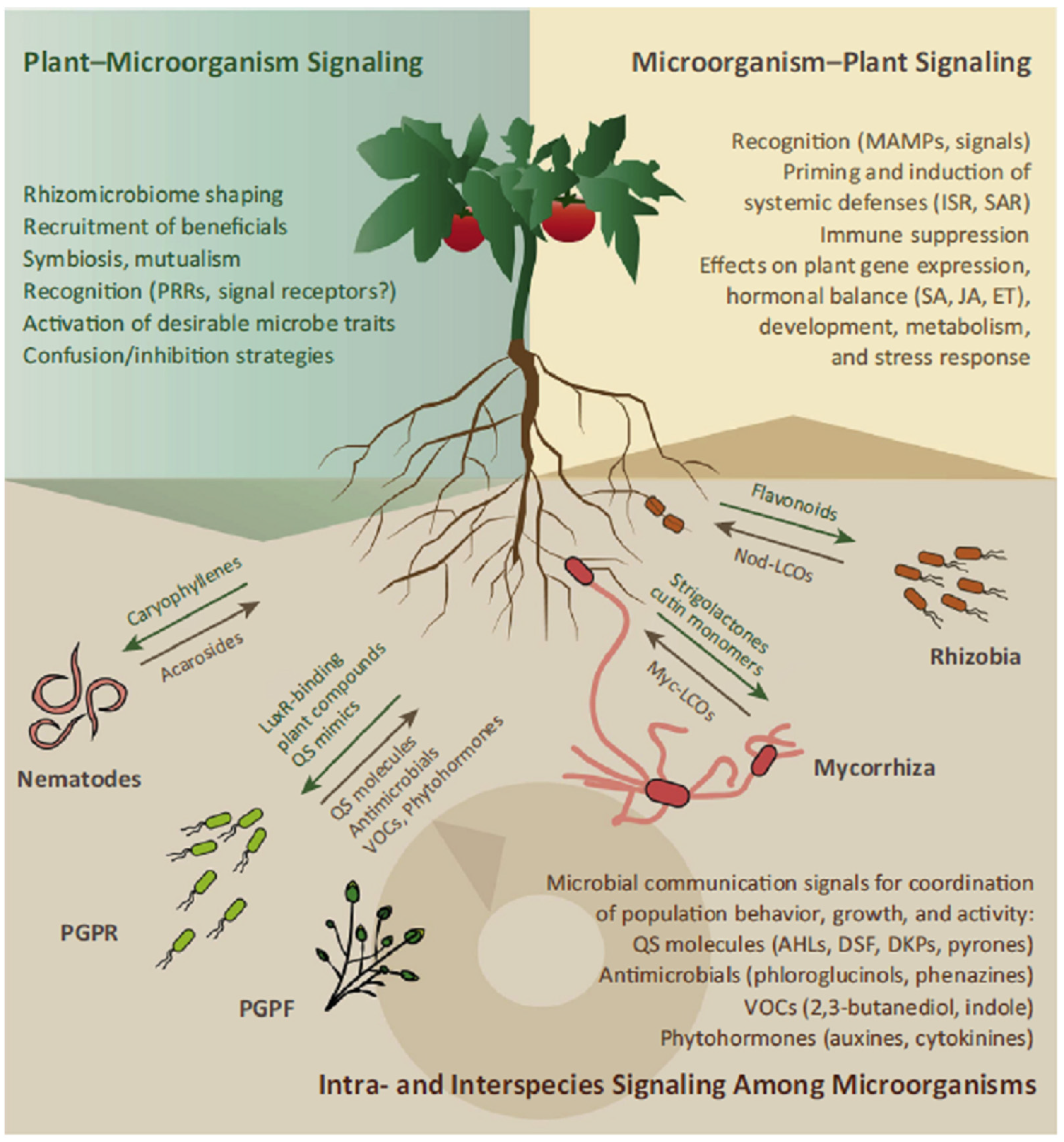
2. Signaling in Rhizosphere Plant–Microbe Interactions
2.1. Microbe–Microbe and Soil–Microbe Signaling
2.2. Plant–Microbe Signaling
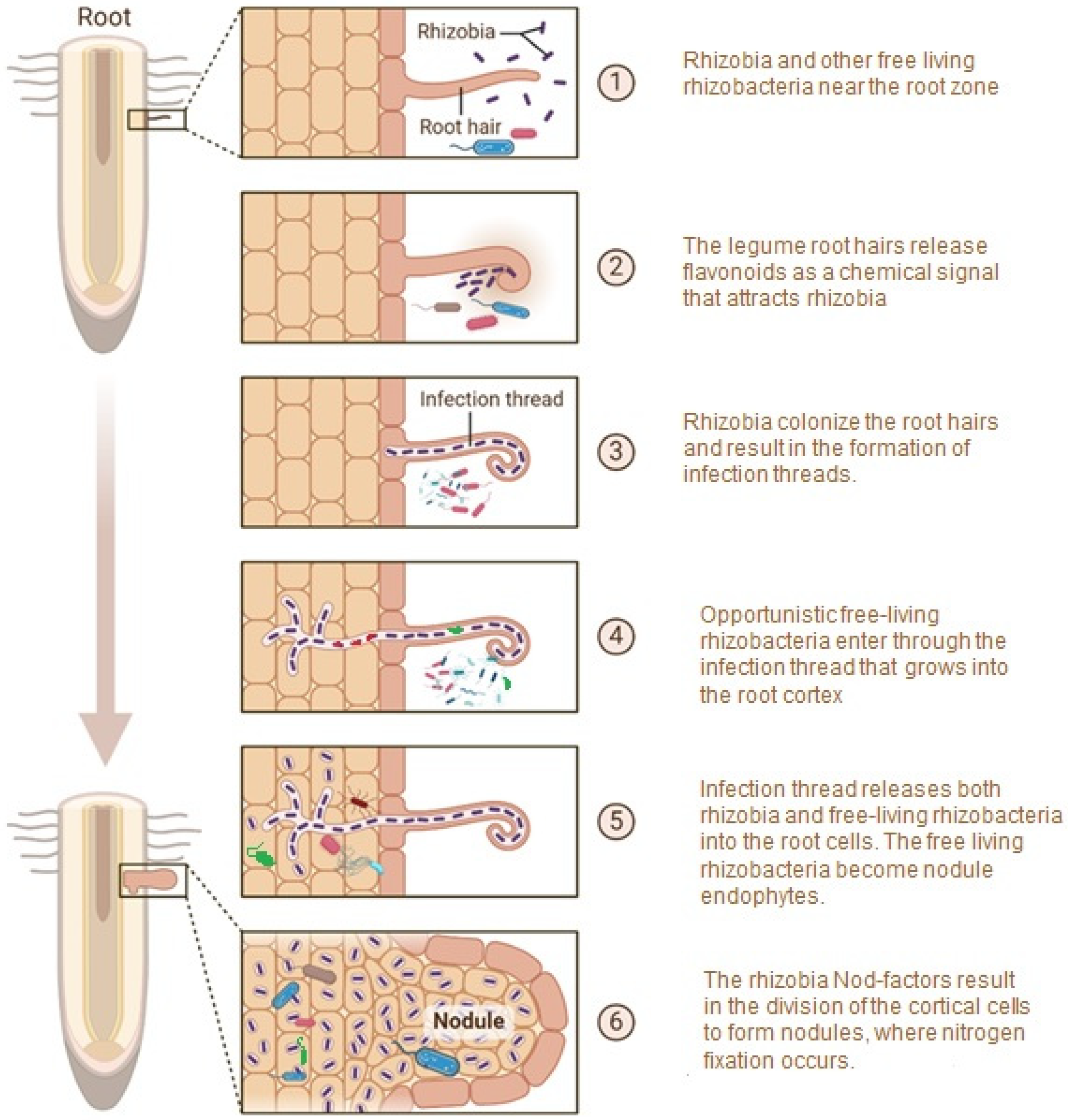
3. How Do NREs Enter the Nodules?
4. Metagenomics and Molecular Studies of the Nodule Microbiome
5. Role of NREs in Plant Growth and Mitigation of Abiotic Stress
5.1. ACC Deaminase Activity
5.2. Other NRE Traits for Mitigation of Abiotic Stress
6. Plant Growth-Promoting Traits of NREs in Legumes
6.1. Indoleacetic Acid (IAA)
6.2. Phosphorus
6.3. Siderophores
6.4. Nitrogen Fixation
7. Inoculation Using Consortia of Rhizobia and NREs
| Species | Phylum | Original Host | Host Tested on | PGP Mechanism | Parameters Promoted | Reference |
|---|---|---|---|---|---|---|
| Paenibacillus taichungensis | Bacillota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [4] |
| Novosphingobium spp. | Pseudomonadota | Glycine max | ND | ND | ND | [10] |
| Variovorax spp. | Pseudomonadota | Glycine max | ND | ND | ND | [10] |
| Flavobacterium spp. | Bacteroidota | Glycine max | ND | ND | ND | [10] |
| Stenotrophomonas spp. | Pseudomonadota | Glycine max | ND | ND | ND | [10] |
| Nitrospira spp. | Pseudomonadota | Glycine max | ND | ND | ND | [10] |
| Arthrobacter spp. | Actinomycetota | Glycine max | ND | ND | ND | [10] |
| Sporosarcina spp. | Bacillota | Glycine max | ND | ND | ND | [10] |
| Pseudomonas spp. | Pseudomonadota | Pisum sativum, Trifolium sp., Glycine max | Peanut, ND, ND | -Heavy metals tolerance | -Root length -Fresh weight | [10] |
| Nitrobacter spp. | Pseudomonadota | Glycine max | ND | ND | ND | [10] |
| Tardiphaga spp. | Pseudomonadota | Glycine max | ND | ND | ND | [10] |
| Bacillus sp. AAU B6 | Bacillota | Vigna radiata (mung bean) | Vigna radiata | -Phosphorus solubilization -Potash mobilization -Production of IAA -1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity -Nitrogen fixation | -Germination plant height -Number of nodules per plant -Root length -Fresh biomass -Dry biomass -Seed yields | [70] |
| Bacillus sp. AAU B12 | Bacillota | Vigna radiata (mung bean) | Vigna radiata | -Phosphorus solubilization -Potash mobilization -Production of IAA -1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity -Nitrogen fixation | -Germination plant height -Number of nodules per plant -Root length -Fresh biomass -Dry biomass -Seed yields | [70] |
| Bacillus sp. AAU B6 | Bacillota | Vigna radiata (mung bean) | Vigna radiata | -Phosphorus solubilization -Potash mobilization -Production of IAA -1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity -Nitrogen fixation | -Germination plant height -Number of nodules per plant -Root length -Fresh biomass -Dry biomass -Seed yields | [70] |
| Comamonas terrigena NSB15 | Pseudomonadota/Proteobacteria | Glycine max | Glycine max | -Production of IAA -Phosphorous solubilization -Biofilm formation -Cellulase activity -Nitrogen fixation | -Plant dry weight | [74] |
| Actinomycetes spp. | Actinomycetota | Vicia sativa, Glycine max | Vicia faba and Pisum sativum, ND | -Production of IAA -Phosphorous solubilization -Protease or cellulase or amylase or chitinase -Antifungal abilities against soil-borne pathogenic fungi | -Shoot fresh weight -Root fresh weight -Root length -Shoot length -Pods -Fresh weight/plant, -Seeds fresh weight/plant -Seeds number | [75] |
| Streptomyces variabilis | Actinomycetota | Vicia sativa | Vicia faba and Pisum sativum | -Production of IAA -Phosphorous solubilization -Protease or cellulase or amylase or chitinase -Antifungal abilities against soil-borne pathogenic fungi | -Shoot fresh weight -Root fresh weight -Root length -Shoot length -Pods weight -Fresh weight/plant -Seeds fresh weight/plant -Seeds number | [75] |
| Streptomyces tendae | Actinomycetota | Vicia sativa, Glycine max | Vicia faba and Pisum sativum, ND | -Production of IAA -Phosphorous solubilization -Protease or cellulase or amylase or chitinase -Antifungal abilities against soil-borne pathogenic fungi | -Shoot fresh weight -Root fresh weight -Root length -Shoot length -Pods weight -Fresh weight/plant -Seeds fresh weight/number of seeds - | [75] |
| Phyllobacterium ifriqiyense | Pseudomonadota | Calobota saharae, Calicotome villosa | Cicer arietinum | -Production of IAA -Siderophores production | -Shoot dry weight -Root dry weight -Nodules number -Nodules dry weight -Dry weight/nodule -Total nitrogen | [76] |
| Xanthomonas translucens | Pseudomonadota | Calobota saharae and Calicotome villosa | Cicer arietinum | -Siderophores production -Production of IAA -Biofilm formation | -Shoot dry weight -Root dry weight -Nodules number -Nodules dry weight -Dry weight/nodule -Total nitrogen | [76] |
| Cupriavidus respiraculi | Pseudomonadota | Calobota saharae and Calicotome Villosa, Trifolium sp. | Cicer arietinum, ND | -Production of IAA -Siderophores production | -Shoot dry weight -Root dry weight -Nodules number -Nodules dry weight -Dry weight/nodule -Total nitrogen | [76] |
| Pantoea dispersa YBB19B | Pseudomonadota | Groundnut | Groundnut | -Production of IAA, -Siderophore production -Phosphorus solubilization -ACC deaminase activity -Catalase and ascorbate peroxidase activity (antioxidant enzymes) | -Shoot length -Root length -Dry weight of plant -Pod number -Nodule number per plant | [77] |
| Bacillus tequilensis NBB13 | Bacillota | Groundnut | Groundnut | -Production of IAA -Siderophore production, -Phosphorus solubilization -ACC deaminase activity -Catalase and ascorbate peroxidase activity (antioxidant enzymes) | -Shoot length -Root length -Dry weight of plant -Pod number -Nodule number per plant | [77] |
| Pantoea spp. | Pseudomonadota | Peanut | Peanut | -Production of IAA, -Siderophore production -Phosphorus solubilization -ACC deaminase activity -Nitrogen-fixation | -Root length -Fresh weights | [78] |
| Herbaspirillum sp. | Pseudomonadota | Peanut | Peanut | -Production of IAA -Siderophore production -Phosphorus solubilization -ACC deaminase activity -Nitrogen fixation | -Root length -Fresh weight | [78] |
| Blastobacter aggregatus | Pseudomonadota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Chitinophaga filiformis | Bacteroidota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Dyadobacter fermentans | Bacteroidota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Macrophomina phaseolina | Ascomycota (fungi) | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Blastobacter aggregatus | Pseudomonadota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Chitinophaga filiformis | Bacteroidota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Dyadobacter fermentans | Bacteroidota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Paenibacillus xyla_ nilyticus | Bacillota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Bacillus anthracis | Bacillota | Vigna radiata | Vigna radiata | -Production of IAA -Siderophore production -Phosphorus solubilization | -Seedling vigor -Root length -Hypocotyl length -Shoot length -Number of lateral roots -Dry weight | [80] |
| Serratia sp. R6 | Pseudomonadota | Lentils | Lentils | -Production of IAA -Siderophore production -Phosphorus solubilization -Potassium solubilization -ACC deaminase activity -Nitrogen fixation -Hydrogen cyanide (HCN) -Biofilm production -Protease activity | -Fresh weight -Dry weight -Number of nodules per plant -Nodule fresh weight -Total nitrogen content | [81] |
| Bacillus sp. | Bacillota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Staphylococcus sp. | Bacillota | Trifolium sp. | ND | -Heavy metals tolerance | [110] | |
| Enterobacter sp. | Pseudomonadota | Trifolium sp. | ND | -Heavy metals tolerance | [110] | |
| Acinetobacter sp. | Pseudomonadota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Roseomonas sp. | Pseudomonadota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Frondihabitans sp. | Actinomycetota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Microbacterium sp. | Actinomycetota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Kocuria sp. | Actinomycetota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Providencia sp. | Pseudomonadota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Micrococcus sp. | Actinomycetota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Rhodotorula mucilaginosa | Basidiomycota (fungi) | Trifolium sp. | ND | -Heavy metals tolerance | ND | [110] |
| Staphylococcus sp. | Bacillota | Trifolium sp. | ND | -Heavy metals tolerance | [110] | |
| Cupriavidus sp. | Pseudomonadota | Trifolium sp. | ND | -Heavy metals tolerance | ND | [111] |
| Bacillus spp. ESA 417, ESA 418 | Bacillota | Cowpea | Cowpea | -Production of auxin -Siderophores -Biofilm formation -Nitrogen fixation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Bacillus sp. ESA 420 | Bacillota | Cowpea | Cowpea | -Production of auxin -Siderophores -Biofilm formation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Chryseobacterium spp. 29, 23, 19, 412 | Bacteroidota | Cowpea | Cowpea | -Production of auxin -Siderophores -Biofilm formation -Nitrogen fixation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Microbacterium sp. ESA 413 | Actinomycetota | Cowpea | Cowpea | -Production of auxin -Siderophores production -Biofilm formation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Agrobacterium sp. ESA 422 | Pseudomonadota | Cowpea | Cowpea | -Production of auxin -Siderophores production -Biofilm formation -Nitrogen fixation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Delftia ESA 421 | Pseudomonadota | Cowpea | Cowpea | -Production of auxin -Siderophores -Biofilm formation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Bacillus spp. | Bacillota | Cowpea | Cowpea | -Production of auxin -Siderophores -Biofilm formation -Nitrogen fixation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Sphingomonas sp. ESA 423 | Pseudomonadota | Cowpea | Cowpea | -Production of auxin -Siderophores production -Biofilm formation -Nitrogen fixation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
| Pelomonas sp. ESA 424 | Pseudomonadota | Cowpea | Cowpea | -Production of auxin -Siderophores production -Biofilm formation -Nitrogen fixation | -Grain yield -Root and shoot dry matter -Shoot N content -Nodulation (number and dry matter of nodules) | [111] |
8. Concluding Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flavell-While, C. Fritz-Haber and Carl-Bosch Feed the World. The Chemical Engineer 2010. Available online: https://www.thechemicalengineer.com/features/cewctw-fritz-haber-and-carl-bosch-feed-the-world/ (accessed on 22 May 2024).
- Soria-Lopez, A.; Garcia-Perez, P.; Carpena, M.; Garcia-Oliveira, P.; Otero, P.; Fraga-Corral, M.; Cao, H.; Prieto, M.A.; Simal-Gandar, J. Challenges for future food systems: From the Green Revolution to food supply chains with a special focus on sustainability. Food Front. 2023, 4, 9–20. [Google Scholar] [CrossRef]
- Hungria, M.; Eaglesham, A.R.; Hardy, R.W. Physiological comparisons of root and stem nodules of Aeschynomene scabra and Sesbania rostrata. Plant Soil 1992, 139, 7–13. [Google Scholar] [CrossRef]
- Saraswati, R.; Matoh, T.; Sekiya, J. Nitrogen fixation of Sesbania rostrata: Contribution of stem nodules to nitrogen acquisition. Soil Sci. Plant Nutr. 1992, 38, 775–780. [Google Scholar] [CrossRef]
- Hassen, A.I.; Lamprecht, S.C.; Bopape, F.L. Emergence of β-rhizobia as new root nodulating bacteria in legumes and current status of the legume–rhizobium host specificity dogma. World J. Microbiol. Biotechnol. 2020, 36, 40. [Google Scholar] [CrossRef]
- De Meyer, S.E.; De Beuf, K.; Vekeman, B.; Willems, A. A large diversity of non-rhizobial endophytes found in legume root nodules in Flanders (Belgium). Soil Biol. Biochem. 2015, 83, 1–11. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303.79. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.L.; Simms, E.L. The origins of uncooperative rhizobia. Oikos 2008, 117, 961–966. [Google Scholar] [CrossRef]
- Lu, J.; Yang, F.; Wang, S.; Ma, H.; Liang, J.; Chen, Y. Co-existence of Rhizobia and diverse non-rhizobial bacteria in the rhizosphere and nodules of Dalbergia odorifera seedlings inoculated with Bradyrhizobium elkani, Rhizobium multihospitum like and Bradyrhizobium pyrrocinia like strains. Front. Microbiol. 2017, 8, 2255. [Google Scholar] [CrossRef]
- Mayhood, P.; Mirza, B.S. Soybean root nodule and rhizosphere microbiome: Distribution of rhizobial and non-rhizobial endophytes. Appl. Environ. Microbiol. 2021, 87, e02884-20. [Google Scholar] [CrossRef]
- Nichols, D. Cultivation gives context to the microbial ecologist. FEMS Microbiol. Ecol. 2007, 60, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.; Mirzac, B.S.; Imrana, A.; Zaheera, A.; Yasmina, S.; Mubeena, F.; Mcleane, J.E.M.; Mirzaa, M.S. Illumina sequencing of 16S rRNA tag shows disparity in rhizobial and non rhizobial diversity associated with root nodules of mung bean (Vigna radiata L.) growing in different habitats in Pakistan. Microbiol. Res. 2020, 231, 126356. [Google Scholar] [CrossRef] [PubMed]
- Lui, L.M.; Nielsen, T.N.; Arkin, A.P. A method for achieving complete microbial genomes and improving bins from metagenomics data. PLoS Comput. Biol. 2021, 17, e1008972. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Grillo, M.A.; Podowski, J.C.; Heath, K.D. Soil origin and plant genotype structure distinct microbiome compartments in the model legume Medicago truncatula. Microbiome 2020, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Iyer, B.; Rajkumar, S. A Metagenomic approach to identify distinct rhizospheric and endophytic bacterial communities from roots and root nodules of Vigna radiata. In Understanding Host-Microbiome Interactions—An Omics approach: Omics of Host-Microbiome Association; Springer: Singapore, 2017; pp. 173–191. [Google Scholar]
- Zhang, B.; Du, N.; Li, Y.; Shi, P.; Wei, G. Distinct biogeographic patterns of rhizobia and non-rhizobial endophytes associated with soybean nodules across China. Sci. Total Environ. 2018, 643, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Hnini, M.; Aurag, J. Prevalence, diversity and applications potential of nodules endophytic bacteria: A systematic review. Front. Microbiol. 2024, 15, 1386742. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, C.; Parsek, M.R.; Greenburg, E.P. Regulation of gene expression by cell-to-cell communication Acyl-Homoserine Lactone Quorum sensing. Ann. Rev. Gen. 2001, 35, 439–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Vivano, J.M.; Shen, Q. The unseen rhizosphere root –soil-microbe interactions for crop production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef]
- Redmond, J.W.; Batley, M.; Djordjevic, M.A.; Innes, R.W.; Kuempel, P.L.; Rolfe, B.G. Flavones induce expression of nodulation genes in Rhizobium. Nature 1986, 323, 632–635. [Google Scholar] [CrossRef]
- Oldroyd, G.E.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Mougel, C.; Jaillard, B.; Hinsinger, P. Plant-microbe-soil interactions in the rhizosphere: An evolutionanry perspective. Plant Soil 2009, 321, 83–115. [Google Scholar] [CrossRef]
- Beauregard, P.B.; Chai, Y.; Vlamakis, H.; Losick, R.; Kolter, R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. USA 2016, 110, E1621–E1630. [Google Scholar]
- Martinez-Hidalgo, P.; Hirsh, A.M. The nodule microbiome: N2-fixing rhizobia do not live alone. Phytobiomes 2017, 1, 70–82. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R.; Matheson, B.G.; Nowak, J. Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol. Fertil. Soils 1997, 25, 13–19. [Google Scholar] [CrossRef]
- Chen, W.-M.; de Faria, S.M.; Straliotto, R.; Pitard, R.M.; Simoes-Araujo, J.L.; Chou, J.-H.; Chou, Y.-J.; Barrios, E.; Prescott, A.R.; Elliot, G.N.; et al. Proof that Burkholderia strains form effective symbioses with legumes: A study of novel Mimosa-nodulating strains from South America. Appl. Environ. Microbiol. 2005, 71, 7461–7471. [Google Scholar] [CrossRef]
- Annapurna, K.; Ramadoss, D.; Bose, P.; VithalKumar, L. In situ localization of Paenibacillus polymyxa HKA-15 in roots and root nodules of soybean (Glycine max.L.). Plant Soil 2013, 373, 641–648. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Doring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional characteristic of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Ann. Rev. Plant Biol. 2013, 64, 807–836. [Google Scholar] [CrossRef] [PubMed]
- Scientific Image and Illustration Software. Available online: https://www.biorender.com/ (accessed on 10 May 2024).
- Subba-Rao, N.S.; Mateos, P.F.; Baker, D.; Stuart Pankratz, H.; Palma, J.; Dazzo, F.B.; Sprent, J.I. The unique root-nodule symbiosis between Rhizobium and the aquatic legume, Neptunia natans (L. f.) Druce. Planta 1995, 196, 311–320. [Google Scholar] [CrossRef]
- Leite, J.; Fischer, D.; Rous, L.F.; Fernandes-Junior, P.I.; Hofmann, A.; Kublik, S.; Schloter, M.; Xavier, G.R.; Radl, V. Cow pea nodules harbour non-rhizobial bacterial communities that are shaped by soil type rather than plant genotypes. Front. Plant Sci. 2017, 7, 2064. [Google Scholar] [CrossRef] [PubMed]
- Zgadzaj, R.; James, E.K.; Kelly, S.; Kawaharada, Y.; de Jonge, N.; Jensen, D.B.; Madsen, L.H.; Radutoiu, S. A legume genetic framework controls infection of nodules by symbiotic and endophytic bacteria. PLoS Genet. 2015, 11, 1–21. [Google Scholar] [CrossRef]
- Rocha, S.M.B.; Mendes, L.W.; de Sousa Oliveria, L.M.; Melo, V.M.M.; Antunes, J.E.L.; Araujo, F.F.; Hungria, M.; Araujo, A.S.F. Nodule microbiome from cowpea and lima bean grown in composted tannery sludge treated soil. Appl. Soil Ecol. 2020, 151, 103542. [Google Scholar] [CrossRef]
- Ellen, F. The Metagenomes of Root Nodules in Actinorhizal Plants: A Bioinformatics Study of Endophytic Bacterial Communities. Bachelor’s Thesis, UMEA Universitet, Umeå, Sweden, 2021. [Google Scholar]
- Jesus, J.G.; Máguas, C.; Dias, R.; Nunes, M.; Pascoal, P.; Pereira, M.; Trindade, H. What If Root Nodules Are a Guesthouse for a Microbiome? The Case Study of Acacia longifolia. Biology 2023, 12, 1168. [Google Scholar] [CrossRef]
- Muindi, M.M.; Muthini, M.; Njeru, E.M.; Maingi, J. Symbiotic efficiency and genetic characterization of rhizobia and non rhizobial endophytes associated with cowpea grown in semi-arid tropics of Kenya. Heliyon 2021, 7, e06867. [Google Scholar] [CrossRef]
- Bakhtiyarifar, M.; Enayatizamir, N.; Khanlou, K.M. Biochemical and molecular investigation of non-rhizobial endophytic bacteria as potential biofertilisers. Arch. Microbiol. 2021, 203, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Co-inoculation of soybeans and common beans with Rhizobia and Azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 2023, 49, 791–801. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Humm, E.A.; Still, D.W.; Shi, B.; Pellegrini, M.; de la Roca, G.; Veliz, E.; Maymon, M.; Bru, P.; Huntemann, M.; et al. Medicago root nodule microbiomes: Insights into a complex ecosystem with potential candidates for plant growth promotion. Plant Soil 2022, 471, 507–526. [Google Scholar] [CrossRef]
- Saikia, S.P.; Dutta, S.P.; Goswami, A.; Bhau, B.S.; Kanjilal, P.B. Role of Azospirillum in the Improvement of Legumes. In Microbes for Legume Improvement; Khan, M.S., Musarrat, J., Zaidi, A., Eds.; Springer: Vienna, Austria, 2010. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martínez, C.R.; Chanwa, C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. Unravelling the biochemistry and genetics of ACC deaminase—An enzyme alleviating the biotic and abiotic stress in plants. Plant Gene 2019, 18, 100175. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Brígido, C.; Glick, B.R.; Rossi, M.J. The Role of Rhizobial ACC Deaminase in the nodulation process of leguminous plants. Int. J. Agron. 2016, 1369772. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bateria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Glick, B.R. Bacterial ACC deaminase can promote and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Youseif, S.H.; Abd El-Megeed, F.H.; Abdelaal, A.S.; Ageez, A.; Martínez-Romero, E. Plant–microbe–microbe interactions influence the faba bean nodule colonization by diverse endophytic bacteria. FEMS Microbiol. Ecol. 2021, 97, fiab138. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Paço, A.; da-Silva, J.R.; Torres, D.P.; Glick, B.R.; Brígido, C. Exogenous ACC Deaminase is key to improving the performance of pasture legume-rhizobial symbioses in the presence of a high Manganese concentration. Plants 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Yin, X.; Zeng, I. Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef]
- Daur, I.; Saad, M.M.; Eida, A.A.; Ahmad, S.; Shah, Z.H.; Ihsan, M.Z. Boosting Alfalfa (Medicago sativa L.) production with rhizobacteria from various plants in Saudi Arabia. Front. Microbiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.F.S.; McConkey, B.J.; Glick, B.R. New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef]
- Singh, R.P.; Ma, Y.; Shadan, A. Perspective of ACC-deaminase producing bacteria in stress agriculture. J. Biotech. 2022, 352, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.J.; Nascimento, F.X.; Glick, B.R.; Rossi, M.J. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Lett. Appl. Microbiol. 2018, 66, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Khan, M.S.; Zaidi, A. ACC deaminase producing Pseudomonas putida strain PSE3 and Rhizobium leguminosarum strain RP2 in synergism improves growth, nodulation and yield of pea grown in alluvial soils. Symbiosis 2013, 61, 93–104. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, S.; Singh, M.; Srivastava, P.K.; Singh, V.P. Transcriptional regulation of salinity stress in plants: A short review. Plant Gene 2017, 11, 160–169. [Google Scholar] [CrossRef]
- Arrese-Igor, C.; Royuela, M.; De Lorenzo, C.; De Felipe, M.R.; Aparicio-Tejo, P.M. Effect of low rhizosphere oxygen on growth, nitrogen fixation and nodule morphology in lucerne C. Physiol. Plantarum 1993, 89, 55–63. [Google Scholar] [CrossRef]
- Sung, F.J.M. Waterlogging effect on nodule nitrogenase and leaf nitrate reductase activities in soybean. Field Crops Res. 1993, 35, 183–189. [Google Scholar] [CrossRef]
- Elmsehli, S.; Jihene, J.; Samira, S.-A. Physiological responses of Medicago truncatula growth under prolonged hypoxia stress. Afr. J. Agric. Res. 2015, 31, 3073–3079. [Google Scholar] [CrossRef]
- Wang, Q.; Dodd, I.C.; Belimov, A.A.; Jiang, E. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting NaI accumulation. Funct. Plant Biol. 2016, 43, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Mohammed, O.A.; Deng, Z.; Liu, X.; Glick, B.R.; Wie, G. Rhizobial symbiosis effect on the growth, metal uptake and antioxidant response of Medicago lupulina under copper stress. Environ. Sci. Pollut. Res. 2015, 22, 12479–12489. [Google Scholar] [CrossRef]
- Płociniczaka, L.T.; Sinkkonenb, A.; Romantschukb, M.; Piotrowska-Segeta, Z. Characterization of Enterobacter intermedius MH8b and its use for the enhancement of heavy metals uptake by Sinapis alba. Appl. Soil Ecol. 2013, 63, 1–7. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Tren. Plant. Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Jabbarova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef] [PubMed]
- Chandawani, S.; Amaresan, N. Role of ACC deaminase producing bacteria for abiotic stress mangement and sustainable agriculture production. Environ. Sci. Poll. Res. 2002, 29, 22843–22859. [Google Scholar] [CrossRef]
- Yaghoubian, I.; Ghassemi, S.; Nazari, M.; Raei, Y.; Smith, D.L. Response of physiological traits, antioxidant enzymes and nutrient uptake of soybean to Azotobacter Chroococcum and zinc sulfate under salinity. S. Afr. J. Bot. 2021, 143, 42–51. [Google Scholar] [CrossRef]
- Kumar, V.; Raghuvanshi, N.; Pandey, A.K.; Kumar, A.; Thoday-Kennedy, E.T.; Kant, S. Role of halotolerant plant growth-promoting Rhizobacteria in Mitigating Salinity Stress: Recent Advances and Possibilities. Agriculture 2023, 13, 168. [Google Scholar] [CrossRef]
- Hassen, A.I.; Babalola, O.O.; Carlson, R. Rhizobacterial mediated interactions for enhanced symbiotic performance of the root nodule rhizobia in nodules. In Sustsinable Agrobiology, Microorganisms for Sustainability; Maheshwari, D.K., Dheeman, S., Eds.; Springer: Singapore, 2023; Volume 43. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, U.B.; Khan, M.S.; Singh, P.; Kumar, R.; Singh, R.N.; Kumar, A.; Singh, H.V. Baterial ACC deaminase: Insigts into enzymology, biochemistry, genetics and potential role in amelioration of environmental stress in plants. Front. Microbiol. 2023, 14, 1132770. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Kucharova, Z. Selection for root colonizing bacteria stimulating wheat growth in saline soils. Biol. Fert. Soil 2009, 45, 561–573. [Google Scholar] [CrossRef]
- Tariq, M.; Tahreem, N.; Zafar, M.; Raza, G.; Shahid, M.; Zunair, M.; Iram, W.; Zahra, S.T. Occurrence of diverse plant growth promoting bacteria in soybean [Glycine max (L.) Merrill] root nodules and their prospective role in enhancing crop yield. Biocatal. Agric. Biotechnol. 2024, 57, 103072. [Google Scholar] [CrossRef]
- Badawy, A.M. Impact of antagonistic endophytic bacteria on productivity of some economically important legumes. Braz. J. Microbiol. 2024, 55, 749–757. [Google Scholar] [CrossRef]
- Ben Gaied, R.; Sbissi, I.; Tarhouni, M.; Brígido, C. Bacterial endophytes from legumes native to arid environments are promising tools to improve Mesorhizobium–Chickpea symbiosis under salinity. Biology 2024, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Oviya, G.; Rangasamy, A.; Ariyan, M.; Krishnamoorthy, R.; Senthilkumar, M.; Gopal, N.; Thiyageshwari, S.; Meena, S.; Vincent, S. Halotolerant nodule rhizobial and passenger endophytes alleviates salinity stress in groundnut (Arachis hypogaea L.). J. Plant Growth Regul. 2003, 42, 6620–6635. [Google Scholar] [CrossRef]
- Hossain, M.S.; Frith, C.; Bhattacharyya, S.S.; DeLaune, P.B.; Gentry, T.J. Isolation and Characterization of Bacterial Endophytes from Small Nodules of Field-Grown Peanut. Microorganisms 2023, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Dhole, A.M.; Shelat, H.N.; Patel, H.K.; Jhala, Y.K. Evaluation of the co-inoculation effect of Rhizobium and plant growth promoting non-rhizobial endophytes on Vigna radiata. Curr. Microbiol. 2023, 80, 167. [Google Scholar] [CrossRef]
- Pandya, M.; Rajput, M.; Rajkumar, S. Exploring plant growth promoting potential of non rhizobial root nodules endophytes of Vigna radiata. Microbiology 2015, 84, 80–89. [Google Scholar] [CrossRef]
- Debnath, S.; Chakraborty, S.; Langthasa, M.; Choure, K.; Agnihotri, V.; Srivastava, A.; Rai, P.K.; Tilwari, A.; Maheshwari, D.K.; Pandey, P. Non-rhizobial nodule endophytes improve nodulation, change root exudation pattern and promote the growth of lentil, for prospective application in fallow soil. Front. Plant Sci. 2023, 14, 1152875. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Sánchez-Yáñez, J.M.; de los Santos-Villalobos, S. Methods for detecting biocontrol and plant growth-promoting traits in Rhizobacteria. In Methods in Rhizosphere Biology Research; Springer: Singapore, 2019; pp. 133–149. [Google Scholar]
- Fernandez, L.A.; Zalba, P.; Gomez, M.A.; Sagardoy, M.A. Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biol. Fertil. Soils 2007, 43, 805–809. [Google Scholar] [CrossRef]
- Bandeppaa, S.; Kumar, K.; Latha, P.C.; Manjusha, P.G.S.; Phulea, A.; Chandrakala, C. Phosphate-solubilizing microbial inoculants for sustainable agriculture. In Trends of Applied Microbiology for Sustainable Economy; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Multifunctional Pseudomonas putida strain FBKV2 from arid rhizosphere soil and its growth promotional effects on maize under drought stress. Rhizosphere 2016, 1, 4–13. [Google Scholar] [CrossRef]
- Rios-Ruiz, R.; Castro-Tuanama, R.; Valdez-Nunez, R.A.; Torez-Bernal, L.; Jave-Concepcion, H.G.; Daza-Perez, A.C.; Barrera-Lozano, M.; Archentti-Reategui, F. Co-inoculation of phosphate solubilizing bacteria and rhizobia increase phosphorous availability and promotes the development of forage legumes. Agronomy 2024, 14, 2498. [Google Scholar] [CrossRef]
- Dinic, Z.; Ugrinović, M.; Bosnić, P.; Mijatović, M.; Zdravković, J.; Miladinović, M.; Jošić, D. Solubilization of inorganic phosphate by endophytic Pseudomonas sp. from French bean nodules. Ratarstvo i Povrtarstvo 2014, 51, 100–105. [Google Scholar] [CrossRef]
- Dhole, A.; Shelat, H.; Vyas, R.; Jhala, Y.; Bhange, M. Endophytic occupation of legume root nodules by nifH-positive non-rhizobial bacteria, and their efficacy in the groundnut (Arachis hypogaea). Ann. Microbiol. 2016, 66, 1397–1407. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef]
- Grobelak, A.; Hiller, J. Bacterial siderophores promote plant growth: Screening of catechol and hydroxamate siderophores. Int. J. Phytoremed. 2017, 19, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using plant growth-promoting rhizobacteria (PGPR) to improve plant growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Prasad, M.N.; Freitas, H.; Fraenzle, S.; Wuenschmann, S.; Markert, B. Knowledge explosion in phytotechnologies for environmental solutions. Environ. Pollut. 2010, 158, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M.; Ran, L.X.; Pieterse, C.M.J.; van Loon, L. C Understanding the involvement of rhizobacteria-mediated induction of systemic resistance in biocontrol of plant diseases. Can. J. Plant Pathol. 2003, 25, 5–9. [Google Scholar] [CrossRef]
- Hassen, A.I.; Bopape, F.L.; Lamprecht, S.C.; Habig, J. Nodulation of rooibos (Aspalathus linearis burm f.), an indigenous South African legume by members of both the α-proteobacteria and β-proteobacteria. Biol. Fertil. Soils 2012, 48, 295–303. [Google Scholar] [CrossRef]
- Dhole, A.; Shelat, H.; Panpatte, D. Chryseobacterium indologenes: A Novel Root Nodule Endophyte in Vigna radiata. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 836–844. [Google Scholar]
- Aeron, A.; Chauhan, P.S.; Dubey, R.C.; Maheshwari, D.K.; Bajpa, V.K. Root nodule bacteria from Clitoria ternatea L. are putative invasive non-rhizobial endophytes. Can. J. Microbiol. 2015, 61, 131–142. [Google Scholar] [CrossRef]
- Rana, A.K.; Vyas, P.; Sharma, S.; Sardana, V. Groundnut harbours non-nodulating non-rhizobial plant growth-promoting bacterial endophytes. 3 Biotech 2023, 13, 420. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Asis, C.A., Jr.; Akao, S. Production of growth-promoting substances and high colonization ability of rhizobacteria enhance the nitrogen fixation of soybean when co-inoculated with Bradyrhizobium japonicum. Biol. Fertil. Soils 2001, 34, 427–432. [Google Scholar] [CrossRef]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-inoculation effect of Rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front. Plant Sci. 2017, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Mishra, S.; Selvakumar, G.; Bisht, J.K.; Kundu, S.; Gupta, H.S. Co-inoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J. Microbiol. Biotechnol. 2009, 25, 753–761. [Google Scholar] [CrossRef]
- Chuong, N.V.; Tri, T.L.K. Isolation, characterization and identification of edophytic nitrogen-fixing bacteria from peanut nodules. Int. J. Microbiol. 2024, 8973718. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, X.; Smith, D.L. Enhanced soybean plant growth resulting from co-inoculation of Bacillus strains with Bradyrhizobium japonicum. Crop. Sci. 2003, 43, 1774–1781. [Google Scholar] [CrossRef]
- Rajendran, G.; Sing, F.; Desai, A.J.; Archana, G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium sp. Bioresour. Technol. 2008, 99, 4544–4550. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; O’ Hara, G.W.; Brau, L. Enhanced nodulation and symbiotic effectiveness of Medicago truncatula when co-inoculated with Pseudomonas flourescens WSM3457 and Ensifer (Sinorhizobium) medicae WSM419. Plant Soil 2011, 348, 245–254. [Google Scholar] [CrossRef]
- Barea, J.-M.; Pozo, M.J.; Azcon, R.; Azcon-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.; da Silva Oliveira, C.E.; Zoza, T.; Zuffob, A.M.; de Freitas, R.S. Co-Inoculation of Common Bean with Rhizobium and Azospirillum enhance the drought tolerance. Russ. J. Plant Physiol. 2020, 67, 923–932. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; da Silva, E.C.; Silva, V.M.; Fernandes, G.C.; Rodrigues, W.L.; Céu, E.G.O.; de Lima, B.H.; Jalal, A.; Muraoka, T.; et al. Co-Inoculation with Azospirillum brasilense and Bradyrhizobium sp. enhances nitrogen uptake and yield in field-grown cowpea and did not change N-fertilizer recovery. Plants 2022, 11, 1847. [Google Scholar] [CrossRef]
- Rahal, S.; Chekireb, D. Diversity of rhizobia and non-rhizobia endophytes isolated from the root nodules of Trifolium sp. growing in lead and zinc mine sites Gueima, Algeria. Arch. Microbiol. 2021, 203, 3839–3849. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.R.; Rodrigues, R.T.; Jovino, R.S.; Carvalho, J.R.d.S.; Leite, J.; Hoffman, A.; Fischer, D.; Ribeiro, P.R.d.A.; Rouws, L.F.M.; Radl, V. Not just passengers, but co-pilots! Non-rhizobial nodule-associated bacteria promote cowpea growth and symbiosis with (brady) rhizobia. J. Appl. Microbiol. 2023, 134, lxac013. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassen, A.I.; Muema, E.K.; Diale, M.O.; Mpai, T.; Bopape, F.L. Non-Rhizobial Endophytes (NREs) of the Nodule Microbiome Have Synergistic Roles in Beneficial Tripartite Plant–Microbe Interactions. Microorganisms 2025, 13, 518. https://doi.org/10.3390/microorganisms13030518
Hassen AI, Muema EK, Diale MO, Mpai T, Bopape FL. Non-Rhizobial Endophytes (NREs) of the Nodule Microbiome Have Synergistic Roles in Beneficial Tripartite Plant–Microbe Interactions. Microorganisms. 2025; 13(3):518. https://doi.org/10.3390/microorganisms13030518
Chicago/Turabian StyleHassen, Ahmed Idris, Esther K. Muema, Mamonokane O. Diale, Tiisetso Mpai, and Francina L. Bopape. 2025. "Non-Rhizobial Endophytes (NREs) of the Nodule Microbiome Have Synergistic Roles in Beneficial Tripartite Plant–Microbe Interactions" Microorganisms 13, no. 3: 518. https://doi.org/10.3390/microorganisms13030518
APA StyleHassen, A. I., Muema, E. K., Diale, M. O., Mpai, T., & Bopape, F. L. (2025). Non-Rhizobial Endophytes (NREs) of the Nodule Microbiome Have Synergistic Roles in Beneficial Tripartite Plant–Microbe Interactions. Microorganisms, 13(3), 518. https://doi.org/10.3390/microorganisms13030518







