ZIKA Virus, an Emerging Arbovirus in India: A Glimpse of Global Genetic Lineages
Abstract
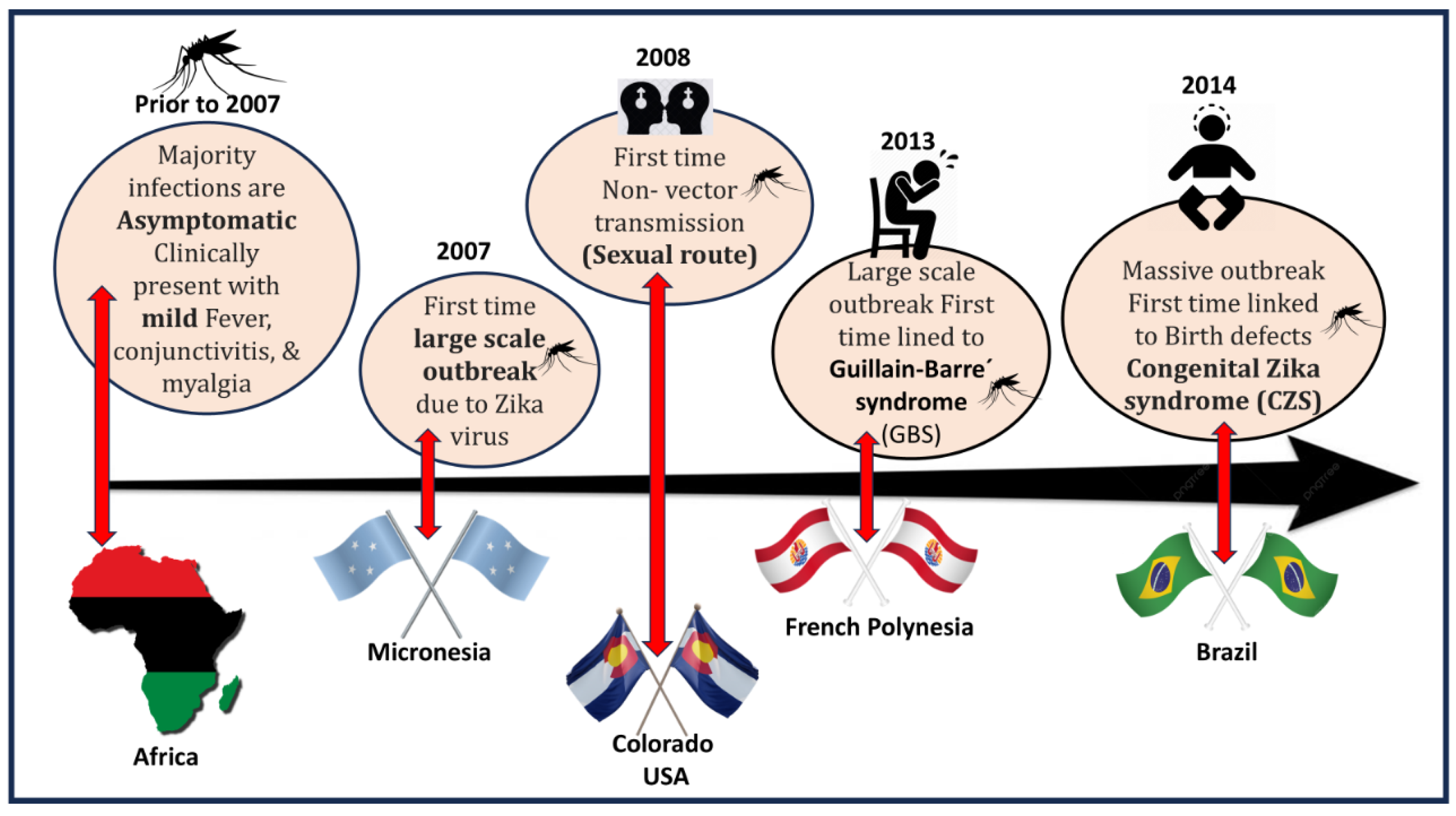
1. Introduction
2. Genomic Organization of ZIKAV
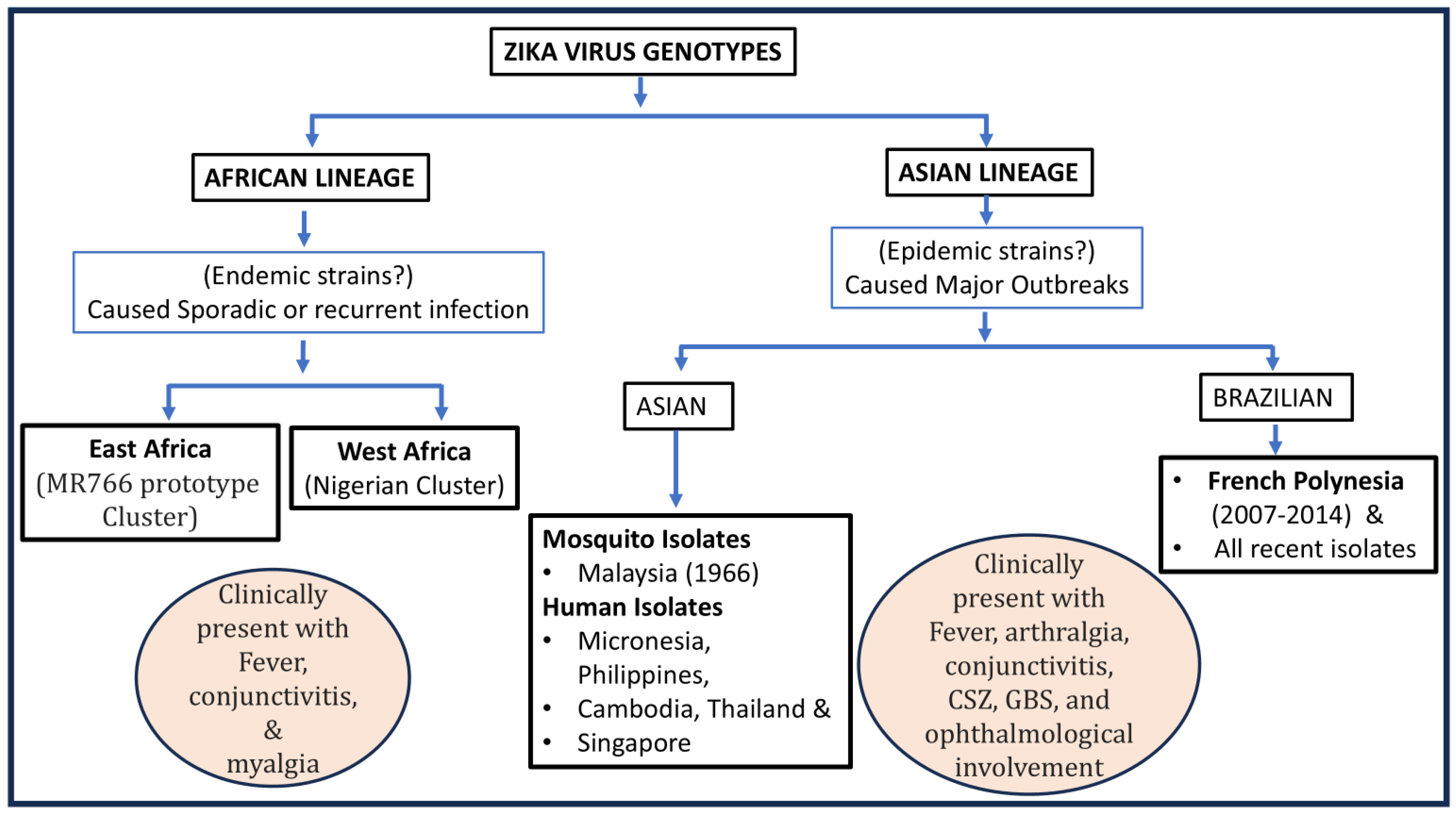
3. A Global Picture of Genetic Lineages
4. Mutation of ZIKAV and Its Impact on Phenotypes
| Reference Cited | Location | Substitution | Phenotypic Change |
|---|---|---|---|
| Shan et al. (2020) [33] | Envelop | EV-473M | -Increases neurovirulence -Undergoes maternal-to-fetal transmission -Causes viremia to increase urban transmission |
| Fontes-Garfias, Camila R 2017 et al. [36] | Envelop | Asn 154 | - Mosquito-cell infectivity -Virus assembly |
| Liu et al. (2021) [37] | Capsid | C-T106A | -Virus fitness advantage -Accelerates the spread in both mosquitoes and rodents -Enhances transmissibility between vectors and hosts |
| Phumee et al. (2023) [38] | Pre-Membrane | prM-V1A | -Linked with high mortality rate |
| Yuan et al. (2017) [39] | Pre-Membrane | prM-S17N | -Increased microcephaly in fetus? |
| Liu et al. (2021) [37] | Pre-Membrane | prM-V123A | -Virus fitness advantage |
| Yuan et al. (2017) [39] | Pre-Membrane | PrM-S139N | -Accelerates virus infectivity for mouse and human neural progenitor cells -Enhances apoptosis |
| Xia et al. (2018) [40] Liu et al. (2017) [41] | NS1 | NS1-A188V | -Enhances virus infectivity in Aedes aegypti -Suppresses Type-T interferon |
| Liu et al. (2021) [37] | NS1 | NS1-A982V | -Virus fitness advantage |
| Zhang et al. (2023) [42] | NS2A | NS2A-A1204T | -Associated with neurovirulence |
| Regla Nava et al. (2022) [43] | NS2B | NS2B-139V | -Enhances virus virulence -Escapes from pre-immune dengue antibody |
| NS5 | NS5-M872 | -To be determined | |
| Peng et al. (2022) [44] | NS5 | NS5-M114V | -No role in virus replication and transmission potential. |
| Liu et al. (2021) [37] | NS5 | NS5-M3392V | -Virus fitness advantage |
5. Indian Scenario
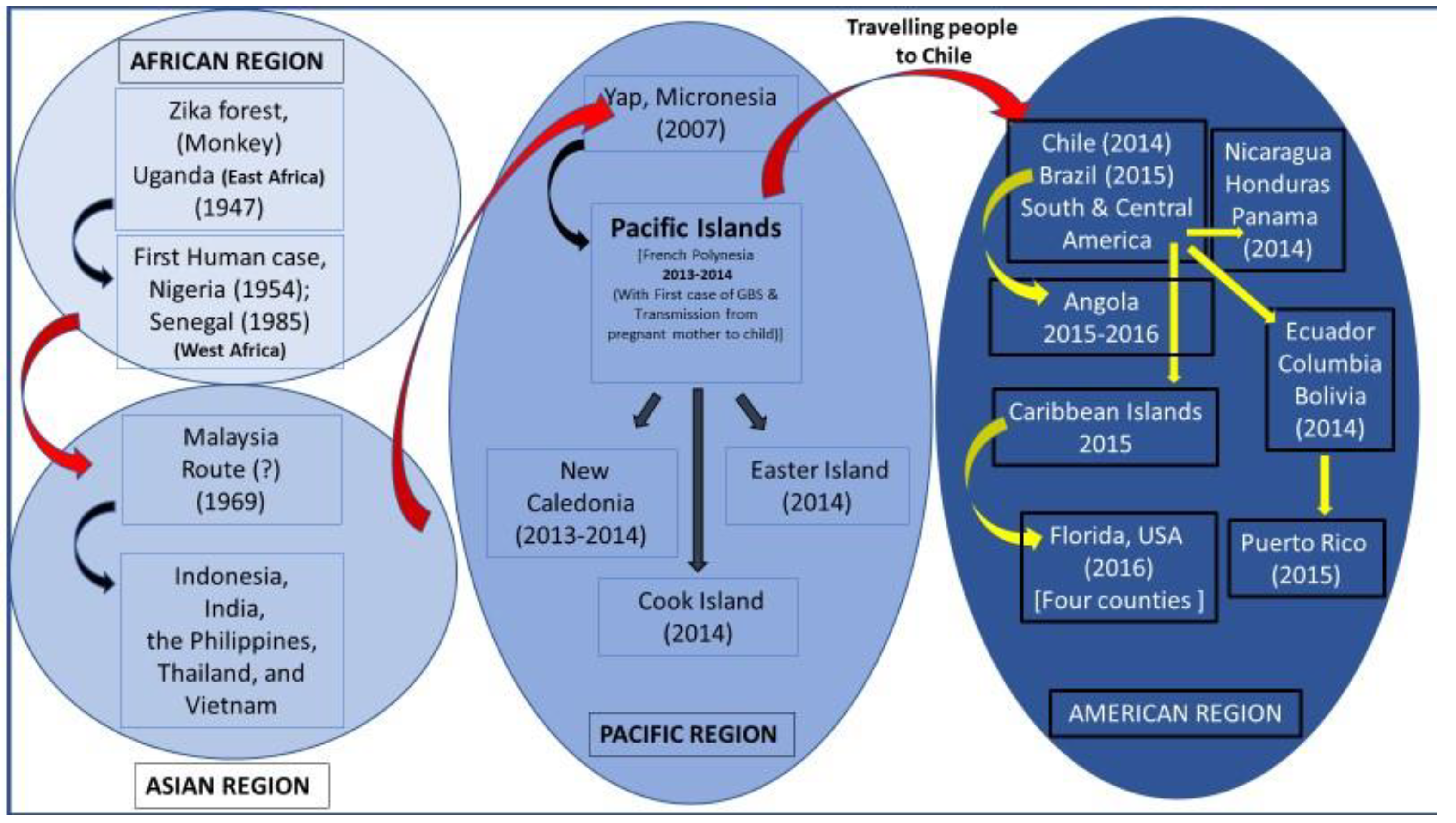
6. Origin and Global Dispersal of ZIKAV
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Bossin, H.; Mallet, H.P.; Besnard, M.; Broult, J.; Baudouin, L.; Levi, J.E.; Sabino, E.C.; Ghawche, F.; Lanteri, M.C.; et al. Zika virus in French Polynesia 2013-14: Anatomy of a completed outbreak. Lancet Infect. Dis. 2018, 18, e172–e182. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; Melo, V.C.A.; Mosimann, A.L.P.; Santos, G.I.V.D.; Santos, C.N.D.S.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Vaziri, S.; Pour, S.H.; Akrami-Mohajeri, F. Zika virus as an emerging arbovirus of international public health concern. Osong Public Health Res. Perspect. 2022, 13, 341–351. [Google Scholar] [CrossRef]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.M. Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef]
- Besnard, M.; Lastere, S.; Teissier, A.; Cao-Lormeau, V.; Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Eurosurveillance 2014, 19, 20751. [Google Scholar] [CrossRef]
- Westrich, J.A.; McNulty, E.E.; Edmonds, M.J.; Nalls, A.V.; Miller, M.R.; Foy, B.D.; Rovnak, J.; Perera, R.; Mathiason, C.K. Characterization of subclinical ZIKV infection in immune-competent guinea pigs and mice. J. Gen. Virol. 2021, 102, 001641. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Tesla, B.; Bonds, M.H.; Ngonghala, C.N.; Mordecai, E.A.; Johnson, L.R.; Murdock, C.C. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob. Change Biol. 2021, 27, 84–93. [Google Scholar] [CrossRef]
- Karkhah, A.; Nouri, H.R.; Javanian, M.; Koppolu, V.; Masrour-Roudsari, J.; Kazemi, S.; Ebrahimpour, S. Zika virus: Epidemiology, clinical aspects, diagnosis, and control of infection. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2035–2043. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Zhang, Z.; Marin-Lopez, A. Current Advances in Zika Vaccine Development. Vaccines 2022, 10, 1816. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C.; Kerr, J.A.; Gatne, P.B. Neutralizing antibodies against certain viruses in the sera of residents of India. J. Immunol. 1954, 72, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Wiley, M.R.; Prieto, K.; Yasuda, C.Y.; Nagle, E.; Kasper, M.R.; Reyes, D.; Vasilakis, N.; Heang, V.; Weaver, S.C.; et al. Complete Genome Sequences of Five Zika Virus Isolates. Genome Announc. 2016, 4, e00377-16. [Google Scholar] [CrossRef]
- Aziz, A.; Suleman, M.; Shah, A.; Ullah, A.; Rashid, F.; Khan, S.; Iqbal, A.; Luo, S.; Xie, L.; Xie, Z. Comparative mutational analysis of the Zika virus genome from different geographical locations and its effect on the efficacy of Zika virus-specific neutralizing antibodies. Front. Microbiol. 2023, 14, 1098323. [Google Scholar] [CrossRef]
- Pettersson, J.H.; Bohlin, J.; Dupont-Rouzeyrol, M.; Brynildsrud, O.B.; Alfsnes, K.; Cao-Lormeau, V.M.; Gaunt, M.W.; Falconar, A.K.; de Lamballerie, X.; Eldholm, V.; et al. Re-visiting the evolution, dispersal and epidemiology of Zika virus in Asia. Emerg. Microbes. Infect. 2018, 7, 79. [Google Scholar] [CrossRef]
- Shrivastava, S.; Puri, V.; Dilley, K.A.; Ngouajio, E.; Shifflett, J.; Oldfield, L.M.; Fedorova, N.B.; Hu, L.; Williams, T.; Durbin, A.; et al. Whole genome sequencing, variant analysis, phylogenetics, and deep sequencing of Zika virus strains. Sci. Rep. 2018, 8, 15843. [Google Scholar] [CrossRef]
- Barzilai, L.; Schrago, C.G. The range of sampling times affects Zika virus evolutionary rates and divergence times. Arch. Virol. 2019, 164, 3027–3034. [Google Scholar] [CrossRef]
- Simonin, Y.; van Riel, D.; Van de Perre, P.; Rockx, B.; Salinas, S. Differential virulence between Asian and African lineages of Zika virus. PLoS Negl. Trop. Dis. 2017, 11, e0005821. [Google Scholar] [CrossRef]
- de Araújo, T.V.B.; Rodrigues, L.C.; de Alencar Ximenes, R.A.; de Barros Miranda-Filho, D.; Montarroyos, U.R.; de Melo, A.P.L.; Valongueiro, S.; de Albuquerque, M.D.F.P.M.; Souza, W.V.; Braga, C.; et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 2016, 16, 1356–1363. [Google Scholar] [CrossRef]
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika outbreak of the 21st century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef]
- Fisher, D.; Cutter, J. The inevitable colonisation of Singapore by Zika virus. BMC Med. 2016, 14, 188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Woon, Y.L.; Lim, M.F.; Abd Rashid, T.R.T.; Thayan, R.; Chidambaram, S.K.; Abdul Rahim, S.S.S.; Mudin, R.N.; Sivasampu, S. Zika virus infection in Malaysia: An epidemiological, clinical and virological analysis. BMC Infect. Dis. 2019, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Fabien, A.; Sofie, J.; Maïlis, D.; Sebastian, L.; Leen, D.; Albin, F.; Natapong, J.; Elliott, F.; Miot, S.D.; Caroline, M.; et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asianstrains. Nat. Commun. 2021, 12, 916. [Google Scholar] [CrossRef]
- Haddow, A.D.; Nasar, F.; Guzman, H.; Ponlawat, A.; Jarman, R.G.; Tesh, R.B.; Weaver, S.C. Genetic Characterization of Spondweni and Zika Viruses and Susceptibility of Geographically Distinct Strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005083. [Google Scholar] [CrossRef]
- Neal, J.W. Flaviviruses are neurotropic, but how do they invade the CNS? J. Infect. 2014, 69, 203–215. [Google Scholar] [CrossRef]
- Khongwichit, S.; Chuchaona, W.; Vongpunsawad, S.; Poovorawan, Y. Molecular epidemiology, clinical analysis, and genetic characterization of Zika virus infections in Thailand (2020–2023). Sci. Rep. 2023, 13, 21030. [Google Scholar] [CrossRef]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Hilda, G.; Robert, B.T.; Scott, C.W. Genetic Characterization of Zika Virus Strains: Geographic Expansion of the Asian Lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef]
- Yokoyama, S.; Starmer, W.T. Possible roles of new mutations shared by Asian and American zika viruses. Mol. Biol. Evol. 2017, 34, 525–534. [Google Scholar] [CrossRef]
- Smith, D.R.; Sprague, T.R.; Hollidge, B.S.; Valdez, S.M.; Padilla, S.L.; Bellanca, S.A.; Golden, J.W.; Coyne, S.R.; Kulesh, D.A.; Miller, L.J.; et al. African and Asian Zika virus isolates display phenotypic differences both in vitro and in vivo. Am. J. Trop. Med. Hyg. 2018, 98, 432–444. [Google Scholar] [CrossRef]
- Logan, I.S. ZIKA—How fast does this virus mutate? Dongwuxue Yanjiu 2016, 37, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Jiang, M.; Westenius, V.; Kuivanenm, S.; Jarvi, R.; Kokkola, L.; Lundberg, R.; Kelen, K.; Korva, T.A.; Avsic-Zupanc, T.; et al. Asian and African lineage Zika viruses show differential replication and innate immune responses in human dendritic cells and macrophages. Sci. Rep. 2019, 9, 15710. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xia, H.; Haller, S.L.; Azar, S.R.; Liu, Y.; Liu, J.; Muruato, A.E.; Chen, R.; Rossi, S.L.; Wakamiya, M.; et al. A Zika virus envelope mutation preceding the 2015 epidemic enhances virulence and fitness for transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 20190–20197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chan, J.F.; Tee, K.M.; Choi, G.K.; Lau, S.K.; Woo, P.C.; Tse, H.; Yuen, K.Y. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg. Microbes Infect. 2016, 5, e22. [Google Scholar] [CrossRef]
- Jun, S.R.; Wassenaar, T.M.; Wanchai, V.; Patumcharoenpol, P.; Nookaew, I.; Ussery, D.W. Suggested mechanisms for Zika virus causing microcephaly: What do the genomes tell us? BMC Bioinform. 2017, 18, 471. [Google Scholar] [CrossRef]
- Fontes-Garfias, C.R.; Shan, C.; Luo, H.; Muruato, A.E.; Medeiros, D.B.; Mays, E.; Xie, X.; Zou, J.; Roundy, C.M.; Wakamiya, M.; et al. Functional Analysis of Glycosylation of Zika Virus Envelope Protein. Cell Rep. 2017, 21, 1180–1190. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Shan, C.; Nunes, B.T.D.; Yun, R.; Haller, S.L.; Rafael, G.H.; Azar, S.R.; Andersen, C.R.; Plante, K.; et al. Role of mutational reversions and fitness restoration in Zika virus spread to the Americas. Nat. Commun. 2021, 12, 595. [Google Scholar] [CrossRef]
- Phumee, A.; Chitcharoen, S.; Sutthanont, N.; Intayot, P.; Wacharapluesadee, S.; Siriyasatien, P. Genetic diversity and phylogenetic analyses of Asian lineage Zika virus whole genome sequences derived from Culex quinquefasciatus mosquitoes and urine of patients during the 2020 epidemic in Thailand. Sci. Rep. 2023, 13, 18470. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, X.Y.; Liu, Z.Y.; Zhang, F.; Zhu, X.L.; Yu, J.Y.; Ji, X.; Xu, Y.-P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Daniele, B.A.; Zou, J.; Xie, X.; Girldo, M.I.; Vasconcelos, P.F.C.; et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat. Commun. 2018, 9, 414. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Du, S.; Shan, C.; Nie, K.; Zhang, R.; Li, X.F.; Zhang, R.; Wang, T.; Qin, C.F.; et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 2017, 545, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Guo, J.J.; Yang, F.; Zhou, H.Y.; Zhang, N.N.; Xiong, X.C.; Feng, Y.; Deng, Y.Q.; Qin, C.F. Characterization and phylogenetic analysis of a neurovirulent Zika virus isolated from Cambodia in 2019. J. Med. Virol. 2023, 95, e28290. [Google Scholar] [CrossRef] [PubMed]
- Regla-Nava, J.A.; Wang, Y.T.; Fontes-Garfias, C.R.; Liu, Y.; Syed, T.; Susantono, M.; Gonzalez, A.; Viramontes, K.M.; Verma, S.K.; Kim, K.; et al. A Zika virus mutation enhances transmission potential and confers escape from protective dengue virus immunity. Cell Rep. 2022, 39, 110655. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.Y.G.; Amarilla, A.A.; Hugo, L.E.; Modhiran, N.; Sng, J.D.J.; Slonchak, A.; Watterson, D.; Setoh, Y.X.; Khromykh, A.A. The distinguishing NS5-M114V mutation in American Zika virus isolates has negligible impacts on virus replication and transmission potential. PLoS Negl. Trop. Dis. 2022, 16, e0010426. [Google Scholar] [CrossRef] [PubMed]
- Sapkal, G.N.; Yadav, P.D.; Vegad, M.M.; Viswanathan, R.; Gupta, N.; Mourya, D.T. First laboratory confirmation on the existence of Zika virus disease in India. J. Infect. 2017, 76, 314–317. [Google Scholar] [CrossRef]
- Pradeep Kumar, N.; Ajithlal, P.M.; Saini, P.; Abidha, S.; Samuel, P.; Balasubramaniam, M.; Jessu, M.; Sonia, T.; Amju, K.P.; Aiswarya, R.S.; et al. Recent Outbreak of Zika in Kerala State, India; is Zika Virus Re-Emerging as a Distinct Genetic Lineage in India? Lancet 2023. Preprint. [Google Scholar] [CrossRef]
- Scroll.in. Inside Uttar Pradesh’s Zika Outbreak: Can India’s Most Populous State Contain the Virus Spread? 2021. Available online: https://scroll.in/article/1010555/inside-uttar-pradeshs-zika-outbreak-can-indias-most-populous-state-contain-the-virus-spread (accessed on 30 November 2021).
- Yadav, P.D.; Niyas, V.K.M.; Arjun, R.; Sahay, R.R.; Shete, A.M.; Sapkal, G.N.D.; Pawar, S.; Patil, D.Y.; Gupta, N.; Abraham, P. Detection of Zika virus disease in Thiruvananthapuram, Kerala, India 2021 during the second wave of COVID-19 pandemic. J. Med. Virol. 2022, 94, 2346–2349. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085. [Google Scholar] [CrossRef]
- Faria, N.R.; Azevedo, R.D.S.D.S.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, L.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Hill, S.C.; Vasconcelos, J.; Neto, Z.; Jandondo, D.; Zé-Zé, L.; Aguiar, R.S.; Xavier, J.; Thézé, J.; Mirandela, M.; Micolo Cândido, A.L.; et al. Emergence of the Asian lineage of Zika virus in Angola: An outbreak investigation. Lancet Infect. Dis. 2019, 19, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Perkins, T.A.; Siraj, A.S.; Ruktanonchai, C.W.; Kraemer, M.U.G.; Tatem, A.J. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat. Microbiol. 2016, 1, 16126. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V. Zika virus associated with microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L. Guillain-Barré syndrome associated with Zika virus infection in Colombia. N. Engl. J. Med. 2016, 375, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.; Kalinich, C.C.; Cruz-López, F.; Gonzalez, G.L.; Flores, B.; Hentoff, A.; Charriez, K.N.; Fauver, J.R.; Adam, L.E.; Sharp, T.M.; et al. Tracing the Origin, Spread, and Molecular Evolution of Zika Virus in Puerto Rico, 2016–2017. Emerg. Infec. Dis. 2021, 27, 2971–2973. [Google Scholar] [CrossRef]
- Yadav, P.D.; Malhotra, B.; Sapkal, G.; Nyayanit, D.A.; Deshpande, G.; Gupta, N.; Padinjaremattathil, U.T.; Sharma, H.; Sahay, R.R.; Sharma, P.; et al. Zika virus outbreak in Rajasthan, India in 2018 was caused by a virus endemic to Asia. Infect. Genet. Evol. 2019, 69, 199–202. [Google Scholar] [CrossRef]


| Accession no: | Place | Source | Year | Reference |
|---|---|---|---|---|
| MCL-21-H-8900 and MCL-21-H-8901 | Thiruvananthapuram, Kerala, India | Human | 2021 | Yadav et al. (2022) [48] |
| MK238037.1 | Rajasthan, India | Human | 2018 | Yadav et al. (2019) [57] |
| OP678998 | Thiruvananthapuram, Kerala, India | Human | 2021 | Pradeep Kumar et al. (2023) [46] |
| OP678999 | Thiruvananthapuram, Kerala | Aedes albopictus | 2021 | Pradeep Kumar et al. (2023) [46] |
| OM666892.1 | Maharashtra | 2021 | ||
| NIV1720741/1845ZKV | Gujarat | Human | 2016 | Sapkal et al. (2017) [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajaiah, P.; Gupta, B.; Mayilsamy, M. ZIKA Virus, an Emerging Arbovirus in India: A Glimpse of Global Genetic Lineages. Microorganisms 2025, 13, 544. https://doi.org/10.3390/microorganisms13030544
Rajaiah P, Gupta B, Mayilsamy M. ZIKA Virus, an Emerging Arbovirus in India: A Glimpse of Global Genetic Lineages. Microorganisms. 2025; 13(3):544. https://doi.org/10.3390/microorganisms13030544
Chicago/Turabian StyleRajaiah, Paramasivan, Bhavna Gupta, and Muniyaraj Mayilsamy. 2025. "ZIKA Virus, an Emerging Arbovirus in India: A Glimpse of Global Genetic Lineages" Microorganisms 13, no. 3: 544. https://doi.org/10.3390/microorganisms13030544
APA StyleRajaiah, P., Gupta, B., & Mayilsamy, M. (2025). ZIKA Virus, an Emerging Arbovirus in India: A Glimpse of Global Genetic Lineages. Microorganisms, 13(3), 544. https://doi.org/10.3390/microorganisms13030544






