Variation of Microorganisms and Water Quality, and Their Impacts on the Production of Penaeus vannamei in Small-Scale Greenhouse Ponds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Water Quality Factors Testing
2.3. Pathogen Detection
2.4. Analysis of Bacterial Community Composition
2.5. Shrimp Growth Indicators
2.6. Data Analysis
3. Results
3.1. Shrimp Growth Indicators and Culture Production
3.2. Water Quality Indicators
3.3. Bacterial Community Composition in the Pond Water and Intestinal of Shrimp
3.3.1. Bacterial Community Structure at the Phylum Level
3.3.2. Bacterial Community Structure at the Genus Level
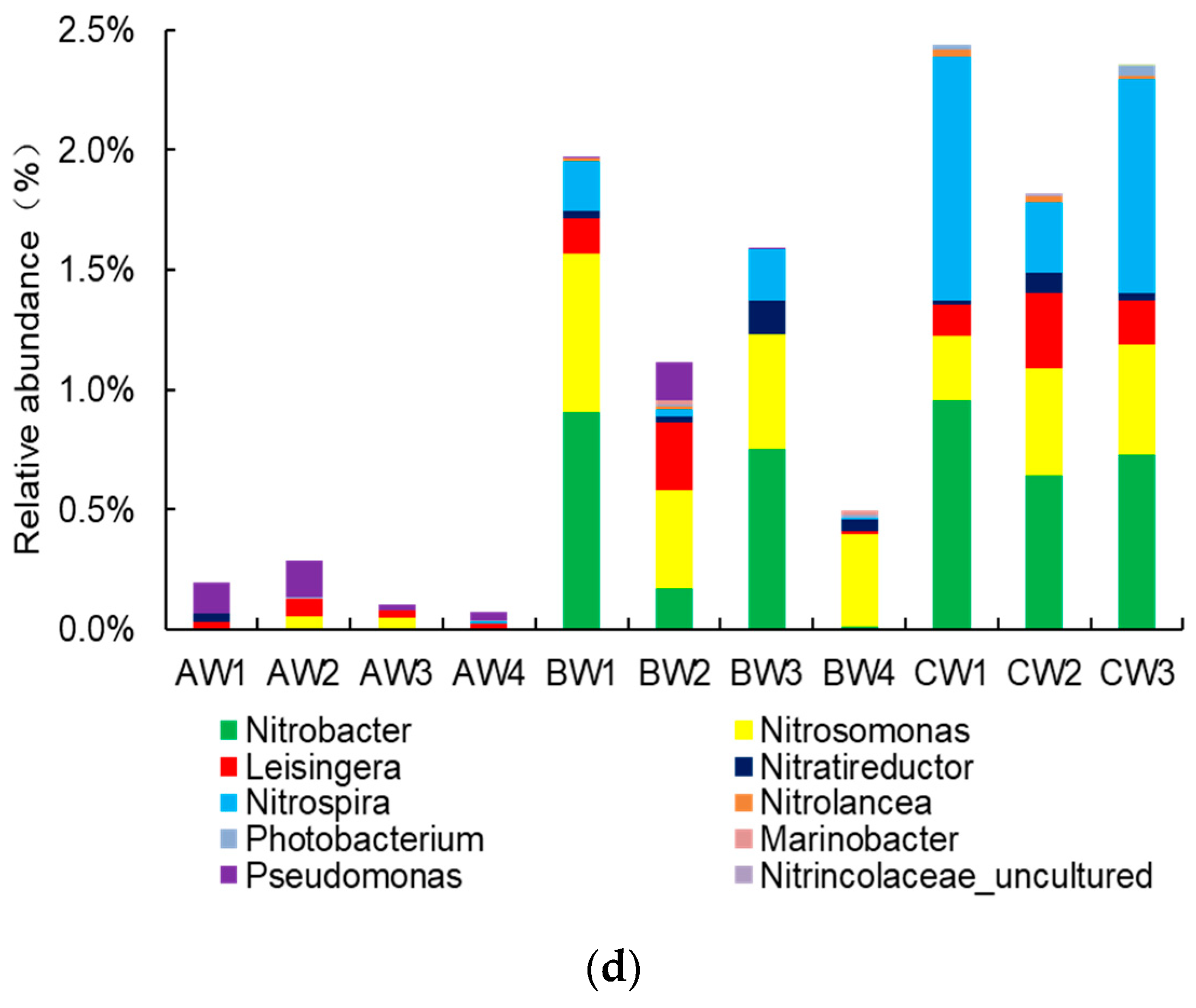
3.3.3. Nitrifying Bacterial Community in Pond Water at the Genus Level
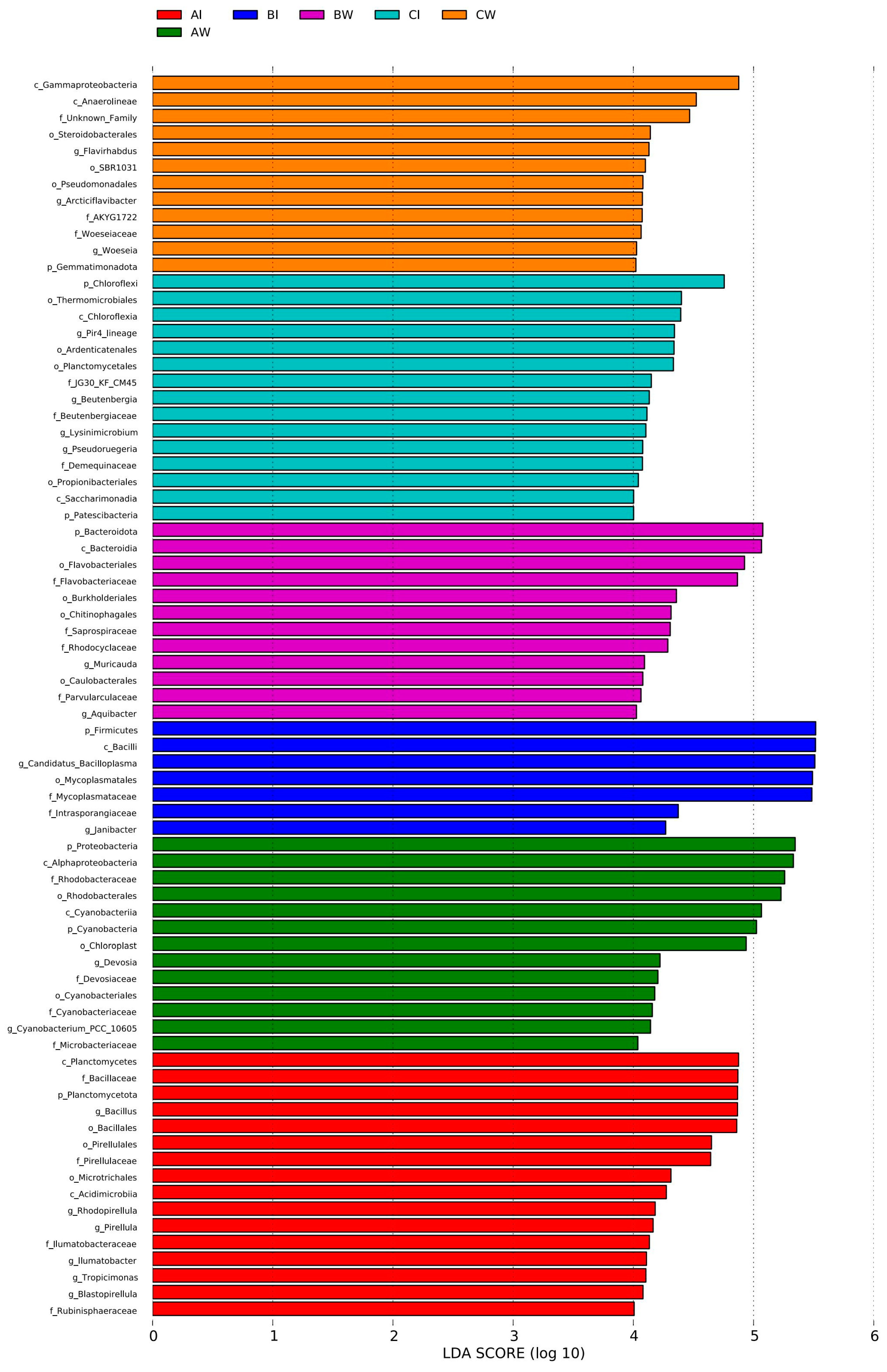
3.3.4. Microbial Differences
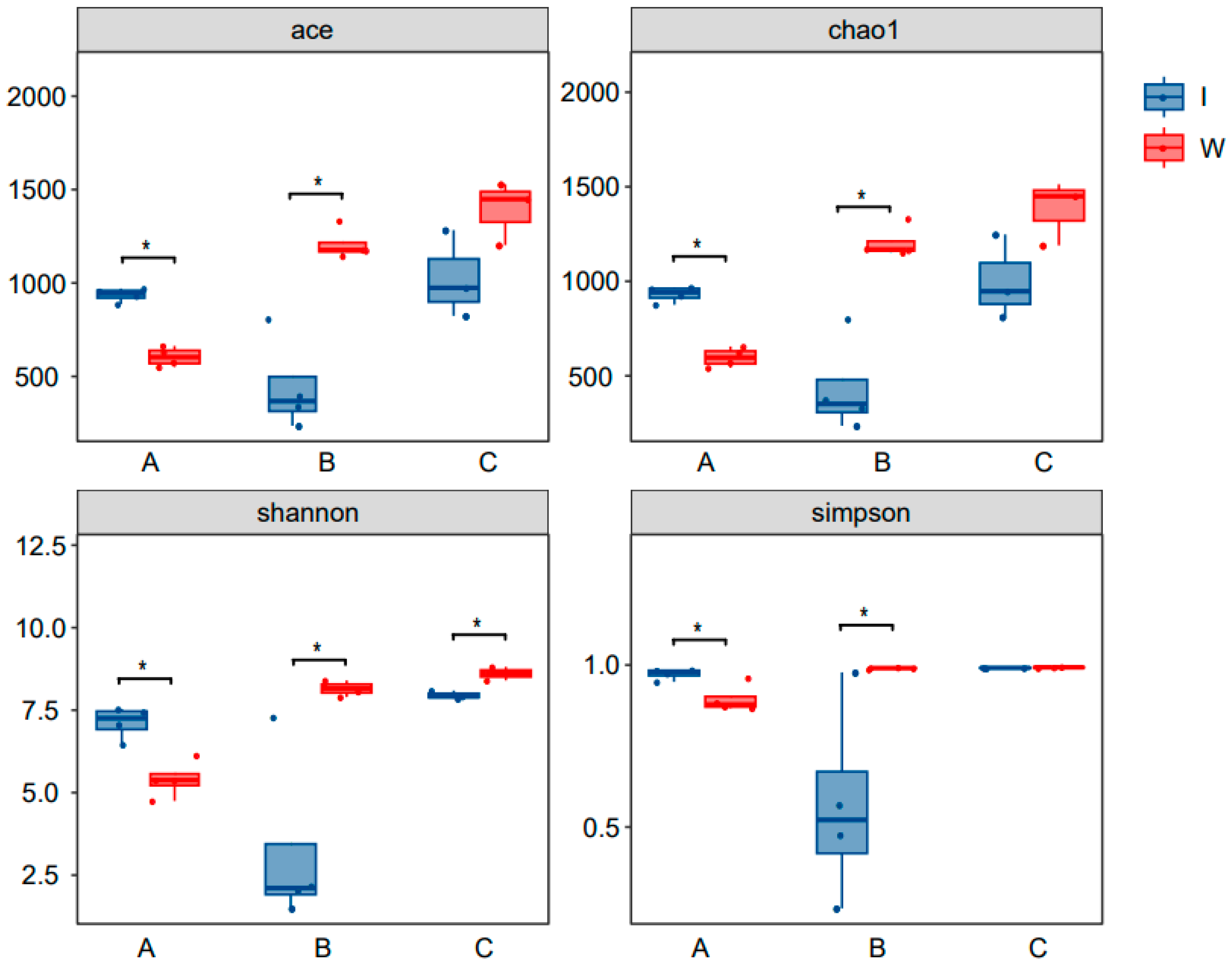
3.3.5. α Diversity Index Analysis
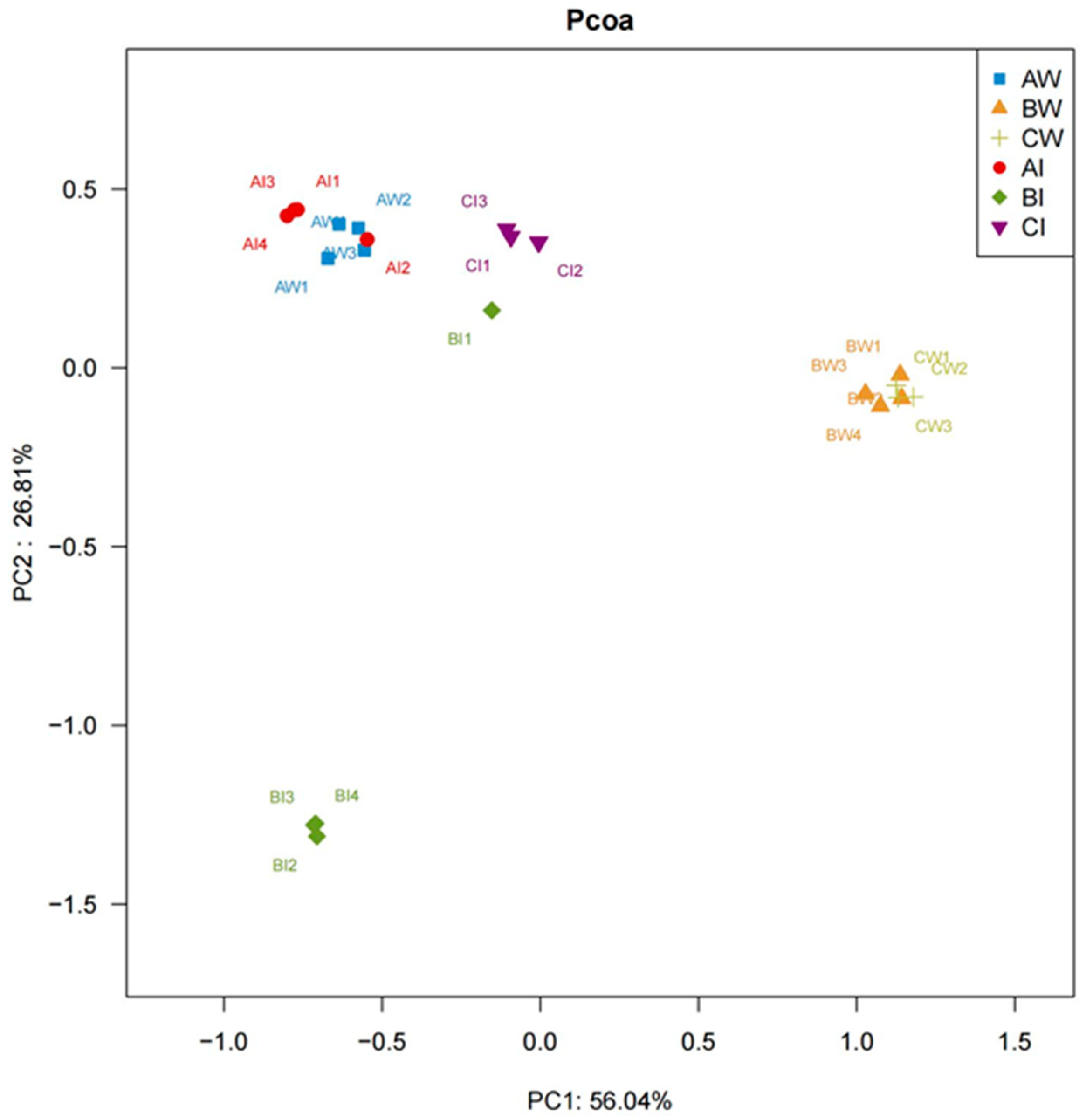
3.3.6. PCoA Analysis
3.4. Pathogen Quantification
3.5. The Relationship Between Nitrifying Bacterial Communities and Water Quality Factors
3.6. The Relationship Between Bacterial Communities, Pathogens, Water Quality Factors, and Shrimp Production
4. Discussion
4.1. The Impact of the Pond Water Environment on Shrimp Culture
4.2. The Impact of Microorganisms on Shrimp Culture
4.3. The Impact of Microbial and Water Quality Factor Regulation on Shrimp Culture Ponds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, C.Y.; Liu, J.Y. Evaluation of genetic parameters for growth and comprehensive stress tolerance traits of Litopenaeus vannamei. South China Fish. Sci. 2022, 18, 95–102. [Google Scholar]
- Hu, X.J.; Xu, Y.; Cao, Y.C. Full-Color Illustrated Guide to Efficient Culture and Disease Control of the Litopenaeus vannamei; Chemical Industry Press: Beijing, China, 2021; Volume 1, pp. 130–219. ISBN 978-7-122-39282-4. [Google Scholar]
- Hang, Z.Q.; Neori, A.; He, Y.Y.; Li, J.T.; Qiao, L.; Preston, S.I.; Liu, P.; Li, J. Development and current state of seawater shrimp farming, with an emphasis on integrated multi-trophic pond aquaculture farms, in China—A review. Rev. Aquac. 2020, 12, 2544–2558. [Google Scholar]
- Jifiriya, M.J.; Preena, P.G.; Rejish Kumar, V.J.; Nair, A.J.; Joseph, V. Role of archaea in aquaculture: Prospects and challenges. Aquac. Int. 2024, 32, 3169–3194. [Google Scholar] [CrossRef]
- Qi, F.; Rebiguli, S.; Xian, Y.L.; Han, J.J.; Mina’er, W.; Chen, H.F.; Sun, S.P.; Ma, Y.W.; Chen, P. Spatio-temporal changes of bacterioplankton communities in Litopenaeus vannamei desalinated ponds and their responses to physicochemical factors. South China Fish. Sci. 2023, 19, 50–61. [Google Scholar]
- Yan, M.; Zhang, X.; Hu, L.H.; Huang, X.K.; Zhou, Q.J.; Zeng, G.Q.; Zhang, J.M.; Xiao, G.Q.; Chai, X.L.; Chen, J. Bacterial community dynamics during nursery rearing of pacific white shrimp (Litopenaeus vannamei) revealed via high-throughput Sequencing. Indian J. Microbiol. 2020, 60, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, Y.; Yin, D.D.; Song, C.D.; Mo, Z.J.; Xiao, J.F.; Yi, H.; Zhang, H.Y.; Zhang, B.; Shen, N.K. Screening, condition optimization and preliminary application of nitrite degrading Bacillus velezensis GXMZU-B1. South China Fish. Sci. 2024, 20, 133–143. [Google Scholar]
- Xu, W.J.; Huang, F.; Zhao, Y.Z.; Su, H.C.; Hu, X.J.; Xu, Y.; Wen, G.L.; Cao, Y.C. Carbohydrate addition strategy affects nitrogen dynamics, budget and utilization, and its microbial mechanisms in biofloc-based Penaeus vannamei culture. Aquaculture 2024, 589, 66–75. [Google Scholar] [CrossRef]
- Gao, F.; Gan, E.L.; Liu, W.; Guo, H.P.; Wang, Y.T.; Wang, R.-Y.; Yan, M.C.; Dong, P.S.; Zhang, D.M. Screening of carbon sources for enrichment and directional isolation of Rhodobacteraceae from the gut of Litopenaeus vannamei. Acta Microbiol. Sin. 2022, 62, 1805–1818. [Google Scholar]
- Huang, Z.J.; Zeng, S.Z.; Xiong, J.B.; Hou, D.W.; Zhou, R.J.; Xing, C.G.; Wei, D.D.; Deng, X.S.; Yu, L.F.; Wang, H.; et al. Microecological Koch’s postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome 2020, 8, 32. [Google Scholar] [CrossRef]
- Liao, G.W.; Wu, Q.P.; Mo, B.H.; Zhou, J.; Li, J.Y.; Zou, J.X.; Fan, L.F. Intestinal morphology and microflora to Vibrio alginolyticus in pacific white shrimp (Litopenaeus vannamei). Fish Shellfish. Immunol. 2022, 121, 437–445. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, T.; Li, S.W.; Zhu, C.B.; Zhang, B.; Su, J.Q.; Ruan, G.-L.; Zhang, X.Y. Purification effect of searice paddy field on brackish water environment of shrimp culture. South China Fish. Sci. 2023, 19, 19–28. [Google Scholar]
- See, K.F.; Ibrahim, R.A.; Goh, K.H. Aquaculture efficiency and productivity: A comprehensive review and bibliometric analysis. Aquaculture 2021, 544, 736881. [Google Scholar] [CrossRef]
- Devadas, S.; Bhassu, S.; Soo, T.C.C.; Yusoff, F.M.; Shariff, M. A new 5-plex PCR detection method for acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus strains. Aquaculture 2019, 503, 373–380. [Google Scholar] [CrossRef]
- Rajeshwar, B.N.; Kumar, T.S.; Jithendran, K.P.; Bedekar, M.K.; Rajendran, K.V. Prevalence of enterocytozoon hepatopenaei (EHP) in Penaeus monodon farms in India. Aquac. Int. 2024, 32, 9393–9414. [Google Scholar] [CrossRef]
- Liao, M.Z.; Liao, X.Z.; Long, X.X.; Zhao, J.C.; He, Z.H.; Zhang, J.Y.; Wu, T.-F.; Sun, C.-B. Host-microbiota interactions and responses of Metapenaeus ensis infected with decapod iridescent virus 1. Front. Microbiol. 2023, 13, 1097931. [Google Scholar] [CrossRef]
- Li, X.P.; Luan, S.; Luo, K.; Cao, B.X.; Chen, B.L.; Kong, J.; Meng, X.H. Comparative transcriptomic analysis of Chinese shrimp Fenneropenaeus chinensis infected with white spot syndrome virus. Aquac. Rep. 2022, 22, 100986. [Google Scholar] [CrossRef]
- Fang, Y.; Li, H.; Wang, L.B.; Wan, X.H.; Shi, W.J.; Yang, Z.Y.; Jiang, Q.; Shen, H.; Hu, R.H.; Guan, X.P.; et al. Study on bacterial community structure in rearing water in small green-house of Litopenaeus vannamei. South China Fish. Sci. 2023, 19, 29–41. [Google Scholar]
- John, E.M.; Krishnapriya, K.; Sankar, T.V. Treatment of ammonia and nitrite in aquaculture wastewater by an assembled bacterial consortium. Aquaculture 2020, 526, 735390. [Google Scholar] [CrossRef]
- Gao, S.; Dai, L.X.; Wang, Y.; Liang, Z.L.; Zhou, Y.H.; Sun, C.B. Effects of WSSV and ammonia nitrogen stress on the enzyme activity and transcriptome of Litopenaeus vannamei. Aquacult. Rep. 2024, 39, 102412. [Google Scholar] [CrossRef]
- Xing, Y.F.; Zhu, X.Y.; Duan, Y.F.; Huang, J.H.; Nan, Y.X.; Zhang, J.S. Toxic effects of nitrite and microplastics stress on histology, oxidative stress, and metabolic function in the gills of Pacific white shrimp, Litopenaeus Vannamei. Mar. Pollut. Bull. 2023, 187, 114531. [Google Scholar] [CrossRef]
- Xu, W.J.; Xu, Y.; Su, H.C.; Hu, X.J.; Xu, Y.N.; Li, Z.J.; Wen, G.L.; Cao, Y.C. Production performance, inorganic nitrogen control and bacterial community characteristics in a controlled biofloc-based system for indoor and outdoor super-intensive culture of Litopenaeus vannamei. Aquaculture 2021, 531, 735749. [Google Scholar] [CrossRef]
- Li, H.; Gu, S.W.; Wang, L.B.; Shi, W.J.; Jiang, Q.; Wan, X.H. Dynamic changes of environment and gut microbial community of Litopenaeus vannamei in greenhouse farming and potential mechanism of gut microbial community construction. Fishes 2024, 9, 155. [Google Scholar] [CrossRef]
- Wang, L.W.; Li, X.N.; Xie, J.P.; Feng, Q.F.; Wang, C.Y.; Dai, X.L. Effects ofsalinity, temperature, ammonia nitrogen and nitrite stress on growth, survival, physiological and biochemical indexes of Penaeus vanname. J. Shanghai Ocean Univ. 2024, 33, 1084–1097. [Google Scholar]
- Song, G.L.; Zhao, Y.C.; Liu, Z. Acute toxicity test of ammonia nitrogen to Litopenaeus annamei juveniles under low salinity and high alkalinity. J. Fish. Res. 2022, 44, 374–379. [Google Scholar]
- Wang, A.L.; Jiang, Y.S.; Xing, Y.L.; Wang, C.J.; Jiao, N.; Sun, N. Effects of low-salinity nitrite exposure on the toxicity and antioxidant ability of Litopenaeus vannamei. Fish. Sci. Technol. Inf. 2024, 1–11. [Google Scholar]
- Lin, Y.C.; Chen, J.C. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 2001, 25, 109–119. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C. Acute toxicity of nitrite on Litopenaeus vannamei (Boone) juveniles at different salinity levels. Aquaculture 2003, 224, 193–201. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Sim, S.S.; Chiew, S.L.; Yeh, S.T.; Liou, C.H.; Chen, J.C. Dietary administration of a Gracilaria tenuistipitata extract produces protective immunity of white shrimp Litopenaeus vannamei in response to ammonia stress. Aquaculture 2012, 370, 26–31. [Google Scholar] [CrossRef]
- Perez-Sanchez, T.; Mora-Sanchez, B.; Luis Balcazar, J. Biological approaches for disease control in aquaculture: Advantages, limitations and challenges. Trends Microbiol. 2018, 26, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Li, P.; Liu, L.; Li, Z.H. Exploring the interactions between the gut microbiome and the shifting surrounding aquatic environment in fisheries and aquaculture: A review. Environ. Res. 2022, 214, 114202. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Guo, H.P.; Zhang, D.M. Effects of different culture patterns on the intestinal microbiota of Litopenaeus vannamei. J. Fish. Sci. 2021, 45, 221–234. [Google Scholar]
- Zhan, P.F.; Li, L.; Tang, C.; Yang, P.; Hu, H.W.; Penuelas, J.; Tong, C. Successional patterns of bacterial communities and their functions in shrimp aquaculture pond water across farming phases. Aquac. Res. 2021, 52, 6322–6337. [Google Scholar] [CrossRef]
- Duc, H.L.; Nam Trung, N.; Oanh Hoang Thi, D.; Steinert, G.; Thanh Trung, T.; Trang Huyen, V.; Sipkema, D.; Ha Hoang, C. Characterization of bacterial community in the gut of Penaeus monodon and its culture water in shrimp ponds. Turk. J. Fish. Aquat. Sci. 2019, 19, 977–986. [Google Scholar]
- He, Z.; Pan, L.; Zhang, M.; Zhang, M.; Huang, F.; Gao, S. Metagenomic comparison of structure and function of microbial community between water, effluent and shrimp intestine of higher place Litopenaeus vannamei ponds. J. Appl. Microbiol. 2020, 129, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Wang, Y.; Yang, L.K.; Kong, Q.; Zhang, H.X. Microbial degradation mechanisms of surface petroleum contaminated seawater in a typical oil trading port. Environ. Pollut. 2023, 324, 121420. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidou, A.; Gutleben, J.; Versluis, D.; Forgiarini, F.; van Passel, M.W.J.; Ingham, C.J.; Smidt, H.; Sipkema, D. Comparative genomic analysis of Flavobacteriaceae: Insights into carbohydrate metabolism, gliding motility and secondary metabolite biosynthesis. BMC Genom. 2020, 21, 569. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, M.; Cho, J.C. Rhodobacteraceae are prevalent and ecologically crucial bacterial members in marine biofloc aquaculture. J. Microbiol. 2024, 62, 985–997. [Google Scholar] [CrossRef]
- Cao, H.Z.; Chen, D.D.; Guo, L.F.; Jv, R.; Xin, Y.T.; Mo, W.; Wang, C.; Li, P.-F.; Wang, H. Effects of Bacillus subtilis on growth performance and intestinal flora of Penaeus vannamei. Aquac. Rep. 2022, 23, 101070. [Google Scholar] [CrossRef]
- Hou, D.W.; Huang, Z.J.; Zeng, S.Z.; Liu, J.; Wei, D.D.; Deng, X.S.; Weng, S.-P.; Yan, Q.-Y.; He, J.G. Intestinal bacterial signatures of white feces syndrome in shrimp. Appl. Microbiol. Biotechnol. 2018, 102, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Xu, W.J.; Xu, Y.; Su, H.C.; Hu, X.J.; Cao, Y.C.; Zhang, J.S.; Wen, G.L. Impact of organic carbons addition on the enrichment culture of nitrifying biofloc from aquaculture water: Process, efficiency, and microbial community. Microorganisms 2024, 12, 703. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhu, Y.L.; Wu, D.S.; Wang, Z.Y.; Wang, Y.J.; Wang, G.; Zhou, X.; Sun, H.W. Effect of free nitrous acid on nitritation process: Microbial community, inhibitory kinetics, and functional biomarker. Bioresour. Technol. 2023, 371, 128595. [Google Scholar] [CrossRef] [PubMed]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Sun, W.F.; Huang, X.S.; Hu, X.J.; Wen, G.L.; Cao, Y.C.; Zhang, J.S. Detection and analysis of Enterocytozoon hepatopenaei (EHP), Vibrio parahaemolyticus acute hepatopancreatic necrosis disease (VPAHPND) and shrimp hemocyte iridescent virus (SHIV) from Litopenaeus vannamei in coastal areas of Guangdong Province. J. South. Agric. Sci. 2019, 50, 2343–2349. [Google Scholar]
- Muthu, C.M.M.; Vickram, A.S.; Sowndharya, B.B.; Saravanan, A.; Kamalesh, R.; Dinakarkumar, Y. A comprehensive review on the utilization of probiotics in aquaculture towards sustainable shrimp farming. Fish Shellfish. Immun. 2024, 147, 109459. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Wang, M.Y.; Tian, X.L.; Liu, L.Z.; Li, H.D.; Li, L.; Dong, S.L.; Xue, L. Effects of three different lactic acid bacteria on the growth, disease resistance, and intestinal microbiota structure of Litopenaeus vannamei. J. Ocean U China 2021, 51, 44–54. [Google Scholar]
- Hu, X.J.; Xu, Y.; Su, H.C.; Xu, W.J.; Wen, G.L.; Xu, C.W.; Yang, K.; Zhang, S.; Cao, Y.C. Effect of a Bacillus probiotic compound on Penaeus vannamei survival, water quality, and microbial communities. Fishes 2023, 8, 362. [Google Scholar] [CrossRef]
- Peng, F.Q.; Gao, Y.X.; Zhu, X.; Pang, Q.Q.; Wang, L.M.; Xu, W.W.; Yu, J.H.; Gao, P.C.; Huang, J.-X.; Cui, Y.-B. Removal of high-strength ammonia nitrogen in biofilters: Nitrifying bacterial community compositions and their effects on nitrogen transformation. Water 2020, 12, 712. [Google Scholar] [CrossRef]
- Patil, P.K.; Geetha, R.; Ravisankar, T.; Avunje, S.; Solanki, H.G.; Abraham, T.J.; Vinoth, S.P.; Jithendran, K.P.; Alavandi, S.V.; Vijayan, K.K. Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 2021, 533, 736231. [Google Scholar] [CrossRef]
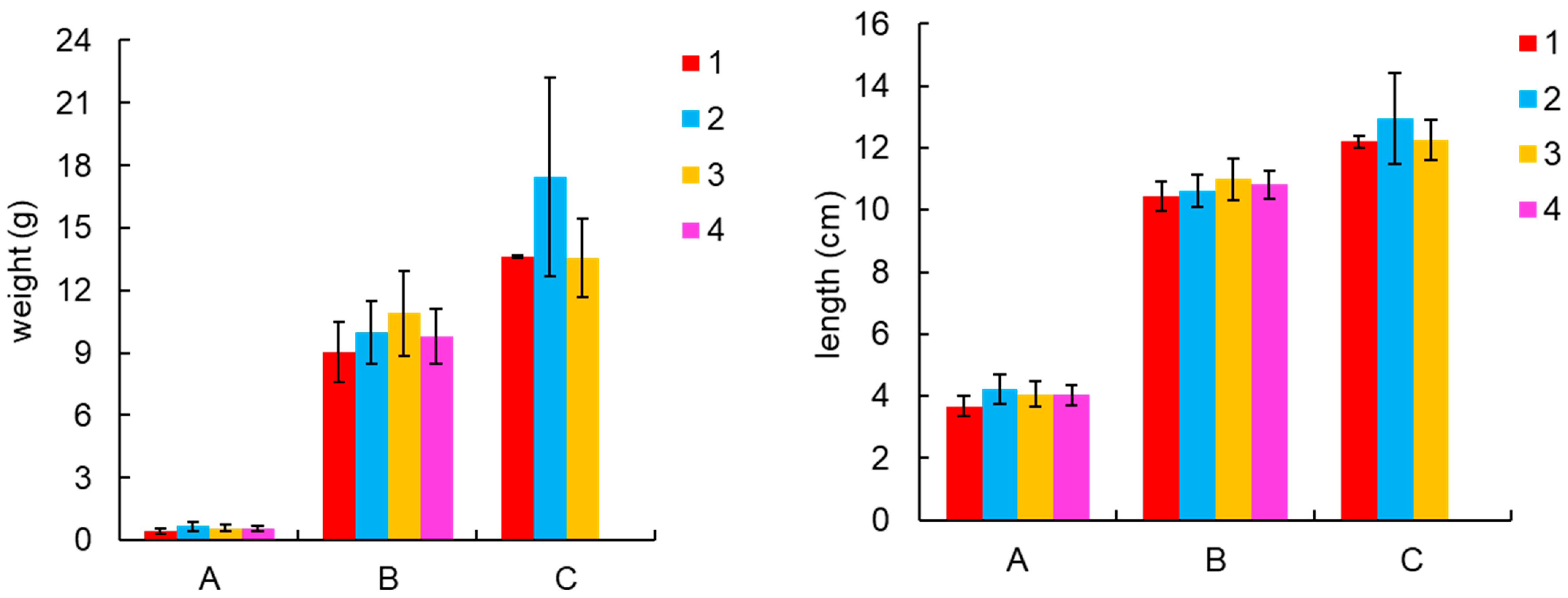
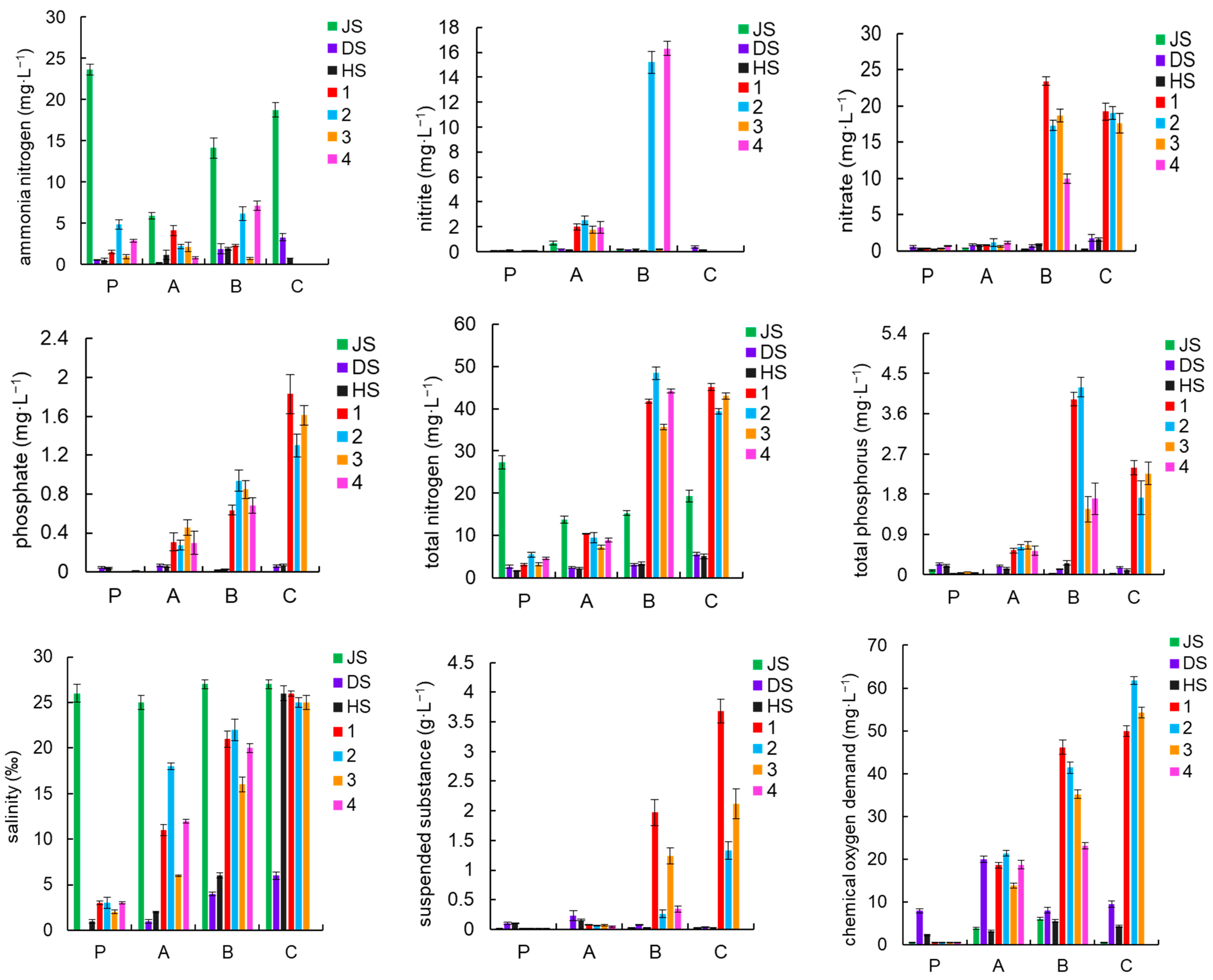

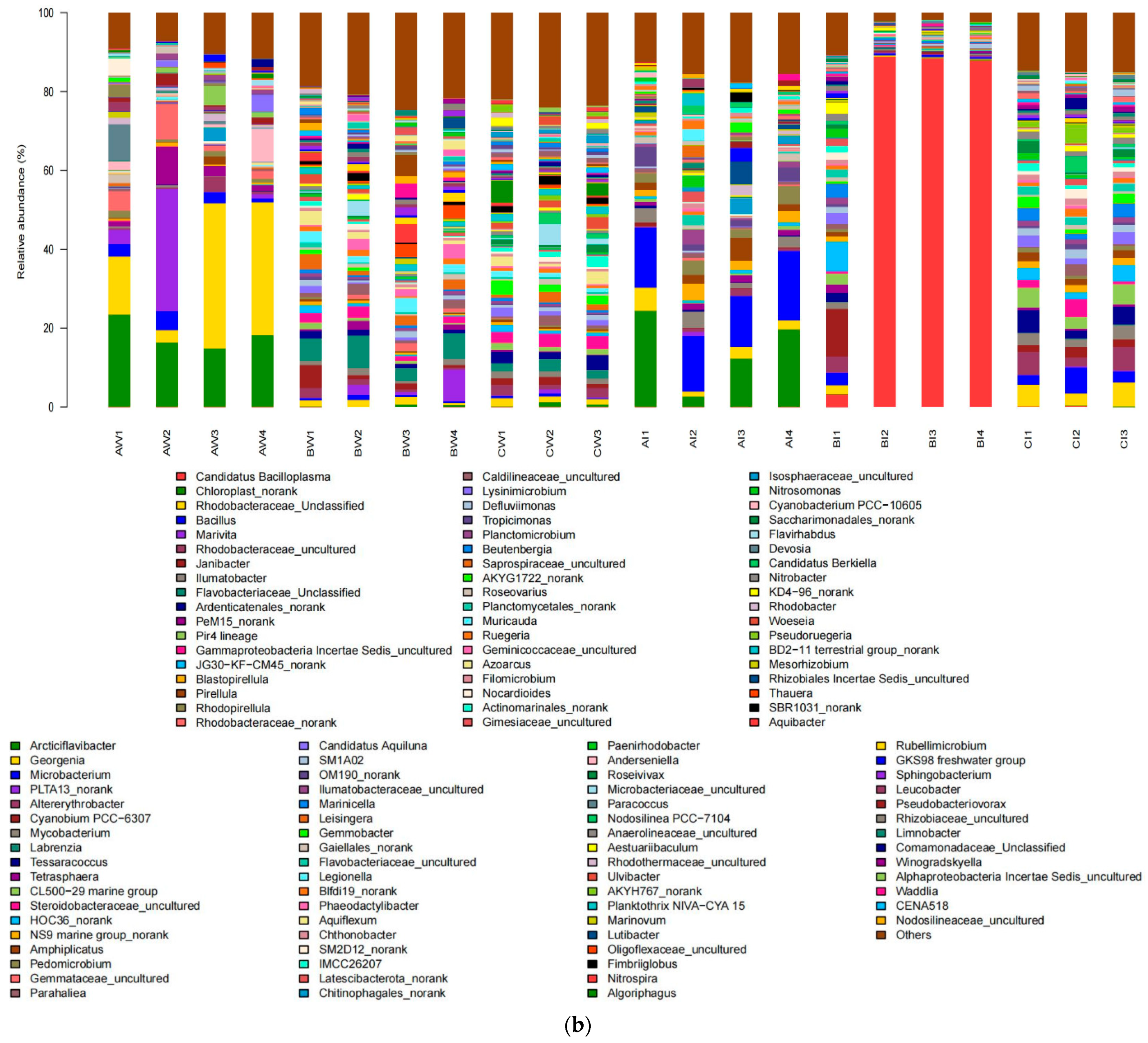
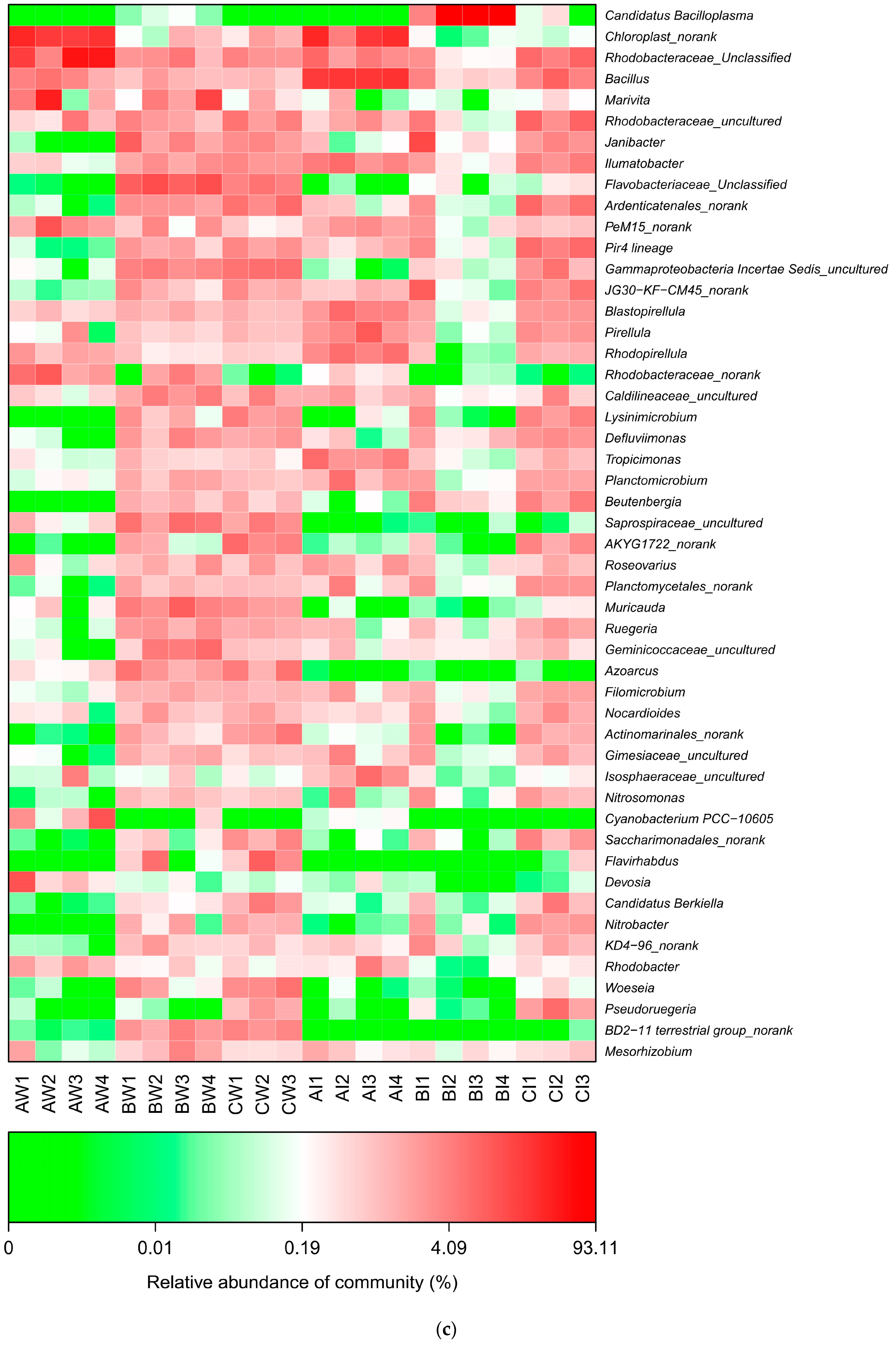

| Items | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Production(kg·m−2) | 2.50 | 2.62 | 2.70 | 2.12 |
| Specific Growth Rate (%) | 1.34 | 1.36 | 1.35 | 1.36 |
| Feed Conversion Ratio (%) | 4.79 | 4.53 | 4.38 | / |
| Breeding Stage | No. | AHPND(W) | EHP(W) | WSSV(W) | DIV1(W) | AHPND(S) | EHP(S) | WSSV(S) | DIV1(S) |
|---|---|---|---|---|---|---|---|---|---|
| A | JS | / | nd | / | nd | - | - | nd | - |
| HS | / | nd | / | nd | - | - | nd | - | |
| DS | nd | nd | nd | nd | - | - | nd | - | |
| 1 | / | nd | nd | nd | nd | nd | nd | nd | |
| 2 | / | nd | nd | nd | / | nd | nd | nd | |
| 3 | nd | nd | / | nd | / | nd | nd | nd | |
| 4 | nd | nd | / | nd | nd | nd | nd | nd | |
| B | JS | / | / | nd | nd | - | - | nd | - |
| HS | / | / | nd | nd | - | - | nd | - | |
| DS | / | / | nd | nd | - | - | nd | - | |
| 1 | / | / | nd | nd | nd | / | nd | nd | |
| 2 | nd | / | nd | nd | / | / | nd | nd | |
| 3 | nd | / | / | nd | / | / | nd | nd | |
| 4 | 78.93 | 1.09 × 103 | / | nd | 2.18 × 103 | 7.88 × 104 | nd | nd | |
| C | JS | nd | nd | nd | nd | - | - | nd | - |
| HS | nd | nd | nd | nd | - | - | nd | - | |
| DS | nd | nd | nd | nd | - | - | nd | - | |
| 1 | nd | nd | nd | nd | nd | nd | nd | nd | |
| 2 | nd | nd | / | nd | nd | nd | nd | nd | |
| 3 | nd | nd | / | nd | nd | nd | nd | nd |
| Model a | Unstandardised Coefficients | Standardised Coefficients | T | Sig. | Collinearity Statistics | |||
|---|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Tolerance | VIF | ||||
| (Constant) | 3.146 | 0.029 | 109.116 | 0.000 | ||||
| EHP-W | −1.4 × 10−6 | 0.006 | −0.871 | −6.145 | 0.000 | 0.768 | 1.302 | |
| Ardenticatenales_norank-W | 0.036 | 0.006 | 1.001 | 5.630 | 0.001 | 0.488 | 2.050 | |
| Marivita-W | 0.029 | 0.008 | 0.684 | 3.586 | 0.009 | 0.424 | 2.361 | |
| a. Dependent: production | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Su, H.; Su, L.; Cao, Y.; Wen, G.; Xu, Y.; Shen, B.; Wu, S.; Su, Y.; Hu, X. Variation of Microorganisms and Water Quality, and Their Impacts on the Production of Penaeus vannamei in Small-Scale Greenhouse Ponds. Microorganisms 2025, 13, 546. https://doi.org/10.3390/microorganisms13030546
Wu S, Su H, Su L, Cao Y, Wen G, Xu Y, Shen B, Wu S, Su Y, Hu X. Variation of Microorganisms and Water Quality, and Their Impacts on the Production of Penaeus vannamei in Small-Scale Greenhouse Ponds. Microorganisms. 2025; 13(3):546. https://doi.org/10.3390/microorganisms13030546
Chicago/Turabian StyleWu, Siyu, Haochang Su, Lei Su, Yucheng Cao, Guoliang Wen, Yu Xu, Bin Shen, Shanshan Wu, Yuting Su, and Xiaojuan Hu. 2025. "Variation of Microorganisms and Water Quality, and Their Impacts on the Production of Penaeus vannamei in Small-Scale Greenhouse Ponds" Microorganisms 13, no. 3: 546. https://doi.org/10.3390/microorganisms13030546
APA StyleWu, S., Su, H., Su, L., Cao, Y., Wen, G., Xu, Y., Shen, B., Wu, S., Su, Y., & Hu, X. (2025). Variation of Microorganisms and Water Quality, and Their Impacts on the Production of Penaeus vannamei in Small-Scale Greenhouse Ponds. Microorganisms, 13(3), 546. https://doi.org/10.3390/microorganisms13030546





