Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract
Abstract
1. Introduction
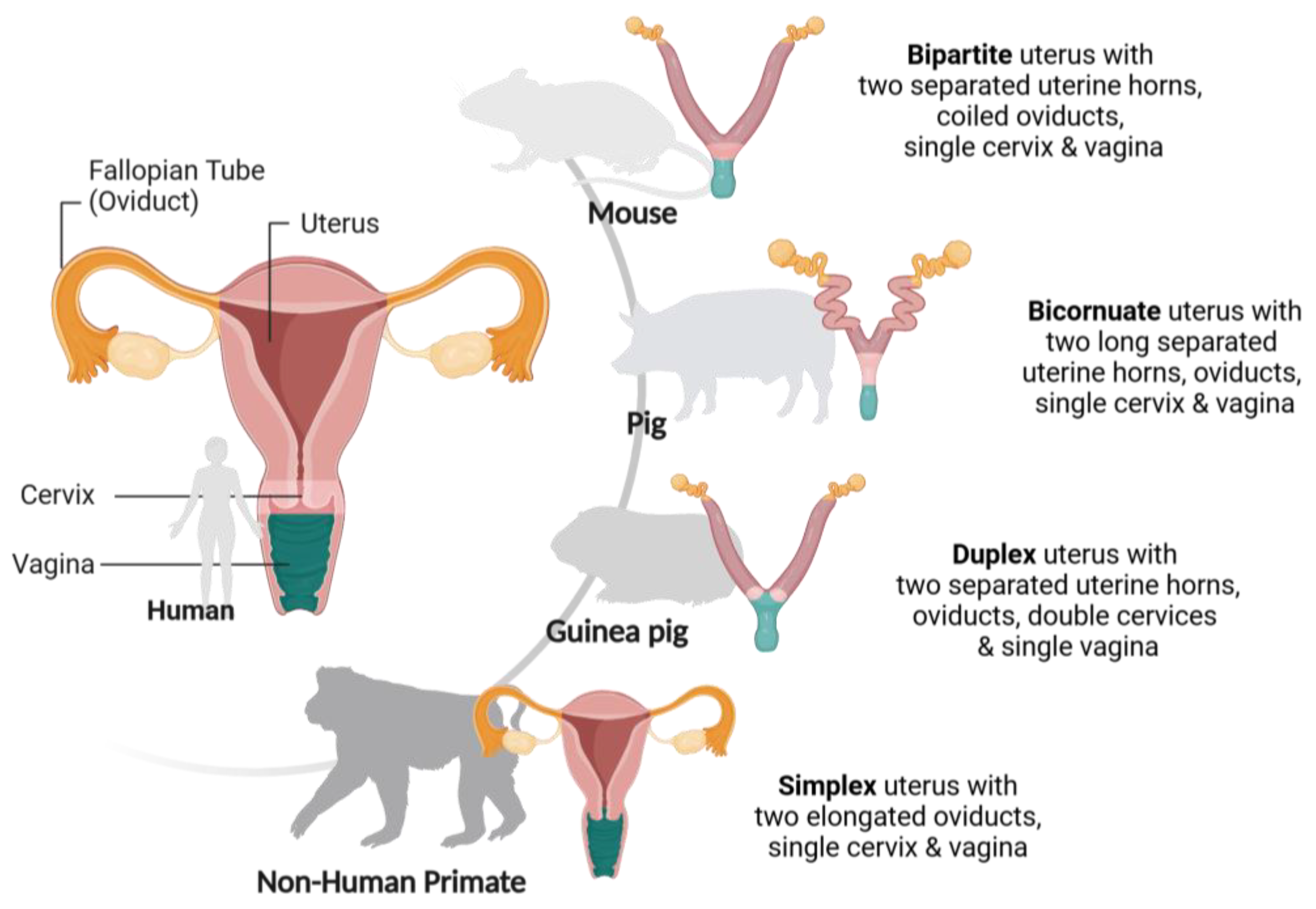
2. In Vivo Models
2.1. Mouse Models
2.2. Porcine (Pig) Models
2.3. Guinea Pig Models
2.4. Non-Human Primate Models
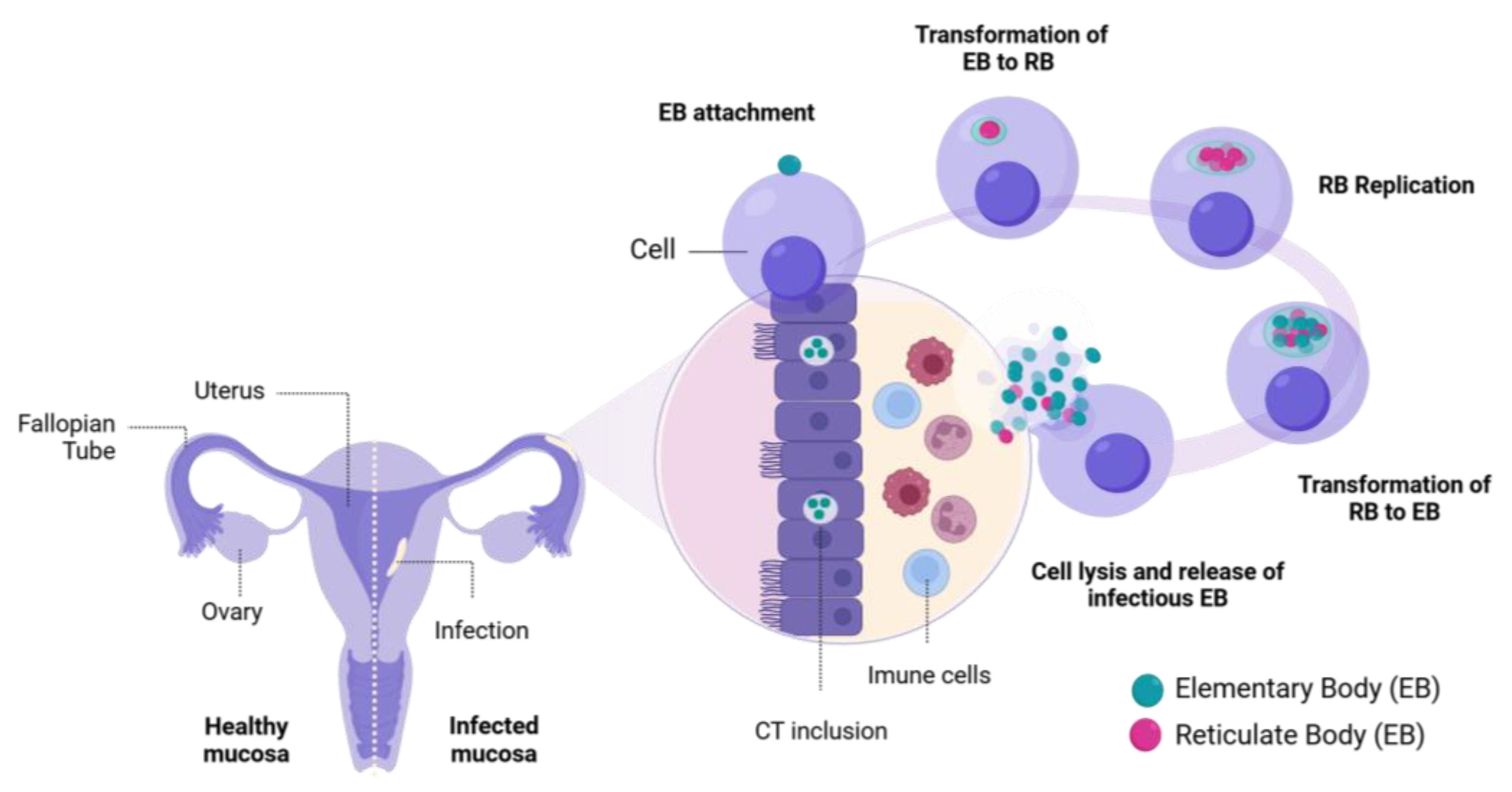
3. In Vitro Models
3.1. Cell Sources
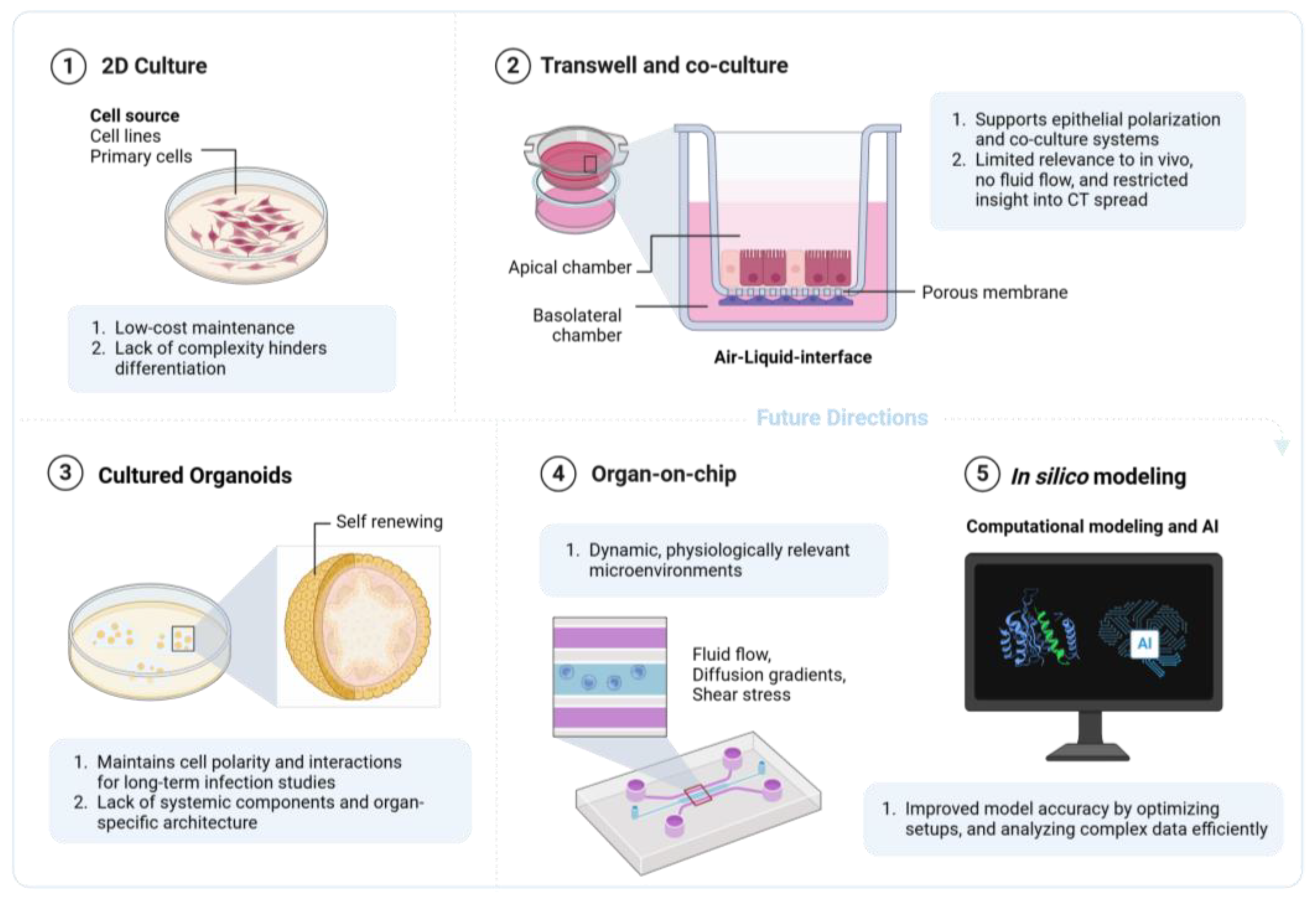
3.2. Two-Dimensional Models
3.2.1. Traditional Mono-Cell Culture
3.2.2. Membrane Inserts, Including Corning Transwell® and the Greiner ThinCert™
3.2.3. Co-Culture
3.3. Three-Dimensional Models and Organoids
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jury, B.; Fleming, C.; Huston, W.M.; Luu, L.D.W. Molecular Pathogenesis of Chlamydia trachomatis. Front. Cell. Infect. Microbiol. 2023, 13, 1281823. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, K.; Vollmuth, N.; Rudel, T. Intracellular Lifestyle of Chlamydia trachomatis and Host–Pathogen Interactions. Nat. Rev. Microbiol. 2023, 21, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Chlamydia; World Health Organization. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chlamydia (accessed on 20 September 2024).
- Chauhan, V.; Shah, M.; Thakkar, S.; Patel, S.; Marfatia, Y. Sexually Transmitted Infections in Women: A Correlation of Clinical and Laboratory Diagnosis in Cases of Vaginal Discharge Syndrome. Indian Dermatol. Online J. 2014, 5, 1. [Google Scholar] [CrossRef]
- Lane, A.B.; Decker, C.F. Chlamydia trachomatis Infections. Dis. A-Mon. 2016, 62, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, N.; Daniel, J.; Forsyth, S. The Risk of Pelvic Inflammatory Disease in Women Infected with Chlamydia (Chlamydia trachomatis): A Literature Review. Cureus 2024, 16, e66316. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, Z.W.; Hoenderboom, B.M.; Hoebe, C.J.P.A.; Dukers-Muijrers, N.H.T.M.; Götz, H.M.; Van Der Sande, M.A.B.; De Vries, H.J.C.; Den Hartog, J.E.; Morré, S.A.; Van Benthem, B.H.B. Reproductive Tract Complication Risks Following Chlamydia trachomatis Infections: A Long-Term Prospective Cohort Study from 2008 to 2022. Lancet Reg. Health—Eur. 2024, 45, 101027. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.; Luo, L.; Min, S.; Wang, L.; Peng, L.; Hou, Y.; He, P.; He, S.; Tang, S.; et al. Characterization of Genital Chlamydia trachomatis Infection among Women Attending Infertility and Gynecology Clinics in Hunan, China. BMC Infect. Dis. 2024, 24, 405. [Google Scholar] [CrossRef]
- Beatty, W.L.; Morrison, R.P.; Byrne, G.I. Persistent Chlamydiae: From Cell Culture to a Paradigm for Chlamydial Pathogenesis. Microbiol. Rev. 1994, 58, 686–699. [Google Scholar] [CrossRef]
- Kozusnik, T.; Adams, S.E.; Greub, G. Aberrant Bodies: An Alternative Metabolic Homeostasis Allowing Survivability? Microorganisms 2024, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Hocking, J.S.; Geisler, W.M.; Kong, F.Y.S. Update on the Epidemiology, Screening, and Management of Chlamydia trachomatis Infection. Infect. Dis. Clin. N. Am. 2023, 37, 267–288. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C. Screening for Chlamydia trachomatis Infections in Women. N. Engl. J. Med. 2017, 376, 765–773. [Google Scholar] [CrossRef]
- Poston, T.B. Advances in Vaccine Development for Chlamydia Trachomatis. Pathog. Dis. 2024, 82, ftae017. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Fang, C.; Li, Z. Insights into Innate Immune Cell Evasion by Chlamydia trachomatis. Front. Immunol. 2024, 15, 1289644. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Sousa, C.; Vale, N. Chlamydia trachomatis as a Current Health Problem: Challenges and Opportunities. Diagnostics 2022, 12, 1795. [Google Scholar] [CrossRef]
- Toumasis, P.; Vrioni, G.; Tsinopoulos, I.T.; Exindari, M.; Samonis, G. Insights into Pathogenesis of Trachoma. Microorganisms 2024, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Morré, S.A.; Rozendaal, L.; van Valkengoed, I.G.M.; Boeke, A.J.P.; van Voorst Vader, P.C.; Schirm, J.; de Blok, S.; van den Hoek, J.A.R.; van Doornum, G.J.J.; Meijer, C.J.L.M.; et al. Urogenital Chlamydia trachomatis Serovars in Men and Women with a Symptomatic or Asymptomatic Infection: An Association with Clinical Manifestations? J. Clin. Microbiol. 2000, 38, 2292–2296. [Google Scholar] [CrossRef] [PubMed]
- Taraktchoglou, M.; Pacey, A.A.; Turnbull, J.E.; Eley, A. Infectivity of Chlamydia trachomatis Serovar LGV but Not E Is Dependent on Host Cell Heparan Sulfate. Infect. Immun. 2001, 69, 968–976. [Google Scholar] [CrossRef]
- Marangoni, A.; Amadesi, S.; Djusse, M.E.; Foschi, C.; Gaspari, V.; Lazzarotto, T.; Gaibani, P. Whole Genome Sequencing of a Chlamydia trachomatis Strain Responsible for a Case of Rectal Lymphogranuloma Venereum in Italy. Curr. Issues Mol. Biol. 2023, 45, 1852–1859. [Google Scholar] [CrossRef]
- Nieuwenhuis, R.F.; Ossewaarde, J.M.; Götz, H.M.; Dees, J.; Thio, H.B.; Thomeer, M.G.J.; den Hollander, J.C.; Neumann, M.H.A.; van der Meijden, W.I. Resurgence of Lymphogranuloma Venereum in Western Europe: An Outbreak of Chlamydia trachomatis Serovar L2 Proctitis in The Netherlands among Men Who Have Sex with Men. Clin. Infect. Dis. 2004, 39, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Hu, J.; Billig, H. Toward Understanding Chlamydia Infection–Induced Infertility Caused by Dysfunctional Oviducts. J. Infect. Dis. 2013, 208, 707–709. [Google Scholar] [CrossRef][Green Version]
- Morrison, R.P. Differential Sensitivities of Chlamydia trachomatis Strains to Inhibitory Effects of Gamma Interferon. Infect. Immun. 2000, 68, 6038–6040. [Google Scholar] [CrossRef]
- Sturdevant, G.L.; Kari, L.; Gardner, D.J.; Olivares-Zavaleta, N.; Randall, L.B.; Whitmire, W.M.; Carlson, J.H.; Goheen, M.M.; Selleck, E.M.; Martens, C.; et al. Frameshift Mutations in a Single Novel Virulence Factor Alter the In Vivo Pathogenicity of Chlamydia trachomatis for the Female Murine Genital Tract. Infect. Immun. 2010, 78, 3660–3668. [Google Scholar] [CrossRef]
- Phillips, S.; Quigley, B.L.; Timms, P. Seventy Years of Chlamydia Vaccine Research—Limitations of the Past and Directions for the Future. Front. Microbiol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, T.; Shu, C.; Han, Z.; Huang, Y.; Wan, J.; Wang, L.; Sun, X. Diverse Animal Models for Chlamydia Infections: Unraveling Pathogenesis through the Genital and Gastrointestinal Tracts. Front. Microbiol. 2024, 15, 1386343. [Google Scholar] [CrossRef]
- Moysidou, C.-M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Sessa, R. Better In Vitro Tools for Exploring Chlamydia trachomatis Pathogenesis. Life 2022, 12, 1065. [Google Scholar] [CrossRef]
- Zhong, G. Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract—A Two-Hit Hypothesis. Trends Microbiol. 2018, 26, 611–623. [Google Scholar] [CrossRef] [PubMed]
- De La Maza, L.M.; Zhong, G.; Brunham, R.C. Update on Chlamydia trachomatis Vaccinology. Clin. Vaccine Immunol. 2017, 24, e00543-16. [Google Scholar] [CrossRef]
- De Clercq, E.; Kalmar, I.; Vanrompay, D. Animal Models for Studying Female Genital Tract Infection with Chlamydia trachomatis. Infect. Immun. 2013, 81, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.H.; Behar, A.R.; Snider, T.A.; Allen, N.A.; Lutter, E.I. Comparison of Murine Cervicovaginal Infection by Chlamydial Strains: Identification of Extrusions Shed In Vivo. Front. Cell. Infect. Microbiol. 2017, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.H.; Sigar, I.M.; Schripsema, J.H.; Denman, C.J.; Bowlin, A.K.; Myers, G.A.S.; Rank, R.G. Strain and Virulence Diversity in the Mouse Pathogen Chlamydia Muridarum. Infect. Immun. 2009, 77, 3284–3293. [Google Scholar] [CrossRef] [PubMed]
- Dockterman, J.; Coers, J. Immunopathogenesis of Genital Chlamydia Infection: Insights from Mouse Models. Pathog. Dis. 2021, 79, ftab012. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.M.; Ito, J.I.; Morré, S.A. Chlamydia trachomatis Serovar E Isolates from Patients with Different Clinical Manifestations Have Similar Courses of Infection in a Murine Model: Host Factors as Major Determinants of C. trachomatis Mediated Pathogenesis. J. Clin. Pathol. 2004, 57, 657–659. [Google Scholar] [CrossRef]
- Gondek, D.C.; Olive, A.J.; Stary, G.; Starnbach, M.N. CD4+ T Cells Are Necessary and Sufficient To Confer Protection against Chlamydia trachomatis Infection in the Murine Upper Genital Tract. J. Immunol. 2012, 189, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Sturdevant, G.L.; Caldwell, H.D. Innate Immunity Is Sufficient for the Clearance of Chlamydia trachomatis from the Female Mouse Genital Tract. Pathog. Dis. 2014, 72, 70–73. [Google Scholar] [CrossRef]
- Nguyen, N.D.N.T.; Olsen, A.W.; Lorenzen, E.; Andersen, P.; Hvid, M.; Follmann, F.; Dietrich, J. Parenteral Vaccination Protects against Transcervical Infection with Chlamydia trachomatis and Generate Tissue-Resident T Cells Post-Challenge. npj Vaccines 2020, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, T.; Melero, J.; Huang, Y.; Liu, Y.; Liu, Q.; He, C.; Nelson, D.E.; Zhong, G. The Genital Tract Virulence Factor pGP3 Is Essential for Chlamydia Muridarum Colonization in the Gastrointestinal Tract. Infect. Immun. 2018, 86, e00429-17. [Google Scholar] [CrossRef]
- Ramsey, K.H.; Schripsema, J.H.; Smith, B.J.; Wang, Y.; Jham, B.C.; O’Hagan, K.P.; Thomson, N.R.; Murthy, A.K.; Skilton, R.J.; Chu, P.; et al. Plasmid CDS5 Influences Infectivity and Virulence in a Mouse Model of Chlamydia trachomatis Urogenital Infection. Infect. Immun. 2014, 82, 3341–3349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. A Mucosal Vaccine against Chlamydia trachomatis Generates Two Waves of Protective Memory T Cells. Science 2015, 348, aaa8205. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tifrea, D.F.; Zhong, G.; De La Maza, L.M. Transcervical Inoculation with Chlamydia trachomatis Induces Infertility in HLA-DR4 Transgenic and Wild-Type Mice. Infect. Immun. 2018, 86, e00722-17. [Google Scholar] [CrossRef]
- Gros, P.; Casanova, J.-L. Reconciling Mouse and Human Immunology at the Altar of Genetics. Annu. Rev. Immunol. 2023, 41, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S.; Greub, G.; Nardelli-Haefliger, D.; Baud, D. Genital Chlamydia trachomatis: Understanding the Roles of Innate and Adaptive Immunity in Vaccine Research. Clin. Microbiol. Rev. 2014, 27, 346–370. [Google Scholar] [CrossRef] [PubMed]
- Käser, T.; Cnudde, T.; Hamonic, G.; Rieder, M.; Pasternak, J.A.; Lai, K.; Tikoo, S.K.; Wilson, H.L.; Meurens, F. Porcine Retinal Cell Line VIDO R1 and Chlamydia Suis to Modelize Ocular Chlamydiosis. Vet. Immunol. Immunopathol. 2015, 166, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Rahman, K.S.; Kick, A.R.; Cortes, L.M.; Robertson, J.; Kaltenboeck, B.; Gerdts, V.; O’Connell, C.M.; Poston, T.B.; Zheng, X.; et al. Mucosal Vaccination with UV-Inactivated Chlamydia Suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-Γ+ Effector Memory Cells. Vaccines 2020, 8, 353. [Google Scholar] [CrossRef]
- Häcker, G. Chlamydia in Pigs: Intriguing Bacteria Associated with Sub-Clinical Carriage and Clinical Disease, and with Zoonotic Potential. Front. Cell Dev. Biol. 2024, 12, 1301892. [Google Scholar] [CrossRef] [PubMed]
- Käser, T.; Renois, F.; Wilson, H.L.; Cnudde, T.; Gerdts, V.; Dillon, J.-A.R.; Jungersen, G.; Agerholm, J.S.; Meurens, F. Contribution of the Swine Model in the Study of Human Sexually Transmitted Infections. Infect. Genet. Evol. 2018, 66, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Käser, T.; Pasternak, J.A.; Delgado-Ortega, M.; Hamonic, G.; Lai, K.; Erickson, J.; Walker, S.; Dillon, J.R.; Gerdts, V.; Meurens, F. Chlamydia Suis and Chlamydia trachomatis Induce Multifunctional CD4 T Cells in Pigs. Vaccine 2017, 35, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, E.; Follmann, F.; Jungersen, G.; Agerholm, J.S. A Review of the Human vs. Porcine Female Genital Tract and Associated Immune System in the Perspective of Using Minipigs as a Model of Human Genital Chlamydia Infection. Vet. Res. 2015, 46, 116. [Google Scholar] [CrossRef]
- Erneholm, K.; Lorenzen, E.; Bøje, S.; Olsen, A.W.; Andersen, P.; Cassidy, J.P.; Follmann, F.; Jensen, H.E.; Agerholm, J.S. Genital Tract Lesions in Sexually Mature Göttingen Minipigs during the Initial Stages of Experimental Vaginal Infection with Chlamydia trachomatis Serovar D. BMC Vet. Res. 2016, 12, 200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorenzen, E.; Follmann, F.; Secher, J.O.; Goericke-Pesch, S.; Hansen, M.S.; Zakariassen, H.; Olsen, A.W.; Andersen, P.; Jungersen, G.; Agerholm, J.S. Intrauterine Inoculation of Minipigs with Chlamydia trachomatis during Diestrus Establishes a Longer Lasting Infection Compared to Vaginal Inoculation during Estrus. Microbes Infect. 2017, 19, 334–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Clercq, E.; Van Gils, M.; Schautteet, K.; Devriendt, B.; Kiekens, C.; Chiers, K.; Van Den Broeck, W.; Cox, E.; Dean, D.; Vanrompay, D. Chlamydia trachomatis L2c Infection in a Porcine Model Produced Urogenital Pathology and Failed to Induce Protective Immune Responses Against Re-Infection. Front. Immunol. 2020, 11, 555305. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.; Li, J.; Jin, Y.; Qiu, Z. Application of the Transgenic Pig Model in Biomedical Research: A Review. Front. Cell Dev. Biol. 2022, 10, 1031812. [Google Scholar] [CrossRef] [PubMed]
- Rank, R.G.; Sanders, M.M. Pathogenesis of Endometritis and Salpingitis in a Guinea Pig Model of Chlamydial Genital Infection. Am. J. Pathol. 1992, 140, 927. [Google Scholar]
- Mount, D.T.; Bigazzi, P.E.; Barron, A.L. Experimental Genital Infection of Male Guinea Pigs with the Agent of Guinea Pig Inclusion Conjunctivitis and Transmission to Females. Infect. Immun. 1973, 8, 925–930. [Google Scholar] [CrossRef]
- Rank, R.G.; Bowlin, A.K.; Reed, R.L.; Darville, T. Characterization of Chlamydial Genital Infection Resulting from Sexual Transmission from Male to Female Guinea Pigs and Determination of Infectious Dose. Infect. Immun. 2003, 71, 6148–6154. [Google Scholar] [CrossRef]
- Rank, R.G.; White, H.J.; Hough, A.J.; Pasley, J.N.; Barron, A.L. Effect of Estradiol on Chlamydial Genital Infection of Female Guinea Pigs. Infect. Immun. 1982, 38, 699–705. [Google Scholar] [CrossRef]
- Agrawal, T.; Vats, V.; Wallace, P.K.; Salhan, S.; Mittal, A. Role of Cervical Dendritic Cell Subsets, Co-Stimulatory Molecules, Cytokine Secretion Profile and Beta-Estradiol in Development of Sequalae to Chlamydia trachomatis Infection. Reprod. Biol. Endocrinol. 2008, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Carlin, D.J.; McMurray, D.N.; Hickey, A.J. The Guinea Pig as a Model of Infectious Diseases. Comp. Med. 2008, 58, 324–340. [Google Scholar] [PubMed]
- De Jonge, M.I.; Keizer, S.A.S.; El Moussaoui, H.M.; Van Dorsten, L.; Azzawi, R.; Van Zuilekom, H.I.; Peters, P.P.W.; Van Opzeeland, F.J.H.; Van Dijk, L.; Nieuwland, R.; et al. A Novel Guinea Pig Model of Chlamydia trachomatis Genital Tract Infection. Vaccine 2011, 29, 5994–6001. [Google Scholar] [CrossRef] [PubMed]
- Wali, S.; Gupta, R.; Yu, J.-J.; Mfuh, A.; Gao, X.; Guentzel, M.N.; Chambers, J.P.; Bakar, S.A.; Zhong, G.; Arulanandam, B.P. Guinea Pig Genital Tract Lipidome Reveals in Vivo and in Vitro Regulation of Phosphatidylcholine 16:0/18:1 and Contribution to Chlamydia trachomatis Serovar D Infectivity. Metabolomics 2016, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Wali, S.; Gupta, R.; Yu, J.; Lanka, G.K.K.; Chambers, J.P.; Guentzel, M.N.; Zhong, G.; Murthy, A.K.; Arulanandam, B.P. Chlamydial Protease-like Activity Factor Mediated Protection against C. Trachomatis in Guinea Pigs. Immunol. Cell Biol. 2017, 95, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Neuendorf, E.; Gajer, P.; Bowlin, A.K.; Marques, P.X.; Ma, B.; Yang, H.; Fu, L.; Humphrys, M.S.; Forney, L.J.; Myers, G.S.A.; et al. Chlamydia Caviae Infection Alters Abundance but Not Composition of the Guinea Pig Vaginal Microbiota. Pathog. Dis. 2015, 73, ftv019. [Google Scholar] [CrossRef] [PubMed]
- Adapen, C.; Réot, L.; Menu, E. Role of the Human Vaginal Microbiota in the Regulation of Inflammation and Sexually Transmitted Infection Acquisition: Contribution of the Non-Human Primate Model to a Better Understanding? Front. Reprod. Health 2022, 4, 992176. [Google Scholar] [CrossRef]
- Hargaden, M.; Singer, L. Chapter 20—Anatomy, Physiology, and Behavior. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012; pp. 575–602. [Google Scholar]
- Morrison, J.L.; Botting, K.J.; Darby, J.R.T.; David, A.L.; Dyson, R.M.; Gatford, K.L.; Gray, C.; Herrera, E.A.; Hirst, J.J.; Kim, B.; et al. Guinea Pig Models for Translation of the Developmental Origins of Health and Disease Hypothesis into the Clinic. J. Physiol. 2018, 596, 5535–5569. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S. Advantages and Limitations of Nonhuman Primates as Animal Models in Genetic Research on Complex Diseases. J. Med. Primatol. 1997, 26, 113–119. [Google Scholar] [CrossRef]
- Bell, J.D.; Bergin, I.L.; Schmidt, K.; Zochowski, M.K.; Aronoff, D.M.; Patton, D.L. Nonhuman Primate Models Used to Study Pelvic Inflammatory Disease Caused by Chlamydia trachomatis. Infect. Dis. Obstet. Gynecol. 2011, 2011, 675360. [Google Scholar] [CrossRef] [PubMed]
- Catalini, L.; Fedder, J. Characteristics of the Endometrium in Menstruating Species: Lessons Learned from the Animal Kingdom†. Biol. Reprod. 2020, 102, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Brokaw, A.; Furuta, A.M.; Coler, B.; Obregon-Perko, V.; Chahroudi, A.; Wang, H.-Y.; Permar, S.R.; Hotchkiss, C.E.; Golos, T.G.; et al. Non-Human Primate Models to Investigate Mechanisms of Infection-Associated Fetal and Pediatric Injury, Teratogenesis and Stillbirth. Front. Genet. 2021, 12, 680342. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.L.; Teng, A.; Randall, A.; Liang, X.; Felgner, P.L.; De La Maza, L.M. Whole Genome Identification of C. trachomatis Immunodominant Antigens after Genital Tract Infections and Effect of Antibiotic Treatment of Pigtailed Macaques. J. Proteom. 2014, 108, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Frazer, L.C.; O’Connell, C.M.; Tarantal, A.F.; Andrews, C.W.; O’Connor, S.L.; Russell, A.N.; Sullivan, J.E.; Poston, T.B.; Vallejo, A.N.; et al. Comparable Genital Tract Infection, Pathology, and Immunity in Rhesus Macaques Inoculated with Wild-Type or Plasmid-Deficient Chlamydia trachomatis Serovar D. Infect. Immun. 2015, 83, 4056–4067. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, S.A.; Aubert, R.D.; Morris, M.R.; Zhao, C.; Philips, C.; Khalil, G.M.; Deyounks, F.; Kelley, K.; Ritter, J.M.; Chen, C.Y.; et al. A Macaque Model for Rectal Lymphogranuloma Venereum and Non-Lymphogranuloma Venereum Chlamydia trachomatis: Impact on Rectal Simian/Human Immunodeficiency Virus Acquisition. Sex. Transm. Dis. 2017, 44, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.L.; Sweeney, Y.C.; Baldessari, A.E.; Cles, L.; Kari, L.; Sturdevant, G.L.; Yang, C.; Caldwell, H.D. The Chlamydia trachomatis Plasmid and CT135 Virulence Factors Are Not Essential for Genital Tract Infection or Pathology in Female Pig-Tailed Macaques. Infect. Immun. 2018, 86, e00121-18. [Google Scholar] [CrossRef] [PubMed]
- Randall, A.; Teng, A.; Liang, X.; Pal, S.; Tarantal, A.F.; Fike, J.; Barry, P.A.; De La Maza, L.M. A Primary Chlamydia trachomatis Genital Infection of Rhesus Macaques Identifies New Immunodominant B-Cell Antigens. PLoS ONE 2021, 16, e0250317. [Google Scholar] [CrossRef]
- Lorenzen, E.; Contreras, V.; Olsen, A.W.; Andersen, P.; Desjardins, D.; Rosenkrands, I.; Juel, H.B.; Delache, B.; Langlois, S.; Delaugerre, C.; et al. Multi-Component Prime-Boost Chlamydia trachomatis Vaccination Regimes Induce Antibody and T Cell Responses and Accelerate Clearance of Infection in a Non-Human Primate Model. Front. Immunol. 2022, 13, 1057375. [Google Scholar] [CrossRef]
- Koene, M.G.; Mulder, H.A.; Stockhofe-Zurwieden, N.; Kruijt, L.; Smits, M.A. Serum Protein Profiles as Potential Biomarkers for Infectious Disease Status in Pigs. BMC Vet. Res. 2012, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Cauvin, A.J.; Peters, C.; Brennan, F. Advantages and Limitations of Commonly Used Nonhuman Primate Species in Research and Development of Biopharmaceuticals. In The Nonhuman Primate in Nonclinical Drug Development and Safety Assessment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 379–395. [Google Scholar] [CrossRef]
- Schroeder, D.; Cook, J.; Hirsch, F.; Fenet, S.; Muthuswamy, V. (Eds.) Ethics Dumping: Case Studies from North-South Research Collaborations; SpringerBriefs in Research and Innovation Governance; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Kobayashi, A.; Behringer, R.R. Developmental Genetics of the Female Reproductive Tract in Mammals. Nat. Rev. Genet. 2003, 4, 969–980. [Google Scholar] [CrossRef]
- Nogueira, A.T.; Braun, K.M.; Carabeo, R.A. Characterization of the Growth of Chlamydia trachomatis in In Vitro-Generated Stratified Epithelium. Front. Cell. Infect. Microbiol. 2017, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Dolat, L.; Valdivia, R.H. A Renewed Tool Kit to Explore Chlamydia Pathogenesis: From Molecular Genetics to New Infection Models. F1000Research 2019, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Vitali, D.; Wessels, J.M.; Kaushic, C. Role of Sex Hormones and the Vaginal Microbiome in Susceptibility and Mucosal Immunity to HIV-1 in the Female Genital Tract. AIDS Res. Ther. 2017, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.M.; Felker, A.M.; Dupont, H.A.; Kaushic, C. The Relationship between Sex Hormones, the Vaginal Microbiome and Immunity in HIV-1 Susceptibility in Women. Dis. Models Mech. 2018, 11, dmm035147. [Google Scholar] [CrossRef] [PubMed]
- Plesniarski, A.; Siddik, A.B.; Su, R.-C. The Microbiome as a Key Regulator of Female Genital Tract Barrier Function. Front. Cell. Infect. Microbiol. 2021, 11, 790627. [Google Scholar] [CrossRef] [PubMed]
- Buckner, L.R.; Amedee, A.M.; Albritton, H.L.; Kozlowski, P.A.; Lacour, N.; McGowin, C.L.; Schust, D.J.; Quayle, A.J. Chlamydia trachomatis Infection of Endocervical Epithelial Cells Enhances Early HIV Transmission Events. PLoS ONE 2016, 11, e0146663. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.J.; Sztupecki, W.; Delayre-Orthez, C.; Rhazi, L.; Barbezier, N.; Depeint, F.; Anton, P.M. Complexification of In Vitro Models of Intestinal Barriers, A True Challenge for a More Accurate Alternative Approach. Int. J. Mol. Sci. 2023, 24, 3595. [Google Scholar] [CrossRef]
- Ekka, R.; Gutierrez, A.; Johnson, K.A.; Tan, M.; Sütterlin, C. Chlamydia trachomatis Induces Disassembly of the Primary Cilium to Promote the Intracellular Infection. PLoS Pathog. 2024, 20, e1012303. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell Lines: Valuable Tools or Useless Artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Suchland, R.J.; Geisler, W.M.; Stamm, W.E. Methodologies and Cell Lines Used for Antimicrobial Susceptibility Testing of Chlamydia spp. Antimicrob. Agents Chemother. 2003, 47, 636–642. [Google Scholar] [CrossRef]
- Malathi, J.; Shyamala, G.; Madhavan, H. Relative susceptibility of six continuous cell lines for cultivation of chlamydia trachomatis strains. Indian J. Med. Microbiol. 2004, 22, 169–171. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Wheelhouse, N.; Cameron, S.; McDonald, S.E.; Lee, K.-F.; Entrican, G.; Critchley, H.O.D.; Horne, A.W. Expression of Secretory Leukocyte Protease Inhibitor and Elafin in Human Fallopian Tube and in an In-Vitro Model of Chlamydia trachomatis Infection. Hum. Reprod. 2008, 24, 679–686. [Google Scholar] [CrossRef]
- Shaw, J.L.V.; Wills, G.S.; Lee, K.-F.; Horner, P.J.; McClure, M.O.; Abrahams, V.M.; Wheelhouse, N.; Jabbour, H.N.; Critchley, H.O.D.; Entrican, G.; et al. Chlamydia trachomatis Infection Increases Fallopian Tube PROKR2 via TLR2 and NFκB Activation Resulting in a Microenvironment Predisposed to Ectopic Pregnancy. Am. J. Pathol. 2011, 178, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Brown, J.K.; Campbell, L.L.; Koscielniak, M.; Oliver, C.; Wheelhouse, N.; Entrican, G.; McFee, S.; Wills, G.S.; McClure, M.O.; et al. Pelvic Chlamydial Infection Predisposes to Ectopic Pregnancy by Upregulating Integrin Β1 to Promote Embryo-Tubal Attachment. EBioMedicine 2018, 29, 159–165. [Google Scholar] [CrossRef] [PubMed]
- N’Gadjaga, M.D.; Perrinet, S.; Connor, M.G.; Bertolin, G.; Millot, G.A.; Subtil, A. Chlamydia trachomatis Development Requires Both Host Glycolysis and Oxidative Phosphorylation but Has Only Minor Effects on These Pathways. J. Biol. Chem. 2022, 298, 102338. [Google Scholar] [CrossRef]
- Walker, F.C.; Derré, I. Contributions of Diverse Models of the Female Reproductive Tract to the Study of Chlamydia trachomatis-Host Interactions. Curr. Opin. Microbiol. 2024, 77, 102416. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, N.; Tyurin-Kuzmin, P.; Karagyaur, M.; Akopyan, Z.; Kulebyakin, K. Practical Use of Immortalized Cells in Medicine: Current Advances and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 12716. [Google Scholar] [CrossRef] [PubMed]
- Goodspeed, A.; Heiser, L.M.; Gray, J.W.; Costello, J.C. Tumor-Derived Cell Lines as Molecular Models of Cancer Pharmacogenomics. Mol. Cancer Res. 2016, 14, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From Donor to the Lab: A Fascinating Journey of Primary Cell Lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmueller, U.; Mann, M. Comparative Proteomic Phenotyping of Cell Lines and Primary Cells to Assess Preservation of Cell Type-Specific Functions. Mol. Cell Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef]
- Bläuer, M.; Heinonen, P.K.; Martikainen, P.M.; Tomás, E.; Ylikomi, T. A Novel Organotypic Culture Model for Normal Human Endometrium: Regulation of Epithelial Cell Proliferation by Estradiol and Medroxyprogesterone Acetate. Hum. Reprod. 2005, 20, 864–871. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Ding, D.-C.; Chu, T.-Y. Estradiol and Progesterone Induced Differentiation and Increased Stemness Gene Expression of Human Fallopian Tube Epithelial Cells. J. Cancer 2019, 10, 3028–3036. [Google Scholar] [CrossRef]
- Mollerup, S.; Jørgensen, K.; Berge, G.; Haugen, A. Expression of Estrogen Receptors a and b in Human Lung Tissue and Cell Lines. Lung Cancer 2002, 37, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kaushic, C.; Murdin, A.D.; Underdown, B.J.; Wira, C.R. Chlamydia trachomatis Infection in the Female Reproductive Tract of the Rat: Influence of Progesterone on Infectivity and Immune Response. Infect. Immun. 1998, 66, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Hall, J.V. The Complexity of Interactions Between Female Sex Hormones and Chlamydia trachomatis Infections. Curr. Clin. Microbiol. Rep. 2019, 6, 67–75. [Google Scholar] [CrossRef]
- McGlade, E.A.; Miyamoto, A.; Winuthayanon, W. Progesterone and Inflammatory Response in the Oviduct during Physiological and Pathological Conditions. Cells 2022, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.D.; Shen, Y.; Ma, Q.; Yang, K.; Hannum, D.F.; Jones, A.; Machlin, J.; Randolph, J.F.; Smith, Y.R.; Schon, S.B.; et al. Cellular Heterogeneity of Human Fallopian Tubes in Normal and Hydrosalpinx Disease States Identified Using scRNA-Seq. Dev. Cell 2022, 57, 914–929.e7. [Google Scholar] [CrossRef] [PubMed]
- Barrila, J.; Crabbé, A.; Yang, J.; Franco, K.; Nydam, S.D.; Forsyth, R.J.; Davis, R.R.; Gangaraju, S.; Ott, C.M.; Coyne, C.B.; et al. Modeling Host-Pathogen Interactions in the Context of the Microenvironment: Three-Dimensional Cell Culture Comes of Age. Infect. Immun. 2018, 86, e00282-18. [Google Scholar] [CrossRef]
- Steube, K.G.; Koelz, A.-L.; Drexler, H.G. Identification and Verification of Rodent Cell Lines by Polymerase Chain Reaction. Cytotechnology 2008, 56, 49–56. [Google Scholar] [CrossRef][Green Version]
- Shay, J.W.; Wright, W.E. Hayflick, His Limit, and Cellular Ageing. Nat. Rev. Mol. Cell Biol. 2000, 1, 72–76. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Beier, F.; Gusmao, E.G.; Koch, C.M.; Hummel, S.; Charapitsa, I.; Joussen, S.; Benes, V.; Brümmendorf, T.H.; Reid, G.; et al. Replicative Senescence Is Associated with Nuclear Reorganization and with DNA Methylation at Specific Transcription Factor Binding Sites. Clin. Epigenet. 2015, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M. Cellular Aging beyond Cellular Senescence: Markers of Senescence Prior to Cell Cycle Arrest In Vitro and In Vivo. Aging Cell 2021, 20, e13338. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The Metabolic Roots of Senescence: Mechanisms and Opportunities for Intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef]
- Duell, B.L.; Cripps, A.W.; Schembri, M.A.; Ulett, G.C. Epithelial Cell Coculture Models for Studying Infectious Diseases: Benefits and Limitations. BioMed Res. Int. 2011, 2011, 852419. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Dawney, N.S.; Cammarota, C.; Jia, Q.; Shipley, A.; Glichowski, J.A.; Vasandani, M.; Finegan, T.M.; Bergstralh, D.T. A Novel Tool for the Unbiased Characterization of Epithelial Monolayer Development in Culture. Mol. Biol. Cell 2023, 34, ar25. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.H.; Raulston, J.E.; Wyrick, P.B. Protein Disulfide Isomerase, a Component of the Estrogen Receptor Complex, Is Associated with Chlamydia trachomatis Serovar E Attached to Human Endometrial Epithelial Cells. Infect. Immun. 2002, 70, 3413–3418. [Google Scholar] [CrossRef]
- Mihalko, E.P.; Brown, A.C. Material Strategies for Modulating Epithelial to Mesenchymal Transitions. ACS Biomater. Sci. Eng. 2018, 4, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Zadora, P.K.; Chumduri, C.; Imami, K.; Berger, H.; Mi, Y.; Selbach, M.; Meyer, T.F.; Gurumurthy, R.K. Integrated Phosphoproteome and Transcriptome Analysis Reveals Chlamydia-Induced Epithelial-to-Mesenchymal Transition in Host Cells. Cell Rep. 2019, 26, 1286–1302.e8. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, F.A.A.; Morgan, F.L.C.; Roumans, N.; Schumacher, A.; Slaats, G.G.; Moroni, L.; LaPointe, V.L.S.; Baker, M.B. Soft, Dynamic Hydrogel Confinement Improves Kidney Organoid Lumen Morphology and Reduces Epithelial–Mesenchymal Transition in Culture. Adv. Sci. 2022, 9, 2200543. [Google Scholar] [CrossRef] [PubMed]
- Hagelaars, M.J.; Yousef Yengej, F.A.; Verhaar, M.C.; Rookmaaker, M.B.; Loerakker, S.; Bouten, C.V.C. Substrate Stiffness Determines the Establishment of Apical-Basal Polarization in Renal Epithelial Cells but Not in Tubuloid-Derived Cells. Front. Bioeng. Biotechnol. 2022, 10, 820930. [Google Scholar] [CrossRef]
- Abdul Halim, M.S.; Dyson, J.M.; Gong, M.M.; O’Bryan, M.K.; Nosrati, R. Fallopian Tube Rheology Regulates Epithelial Cell Differentiation and Function to Enhance Cilia Formation and Coordination. Nat. Commun. 2024, 15, 7411. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, A.A.; Agu, R.U.; Massoud, E. Models for the Study of Nasal and Sinus Physiology in Health and Disease: A Review of the Literature. Laryngoscope Investig. Otolaryngol. 2017, 2, 398–409. [Google Scholar] [CrossRef]
- Reischl, J.; Prelle, K.; Schöl, H.; Neumüller, C.; Einspanier, R.; Sinowatz, F.; Wolf, E. Factors Affecting Proliferation and Dedifferentiation of Primary Bovine Oviduct Epithelial Cells In Vitro. Cell Tissue Res. 1999, 296, 371–383. [Google Scholar] [CrossRef]
- Taebnia, N.; Römling, U.; Lauschke, V.M. In Vitro and Ex Vivo Modeling of Enteric Bacterial Infections. Gut Microbes 2023, 15, 2158034. [Google Scholar] [CrossRef] [PubMed]
- Whitcutt, M.J.; Adler, K.B.; Wu, R. A Biphasic Chamber System for Maintaining Polarity of Differentiation of Culture Respiratory Tract Epithelial Cells. In Vitro Cell Dev. Biol. 1988, 24, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Mireles, M.; Kwarta, B.J.; Gaborski, T.R. Use of Porous Membranes in Tissue Barrier and Co-Culture Models. Lab Chip 2018, 18, 1671–1689. [Google Scholar] [CrossRef]
- Whiteley, J.T.; Fernandes, S.; Sharma, A.; Mendes, A.P.D.; Racha, V.; Benassi, S.K.; Marchetto, M.C. Reaching into the Toolbox: Stem Cell Models to Study Neuropsychiatric Disorders. Stem Cell Rep. 2022, 17, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Marie, M.A.; Sanderlin, E.J.; Yang, L.V. Transwell In Vitro Cell Migration and Invasion Assays. In Cell Viability Assays; Friedrich, O., Gilbert, D.F., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2023; Volume 2644, pp. 349–359. [Google Scholar] [CrossRef]
- Vinaiphat, A.; Charngkaew, K.; Thongboonkerd, V. More Complete Polarization of Renal Tubular Epithelial Cells by Artificial Urine. Cell Death Discov. 2018, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Yeste, J.; Illa, X.; Alvarez, M.; Villa, R. Engineering and Monitoring Cellular Barrier Models. J. Biol. Eng. 2018, 12, 18. [Google Scholar] [CrossRef]
- Hackett, T.-L.; Vasse, G.F.; Van Der Does, A.M.; Rae, B.; Nawijn, M.C.; Heijink, I.H. The Air–Liquid Interface Model. In 3D Lung Models for Regenerating Lung Tissue; Elsevier: Amsterdam, The Netherlands, 2022; pp. 51–72. [Google Scholar] [CrossRef]
- McQueen, B.E.; Kiatthanapaiboon, A.; Fulcher, M.L.; Lam, M.; Patton, K.; Powell, E.; Kollipara, A.; Madden, V.; Suchland, R.J.; Wyrick, P.; et al. Human Fallopian Tube Epithelial Cell Culture Model To Study Host Responses to Chlamydia trachomatis Infection. Infect. Immun. 2020, 88, e00105-20. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-Culture Systems and Technologies: Taking Synthetic Biology to the next Level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Mountcastle, S.E.; Cox, S.C.; Sammons, R.L.; Jabbari, S.; Shelton, R.M.; Kuehne, S.A. A Review of Co-Culture Models to Study the Oral Microenvironment and Disease. J. Oral Microbiol. 2020, 12, 1773122. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Jnana, A.; Murali, T.S. Modeling Microbial Community Networks: Methods and Tools for Studying Microbial Interactions. Microb. Ecol. 2024, 87, 56. [Google Scholar] [CrossRef]
- Barreto-Duran, E.; Szczepański, A.; Gałuszka-Bulaga, A.; Surmiak, M.; Siedlar, M.; Sanak, M.; Rajfur, Z.; Milewska, A.; Lenart, M.; Pyrć, K. The Interplay between the Airway Epithelium and Tissue Macrophages during the SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 991991. [Google Scholar] [CrossRef]
- Strand, D.W.; Hayward, S.W. Modeling Stromal-Epithelial Interactions in Disease Progression. Discov. Med. 2010, 9, 504–511. [Google Scholar] [PubMed]
- Hall, J.V.; Schell, M.; Dessus-Babus, S.; Moore, C.G.; Whittimore, J.D.; Sal, M.; Dill, B.D.; Wyrick, P.B. The Multifaceted Role of Oestrogen in Enhancing Chlamydia trachomatis Infection in Polarized Human Endometrial Epithelial Cells: Oestrogen Effects on C. trachomatis Infection. Cell. Microbiol. 2011, 13, 1183–1199. [Google Scholar] [CrossRef]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection. mBio 2019, 10, e01548-19. [Google Scholar] [CrossRef] [PubMed]
- Edwards, V.L.; McComb, E.; Gleghorn, J.P.; Forney, L.; Bavoil, P.M.; Ravel, J. Three-Dimensional Models of the Cervicovaginal Epithelia to Study Host–Microbiome Interactions and Sexually Transmitted Infections. Pathog. Dis. 2022, 80, ftac026. [Google Scholar] [CrossRef] [PubMed]
- Thayanithy, V.; O’Hare, P.; Wong, P.; Zhao, X.; Steer, C.J.; Subramanian, S.; Lou, E. A Transwell Assay That Excludes Exosomes for Assessment of Tunneling Nanotube-Mediated Intercellular Communication. Cell Commun. Signal. 2017, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Maji, S.; Lee, H. Engineering Hydrogels for the Development of Three-Dimensional In Vitro Models. Int. J. Mol. Sci. 2022, 23, 2662. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A Practical Guide to Hydrogels for Cell Culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The Current Status and Biomedical Applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.L.; Ang, L.T.; Loh, K.M. A Critical Look: Challenges in Differentiating Human Pluripotent Stem Cells into Desired Cell Types and Organoids. WIREs Dev. Biol. 2020, 9, e368. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Marino, F.; Salerno, L.; Cianflone, E.; Molinaro, C.; Salerno, N.; De Angelis, A.; Viglietto, G.; Urbanek, K.; Torella, D. From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish. Int. J. Mol. Sci. 2021, 22, 13180. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Huch, M. Disease Modelling in Human Organoids. Dis. Models Mech. 2019, 12, dmm039347. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Z.; Tang, Z.; Chen, Y.; Huang, M.; Liu, H.; Huang, W.; Ye, Q.; Jia, B. Research Progress, Challenges, and Breakthroughs of Organoids as Disease Models. Front. Cell Dev. Biol. 2021, 9, 740574. [Google Scholar] [CrossRef]
- Blutt, S.E.; Estes, M.K. Organoid Models for Infectious Disease. Annu. Rev. Med. 2022, 73, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Hoffmann, K.; Fritsche, K.; Brinkmann, V.; Mollenkopf, H.-J.; Thieck, O.; Teixeira Da Costa, A.R.; Braicu, E.I.; Sehouli, J.; Mangler, M.; et al. Chronic Chlamydia Infection in Human Organoids Increases Stemness and Promotes Age-Dependent CpG Methylation. Nat. Commun. 2019, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.C.; Boretto, M.; Rutkowski, M.R.; Vankelecom, H.; Derré, I. Murine Endometrial Organoids to Model Chlamydia Infection. Front. Cell. Infect. Microbiol. 2020, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Rajeeve, K.; Vollmuth, N.; Janaki-Raman, S.; Wulff, T.F.; Baluapuri, A.; Dejure, F.R.; Huber, C.; Fink, J.; Schmalhofer, M.; Schmitz, W.; et al. Reprogramming of Host Glutamine Metabolism during Chlamydia trachomatis Infection and Its Key Role in Peptidoglycan Synthesis. Nat. Microbiol. 2020, 5, 1390–1402. [Google Scholar] [CrossRef]
- Dolat, L.; Valdivia, R.H. An Endometrial Organoid Model of Interactions between Chlamydia and Epithelial and Immune Cells. J. Cell Sci. 2021, 134, jcs252403. [Google Scholar] [CrossRef]
- Dolat, L.; Carpenter, V.K.; Chen, Y.-S.; Suzuki, M.; Smith, E.P.; Kuddar, O.; Valdivia, R.H. Chlamydia Repurposes the Actin-Binding Protein EPS8 to Disassemble Epithelial Tight Junctions and Promote Infection. Cell Host Microbe 2022, 30, 1685–1700.e10. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; Gurumurthy, R.K.; Kumar, N.; Prakash, P.G.; Dhanraj, J.; Bayer, S.; Berger, H.; Kurian, S.M.; Drabkina, M.; Mollenkopf, H.-J.; et al. Modelling Chlamydia and HPV Co-Infection in Patient-Derived Ectocervix Organoids Reveals Distinct Cellular Reprogramming. Nat. Commun. 2022, 13, 1030. [Google Scholar] [CrossRef]
- Callan, T.; Woodcock, S.; Huston, W.M. Ascension of Chlamydia Is Moderated by Uterine Peristalsis and the Neutrophil Response to Infection. PLoS Comput. Biol. 2021, 17, e1009365. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Hwangbo, S.; Jang, S.; Jo, Y.K. Bioengineered Co-Culture of Organoids to Recapitulate Host-Microbe Interactions. Mater. Today Bio 2022, 16, 100345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Ergir, E.; Bachmann, B.; Redl, H.; Forte, G.; Ertl, P. Small Force, Big Impact: Next Generation Organ-on-a-Chip Systems Incorporating Biomechanical Cues. Front. Physiol. 2018, 9, 1417. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Abouleila, Y.; Si, L.; Ortega-Prieto, A.M.; Mummery, C.L.; Ingber, D.E.; Mashaghi, A. Human Organs-on-Chips for Virology. Trends Microbiol. 2020, 28, 934–946. [Google Scholar] [CrossRef]
- Fois, C.A.M.; Schindeler, A.; Valtchev, P.; Dehghani, F. Dynamic Flow and Shear Stress as Key Parameters for Intestinal Cells Morphology and Polarization in an Organ-on-a-Chip Model. Biomed. Microdevices 2021, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Ferraz, M.A.M.M.; Henning, H.H.W.; Costa, P.F.; Malda, J.; Melchels, F.P.; Wubbolts, R.; Stout, T.A.E.; Vos, P.L.A.M.; Gadella, B.M. Improved Bovine Embryo Production in an Oviduct-on-a-Chip System: Prevention of Poly-Spermic Fertilization and Parthenogenic Activation. Lab Chip 2017, 17, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Young, R.E.; Huh, D.D. Organ-on-a-Chip Technology for the Study of the Female Reproductive System. Adv. Drug Deliv. Rev. 2021, 173, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Francés-Herrero, E.; Lopez, R.; Hellström, M.; De Miguel-Gómez, L.; Herraiz, S.; Brännström, M.; Pellicer, A.; Cervelló, I. Bioengineering Trends in Female Reproduction: A Systematic Review. Hum. Reprod. Update 2022, 28, 798–837. [Google Scholar] [CrossRef]
- Heinz, E.; Tischler, P.; Rattei, T.; Myers, G.; Wagner, M.; Horn, M. Comprehensive in Silico Prediction and Analysis of Chlamydial Outer Membrane Proteins Reflects Evolution and Life Style of the Chlamydiae. BMC Genom. 2009, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Akinlotan, M.D.; Mallet, D.G.; Araujo, R.P. Mathematical Modelling of the Role of Mucosal Vaccine on the Within-Host Dynamics of Chlamydia trachomatis. J. Theor. Biol. 2020, 497, 110291. [Google Scholar] [CrossRef]
- Lees, J.A.; Russell, T.W.; Shaw, L.P.; Hellewell, J. Recent Approaches in Computational Modelling for Controlling Pathogen Threats. Life Sci. Alliance 2024, 7, e202402666. [Google Scholar] [CrossRef] [PubMed]
- Dillard, L.R.; Glass, E.M.; Lewis, A.L.; Thomas-White, K.; Papin, J.A. Metabolic Network Models of the Gardnerella Pangenome Identify Key Interactions with the Vaginal Environment. mSystems 2023, 8, e00689-22. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Zhang, X.; Zhang, X. Artificial Intelligence Applications in the Diagnosis and Treatment of Bacterial Infections. Front. Microbiol. 2024, 15, 1449844. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.H.; Ikram, A.; Dang, L.T.; Bashir, A.; Zohra, T.; Ali, A.; Tanvir, H.; Mudassar, M.; Ravindran, R.; Akhtar, N.; et al. Comparing Machine Learning Screening Approaches Using Clinical Data and Cytokine Profiles for COVID-19 in Resource-Limited and Resource-Abundant Settings. Sci. Rep. 2024, 14, 14892. [Google Scholar] [CrossRef] [PubMed]
| Model | Advantages | Limitations |
|---|---|---|
| Mouse |
|
|
| Pig |
|
|
| Guinea pig |
|
|
| Non-humanprimate |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ślęczkowska, M.; Nobre, B.; Wieringa, P. Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract. Microorganisms 2025, 13, 553. https://doi.org/10.3390/microorganisms13030553
Kim J, Ślęczkowska M, Nobre B, Wieringa P. Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract. Microorganisms. 2025; 13(3):553. https://doi.org/10.3390/microorganisms13030553
Chicago/Turabian StyleKim, Jaehyeon, Milena Ślęczkowska, Beatriz Nobre, and Paul Wieringa. 2025. "Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract" Microorganisms 13, no. 3: 553. https://doi.org/10.3390/microorganisms13030553
APA StyleKim, J., Ślęczkowska, M., Nobre, B., & Wieringa, P. (2025). Study Models for Chlamydia trachomatis Infection of the Female Reproductive Tract. Microorganisms, 13(3), 553. https://doi.org/10.3390/microorganisms13030553








