Anti-Obesity Potential of Barley Sprouts in Dog Diets and Their Impact on the Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Digestibility Analysis
2.3. Blood Samples and Analysis
2.4. Fecal Sample Collection and Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of the Barley Sprout Diet on Body Weight, Digestibility, and Blood Concentrations of Adipose-Associated Hormones
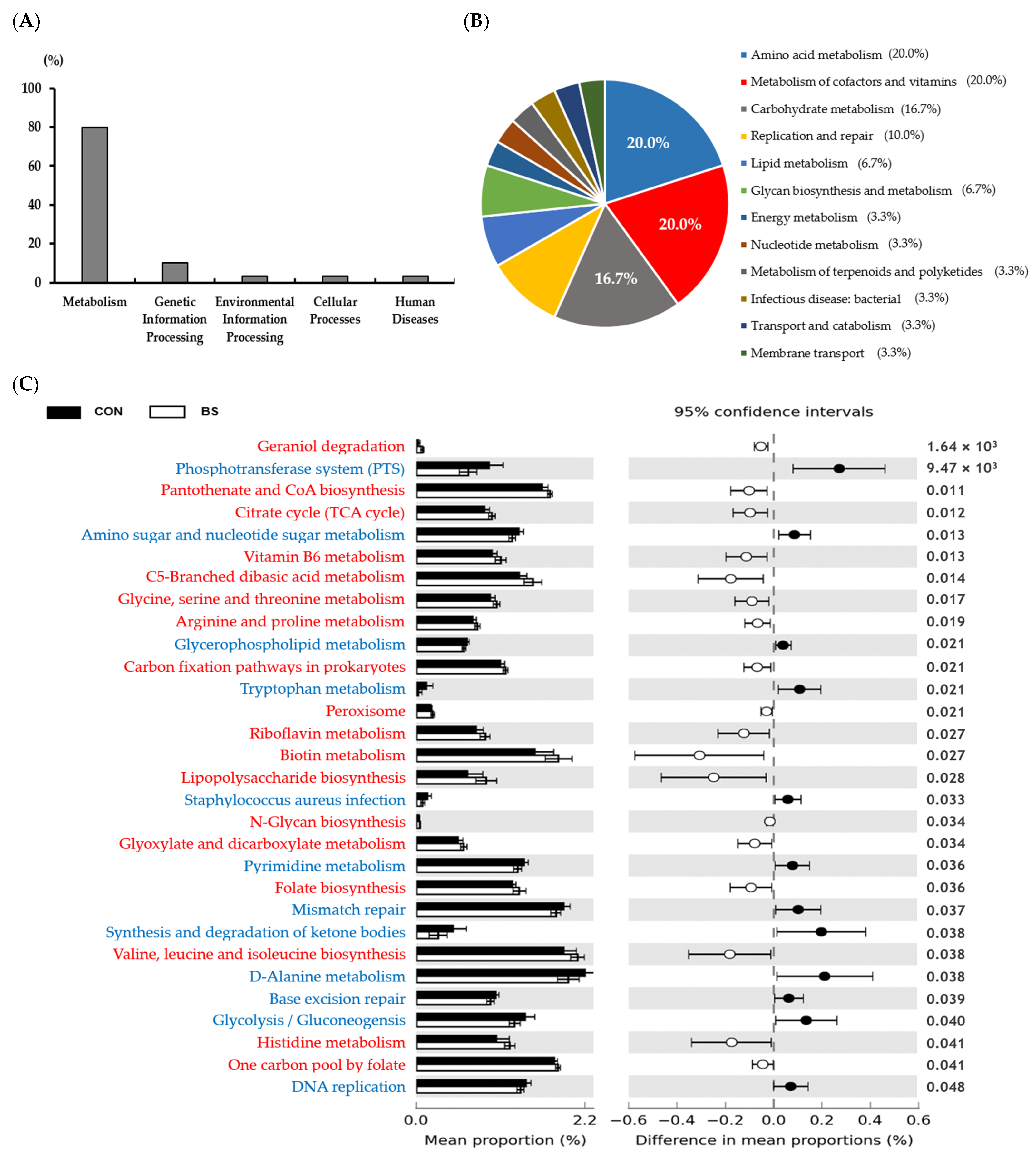
3.2. Analysis of Gut Microbiota Changes in Response to the Barley Sprout Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vučinić, M.M.; Hammond-Seaman, A.A.; Nenadović, K. When the first of the 5Fs for the welfare of dogs goes wrong. Who is responsible?—A review. Vet. Arh. 2023, 93, 191–204. [Google Scholar] [CrossRef]
- German, A. Obesity in companion animals. Practice 2010, 32, 42–50. [Google Scholar] [CrossRef]
- Courcier, E.A.; Thomson, R.M.; Mellor, D.J.; Yam, P.S. An epidemiological study of environmental factors associated with canine obesity. J. Small Anim. Pract. 2010, 51, 362–367. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Woods, G.R.T.; Holden, S.L.; Brennan, L.; Burke, C. Dangerous trends in pet obesity. Vet. Rec. 2018, 182, 25. [Google Scholar] [CrossRef]
- German, A.J. The growing problem of obesity in dogs and cats. J. Nutr. 2006, 136, 1940S–1946S. [Google Scholar] [CrossRef]
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Klausner, J.S. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Int. J. Appl. Res. Vet. Med. 2005, 3, 88–96. [Google Scholar]
- German, A.J.; Ryan, V.H.; German, A.C.; Wood, I.S.; Trayhurn, P. Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet. J. 2010, 185, 4–9. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Ceron, J.J.; Holden, S.L.; Cuthbertson, D.J.; Biourge, V.; Morris, P.J.; German, A.J. Obesity-related metabolic dysfunction in dogs: A comparison with human metabolic syndrome. BMC Vet. Res. 2012, 8, 147. [Google Scholar] [CrossRef]
- Marshall, W.G.; Hazewinkel, H.A.W.; Mullen, D.; De Meyer, G.; Baert, K.; Carmichael, S. The effect of weight loss on lameness in obese dogs with osteoarthritis. Vet. Res. Commun. 2010, 34, 241–253. [Google Scholar] [CrossRef]
- German, A.J.; Hervera, M.; Hunter, L.; Holden, S.L.; Morris, P.J.; Biourge, V.; Trayhurn, P. Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest. Anim. Endocrinol. 2009, 37, 214–226. [Google Scholar] [CrossRef]
- Pan, Y.; Spears, J.K.; Xu, H.; Bhatnagar, S. Effects of a therapeutic weight loss diet on weight loss and metabolic health in overweight and obese dogs. J. Anim. Sci. 2023, 101, skad183. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.S.; Butowski, C.F.; Chitty, J.L.; Naughton, G.; Wiseman-Orr, M.L.; Parkin, T.; Reid, J. Impact of canine overweight and obesity on health-related quality of life. Prev. Vet. Med. 2016, 127, 64–69. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Holden, S.L.; Wiseman-Orr, M.L.; Reid, J.; Nolan, A.M.; Biourge, V.; Morris, P.J.; Scott, E.M.; Scott, E.M. Quality of life is reduced in obese dogs but improves after successful weight loss. Vet. J. 2012, 192, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Pearl, R.L.; Wadden, T.A.; Bach, C.; Leonard, S.M.; Michel, K.E. Who’s a good boy? Effects of dog and owner body weight on veterinarian perceptions and treatment recommendations. Int. J. Obes. 2020, 44, 2455–2464. [Google Scholar] [CrossRef]
- Carciofi, A.C.; Gonçalves, K.N.V.; Vasconcellos, R.S.; Bazolli, R.S.; Brunetto, M.A.; Prada, F. A weight loss protocol and owners participation in the treatment of canine obesity. Ciência Rural. 2005, 35, 1331–1338. [Google Scholar] [CrossRef]
- Lloyd, I.; Furtado, T.; German, A.J.; Watkins, F.; Christley, R.; Westgarth, C. ‘He’d Be Happier if He Wasn’t Chonky’–qualitatively exploring canine obesity perceptions using YouTube™ and discussion fora. Anthrozoös 2023, 36, 513–531. [Google Scholar] [CrossRef]
- Rohlf, V.I.; Toukhsati, S.; Coleman, G.J.; Bennett, P.C. Dog obesity: Can dog caregivers’ (owners’) feeding and exercise intentions and behaviors be predicted from attitudes? J. Appl. Anim. Welf. Sci. 2010, 13, 213–236. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Thomson, P.C.; Pride, C.; Fawcett, A.; Grassi, T.; Jones, B. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet. Rec. 2005, 156, 695–702. [Google Scholar] [CrossRef]
- Degeling, C.; Rock, M. Owning the problem: Media portrayals of overweight dogs and the shared determinants of the health of human and companion animal populations. Anthrozoös 2012, 25, 35–48. [Google Scholar] [CrossRef]
- Cho, H.W.; Seo, K.; Chun, J.L.; Jeon, J.; Kim, C.H.; Lim, S.; Cheon, S.N.; Kim, K.H.; Kim, K.H. Effects of resistant starch on anti-obesity status and nutrient digestibility in dogs. J. Anim. Sci. Technol. 2023, 65, 550–561. [Google Scholar] [CrossRef]
- Axelsson, E.; Ratnakumar, A.; Arendt, M.L.; Maqbool, K.; Webster, M.T.; Perloski, M.; Liberg, O.; Arnemo, J.M.; Hedhammar, A.; Lindblad-Toh, K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 2013, 495, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Arendt, M.; Fall, T.; Lindblad-Toh, K.; Axelsson, E. Amylase activity is associated with AMY 2B copy numbers in dog: Implications for dog domestication, diet and diabetes. Anim. Genet. 2014, 45, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Kore, K.B.; Pattanaik, A.K.; Das, A.; Sharma, K. Evaluation of alternative cereal sources in dog diets: Effect on nutrient utilisation and hindgut fermentation characteristics. J. Sci. Food Agric. 2009, 89, 2174–2180. [Google Scholar] [CrossRef]
- De Godoy, M.R.; Kerr, K.R.; Fahey, G.C., Jr. Alternative dietary fiber sources in companion animal nutrition. Nutrients 2013, 5, 3099–3117. [Google Scholar] [CrossRef]
- Kayser, E.; Finet, S.E.; de Godoy, M.R. The role of carbohydrates in canine and feline nutrition. Anim. Front. 2024, 14, 28–37. [Google Scholar] [CrossRef]
- Murray, S.M.; Fahey, G.C., Jr.; Merchen, N.R.; Sunvold, G.D.; Reinhart, G.A. Evaluation of selected high-starch flours as ingredients in canine diets. J. Anim. Sci. 1999, 77, 2180–2186. [Google Scholar] [CrossRef]
- Cho, H.W.; Seo, K.; Lee, M.Y.; Lee, S.Y.; So, K.M.; Kim, K.H.; Chun, J.L. Nutritional value of common carbohydrate sources used in pet foods. J. Anim. Sci. Technol. 2024, 66, 1282–1290. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T.; Zhou, Y.; et al. Preventive and therapeutic role of functional ingredients of barley grass for chronic diseases in human beings. Oxid. Med. Cell. Longev. 2018, 2018, 3232080. [Google Scholar] [CrossRef]
- Thatiparthi, J.; Dodoala, S.; Koganti, B.; Kvsrg, P. Barley grass juice (Hordeum vulgare L.) inhibits obesity and improves lipid profile in high fat diet-induced rat model. J. Ethnopharmacol. 2019, 238, 111843. [Google Scholar] [CrossRef]
- Márton, M.; Mándoki, Z.; Csapóné Kiss, Z.; Csapó, J. The Role of Sprouts in Human Nutrition. A Review. Acta Univ. Sapientiae Aliment. 2010, 3, 81–117. [Google Scholar]
- Sattar, A.; Badshah, A.; Aurangzeb. Biosynthesis of ascorbic acid in germinating rapeseed cultivars. Plant Foods Hum. Nutr. 1995, 47, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Martínez-Villaluenga, C. Advances in Production, Properties and Applications of Sprouted Seeds. Foods 2020, 9, 790. [Google Scholar] [CrossRef]
- Seo, W.D.; Yuk, H.J.; Curtis-Long, M.J.; Jang, K.C.; Lee, J.H.; Han, S.I.; Kang, H.W.; Nam, M.H.; Lee, S.J.; Lee, J.H.; et al. Effect of the growth stage and cultivar on policosanol profiles of barley sprouts and their adenosine 5′-monophosphate-activated protein kinase activation. J. Agric. Food Chem. 2013, 61, 1117–1123. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeong, E.; Jo, S.M.; Park, J.; Kim, J.Y. Comparative study of the effects of light controlled germination conditions on saponarin content in barley sprouts and lipid accumulation suppression in HepG2 hepatocyte and 3T3-L1 adipocyte cells using barley sprout extracts. Molecules 2020, 25, 5349. [Google Scholar] [CrossRef]
- Yang, J.Y.; Woo, S.Y.; Lee, M.J.; Kim, H.Y.; Lee, J.H.; Kim, S.H.; Seo, W.D. Lutonarin from Barley seedlings inhibits the lipopolysacchride-stimulated inflammatory response of RAW 264.7 macrophages by suppressing nuclear factor-κB signaling. Molecules 2021, 26, 1571. [Google Scholar] [CrossRef]
- Byun, A.R.; Chun, H.; Lee, J.; Lee, S.W.; Lee, H.S.; Shim, K.W. Effects of a dietary supplement with barley sprout extract on blood cholesterol metabolism. Evid. Based Complement. Altern. Med. 2015, 2015, 473056. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.Y.; Kim, B.; Seo, W.D.; Jia, Y.; Wu, C.; Jun, H.; Lee, S.J.; Lee, S.J. Barley sprout extract containing policosanols and polyphenols regulate AMPK, SREBP2 and ACAT2 activity and cholesterol and glucose metabolism in vitro and in vivo. Food Res. Int. 2015, 72, 174–183. [Google Scholar] [CrossRef]
- AOAC, Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Beiko, R.G. Identifying biologically relevant differences between metagenomic communities. Bioinformatics 2010, 26, 715–721. [Google Scholar] [CrossRef]
- Kim, M.J.; Kawk, H.W.; Kim, S.H.; Lee, H.J.; Seo, J.W.; Kim, J.T.; Jang, S.H.; Kim, M.J.; Kim, Y.M.; Kim, M.J.; et al. Anti-obesity effect of hot water extract of barley sprout through the inhibition of adipocyte differentiation and growth. Metabolites 2021, 11, 610. [Google Scholar] [CrossRef]
- Krogdahl, A.; Ahlstrøm, Ø.; Skrede, A. Nutrient digestibility of commercial dog foods using mink as a model. J. Nutr. 2004, 134, 2141S–2144S. [Google Scholar] [CrossRef]
- Daumas, C.; Paragon, B.M.; Thorin, C.; Martin, L.; Dumon, H.; Ninet, S.; Nguyen, P. Evaluation of eight commercial dog diets. J. Nutr. Sci. 2014, 3, e63. [Google Scholar] [CrossRef]
- Pan, H.; Guo, J.; Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef]
- Finet, S.; He, F.; Clark, L.V.; De Godoy, M.R.C. Functional properties of miscanthus fiber and prebiotic blends in extruded canine diets. J. Anim. Sci. 2022, 100, skac078. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef]
- Swanson, K.S.; Dowd, S.E.; Suchodolski, J.S.; Middelbos, I.S.; Vester, B.M.; Barry, K.A.; Nelson, K.E.; Torralba, M.; Henrissat, B.; Coutinho, P.M.; et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011, 5, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ju, X.; Chen, W.; Yuan, J.; Wang, Z.; Aluko, R.E.; He, R. Rice bran attenuated obesity via alleviating dyslipidemia, browning of white adipocytes and modulating gut microbiota in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2406–2417. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P.; Pieraccini, G.; et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Lee, P.S.; Teng, C.Y.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Garcinol reduces obesity in high-fat-diet-fed mice by modulating gut microbiota composition. Mol. Nutr. Food Res. 2019, 63, e1800390. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Parks, B.W.; Nam, E.; Org, E.; Kostem, E.; Norheim, F.; Hui, S.T.; Pan, C.; Civelek, M.; Rau, C.D.; Bennett, B.J.; et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013, 17, 141–152. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.I.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Glenn, T.C. Field guide to next-generation DNA sequencers. Mol. Ecol. Resour. 2011, 11, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.R.; Robeson, M.S.; Hauser, L.J.; Schadt, C.W.; Gorin, A.A. PanFP: Pangenome-based functional profiles for microbial communities. BMC Res. Notes 2015, 8, 479. [Google Scholar] [CrossRef]
- Hou, Y.P.; He, Q.Q.; Ouyang, H.M.; Peng, H.S.; Wang, Q.; Li, J.; Lv, X.F.; Zheng, Y.N.; Li, S.C.; Liu, H.L.; et al. Human gut microbiota associated with obesity in Chinese children and adolescents. BioMed Res. Int. 2017, 2017, 7585989. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Wang, Y.; Ran, L.; Zhang, F.; Li, H.; Cha, Q.; Yang, K.; Wang, H.; Wu, Y.; Yu, Z.; Wu, Y.; et al. Growth hormone attenuates obesity and reshapes gut microbiota in high-fat diet-fed mice. Metab. Open 2024, 24, 100326. [Google Scholar] [CrossRef]
- Del Chierico, F.; Abbatini, F.; Russo, A.; Quagliariello, A.; Reddel, S.; Capoccia, D.; Caccamo, R.; Ginanni Corradini, S.; Nobili, V.; De Peppo, F.; et al. Gut microbiota markers in obese adolescent and adult patients: Age-dependent differential patterns. Front. Microbiol. 2018, 9, 1210. [Google Scholar] [CrossRef]




| Item | CON | BS |
|---|---|---|
| Ingredient, % | ||
| Rice powder | 31.9 | 29.2 |
| Barley sprout powder | N/A | 2.8 |
| Lard | 1.5 | 1.5 |
| Water | 35.0 | 35.0 |
| Salt | 0.2 | 0.2 |
| Vitamin and mineral premix 1 | 0.4 | 0.4 |
| Calcium phosphate | 0.4 | 0.4 |
| Potassium citrate | 0.6 | 0.6 |
| Cabbage powder | 1.0 | 1.0 |
| Calcium carbonate | 1.0 | 1.0 |
| Green laver | 1.0 | 1.0 |
| York powder | 12.0 | 12.0 |
| Chicken breast meal | 15.0 | 15.0 |
| Chemical composition, DM basis (analyzed), % | ||
| Crude protein | 39.5 | 38.9 |
| Crude fat | 18.1 | 18.3 |
| Crude ash | 8.2 | 8.4 |
| Crude fiber | 2.0 | 2.8 |
| Nitrogen-free extract | 32.2 | 31.6 |
| Calcium | 0.83 | 0.81 |
| Phosphorus | 0.59 | 0.57 |
| Metabolizable energy, kcal/kg 2 | 4045 | 4021 |
| CON | BS | p-Value | |
|---|---|---|---|
| Daily DM intake | |||
| DM (g) | 224.2 ± 9.54 | 225.7 ± 12.42 | 0.929 |
| CP (g) | 78.3 ± 3.33 | 77.0 ± 4.24 | 0.814 |
| AHF (g) | 25.6 ± 1.09 | 23.6 ± 1.30 | 0.284 |
| NFE (g) | 110.6 ± 4.71 | 115.3 ± 6.34 | 0.569 |
| OM (g) | 214.4 ± 9.13 | 215.9 ± 11.88 | 0.926 |
| ME (kcal/kg) 1) | 941.3 ± 40.06 | 944.6 ± 51.99 | 0.958 |
| ATTD (%) | |||
| DM | 91.7 ± 0.33 | 91.2 ± 0.53 | 0.576 |
| CP | 81.5 ± 1.03 | 79.3 ± 0.81 | 0.253 |
| AHF | 95.8 ± 0.25 | 93.1 ± 1.30 | 0.170 |
| NFE | 86.2 ± 1.06 | 88.3 ± 2.06 | 0.534 |
| DF | 41.9 ± 2.78 | 40.6 ± 1.75 | 0.779 |
| ME | 83.7 ± 0.69 | 82.0 ± 1.01 | 0.332 |
| Parameter, Unit | Reference Range | CON | BS | p-Value |
|---|---|---|---|---|
| Red blood cell, ×106/μL | 5.65–8.87 | 8.1 ± 0.18 | 8.4 ± 0.12 | 0.217 |
| Hematocrit, % | 37.3–61.7 | 52.3 ± 0.93 | 52.0 ± 1.00 | 0.854 |
| Hemoglobin, g/dL | 13.1–20.5 | 18.2 ± 0.35 | 18.2 ± 0.35 | 0.978 |
| Mean corpuscular volume, fL | 61.6–73.5 | 64.7 ± 0.58 | 62.2 ± 1.06 | 0.058 |
| Mean corpuscular hemoglobin, pg | 21.2–25.9 | 22.5 ± 0.22 | 21.7 ± 0.40 | 0.107 |
| Mean corpuscular hemoglobin concentration, g/dL | 32–37.9 | 34.7 ± 0.14 | 34.9 ± 0.16 | 0.474 |
| Red cell distribution width, % | 13.6–21.7 | 19.6 ± 0.22 | 20.3 ± 0.25 | 0.040 |
| Reticulocytes, ×103/μL | 10–110 | 46.8 ± 11.91 | 42.6 ± 5.77 | 0.622 |
| White blood cell, ×103/μL | 5.1–16.8 | 9.2 ± 0.34 | 8.4 ± 0.82 | 0.366 |
| Neutrophil, ×103/μL | 3.0–11.6 | 6.2 ± 0.38 | 5.3 ± 0.62 | 0.228 |
| Lymphocyte, ×103/μL | 1.1–5.1 | 2.4 ± 0.14 | 2.5 ± 0.21 | 0.486 |
| Monocyte, ×103/μL | 0.2–1.1 | 0.3 ± 0.03 | 0.3 ± 0.03 | 0.651 |
| Eosinophil, ×103/μL | 0.1–1.2 | 0.2 ± 0.02 | 0.2 ± 0.03 | 0.297 |
| Basophil, ×103/μL | 0–0.1 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.786 |
| Platelets, ×103/μL | 184–484 | 295.7 ± 28.78 | 282.6 ± 35.79 | 0.780 |
| Parameter, Unit | Reference Range | CON | BS | p-Value |
|---|---|---|---|---|
| Total protein, g/dL | 5.4–7.7 | 6.6 ± 0.11 | 6.4 ± 0.14 | 0.247 |
| Aspartate transaminase, U/L | 19–42 | 26.3 ± 2.25 | 28.0 ± 3.21 | 0.641 |
| Alanine transaminase, U/L | 19–67 | 32.0 ± 3.38 | 32.8 ± 5.39 | 0.902 |
| Gamma-glutamyl transferase, U/L | 0–6 | 4.3 ± 1.12 | 3.8 ± 0.27 | 0.650 |
| Creatinine, mg/dL | 0.5–1.7 | 0.8 ± 0.04 | 0.8 ± 0.03 | 0.830 |
| Glucose, mg/dL | 76–119 | 103.4 ± 2.46 | 98.8 ± 2.05 | 0.144 |
| Lactate dehydrogenase, U/L | 0–236 | 90.5 ± 11.26 | 109.3 ± 13.61 | 0.275 |
| Cholesterol, mg/dL | 135–361 | 228.4 ± 15.41 | 235.9 ± 21.44 | 0.766 |
| Triglycerides, mg/dL | 19–133 | 61.9 ± 11.45 | 41.1 ± 7.62 | 0.129 |
| Urea nitrogen, mg/dL | 8–28 | 13.4 ± 0.96 | 14.2 ± 0.59 | 0.446 |
| Total bilirubin, mg/dL | 0–0.51 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.070 |
| Creatine kinase, U/L | 52–368 | 147.0 ± 11.33 | 176.5 ± 34.73 | 0.402 |
| Index | CON | BS | p-Value |
|---|---|---|---|
| Chao1 | 151.6 ± 9.60 | 162.2 ± 16.00 | 0.581 |
| Shannon | 4.9 ± 0.07 | 5.0 ± 0.25 | 0.660 |
| Simpson | 0.9 ± 0.00 | 0.9 ± 0.01 | 0.919 |
| Evenness | 0.7 ± 0.00 | 0.7 ± 0.03 | 0.742 |
| Ace | 152.3 ± 9.65 | 163.7 ± 16.15 | 0.556 |
| Observed features | 137.1 ± 14.06 | 161.6 ± 16.06 | 0.275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-W.; Seo, K.; Lee, M.Y.; Lee, S.-Y.; So, K.-M.; Song, S.-Y.; Seo, W.-D.; Chun, J.L.; Kim, K.H. Anti-Obesity Potential of Barley Sprouts in Dog Diets and Their Impact on the Gut Microbiota. Microorganisms 2025, 13, 594. https://doi.org/10.3390/microorganisms13030594
Cho H-W, Seo K, Lee MY, Lee S-Y, So K-M, Song S-Y, Seo W-D, Chun JL, Kim KH. Anti-Obesity Potential of Barley Sprouts in Dog Diets and Their Impact on the Gut Microbiota. Microorganisms. 2025; 13(3):594. https://doi.org/10.3390/microorganisms13030594
Chicago/Turabian StyleCho, Hyun-Woo, Kangmin Seo, Min Young Lee, Sang-Yeob Lee, Kyoung-Min So, Seung-Yeob Song, Woo-Duck Seo, Ju Lan Chun, and Ki Hyun Kim. 2025. "Anti-Obesity Potential of Barley Sprouts in Dog Diets and Their Impact on the Gut Microbiota" Microorganisms 13, no. 3: 594. https://doi.org/10.3390/microorganisms13030594
APA StyleCho, H.-W., Seo, K., Lee, M. Y., Lee, S.-Y., So, K.-M., Song, S.-Y., Seo, W.-D., Chun, J. L., & Kim, K. H. (2025). Anti-Obesity Potential of Barley Sprouts in Dog Diets and Their Impact on the Gut Microbiota. Microorganisms, 13(3), 594. https://doi.org/10.3390/microorganisms13030594






