Bacterial Diversity in Pet Rabbits: Implications for Public Health, Zoonotic Risks, and Antimicrobial Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Microbial Identification
2.3. Antimicrobial Susceptibility Testing
2.4. Statistical Analysis
3. Results
3.1. Clinical Presentation
3.2. Bacterial Identification
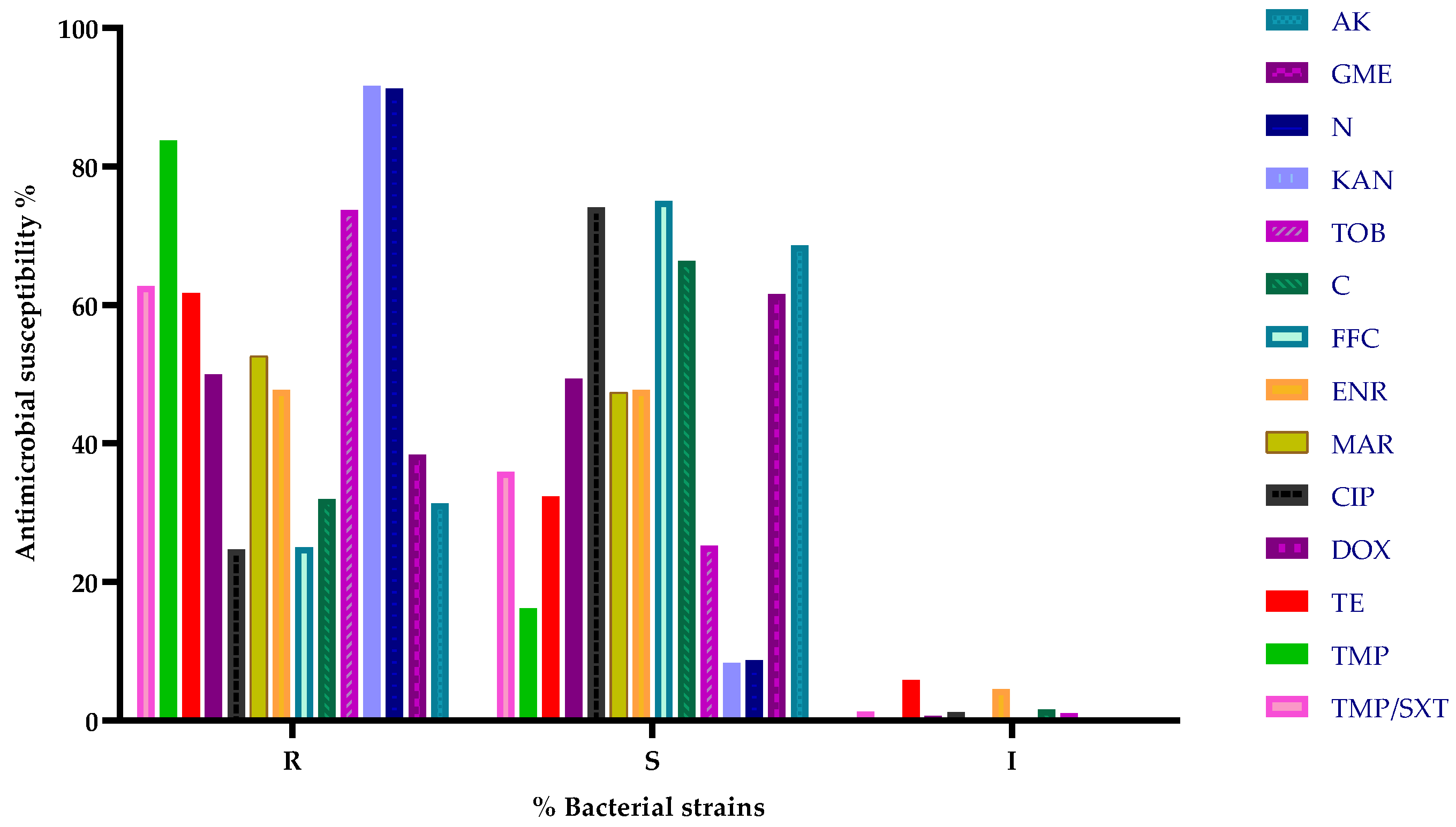
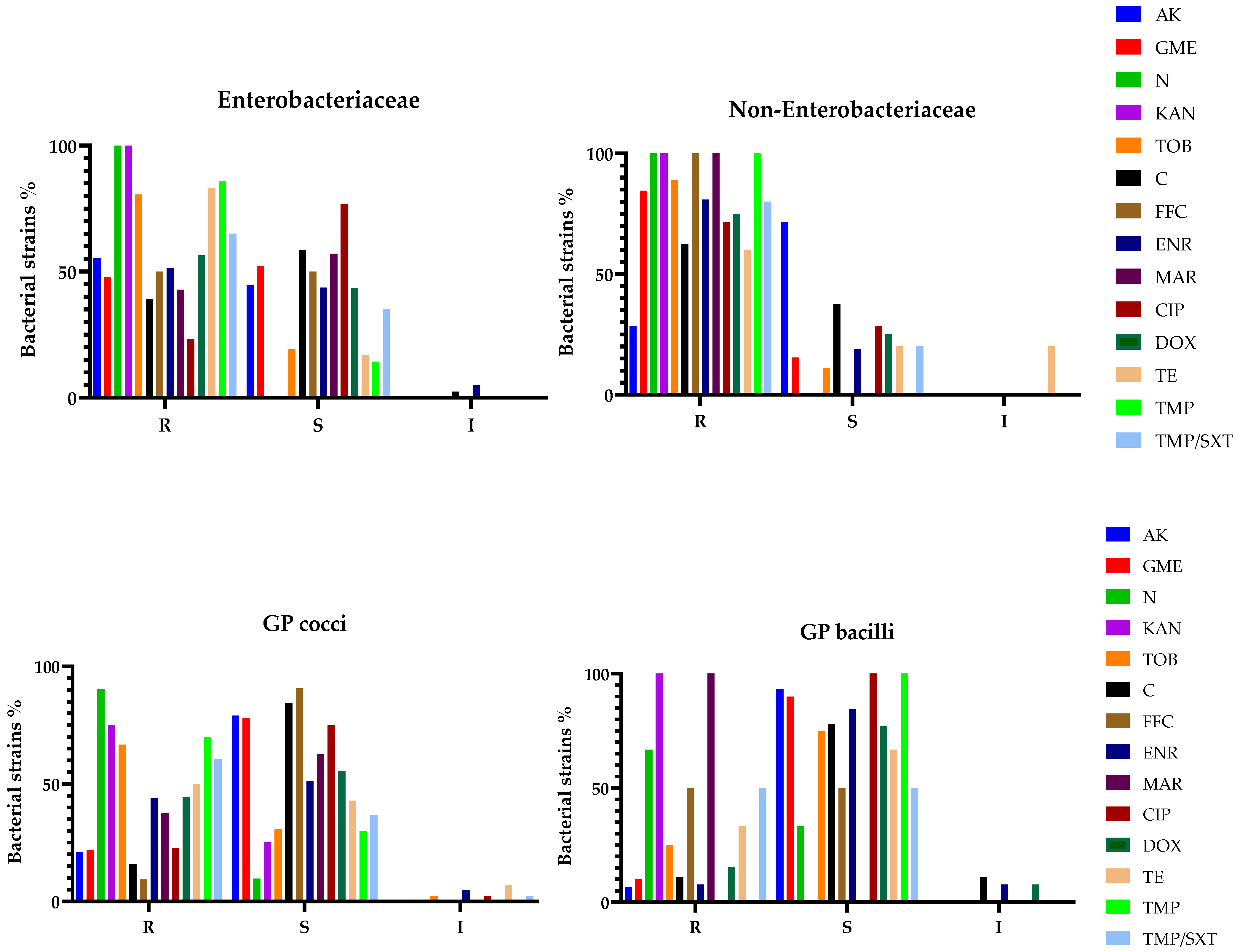
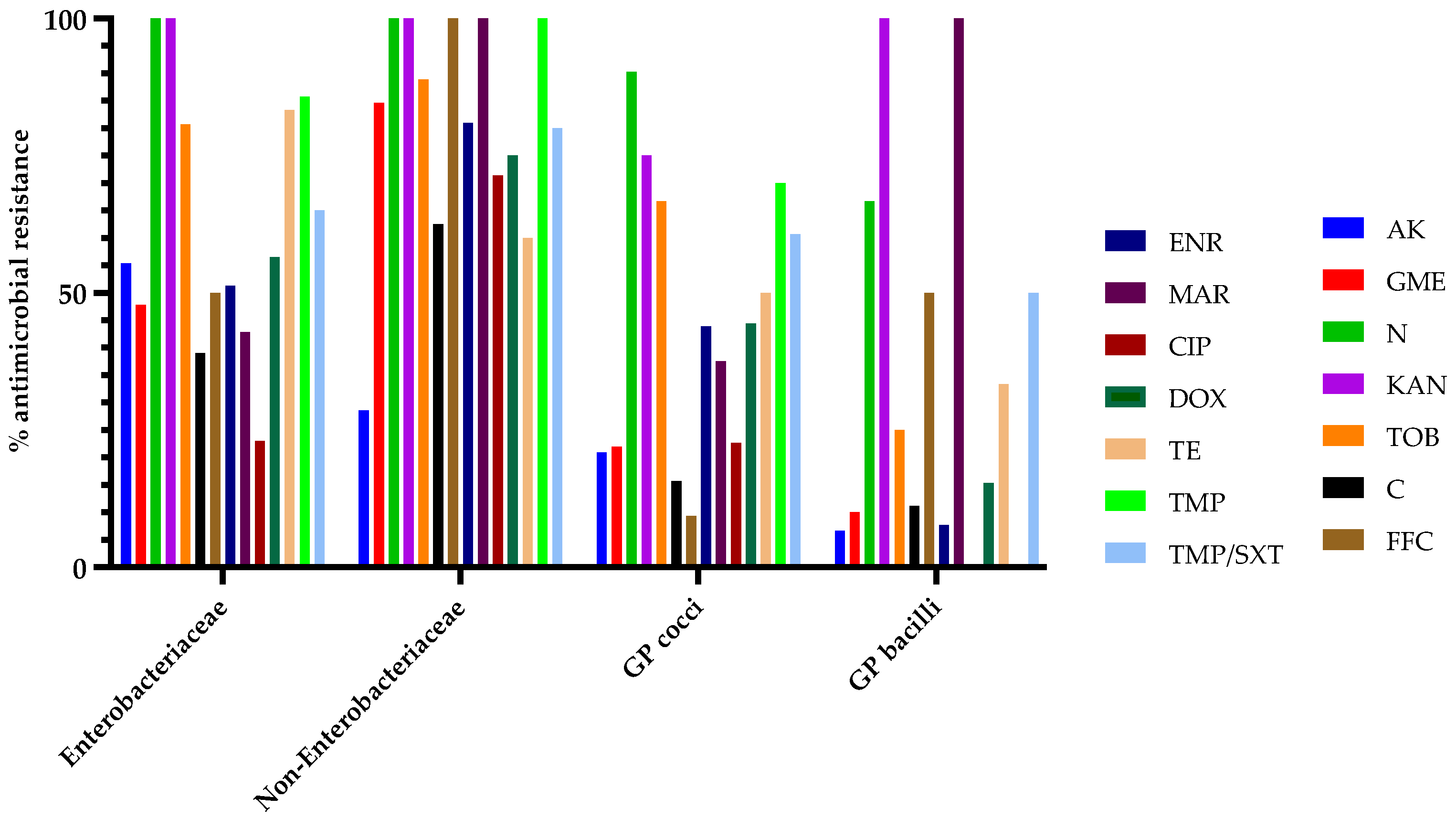
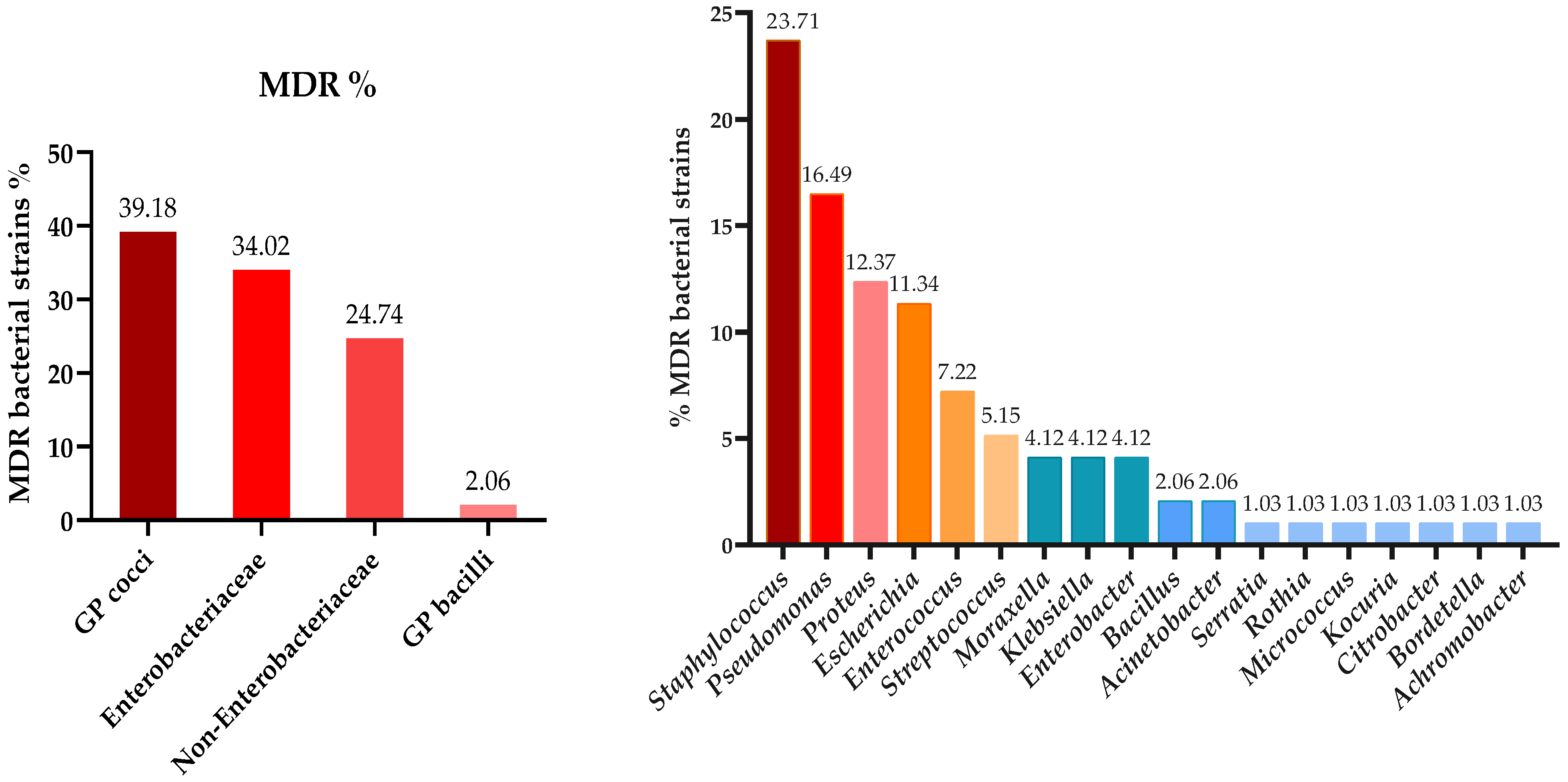
3.3. Antibiotic Susceptibility Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDR | Multidrug resistance |
| GP | Gram-positive |
References
- Skovlund, C.; Forkman, B.; Lund, T.; Mistry, B.; Nielsen, S.; Sandøe, P. Perceptions of the Rabbit as a Low Investment ‘Starter Pet’ Lead to Negative Impacts on Its Welfare: Results of Two Danish Surveys. Anim. Welf. 2023, 32, E45. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Garcias, B.; Duran, I.; Molina-López, R.A.; Darwich, L. Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain. Vet. Sci. 2023, 10, 352. [Google Scholar] [CrossRef]
- Espinosa, J.; Ferreras, M.C.; Benavides, J.; Cuesta, N.; Pérez, C.; García Iglesias, M.J.; García Marín, J.F.; Pérez, V. Causes of Mortality and Disease in Rabbits and Hares: A Retrospective Study. Animals 2020, 10, 158. [Google Scholar] [CrossRef]
- Lennox, A.M.; Kelleher, S. Bacterial and Parasitic Diseases of Rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 519–530. [Google Scholar] [CrossRef]
- Palma-Medel, T.; Marcone, D.; Alegría-Morán, R. Dental Disease in Rabbits (Oryctolagus cuniculus) and Its Risk Factors—A Private Practice Study in the Metropolitan Region of Chile. Animals 2023, 13, 676. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, K.L.; Citron, D.M.; Jenkins, J.R.; Goldstein, E.J.C. Periodontal Bacteria in Rabbit Mandibular and Maxillary Abscesses. J. Clin. Microbiol. 2002, 40, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Harcourt-Brown, F. Textbook of Rabbit Medicine; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Kharitonova, M.; Bokhina, O.; Klyukin, S. Analysis of the Incidence of Dental Pathologies in Ornamental and Agricultural Rabbits. BIO Web Conf. 2022, 43, 03031. [Google Scholar] [CrossRef]
- Gardhouse, S.; Sanchez-Migallon Guzman, D.; Paul-Murphy, J.; Byrne, B.A.; Hawkins, M.G. Bacterial isolates and antimicrobial susceptibilities from odontogenic abscesses in rabbits: 48 cases. Vet. Rec. 2017, 181, 538. [Google Scholar] [CrossRef]
- Benato, L. Odontogenic abscesses in pet rabbits. Vet. Rec. 2017, 181, 536–537. [Google Scholar] [CrossRef]
- Levy, I.; Mans, C. Diagnosis and outcome of odontogenic abscesses in client-owned rabbits (Oryctolagus cuniculus): 72 cases (2011–2022). J. Am. Vet. Med. Assoc. 2024, 262, 658–664. [Google Scholar] [CrossRef]
- Tinelli, A.; Perillo, A.; Galgano, M.; Trotta, A.; Leone, R.; Passantino, G.; Zizzo, N. Pathological findings in a fatal pet rabbit Pasteurellosis. Comp. Clin. Pathol. 2020, 29, 895–898. [Google Scholar] [CrossRef]
- Johnson-Delaney, C.; Orosz, S. Rabbit Respiratory System: Clinical Anatomy, Physiology and Disease. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 257–266. [Google Scholar] [CrossRef]
- Jekl, V. Respiratory Disorders in Rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2021, 24, 459–482. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, E.; Lennox, A. Management of otitis in rabbits. J. Exot. Pet Med. 2016, 26, 63–73. [Google Scholar] [CrossRef]
- Hedley, J.; Ede, V.; Dawson, C. Retrospective study identifying risk factors for dacryocystitis in pet rabbits. Vet. Rec. 2022, 191, e1903. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Rabbit and rodent ophthalmology. Eur. J. Companion Anim. Pract. 2007, 17, 242–252. [Google Scholar]
- Florin, M.; Rusanen, E.; Haessig, M.; Richter, M.; Spiess, B.M. Clinical presentation, treatment, and outcome of dacryocystitis in rabbits: A retrospective study of 28 cases (2003–2007). Vet. Ophthalmol. 2009, 12, 350–356. [Google Scholar] [CrossRef]
- Harvey, N.; Oxley, J.; Miguel-Pacheco, G.; Gosling, E.; Farnworth, M. What makes a rabbit cute? Preference for rabbit faces differs according to skull morphology and demographic factors. Animals 2019, 9, 728. [Google Scholar] [CrossRef]
- Luo, B.; Li, M.; Xiang, N.; Hu, W.; Liu, R.; Yan, X. The microbiologic spectrum of dacryocystitis. BMC Ophthalmol. 2021, 21, 29. [Google Scholar] [CrossRef]
- Keeble, E. Ear disease in pet rabbits. Practice 2023, 45, 87–99. [Google Scholar] [CrossRef]
- Vecere, G.; Malka, S.; Holden, N.; Tang, S.; Krumbeck, J. Comparison of ear canal microbiome in rabbits (Oryctolagus cuniculus domesticus) with and without otitis externa using next-generation DNA sequencing. J. Exot. Pet Med. 2022, 42, 35–41. [Google Scholar] [CrossRef]
- Makri, N.; Ring, N.; Shaw, D.J.; Athinodorou, A.; Robinson, V.; Paterson, G.K.; Richardson, J.; Gow, D.; Nuttall, T. Cytological evaluation, culture and genomics to evaluate the microbiome in healthy rabbit external ear canals. Vet. Dermatol. 2024, 35, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Oglesbee, B.L.; Lord, B. Gastrointestinal diseases of rabbits. In Ferrets, Rabbits, and Rodents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 174–187. [Google Scholar] [CrossRef]
- Panda, A.; Tatarov, I.; Melton-Celsa, A.R.; Kolappaswamy, K.; Kriel, E.H.; Petkov, D.; Coksaygan, T.; Livio, S.; McLeod, C.G.; Nataro, J.P.; et al. Escherichia coli O157:H7 infection in Dutch Belted and New Zealand White rabbits. Comp. Med. 2010, 60, 31–37. [Google Scholar] [PubMed]
- Tzika, E.D.; Saoulidis, K. Rabbit enteritis. J. Hell. Vet. Med. Soc. 2017, 55, 145–155. [Google Scholar] [CrossRef]
- Benato, L. Guide to skin diseases in rabbits. Practice 2019, 41, 488–497. [Google Scholar] [CrossRef]
- Snook, T.S.; White, S.D.; Hawkins, M.G.; Tell, L.A.; Wilson, L.S.; Outerbridge, C.A.; Ihrke, P.J. Skin diseases in pet rabbits: A retrospective study of 334 cases seen at the University of California at Davis, USA (1984–2004). Vet. Dermatol. 2013, 24, 613–617. [Google Scholar] [CrossRef]
- Varga, M. Skin diseases. In Textbook of Rabbit Medicine, 2nd ed.; Butterworth Heinemann: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2014; pp. 271–302. [Google Scholar]
- Broens, E.M.; van Geijlswijk, I.M. Prudent Use of Antimicrobials in Exotic Animal Medicine. Vet. Clin. Exot. Anim. 2018, 21, 341–353. [Google Scholar] [CrossRef]
- Harcourt-Brown, F. Dental Disease in Pet Rabbits 3. Jaw Abscesses. Practice 2009, 31, 496–505. [Google Scholar] [CrossRef]
- Rosenthal, K.L. Therapeutic Contraindications in Exotic Pets. Semin. Avian Exot. Pet Med. 2004, 13, 44–48. [Google Scholar] [CrossRef]
- Hedley, J. Antibiotic Usage in Rabbits and Rodents. Practice 2018, 40, 230–237. [Google Scholar] [CrossRef]
- Giraldi, G.; Montesano, M.; Napoli, C.; Frati, P.; La Russa, R.; Santurro, A.; Scopetti, M.; Orsi, G.B. Healthcare-associated infections due to multidrug-resistant organisms: A surveillance study on extra hospital stay and direct costs. Curr. Pharm. Biotechnol. 2019, 20, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Moruzi, R.F.; Tirziu, E.; Muselin, F.; Dumitrescu, E.; Hutu, I.; Mircu, C.; Tulcan, C.; Doma, A.; Degi, J.; Degi, D.M.; et al. The Importance of Databases to Manage the Phenomenon of Resistance to Antimicrobials for Veterinary Use. Rev. Med. Vet. 2019, 29, 40–57. [Google Scholar] [CrossRef]
- Cardoso, S.; Loc’h, A.L.; Marques, I.; Almeida, A.; Sousa, S.; Saavedra, M.J.; Anastácio, S.; Silveira, E. Unveiling the emergence of multidrug-resistant pathogens in exotic pets from France: A comprehensive study (2017–2019). One Health Implement Res. 2023, 3, 161–176. [Google Scholar] [CrossRef]
- Barbosa, C.K.; Teixeira, V.N.; Pimpão, C.T. Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Vet. J. 2023, 13, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N. Antimicrobial resistance and treatment in companion, food, and exotic animals. Antibiotics 2022, 11, 1360. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Tavares, T.; López, M.; Rojo-Bezares, B.; Pereira, J.E.; Falco, V.; Valentão, P.; Igrejas, G.; Sáenz, Y.; et al. Rabbits as a reservoir of multidrug-resistant Escherichia coli: Clonal lineages and public health impact. Antibiotics 2024, 13, 376. [Google Scholar] [CrossRef]
- Varela, K.; Brown, J.A.; Lipton, B.; Dunn, J.; Stanek, D.; Behravesh, C.B.; Chapman, H.; Conger, T.H.; Vanover, T.; Edling, T.; et al. A review of zoonotic disease threats to pet owners: A compendium of measures to prevent zoonotic diseases associated with non-traditional pets: Rodents and other small mammals, reptiles, amphibians, backyard poultry, and other selected animals. Vector Borne Zoonotic Dis. 2022, 22, 303–360. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Zanen, L.A.; Kusters, J.G.; Overgaauw, P.A.M. Zoonotic Risks of Sleeping with Pets. Pathogens 2022, 11, 1149. [Google Scholar] [CrossRef]
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters 2024. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 15 December 2024).
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; CLSI Supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; ISBN 978-1-68440-211-3. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Hill, F.; Elsohaby, I. Retrospective analysis of antimicrobial resistance in bacterial pathogens from pet rabbits in Hong Kong, 2019–2022. J. Vet. Diagn. Investig. 2024, 36, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Brockmann, M.; Stumpf, P.; Pfabe, J.; Müller, E.; Pees, M.; Marschang, R.E. Isolation of aerobic bacteria from abscesses and wounds in rabbits and antibiotic susceptibility testing of Staphylococcus spp. and Pseudomonas spp. isolates. J. Exot. Pet Med. 2024, 49, 41–47. [Google Scholar] [CrossRef]
- Agnoletti, F.; Brunetta, R.; Bano, L.; Drigo, I.; Mazzolini, E. Longitudinal Study on Antimicrobial Consumption and Resistance in Rabbit Farming. Int. J. Antimicrob. Agents 2018, 51, 197–205. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Li, Y.; Huang, J. Antibiotic resistance spectrums of Escherichia coli and Enterococcus spp. strains against commonly used antimicrobials from commercial meat rabbit farms in Chengdu City, Southwest China. Front. Vet. Sci. 2024, 11, 1369655. [Google Scholar] [CrossRef]
- Silva, N.; Igrejas, G.; Figueiredo, N.; Gonçalves, A.; Radhouani, H.; Rodrigues, J.; Poeta, P. Molecular Characterization of Antimicrobial Resistance in Enterococci and Escherichia coli Isolates from European Wild Rabbit (Oryctolagus cuniculus). Sci. Total Environ. 2010, 408, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, L.; Jouini, A.; Fliss, I.; Torres, C.; Torres, N. Antimicrobial resistance genes and virulence gene encoding intimin in Escherichia coli and Enterococcus isolated from wild rabbits (Oryctolagus cuniculus) in Tunisia. Act. Vet. Hung. 2019, 67, 477–488. [Google Scholar] [CrossRef]
- Sousa, M.; Silva, V.; Silva, A.; Silva, N.; Ribeiro, J.; Tejedor-Junco, M.T.; Capita, R.; Chenouf, N.S.; Alonso-Calleja, C.; Rodrigues, T.M.; et al. Staphylococci among Wild European Rabbits from the Azores: A Potential Zoonotic Issue? J. Food Prot. 2020, 83, 1110–1114. [Google Scholar] [CrossRef]
- Jenckel, M.; Hall, R.N.; Strive, T. Pathogen Profiling of Australian Rabbits by Metatranscriptomic Sequencing. Transbound. Emerg. Dis. 2022, 69, e2629–e2640. [Google Scholar] [CrossRef]
- Suriyakhun, N.; Jangsangthong, A.; Tunyong, W.; Kong-Ngoen, T.; Santajit, S.; Indrawattana, N.; Buranasinsup, S. Investigation of antimicrobial resistance and antimicrobial resistance genes in Staphylococcus aureus and coagulase-negative staphylococci isolated from rabbit. Vet. World 2024, 17, 1328–1335. [Google Scholar] [CrossRef] [PubMed Central]
- Ataya, H.; Soliman, S.; Kayaf, A.; Marouf, S.; Alamry, K. Incidence, bacterial causes and antibiotic resistance patterns of urinary tract infection in pet animals. J. Appl. Vet. Sci. 2023, 8, 35–43. [Google Scholar] [CrossRef]
- Jeong, M.B.; Kim, N.R.; Yi, N.Y.; Park, S.A.; Kim, M.S.; Park, J.H.; Jeong, S.M.; Seo, K.D.; Nam, T.C.; Oh, Y.S.; et al. Spontaneous Ophthalmic Diseases in 586 New Zealand White Rabbits. Exp. Anim. 2005, 54, 395–403. [Google Scholar] [CrossRef]
- Smoglica, C.; Evangelisti, G.; Fani, C.; Marsilio, F.; Trotta, M.; Messina, F.; Di Francesco, C. Antimicrobial Resistance Profile of Bacterial Isolates from Urinary Tract Infections in Companion Animals in Central Italy. Antibiotics 2022, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Ibarra, E.; Molina-López, R.A.; Durán, I.; Garcias, B.; Martín, M.; Darwich, L. Antimicrobial Resistance in Bacteria Isolated from Exotic Pets: The Situation in the Iberian Peninsula. Animals 2022, 12, 1912. [Google Scholar] [CrossRef]
- Marques, C.; Belas, A.; Menezes, J.; Moreira da Silva, J.; Cavaco-Silva, P.; Trigueiro, G.; Gama, L.; Pomba, C. Human and Companion Animal Proteus mirabilis Sharing. Microbiol. Res. 2021, 13, 38–48. [Google Scholar] [CrossRef]
- Foksiński, P.; Blank, A.; Kaczorek-Łukowska, E.; Małaczewska, J.; Wróbel, M.; Wójcik, E.; Sowińska, P.; Pietrzyk, N.; Matusiak, R.; Wojcik, R. Does Every Strain of Pseudomonas aeruginosa Attack the Same? Results of a Study of the Prevalence of Virulence Factors of Strains Obtained from Different Animal Species in Northeastern Poland. Pathogens 2024, 13, 979. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Chen, Y.; Chen, D.; Sang, L.; Xie, X. Characterization of Bordetella bronchiseptica Isolated from Rabbits in Fujian, China. Epidemiol. Infect. 2020, 148, e237. [Google Scholar] [CrossRef]
- Pereira, A.; de Sousa, T.; Silva, C.; Igrejas, G.; Poeta, P. Impact of Antimicrobial Resistance of Pseudomonas aeruginosa in Urine of Small Companion Animals in Global Context: Comprehensive Analysis. Vet. Sci. 2025, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Marco-Fuertes, A.; Marin, C.; Villora-Gonzalez, J.; Gimeno-Cardona, C.; Artal-Muñoz, V.; Vega, S.; Montoro-Dasi, L. Non-traditional small companion mammals in Spain as reservoirs of antimicrobial-resistant Staphylococci. Front. Vet. Sci. 2024, 11, 1378346. [Google Scholar] [CrossRef]
- Li, G.; Walker, M.J.; De Oliveira, D.M.P. Vancomycin Resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2023, 11, 24. [Google Scholar] [CrossRef]
- De Angelis, G.; Del Giacomo, P.; Posteraro, B.; Sanguinetti, M.; Tumbarello, M. Molecular Mechanisms, Epidemiology, and Clinical Importance of β-Lactam Resistance in Enterobacteriaceae. Int. J. Mol. Sci. 2020, 21, 5090. [Google Scholar] [CrossRef] [PubMed]
- Azargun, R.; Gholizadeh, P.; Sadeghi, V.; Hosainzadegan, H.; Tarhriz, V.; Memar, M.Y.; Eyvazi, S. Molecular mechanisms associated with quinolone resistance in Enterobacteriaceae: Review and update. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, A.C.; Palmieri, M.; Mirande, C.; Oliver, A.; Moons, P.; Goossens, H.; van Belkum, A. Pseudomonas aeruginosa: A clinical and genomics update. FEMS Microbiol. Rev. 2021, 45, fuab026. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Cruz, M.Á.; Gonzalez-Avila, L.U.; Martínez-Trejo, A.; Saldaña-Padilla, A.; Hernández-Cortez, C.; Bello-López, J.M.; Castro-Escarpulli, G. ESKAPE and Beyond: The Burden of Coinfections in the COVID-19 Pandemic. Pathogens 2023, 12, 743. [Google Scholar] [CrossRef]

| Rabbits % | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male 58.24 (n = 99) | Female 41.76 (n = 71) | 170 | ||||||
| Age | Young 22.94 (n = 39) | Adult 42.35 (n = 72) | Geriatric 34.71 (59) | 170 | |||||
| Season | Spring 34.12 (n = 58) | Summer 17.06 (n = 29) | Autumn 25.88 (n = 44) | Winter 22.94 (n = 39) | 170 | ||||
| Pathology | Dental 35.29 (n = 60) | Respiratory 28.24 (n = 48) | Otitis 13.53 (n = 23) | Ocular 9.41 (n = 16) | Skin 5.88 (n = 10) | Digestive 5.29 (n = 9) | Urinary 1.76 (n = 3) | Other 0.59 (n = 1) | 170 |
| Antibioticusage | Yes 47.06 (n = 80) | No 52.94 (n = 90) | 170 | ||||||
| Dental (n) | Respiratory (n) | Otitis (n) | Ocular (n) | Skin (n) | Digestive (n) | Urinary (n) | Other (n) | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | 46 * 46.46% ** 76.67% *** | 28 * 28.28% ** 58.33% *** | 9 * 9.09% ** 39.13% *** | 6 * 6.06% ** 37.50% *** | 3 * 3.03% ** 30.00% *** | 6 * 6.06% ** 66.67% *** | 1 * 1.01% ** 33.33% *** | 0 * 0.00% ** 0.00% *** | 99 * 100.00% ** 58.24% *** |
| Female | 14 * 19.72% ** 23.33% *** | 20 * 28.17% ** 41.67% *** | 14 * 19.72% ** 60.87% *** | 10 * 14.08% ** 62.50% *** | 7 * 9.86% ** 70.00% *** | 3 * 4.23% ** 33.33% *** | 2 * 2.82% ** 66.67% *** | 1 * 1.41% ** 100.0% *** | 71 * 100.00% ** 41.76% *** | |
| Total | 60 * 35.29% ** 100.00% *** | 48 * 28.24% ** 100.00% *** | 23 * 13.53% ** 100.00% *** | 16 * 9.41% ** 100.00% *** | 10 * 5.88% ** 100.00% *** | 9 * 5.29% ** 100.00% *** | 3 * 1.76% ** 100.00% *** | 1 * 0.59% ** 100.00% *** | 170 * 100.00% ** 100.00% *** | |
| Age | Young (≤1 year) | 9 * 23.08% ** 15.00% *** | 19 * 48.72% ** 39.58% *** | 1 * 2.56% ** 4.35% *** | 4 * 10.26% ** 25.00% *** | 3 * 7.69% ** 30.00% *** | 1 * 2.56% ** 11.11% *** | 1 * 2.56% ** 33.33% *** | 1 * 2.56% ** 100.0% *** | 39 * 100.0% ** 22.94% *** |
| Adult (1–5 years) | 25 * 34.72% ** 41.67% *** | 15 * 20.83% ** 31.25% *** | 12 * 16.67% ** 52.17% *** | 9 * 12.50% ** 56.25% *** | 4 * 5.56% ** 10.00% *** | 6 * 8.33% ** 66.67% *** | 1 * 1.39% ** 33.33% *** | 0 * 0.00% ** 0.00% *** | 72 * 100.0% ** 42.35% *** | |
| Geriatric (≥5 years) | 26 * 44.07% 43.33% *** | 14 * 23.73% 29.17% *** | 10 * 16.95% 43.48% *** | 3 * 5.08% 18.75% *** | 3 * 5.08% 30.00% *** | 2 * 3.39% 22.22% *** | 1 * 1.69% 33.33% *** | 0 * 0.00% 0.00% *** | 59 * 100.0% 34.71% *** | |
| Total | 60 * 35.29% ** 100.0% *** | 48 * 28.24% ** 100.0% *** | 23 * 13.53% ** 100.0% *** | 16 * 9.41% ** 100.0% *** | 10 * 5.88% ** 100.0% *** | 9 * 5.29% ** 100.0% *** | 3 * 1.76% ** 100.0% *** | 1 * 0.59% ** 100.0% *** | 170 * 100.0% ** 100.0% *** | |
| Season | Spring | 25 * 43.10% ** 41.67% *** | 17 * 29.31% ** 35.42% *** | 9 * 15.52% ** 39.13% *** | 3 * 5.17% ** 18.75% *** | 2 * 3.45% ** 20.00% *** | 1 * 1.72% ** 11.11% *** | 1 * 1.72% ** 33.33% *** | 0 * 0.00% ** 0.00% *** | 58 * 100.0% ** 34.12% *** |
| Summer | 9 * 31.03% ** 15.00% *** | 7 * 24.14% ** 14.58% *** | 4 * 13.79% ** 17.39% *** | 3 * 10.34% ** 18.75% *** | 0 * 0.00% ** 0.00% *** | 4 * 13.79% ** 44.44% *** | 1 * 3.45% ** 33.33% *** | 1 * 3.45% ** 100.0% *** | 29 * 100.0% ** 17.06% *** | |
| Autumn | 10 * 22.73% ** 16.67% *** | 15 * 34.09% ** 31.25% *** | 4 * 9.09% ** 17.39% *** | 5 * 11.36% ** 31.25% *** | 6 * 13.64% ** 60.00% *** | 3 * 6.82% ** 33.33% *** | 1 * 2.27% ** 33.33% *** | 0 * 0.00% ** 0.00% *** | 44 * 100.0% ** 25.88% *** | |
| Winter | 16 * 41.03% ** 26.67% *** | 9 * 23.08% ** 18.75% *** | 6 * 15.38% ** 26.09% *** | 5 * 12.82% ** 31.25% *** | 2 * 5.13% ** 20.00% *** | 1 * 2.56% ** 11.11% *** | 0 * 0.00% ** 0.00% *** | 0 * 0.00% ** 0.00% *** | 39 * 100.0% ** 22.94% *** | |
| Total | 60 * 35.29% ** 100.0% *** | 48 * 28.24% ** 100.0% *** | 23 * 13.53% ** 100.0% *** | 16 * 9.41% ** 100.0% *** | 10 * 5.88% ** 100.0% *** | 9 * 5.29% ** 100.0% *** | 3 * 1.76% ** 100.0% *** | 1 * 0.59% ** 100.0% *** | 170 * 100.0% ** 100.0% *** | |
| Isolate | % | Dental (n) | Respiratory (n) | Otitis (n) | Ocular (n) | Skin (n) | Digestive (n) | Urinary (n) | Other (n) |
|---|---|---|---|---|---|---|---|---|---|
| Achromobacter denitrificans | 0.5 (n = 1) | - | - | - | 1 | - | - | - | - |
| Acinetobacter guillouiae | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Acinetobacter johnsonii | 0.5 (n = 1) | - | - | - | - | - | 1 | - | - |
| Aerococcus urinae | 0.5 (n = 1) | 1 | - | - | - | - | - | - | - |
| Aerococcus viridans | 1 (n = 2) | 1 | - | - | 1 | - | - | - | - |
| Bacillus licheniformis | 3 (n = 6) | 3 | 1 | 1 | 1 | - | - | - | - |
| Bacillus pumilus | 4 (n = 8) | 4 | 1 | 1 | - | - | 2 | - | - |
| Bordetella bronchiseptica | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Citrobacter braakii | 0.5 (n = 1) | 1 | - | - | - | - | - | - | - |
| Enterobacter asburiae | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Enterobacter cloacae complex | 1.5 (n = 3) | - | 2 | 1 | - | - | - | - | - |
| Enterobacter hormaechei | 1 (n = 2) | 1 | 1 | - | - | - | - | - | - |
| Enterobacter kobei | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Enterococcus casseliflavus | 0.5 (n = 1) | - | - | - | - | - | 1 | - | - |
| Enterococcus faecalis | 3 (n = 6) | 4 | 1 | - | - | - | 1 | - | - |
| Enterococcus faecium | 2.5 (n = 5) | 1 | 1 | 1 | - | - | 1 | 1 | - |
| Escherichia coli | 12 (n = 24) | 11 | 4 | 1 | 2 | 1 | 3 | 2 | - |
| Glutamicibacter protophormiae | 0.5 (n = 1) | 1 | - | - | - | - | - | - | - |
| Klebsiella oxytoca | 2.5 (n = 5) | 1 | 3 | - | 1 | - | - | - | - |
| Klebsiella pneumoniae | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Kocuria atrinae | 0.5 (n = 1) | 1 | - | - | - | - | - | - | - |
| Micrococcus luteus | 2.5 (n = 5) | 1 | 2 | 1 | - | 1 | - | - | - |
| Moraxella osloensis | 5 (n = 10) | 6 | 1 | 1 | 2 | - | - | - | - |
| Pantoea agglomerans | 0.5 (n = 1) | - | - | - | - | 1 | - | - | - |
| Pasteurella canis | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Peribacillus simplex | 0.5 (n = 1) | - | - | 1 | - | - | - | - | - |
| Proteus mirabilis | 7 (n = 14) | 10 | 1 | 3 | - | - | - | - | - |
| Proteus vulgaris | 0.5 (n = 1) | - | - | 1 | - | - | - | - | - |
| Pseudomonas aeruginosa | 8 (n = 16) | 6 | 4 | 1 | 3 | 2 | - | - | - |
| Rothia kristinae | 0.5 (n = 1) | - | - | - | - | - | - | 1 | - |
| Serratia marcescens | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Staphylococcus aureus | 1 (n = 2) | - | 2 | - | - | - | - | - | - |
| Staphylococcus cohnii ssp. urealyticus | 0.5 (n = 1) | - | - | - | 1 | - | - | - | - |
| Staphylococcus epidermidis | 5 (n = 10) | 4 | - | 1 | 1 | 3 | 1 | - | - |
| Staphylococcus haemolyticus | 1 (n = 2) | 1 | - | - | 1 | - | - | - | - |
| Staphylococcus hominis | 0.5 (n = 1) | - | - | - | - | 1 | - | - | - |
| Staphylococcus hominis ssp. hominis | 1 (n = 2) | 1 | 1 | - | - | - | - | - | - |
| Staphylococcus saprophyticus | 0.5 (n = 1) | - | 1 | - | - | - | - | - | - |
| Staphylococcus sciuri | 6.5 (n = 13) | 4 | 7 | 1 | - | - | - | - | 1 |
| Staphylococcus simulans | 3.5 (n = 7) | 2 | 3 | 2 | - | - | - | - | - |
| Staphylococcus warneri | 4.5 (n = 9) | 1 | 4 | 2 | 1 | 1 | - | - | - |
| Staphylococcus xylosus | 7 (n = 14) | 2 | 8 | 1 | 3 | - | - | - | - |
| Streptococcus mitis | 5 (n = 10) | 9 | - | - | - | 1 | - | - | - |
| Streptococcus pneumoniae | 1.5 (n = 3) | - | 3 | - | - | - | - | - | - |
| Streptococcus pyogenes | 0.5 (n = 1) | - | - | - | - | 1 | - | - | - |
| TOTAL | 100 (n = 200) | 38.5 (n = 77) | 29 (n = 58) | 10 (n = 20) | 9 (n = 18) | 6 (n = 12) | 5 (n = 10) | 2 (n = 4) | 0.5 (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crăciun, S.; Novac, C.Ş.; Fiţ, N.I.; Bouari, C.M.; Bel, L.V.; Nadăş, G.C. Bacterial Diversity in Pet Rabbits: Implications for Public Health, Zoonotic Risks, and Antimicrobial Resistance. Microorganisms 2025, 13, 653. https://doi.org/10.3390/microorganisms13030653
Crăciun S, Novac CŞ, Fiţ NI, Bouari CM, Bel LV, Nadăş GC. Bacterial Diversity in Pet Rabbits: Implications for Public Health, Zoonotic Risks, and Antimicrobial Resistance. Microorganisms. 2025; 13(3):653. https://doi.org/10.3390/microorganisms13030653
Chicago/Turabian StyleCrăciun, Smaranda, Cristiana Ştefania Novac, Nicodim Iosif Fiţ, Cosmina Maria Bouari, Lucia Victoria Bel, and George Cosmin Nadăş. 2025. "Bacterial Diversity in Pet Rabbits: Implications for Public Health, Zoonotic Risks, and Antimicrobial Resistance" Microorganisms 13, no. 3: 653. https://doi.org/10.3390/microorganisms13030653
APA StyleCrăciun, S., Novac, C. Ş., Fiţ, N. I., Bouari, C. M., Bel, L. V., & Nadăş, G. C. (2025). Bacterial Diversity in Pet Rabbits: Implications for Public Health, Zoonotic Risks, and Antimicrobial Resistance. Microorganisms, 13(3), 653. https://doi.org/10.3390/microorganisms13030653







