Abstract
The biotechnological potential of the lactic acid bacterial genus Weissella has not been fully unearthed. Since Weissella have not been tested against Fusaria and their mycotoxins, newly isolated Weissella confusa strains were characterized and tested for their antifungal capacities on Fusarium plant pathogens. W. confusa BF2 and ML2 successfully inhibited Fusarium verticillioides NCIM 1100, F. verticillioides NCIM 1099, Fusarium graminearum MTCC 2089, and Fusarium oxysporum MTCC 284 in co-cultures. Ethyl acetate extracts of the cell-free culture supernatants (CFCS) of W. confusa also exhibited antifungal activity against the tested fungal cultures. The main mycotoxins of Fusaria were tested against the Weissella isolates. In MRS broth, W. confusa BF2 was resistant to the Fusarium mycotoxins (deoxynivalenol, zearalenone, T2, and fumonisin B1), while the ML2 strain showed 22.1–24.5% growth inhibition. Meanwhile, neither bacterium showed potential in mycotoxin reduction. The study highlighted that W. confusa BF2 and ML2 and their CFCS are suitable for Fusarium growth inhibition, as shown on surface-sterilized peanuts and wheat grains, but not for mycotoxin elimination.
1. Introduction
Phylogenetically, the Weissella genus belongs to the Firmicutes, class Bacilli, order Lactobacillales, and family Leuconostocaceae [1]. Weissella confusa [1] synonyms are Lactobacillus confusus [2] and Lactobacillus coprophilus subsp. confusus [3]. Bacteria ascribed to the genus Weissella are Gram-positive, catalase-negative, non-endospore-forming cells with rod-shaped or coccoid morphology [1,4] and have their place in the group of bacteria usually known as lactic acid bacteria (LAB). They are obligately heterofermentative with either D (−)- or a mixture of D (−)- and L (+)- lactic acid and acetic acid as the primary end products from sugar metabolism. Approximately 22 recognized species are within the genus Weissella [4,5,6].
The Weissella genus is increasingly recognized for its potential applications in diverse fields, from food to health care. However, the biotechnological potential of the genus Weissella has not been fully unearthed. Remarkably, Weissella cibaria and Weissella confusa are regarded as high producers of exo-polysaccharides exhibiting texturizing properties [5,6]. Some Weissella strains resisted low pH and bile salts and were isolated from healthy human feces, indicating their potential as probiotics. These strains also serve an essential role in food fermentation and preservation with various health benefits due to the production of several compounds like bacteriocins, hydrogen peroxide, and organic acids [5,6,7,8,9,10]. Weissella spp. are highly valuable in food preservation due to the production of bacteriocins, which exhibit antimicrobial properties against food spoilage bacteria. W. cibaria strains possess antagonistic activity against pathogens like Streptococcus pyogenes, Staphylococcus aureus, Streptococcus pneumoniae [7] and Listeria monocytogenes [8]. W. confusa strains could fight Helicobacter pylori [9] and multidrug-resistant Escherichia coli [10]. Several bacteriocins have been defined in Weissella hellenica and W. confusa strains (reviewed by [11]) and may be utilized as bio-preservatives. Some Weissella strains can decarboxylate polymeric phenolic compounds, resulting in improved bioavailability. The use of Weissella genus or W. confusa as an industrial starter is unknown because, despite Weissella spp. being prospective good starters for food fermentation, they are not recognized as GRAS (Generally Recognized As Safe) organisms (FDA, US) or given Qualified Presumption of Safety (QPS) status (EFSA, EU). Their antibiotic resistance patterns and the possibility of biogenic amine production or infection risks partially clarify this disregard [5,12,13]. However, Weissella spp. can be considered safe according to international standards due to the lack of virulence genes and toxic metabolite production and may achieve GRAS status in the future [14,15].
A rising challenge for green-labeled food pushes us to avoid applying chemicals in agricultural production systems; thus, identifying biocontrol agents is essential for the sector. Recent research suggested that LAB are the best choice as biocontrols for mitigating fungal contamination and mycotoxins [16,17,18,19,20,21,22,23]. More research is needed to identify this Gram-positive group’s potential for agricultural utilization.
Plant-pathogenic and mycotoxin-producing Fusaria are economically important filamentous fungi that contaminate various crops. Among diverse Fusarium mycotoxins, deoxynivalenol (DON), fumonisins (FUM), zearalenone (ZEA), and T2/HT2 toxins are reported to cause risks [24,25]. While Fusarium verticillioides is a known pathogen, it can also exist within plants as an endophyte, typically without inducing any disease symptoms. In conducive environments, maize is susceptible to seedling blight, stalk, or ear rot when infected with F. verticillioides. Beyond just lowering crop yields, it also generates FUM mycotoxins, posing a risk to humans and other organisms [26,27]. Fusarium graminearum is one of the major fungal pathogens responsible for Fusarium head blight. F. graminearum synthesize DON during infection, a virulence factor that promotes Fusarium head blight spread [28]. Fusarium oxysporum is a fungus living in soil that generates several toxins, including fumonisins and fusaric acid, while it produces other less significant mycotoxins like beauvericin, enniatins and moniliformin [1]. It does not create trichothecenes or ZEA [29] and can attack numerous crops [30].
LAB produce acids and other antifungal agents, inhibiting Fusarium growth in low pH environments [31]. LAB can also positively affect plant cultures; for example, W. cibaria DA2 cell-free culture supernatant (CFCS) positively impacted sweet corn quality and resistance [32]. Weissella paramesenteroides demonstrated intense antifungal activity against Aspergillus flavus and reduced aflatoxin B1 production [33], while Weissella soli strains exhibited low aflatoxin B1 binding potential [34]. It was concluded that galactan exopolysaccharides could increase the aflatoxin B1 binding potential of the genus, such as W. confusa KR780676 [6].
We aimed to isolate LAB from different sources and investigate LAB and Fusarium interactions for Fusarium growth control. Since the literature on the interaction of Fusarium and Fusarium mycotoxins with the Weissella genus was rare, in this study we intended to present our results on W. confusa isolates’ potential as antifungal LAB for Fusarium disease control and our studies on possible Fusarium mycotoxin reduction.
2. Materials and Methods
2.1. Microorganisms
Lactic acid bacteria (8 isolates) were isolated on MRS medium plates at 37 °C from neonatal feces and bovine milk samples collected from Trivandrum, Kerala, India. Pure bacterial cultures were obtained by preparing decimal dilutions, plating them in MRS medium, incubating at 37 °C for 24–48 h and re-streaking isolated colonies. Fusarium oxysporum MTCC 284, Fusarium verticillioides NCIM 1100 (ATCC 14164), Fusarium verticillioides NCIM 1099 (ATCC 12616), and Fusarium graminearum MTCC 2089 were used for the antagonism studies. To grow the fungi on a solid medium, PDB (Hi-Media, Mumbai, Maharashtra, India) grown cultures were streaked on PDA plates (Hi-Media, Mumbai, Maharashtra, India) and incubated at 30 °C for 5–7 days.
2.2. Characterization of the Bacteria
Gram-staining and catalase tests were performed as basic identification. Gram-positive and catalase-negative cultures were then assessed for the zone of inhibition formed on modified MRS-calcium carbonate (0.8% w/v CaCO3) plates (Hi-Media, Mumbai, Maharashtra, India) and incubated at 37 °C for 48 h [35,36]. Based on their inhibition zone, selected colonies were streaked onto MRS agar to produce pure cultures (Hi-Media, Mumbai, Maharashtra, India).
For DNA isolation, strains were grown in MRS broth at 37 °C for 48 h and harvested by centrifugation (13,600× g for 5 min). Genomic DNA was extracted using a bacterial genomic DNA extraction kit (G-Spin Intron Biotechnology, Seongnam, Republic of Korea) according to the manufacturer’s instructions. The absorbance ratio A260/A280 determined the DNA concentration and purity. The PCR amplification of the 16S rRNA bacterial barcode sequence was conducted using 16S rRNA universal bacterial primers [1492R: 5′-GGT TAC CTT GTT ACG ACT T-3′, 515F: 5′-GTG CCA GCM GCC GCG GTA A-3′] (IDT, Leuven, Germany) [36] at 55 °C annealing temperature. PCR reaction with Phusion Hot Start II High–Fidelity DNA Polymerase (Thermo Fisher Scientific Life Technologies Inc., Carlsbad, CA, USA), cleanup and sequencing were performed according to Krishnan et al. [36]. The sequences were analyzed using MEGA 11 (version 11.0.13.) software. A homology-based identification was performed with BLASTn: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 22 March 2024). The sequences were submitted to the NCBI GenBank [37]. Using Advanced mode with the default parameters, a phylogenetic tree was built with PCR product sequences at Phylogeny.fr (http://www.phylogeny.fr, accessed on 23 January 2025). The sequences of Latilactobacillus sakei and Weissella confusa were downloaded from the NCBI database https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 23 January 2025).
2.3. Antagonism Test
A solitary bacterial colony was taken from a fresh MRS plate and mixed with 250 µL of sterile distilled water. The resulting suspension was then spread across half of a fresh MRS plate using a sterile cotton swab, and the plate was incubated at 37 °C for 48 h. The fungi were grown in PDA. To assess antifungal activity, a fungal disc was placed on the other half of the MRS plate, previously inoculated with bacteria, using a sterile cork borer. After incubation at 30 °C for 5–6 days, the plates were compared to control plates to observe any inhibition of fungal growth by the bacteria. Fungal inhibition was calculated as the percentage reduction (I%) in colony diameter compared to untreated controls. All standard deviations were less than 5%.
2.4. Preparation of Bioactive Extracts and Well Diffusion Assay
A 250 mL MRS broth was inoculated with 16–24 h-old bacterial culture (OD600nm = 1) and incubated at 37 °C for 48 h at static conditions. After incubation, the supernatant was collected by centrifugation at 8590× g for 15 min. The supernatant was mixed with ethyl acetate (EA) (in ratio 1:2) in a 2 L conical flask and shaken for an hour at room temperature. The organic layer was collected and concentrated using a rotary evaporator (Heidolph Instruments GmbH, Schwabach, Germany). The semi-viscous concentrated sample was collected and adequately suspended. The concentrated crude extract was tested for antifungal activity against Fusarium spp. using well-diffusion assays.
For the tests, Fusarium spp. were grown in PDB and incubated for 2–3 days at 30 °C [36]. To conduct the well-diffusion test, the fungal suspensions prepared in PBS were evenly spread on MHA plates and 100 µL of the crude extract was added per well (n = 3). After incubation at 30 °C for 72 h, the mean of the inhibition zone and standard deviations were determined (n = 3).
2.5. Mycotoxin Detection with HPLC Method
HPLC measurements were performed on Thermo Scientific Dionex Ultimate 3000 (Dionex Softron Ltd., Germering, Germany) HPLC equipment. For all measurements, Biopure mycotoxin standard solutions (Romer Labs, Tulln, Austria) were used [38]. Mycotoxin sample preparation and measurement in the reverse phase were conducted according to Krishnan et al. [36]. Thermo Scientific Dionex Chromeleon 7.2 Chromatography Data System (CDS) software (Dionex Softron Ltd., Germering, Germany) was used for data collection and evaluation. The LOD was 0.01 mg/kg for DON and 0.001 µg/kg for ZEA. A linear range of up to 50 mg/kg was detected. The relative standard deviation was calculated as the absolute value of the coefficient of variation (RSD), and in all cases, it was found to be below 10% [36].
2.6. Mycotoxin Detection with ELISA Method
Mycotoxin detection in competitive ELISA tests was performed for FB1 and T-2 mycotoxins. For the AgraQuant® Fumonisin ELISA kit, the LOD was 0.2 mg/kg, LOQ was 0.025 mg/kg, and the quantitation range was 0.2–5 mg/kg (Romer Labs, Tulln, Austria). For the AgraQuant® T-2 Toxin ELISA test, the LOD was 0.01 mg/kg, the LOQ was 0.020 mg/kg, and the quantitation range was 0.02–0.5 mg/kg (Romer Labs, Tulln, Austria). The samples were measured at 450 nm (n = 3, RSD < 5%) using a BioTek Synergy HTX Multimode Reader (BioTek, Winooski, VT, USA) [36].
2.7. Mycotoxin Resistance Tests
The resistance tests were performed according to Krishnan et al. [36]. The isolates were inoculated into MRS broth (Scharlab, Barcelona, Spain) and incubated at 37 °C for 16 h. A microtiter plate made with 200 μL MRS medium was inoculated to obtain a low-density culture (OD630nm: 0.1–0.2). BIOPURE mycotoxins (Romer Labs, Tulln, Austria) were added to the cultures to a 2 mg/L final concentration. The bacteria were incubated with mycotoxins at 37 °C for 24 h in a microtiter plate reader (BioTek Synergy HTX Multimode Reader, BioTek, Winooski, VT, USA). The optical density was taken hourly at 630 nm after intense shaking (30 sec). Data (n = 4) were analyzed in Gen5 version 3.05 software (BioTek) and Microsoft Excel. A 5% growth inhibition was considered the minimal level statistically different from the control (p < 0.05).
2.8. Mycotoxin Reduction Tests
Mycotoxins (BIOPURE, Romer Labs, Tulln, Austria) were diluted with MRS broth. All mycotoxin-supplemented 16 h MRS LAB cultures (37 °C) were incubated for 1 h at 25 °C by shaking (250 rpm), then centrifuged (13,600× g for 5 min, 4 °C). The supernatants were removed, treated with methanol in a 1:1 ratio, vortexed at high speed, filtered with a 0.45 μm pore Spartan syringe filter (Whatman GmbH, Dassel, Germany), and analyzed with HPLC (2.5) [36].
2.9. Biocontrol Efficacy of CFCS on Grains
MRS broth was inoculated with 16–24 h old bacterial culture (OD 600nm = 1) and incubated at 37 °C for 48 h at static conditions. Peanuts and wheat were washed with distilled water, surface disinfected with 1 w/v % sodium hypochlorite for 1 min, washed with sterile distilled water, and air dried. A mix of 10 g surface-sterilized peanut, and 2 mL of the CFCS (10× concentrated) of W. confusa were incubated at room temperature for 3 h. Then the peanuts were contaminated with 200 µL of Fusarium conidiospore suspension and incubated at 30 °C for 7 days [39]. The control sample, designed to assess the effect of the bacterial treatment, consisted of 10 g of peanuts treated with 2 mL of sterile MRS broth. Disease incidence (DI) was calculated from the number of infected peanuts (1).
DI = (number of infected peanuts × 100)/total number of peanuts treated
Samples of 40 g of surface-sterilized wheat grains and 5 mL of CFCS (10× concentrated) were incubated at room temperature for 3 h. Then, 1 mL of a fungal suspension was inoculated into each dish and mixed to ensure homogenization. To create the control samples, the substrate was inoculated with 5 mL of sterile MRS broth. The Petri plates were incubated at 30 °C for 7 days and grains were visually checked for fungal growth [40]. Disease incidence was determined based on fungal mycelial coverage in the Petri dish.
2.10. Statistical Analyses
Growth data analysis was performed in Gen5 3.05 software (BioTec, Winooski, VT, USA) and significance analysis was done in Microsoft Excel version 2501 Analysis ToolPac Add-in, where Tukey’s test (p ≤ 0.05) was performed.
3. Results
3.1. Characterization of Bacterial Isolates
From neonatal feces (three isolates) and bovine milk samples (five isolates), we isolated eight LAB. From these isolates, based on their antagonistic activity against the Fusaria strains [Fusarium oxysporum MTCC 284, Fusarium verticillioides NCIM 1100 (ATCC 14164) and Fusarium verticillioides NCIM 1099 (ATCC 12616), Fusarium graminearum MTCC 2089], BF2 and ML2 isolates were selected for further antifungal studies. The isolates were tested using biochemical characterization and Gram staining. ML2 and BF2 isolates were Gram-positive, non-motile, short rod-shaped bacteria with flat, circular, creamy-white colonies (Figure 1).
Figure 1.
Macromorphological characterization of Weissella confusa BF2 and ML2 strains.
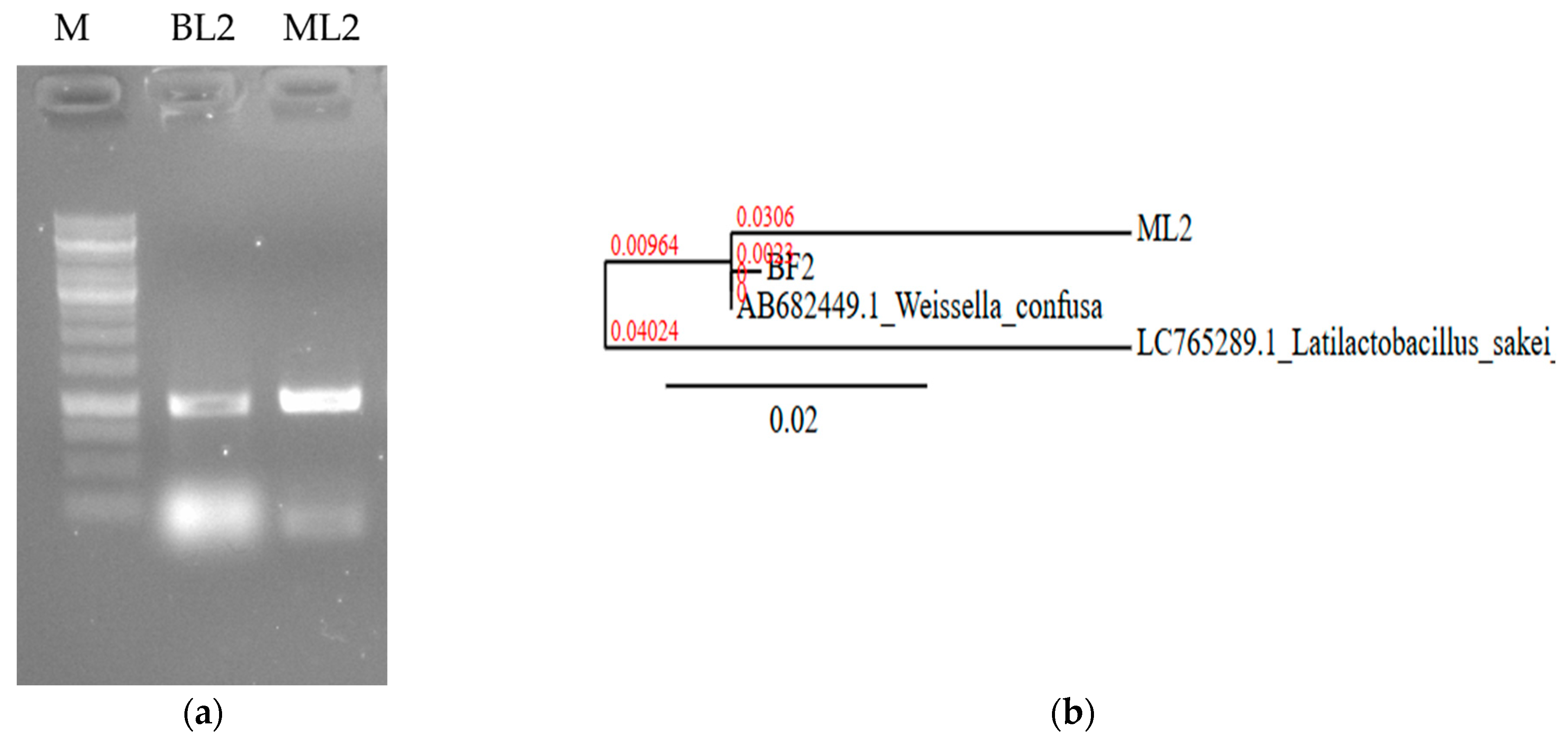
They were non-spore-forming and catalase-negative (Table 1). The isolates were identified based on 16S rRNA nucleic acid PCR (Figure 2a). Sequences were uploaded to the NCBI database under accession number PP535080.2 as W. confusa strain ML2 16S ribosomal RNA gene, partial sequence (688 bp DNA) and PP029339.2 as W. confusa strain BF2 16S ribosomal RNA gene, partial sequence (560 bp DNA). The sequence of ML2 showed 99.47% homology (score: 1018) with 99% coverage with W. confusa HA1 16S RNA partial sequence (accession number: MK255068.1). The BF2 sequence was found to have 100% homology and 100% coverage with several W. confusa strains; e.g., W. confusa 1632 16S rRNA, partial sequence (score: 1035; accession number: MT597518.1). Phylogenetic analysis revealed that the BF2 and the ML2 sequences are closely related (Figure 2b).

Table 1.
Identification and characterization of lactic acid bacteria isolates.
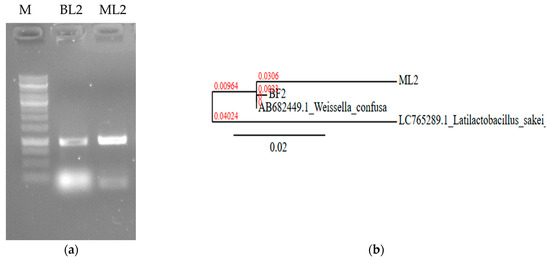
Figure 2.
Nucleic acid-based identification of BL2 and ML2 isolates. (a) Sequence amplification with 16S rRNA specific primers. M: 1 kb DNA marker. (b) Neighbor-joining phylogenetic tree based on 16S rDNA sequence showing the relationship with Weissella confusa NBRC 106469 gene:AB682449.1. Latilactobacillus sakei LC765289.1 was used as an outgroup. The scale bar indicates the evolutionary distance, and the number along the tree branch indicates the bootstrap value.
3.2. Fusarium Antagonism
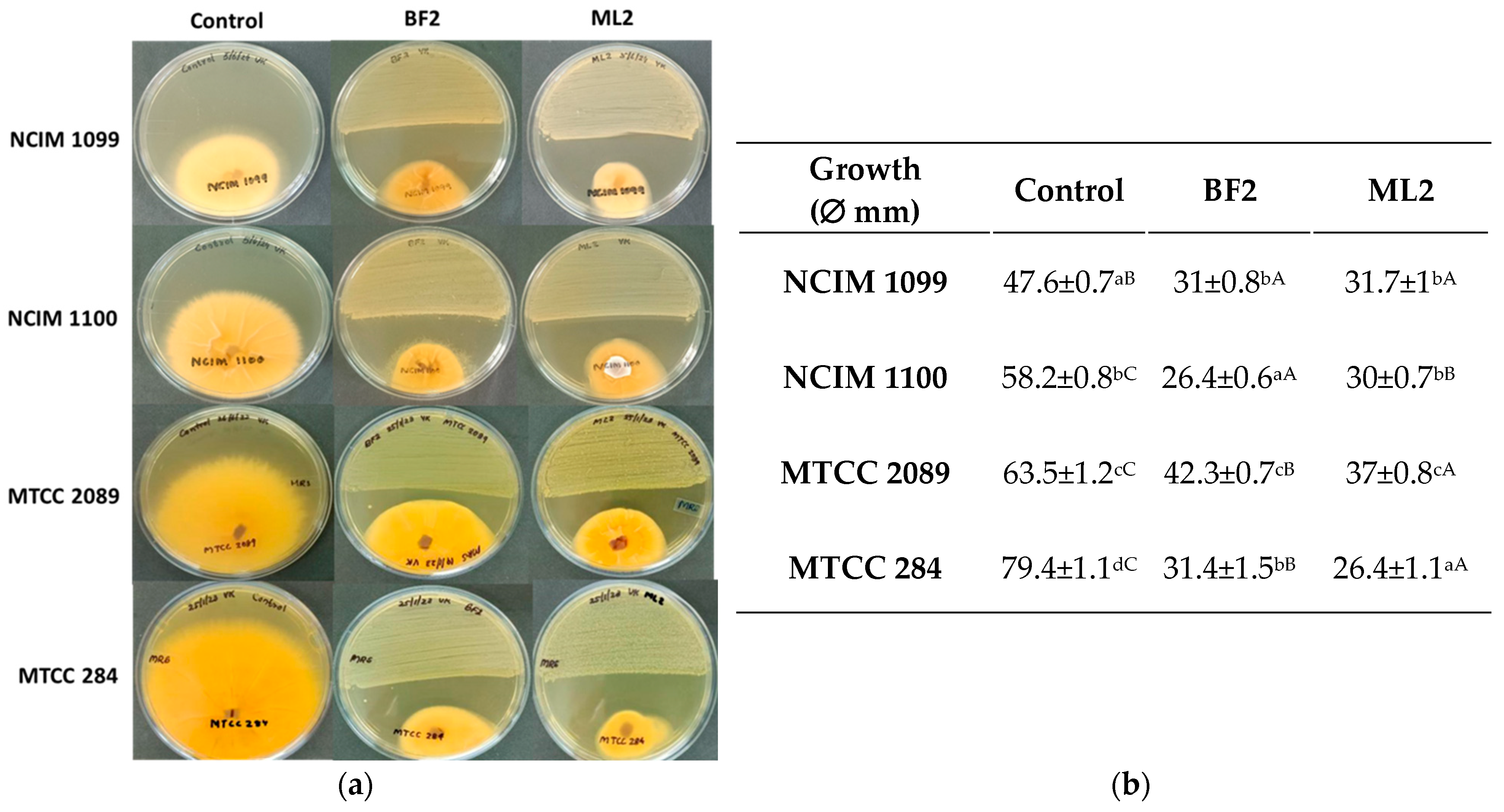
The antagonism test showed significantly different inhibition by W. confusa BF2 and ML2 strains against Fusarium cultures on MRS agar (Figure 3). Against F. verticillioides NCIM 1099, 34% inhibition was shown by both isolates without significant differences. However, F. verticillioides NCIM 1100 was more sensitive as 55% and 46% inhibitions were calculated for BF2 and ML2 strains, respectively. Against F. graminearum MTCC 2089, 34% and 42% inhibitions were found for ML2 and BF2 strains, respectively. F. oxysporum MTCC 284 was the most sensitive to the Weissella strains, as 63% and 69% inhibitions were detected for the two strains.
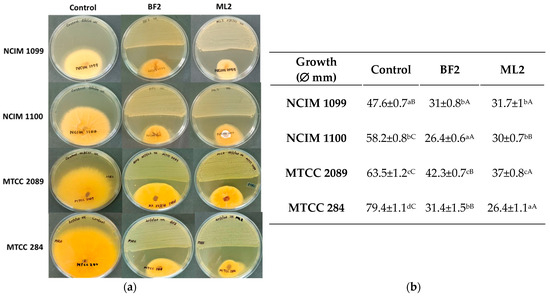
Figure 3.
Weissella confusa isolates show antagonism against Fusarium cultures. (a) The activity of Weissella confusa strains (BF2 and ML2) against Fusarium verticillioides NCIM 1099, F. verticillioides NCIM 1100, F. graminearum MTCC 2089, and F. oxysporum MTCC 284. (b) The mean inhibition-zone diameter and standard deviation are shown (n = 3). Significant differences are shown with different letters (p < 0.05). Lowercase letters compare fungal growth results gained under the same treatment. In contrast, uppercase letters show significant differences between treatments of the same fungal cultures.
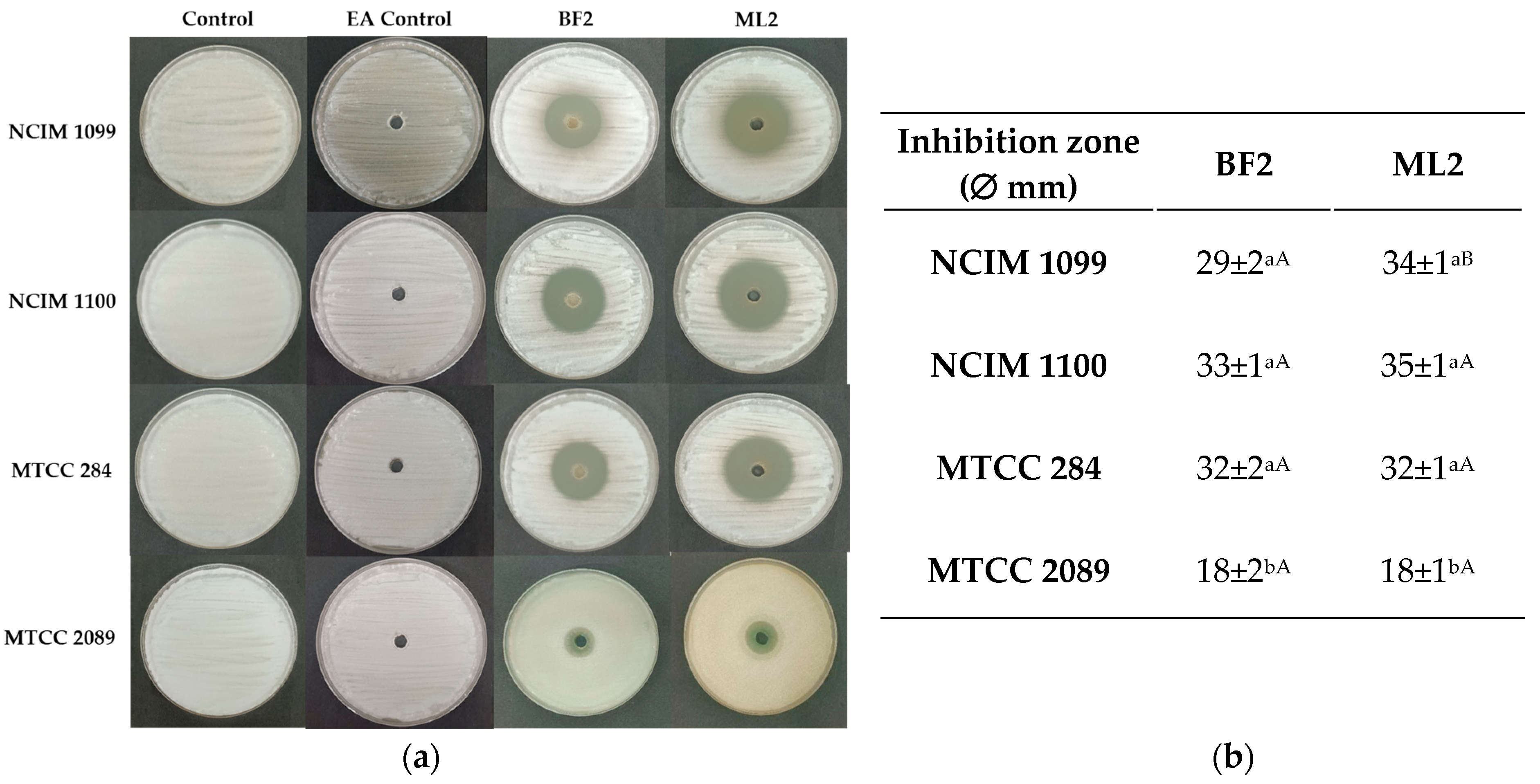
A well-diffusion test with EA extract of the CFCS exhibited the highest antifungal activity against F. verticillioides strains (NCIM 1099 and NCIM 1100), and more against F. oxysporum MTCC 284 than against F. graminearum MTCC 2089 (Figure 4). Between the two strains, no significant difference was detected in antifungal effect against the fungal cultures except against NCIM 1099, while the CFCS EA extract of W. confusa isolate could inhibit both F. verticilloides strains and F. oxysporum without significant differences. F. graminearum MTCC 2089 showed higher resistance against the CFCS extract’s antifungal effect.
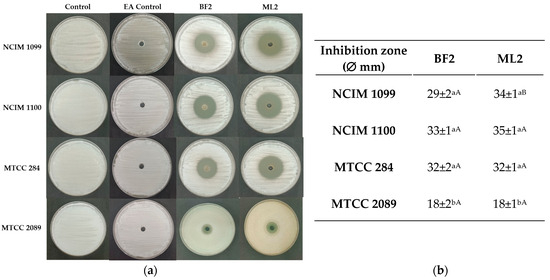
Figure 4.
Diffusion test. (a) Antifungal activity of ethyl acetate extracts of CFCS of Weissella confusa BF2 and ML2 against Fusarium verticillioides NCIM 1099 and NCIM 1100, F. oxysporum MTCC 284, and F. graminearum MTCC 2089 strains. EA: ethyl acetate. (b) The average diameters of the inhibition zones, along with their standard deviations, are presented (n = 3). Statistically significant differences (p < 0.05) are indicated by different letters. Lowercase letters denote differences in inhibition zones produced by varying concentrations of the same strain’s cell-free culture supernatant (CFCS) extract. Uppercase letters signify differences in inhibition zones produced by different strain extracts on the same fungal strain.
3.3. Mycotoxin Resistance and Reduction
The growth inhibition of Weissella confusa strains by different Fusarium mycotoxins and the elimination of the same mycotoxins were also tested. A significant growth reduction (22.1–24.5%) was detected for W. confusa ML2 with all tested mycotoxins (DON, ZEA, T2, FB1) at 2 mg/L toxin concentration, and there were no statistically significant differences within the treatments (p > 0.05). Interestingly, W. confusa BF2 showed resistance to all tested mycotoxins. Mycotoxin reduction was also characterized in liquid cultures, and the same 2 mg/L mycotoxin concentration was tested, but no mycotoxin reduction could be observed.
3.4. Biocontrol Tests
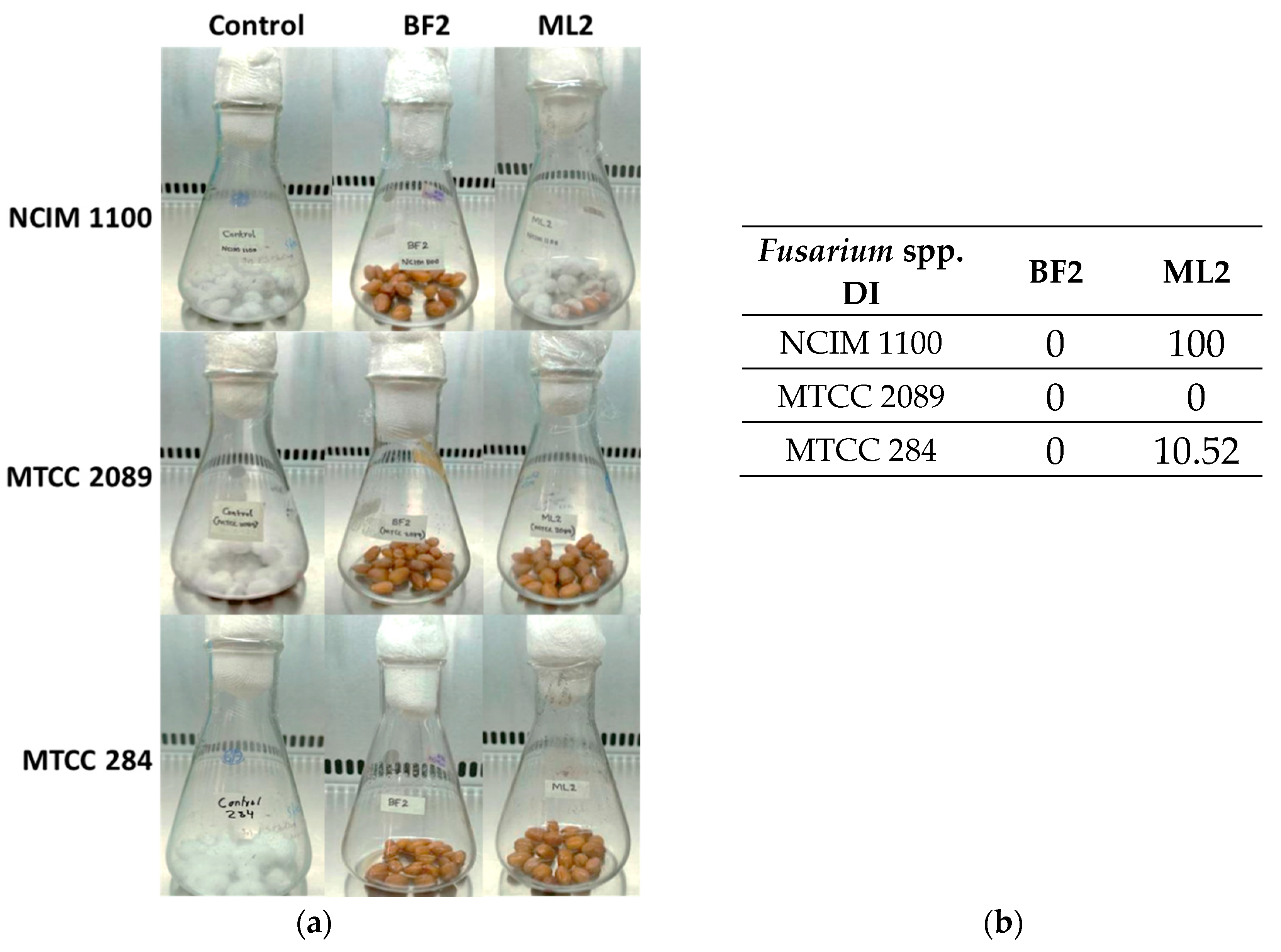
Biocontrol efficacy experiments with peanuts revealed that W. confusa ML2 has limited antifungal activity against F. verticillioides NCIM 1100 (Figure 5a), while applying BF2 in peanut treatment was successful against all three Fusarium species. The disease incidence (DI), which for the controls was 100%, was zero for all of the BF2 isolate treatments, while for the ML2 strain, it was 100% with NCIM 1100, 10.52% with MTCC 284, and zero with MTCC 2089 (Figure 5b).
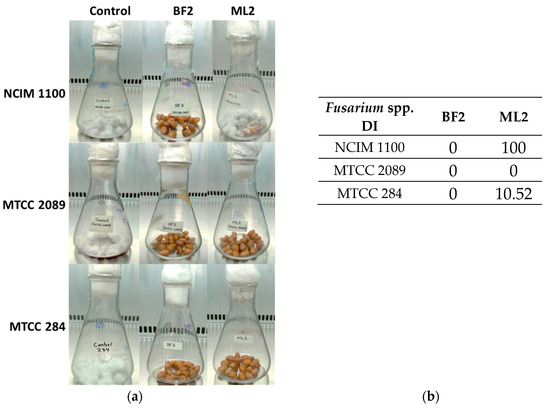
Figure 5.
The antifungal effect was tested on surface-disinfected peanuts. Fusarium verticillioides NCIM 1100, F. oxysporum MTCC 284, and F. graminearum MTCC 2089 strains were tested against cell-free culture supernatants (CFCSs) of Weissella confusa BF2 and ML2 strains. (a) Pictures illustrating Fusaria growth on the surface of peanuts that had been disinfected, taken after a 7–day incubation. (b) Evaluation of Fusarium spp. growth with CFCSs. Disease incidence (DI) was calculated for the peanuts.
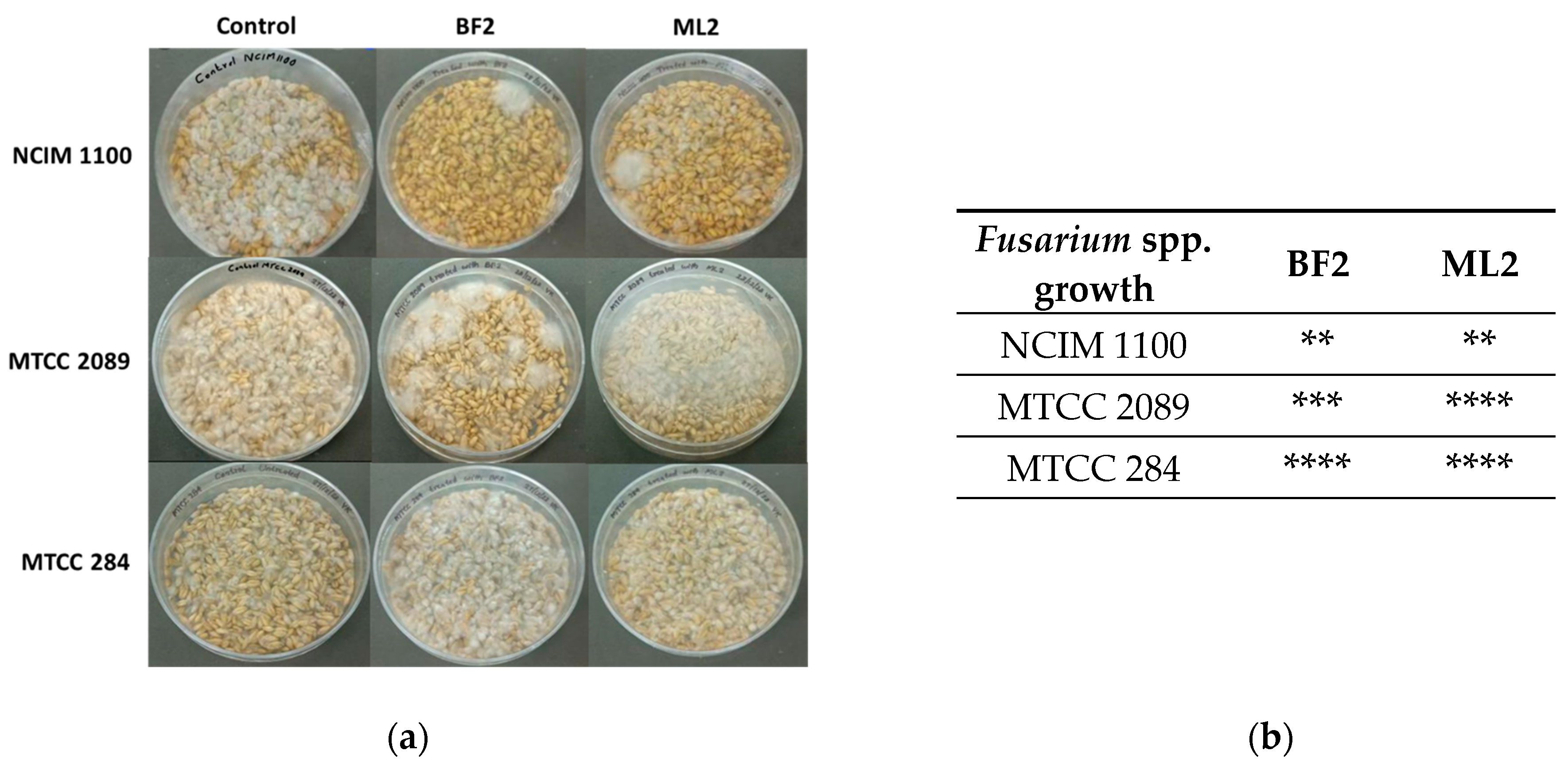
Surface-disinfected wheat grains were also treated with bacterial isolates and infected with Fusarium sp. (Figure 6). It was concluded that the resulting antifungal effect was variable. Contrary to the above findings on peanuts, F. verticillioides NCIM 1100 was sensitive to W. confusa ML2 and BF2. F. graminearum MTCC 2089 was more sensitive to W. confusa BF2, while F. oxysporum MTCC 284 was not sensitive to the CFCS treatments.
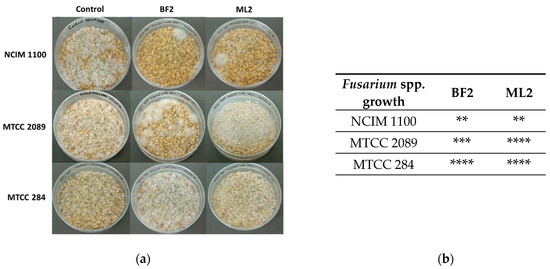
Figure 6.
The antifungal effect was tested on surface-disinfected wheat grains. Fusarium verticillioides NCIM 1100, F. oxysporum MTCC 284, and F. graminearum MTCC 2089 strains were tested against the CFCSs of Weissella confusa BF2 and ML2 strains. (a) After seven days of growth, the presence of contaminating Fusaria was examined. (b) The extent of fungal contamination was then evaluated: **: colony formation by Fusaria (or 25%); ***: colony formation by Fusaria (or 50%); ****: complete contamination by Fusaria compared to controls.
4. Discussion
Weissella confusa is not a well-known industrially applied LAB. It was recently identified from sorghum, wheat semolina, sourdough, etc., [5], and now from fecal matrix and milk. It effectively inhibited Penicillium spp., Aspergillus nidulans, Rhodotorula spp., and Endomyces fibuligera [41,42].
LAB strains can combat mycotoxigenic filamentous fungi (e.g., Fusarium spp., Penicillium spp., Aspergillus spp.) by competing for resources and releasing a range of anti-microbial compounds, specifically small molecules and bacteriocin-like substances [43,44]. Of course, the production of W. confusa’s antifungal compounds depends on environmental conditions such as growth media, temperature and incubation time, pH, and nutritional factors [45,46]. Here, similar experimental settings were applied to compare the strains’ performances.
W. confusa strains were recently found to inhibit Fusarium culmorum spore proliferation [35]. Furthermore, ethyl acetate (EA) extracts of Lactiplantibacillus plantarum CFCS inhibited Fusarium metabolism [47]. W. confusa ML2 showed a similar antagonistic effect to the BF2 strain against all tested Fusaria. The well-diffusion assay using EA extracts of CFCS of W. confusa strains (ML2 and BF2) showed intense antifungal activity against Fusarium strains. Based on the literature, antifungal activity was connected to polylactic acid (PLA), 2-hydroxy-4-methylpentanoic acid, and other organic acid synergism [48] and also to some bacteriocins [11,49]; Weissella cibaria PS2 and certain Lactobacillus species were found to produce antifungal carboxylic acids, including benzoic, vanillic, azelaic, hydrocinnamic, and hydroxybenzoic acids, which were isolated from their culture media [21]. Initial research suggests that certain Weissella strains possess antifungal properties, demonstrating varied levels of effectiveness against fungal diseases like Verticillium wilt and Mucor folium [50]. These studies indicate Weissella’s ability to hinder the growth of filamentous fungi, highlighting its potential as a growth-inhibiting agent. The cell-free supernatant produced by Weissella cibaria BYL4.2 hindered the growth of Penicillium chrysogenum by preventing the germination of its conidiospores. This suggests that the antifungal properties of the CFCS are due to the presence of bioactive compounds within it [51]. However, in our case, further experiments are needed to identify effector molecules against Fusarium spp. in these Weissella strains.
The composition and structure of the plant material were also essential factors in the modelled biocontrol. Peanuts have a different chemical composition than wheat grains, affecting antifungal compound diffusion, bacterial performance, and, presumably, fungal spore attachment to the surface. This could explain the difference in the antifungal effects in peanuts and wheat against the same Fusarium species by the same strain.
The present study investigated W. confusa isolates and concluded that the isolates had potential as antifungal LAB for Fusarium mold control. Also, it was determined for the first time that these Weissella isolates were unsuitable for Fusarium mycotoxin reduction. Therefore, their application could only be suggested at the beginning of mycelial growth before mycotoxin production. It also concluded that the application of the bacteria is matrix-dependent, and other extraction methods also need to be tested before all antifungal factors can be successfully analyzed.
Author Contributions
Conceptualization, K.M.N. and I.P.; investigation, S.V.K., P.A.A., S.K. (Szilvia Kovács), C.A., I.M. and S.K. (Szabina Király); validation, T.P.; funding acquisition, K.M.N. and I.P.; writing—original draft preparation, K.M.N. and T.P.; supervision, K.M.N. and I.P.; writing—review and editing, T.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the Department of Science Technology (DST), India, for financial support for the Indo-Hungary International project [DST/INT/Hun/P-27/2020(G), dated 9 July 2021]. The project, no. 2019-2.1.13-TÉT_IN-2020-00056, was implemented with support provided by the National Research, Development and Innovation Fund of Hungary, financed under the 2019-2.1.13-TÉT_IN funding scheme. Project no. TKP2021-NKTA-32 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CFCS | Cell-Free Culture Supernatant |
| DON | Deoxynivalenol |
| ZEA | Zearalenone |
| FB1 | Fumonisin B1 |
| FDA | U.S. Food and Drug Administration |
| EFSA | European Food Safety Authority |
| LAB | Lactic Acid Bacterium |
| PDA | Potato Dextrose Agar |
| PDB | Potato Dextrose Broth |
| MRS | de-Man-Rogosa-Sharpe |
| MHA | Mueller- Hinton Agar |
| NCIM | National Collection of Industrial Microorganisms |
| ATCC | American Type Culture Collection |
| MTCC | Microbial Type Culture Collection and Gene Bank |
| EA | ethyl acetate |
References
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef]
- Sharpe, M.E.; Garvie, E.I.; Tilbury, R.H. Some slime-forming heterofermentative species of the genus Lactobacillus. Appl. Microbiol. 1972, 23, 389–397. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Kandler, O. Zur Taxonomie der Gattung Lactobacillus Beijerinck. VI. Lactobacillus coprophilus subsp. confusus nov. subsp., eine neue Unterart der Untergattung Betabacterium [Taxonomy of the species Lactobacillus Beijerinck. VI. Lactobacillus coprophilus subsp. confusus nov. subsp., a new variety of the subspecies Betabacterium]. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 1969, 123, 657–666. (In German) [Google Scholar] [PubMed]
- Björkroth, J.; Dicks, L.M.; Endo, A. The genus Weissella. In Lactic Acid Bacteria: Biodiversity and Taxonomy; Holzapfel, W.H., Wood, B.J.B., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 417–428. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Kavitake, D.; Singh, S.P.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Report on aflatoxin-binding activity of galactan exopolysaccharide produced by Weissella confusa KR780676. 3 Biotech 2020, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Yeu, J.E.; Lee, H.G.; Park, G.Y.; Lee, J.; Kang, M.S. Antimicrobial and antibiofilm activities of Weissella cibaria against pathogens of upper respiratory tract infections. Microorganisms 2021, 9, 1181. [Google Scholar] [CrossRef]
- Kariyawasam, K.M.G.M.M.; Jeewanthi, R.K.C.; Lee, N.K.; Paik, H.D. Characterization of cottage cheese using Weissella cibaria D30: Physicochemical, antioxidant, and antilisterial properties. J. Dairy Sci. 2019, 102, 3887–3893. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Ha, M.; Bae, O.; Lee, Y. Effect of Weissella confusa strain PL9001 on the adherence and growth of Helicobacter pylori. Appl. Environ. Microbiol. 2002, 68, 4642–4645. [Google Scholar] [CrossRef]
- Dey, D.K.; Khan, I.; Kang, S.C. Anti-bacterial susceptibility profiling of Weissella confusa DD_A7 against the multidrug-resistant ESBL-positive E. coli. Microbial. Pathogen. 2019, 128, 119–130. [Google Scholar] [CrossRef]
- Singh, J.K.; Devi, P.B.; Reddy, G.B.; Jaiswal, A.K.; Kavitake, D.; Shetty, P.H. Biosynthesis, classification, properties, and applications of Weissella bacteriocins. Front. Microbiol. 2024, 15, 1406904. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef]
- Cupi, D.; Elvig-Jørgensen, S.G. Safety assessment of Weissella confuse—A direct-fed microbial candidate. Reg. Toxicol. Pharmacol. 2019, 107, 104414. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Yeu, J.E.; Hong, S.P. Safety evaluation of oral care probiotics Weissella cibaria CMU and CMS1 by phenotypic and genotypic analysis. Int. J. Mol. Sci. 2019, 20, 2693. [Google Scholar] [CrossRef]
- Bourdichon, F.; Patrone, V.; Fontana, A.; Milani, G.; Morelli, L. Safety demonstration of a microbial species for use in the food chain: Weissella confusa. Int. J. Food Microbiol. 2021, 339, 109028. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Zannini, E.; Arendt, E.K. Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: From crop farming to cereal products. Food Microbiol. 2014, 37, 78–95. [Google Scholar] [CrossRef]
- Gonçalves, A.; Gkrillas, A.; Dorne, J.L.; Dall’Asta, C.; Palumbo, R.; Lima, N.; Battilani, P.; Venâncio, A.; Giorni, P. Pre-and postharvest strategies to minimize mycotoxin contamination in the rice food chain. Comp. Rev. Food Sci. Food Safety 2019, 18, 441–454. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Saari, N.; Meor Hussin, A.S. Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of foods supply. Molecules 2020, 25, 2655. [Google Scholar] [CrossRef]
- Byrne, M.B.; Thapa, G.; Doohan, F.M.; Burke, J.I. Lactic acid bacteria as potential biocontrol agents for Fusarium head blight disease of spring barley. Front. Microbiol. 2022, 13, 912632. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Tarazona, A.; Jiménez, M.; Mateo, F. Lactic acid bacteria as potential agents for biocontrol of aflatoxigenic and ochratoxigenic fungi. Toxins 2022, 14, 807. [Google Scholar] [CrossRef]
- Nasrollahzadeh, A.; Mokhtari, S.; Khomeiri, M.; Saris, P. Mycotoxin detoxification of food by lactic acid bacteria. Intern. J. Food Cont. 2022, 9, 1. [Google Scholar] [CrossRef]
- Hirozawa, M.T.; Ono, M.A.; Suguiura, I.M.D.S.; Bordini, J.G.; Ono, E.Y.S. Lactic acid bacteria and Bacillus spp. as fungal biological control agents. J. Appl. Microbiol. 2023, 134, lxac083. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, I.; Barbosa, J.; Albano, H.; Nogueira, T.; Teixeira, P. Lactic Acid Bacteria isolated from traditional and innovative alheiras as potential biocontrol agents. Food Microbiol. 2024, 119, 104450. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi. Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1542. [Google Scholar] [CrossRef]
- Krishnan, S.V.; Nampoothiri, K.M.; Suresh, A.; Linh, N.T.; Balakumaran, P.A.; Pócsi, I.; Pusztahelyi, T. Fusarium biocontrol: Antagonism and mycotoxin elimination by lactic acid bacteria. Front. Microbiol. 2024, 14, 1260166. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef]
- Hao, G.; McCormick, S.; Usgaard, T.; Tiley, H.; Vaughan, M.M. Characterization of three Fusarium graminearum effectors and their roles during Fusarium head blight. Front. Plant Sci. 2020, 11, 579553. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Abbas, H.K.; Kommedahl, T.; Jarvis, B.B. Mycotoxin production by Fusarium oxysporum and Fusarium sporotrichioides isolated from Baccharis spp. from Brazil. Appl. Environ. Microbiol. 1989, 55, 254–255. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Hera, C.; Sulyok, M.; Schäfer, K.; Capilla, J.; Guarro, J.; Di Pietro, A. The velvet complex governs mycotoxin production and virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Microbiol. 2013, 87, 49–65. [Google Scholar] [CrossRef]
- Cheli, F.; Campagnoli, A.; Dell’Orto, V. Fungal populations and mycotoxins in silages: From occurrence to analysis. Anim. Feed Sci. Technol. 2013, 183, 1–16. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, S.; Xue, Y.; Wu, W.; Zhao, Y.; Li, Y.; Lv, H. Weissella cibaria DA2 cell-free supernatant improves the quality of sweet corn kernels during post-harvest storage. Postharvest Biol. Technol. 2024, 215, 113021. [Google Scholar] [CrossRef]
- Yun, J.; Kim, T.W.; Cho, C.W.; Lee, J.E. Antifungal mechanisms investigation of lactic acid bacteria against Aspergillus flavus: Through combining microbial metabolomics and co-culture system. J. Appl. Microbiol. 2024, 135, lxae112. [Google Scholar] [CrossRef]
- Bata-Vidács, I.; Kosztik, J.; Mörtl, M.; Székács, A.; Kukolya, J. Aflatoxin B1 and sterigmatocystin binding potential of non-Lactobacillus LAB strains. Toxins 2020, 12, 799. [Google Scholar] [CrossRef]
- Lowe, D.P.; Ulmer, H.M.; Graser, K.; Arendt, E.K. The influence of starter cultures on barley contaminated with Fusarium culmorum TMW 4.0754. J. Am. Soc. Brew. Chem. 2006, 64, 158–165. [Google Scholar] [CrossRef]
- Krishnan, S.V.; Anaswara, P.A.; Nampoothiri, K.M.; Kovács, S.; Adácsi, C.; Szarvas, P.; Király, S.; Pócsi, I.; Pusztahelyi, T. Biocontrol activity of new lactic acid bacteria isolates against Fusaria and Fusarium mycotoxins. Toxins 2025, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Adácsi, C.; Kovács, S.; Pócsi, I.; Győri, Z.; Dombrádi, Z.; Pusztahelyi, T. Microbiological and toxicological evaluation of fer-mented forages. Agriculture 2022, 12, 421. [Google Scholar] [CrossRef]
- Poornachandra Rao, K.; Chennappa, G.; Suraj, U.; Nagaraja, H.; Charith Raj, A.P.; Sreenivasa, M.Y. Probiotic potential of Lactobacillus strains isolated from sorghum-based traditional fermented food. Probiot. Antimicrob. Prot. 2015, 7, 146–156. [Google Scholar] [CrossRef]
- Husain, A.; Hassan, Z.; Huda-Faujan, N.; Lani, M.N. Antifungal activity of lactic acid bacteria isolated from soil rhizosphere on Fusarium species infected chilli seeds. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 29, 182–202. [Google Scholar]
- Rouse, S.; Harnett, D.; Vaughan, A.; Sinderen, D.V. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008, 104, 915–923. [Google Scholar] [CrossRef]
- Valerio, F.; Favilla, M.; De Bellis, P.; Sisto, A.; de Candia, S.; Lavermicocca, P. Antifungal activity of strains of lactic acid bacteria isolated from a semolina ecosystem against Penicillium roqueforti, Aspergillus niger and Endomyces fibuliger contaminating bakery products. Syst. Appl. Microbiol. 2009, 32, 438–448. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Singh, T.P.; Özogul, F. Retrospecting the concept and industrial significance of LAB bacteriocins. Food Biosci. 2022, 46, 101607. [Google Scholar] [CrossRef]
- Salman, M.; Javed, M.R.; Ali, H.; Mustafa, G.; Tariq, A.; Sahar, T.; Naheed, S.; Gill, I.; Abid, M.; Tawab, A. Bioprotection of Zea mays L. from aflatoxigenic Aspergillus flavus by Loigolactobacillus coryniformis BCH-4. PLoS ONE 2022, 17, e0271269. [Google Scholar] [CrossRef]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria–Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Belal, J.M.; Zaiton, H.; Kh, S.S.; Aqilah, Z.N.; Azfar, A.A. Effect of pH and heat treatment on antifungal activity of Lactobacillus fermentum Te007, Lactobacillus pentosus G004 and Pediococcus pentosaceus Te010. Inn. Romanian Food Biotech. 2011, 8, 41–53. [Google Scholar]
- Laitila, A.; Alakomi, H.L.; Raaska, L.; Mattila-Sandholm, T.; Haikara, A. Antifungal activities of two Lactobacillus plantarum strains against Fusarium moulds in vitro and in malting of barley. J. Appl. Microbiol. 2002, 93, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Ndagano, D.; Lamoureux, T.; Dortu, C.; Vandermoten, S.; Thonart, P. Antifungal activity of 2 lactic acid bacteria of the Weissella genus isolated from food. J. Food Sci. 2011, 76, M305–M311. [Google Scholar] [CrossRef] [PubMed]
- Goh, H.F.; Philip, K. Purification and characterization of bacteriocin produced by Weissella confusa A3 of dairy origin. PLoS ONE 2015, 10, e0140434. [Google Scholar] [CrossRef]
- Quattrini, M.; Korcari, D.; Ricci, G.; Fortina, M.G. A polyphasic approach to characterise Weissella cibaria and Weissella confusa strains. J. Appl. Microbiol. 2020, 128, 500–512. [Google Scholar] [CrossRef]
- Somashekaraiah, R.; Mottawea, W.; Gunduraj, A.; Joshi, U.; Hammami, R.; Sreenivasa, M.Y. Probiotic and antifungal attributes of Levilactobacillus brevis MYSN105, isolated from an Indian traditional fermented food Pozha. Front. Microbiol. 2021, 12, 696267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).