The Minute Virus of Canines (MVC) Activates the RhoA/ROCK1/MLC2 Signal Transduction Pathway Resulting in the Dissociation of Tight Junctions and Facilitating Occludin-Mediated Viral Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Plasmid Construction
2.3. Immunoprecipitation (IP) and Mass Spectrometry (MS) Analysis
2.4. Fusion Protein Expression and Purification
2.5. GST-Pulldown
2.6. Cytotoxicity Assay
2.7. siRNA Transfection
2.8. Plasmid Transfection
2.9. Western Blot Analysis
2.10. Co-Immunoprecipitation (Co-IP) Assay
2.11. Immunofluorescence Assay (IFA) and Confocal Microscopy
2.12. Quantity Real-Time PCR (qRT-PCR)
2.13. Flow Cytometry for Membrane Permeability
2.14. Statistical Analysis
3. Results
3.1. Identification of VP2-Interacting Host Proteins
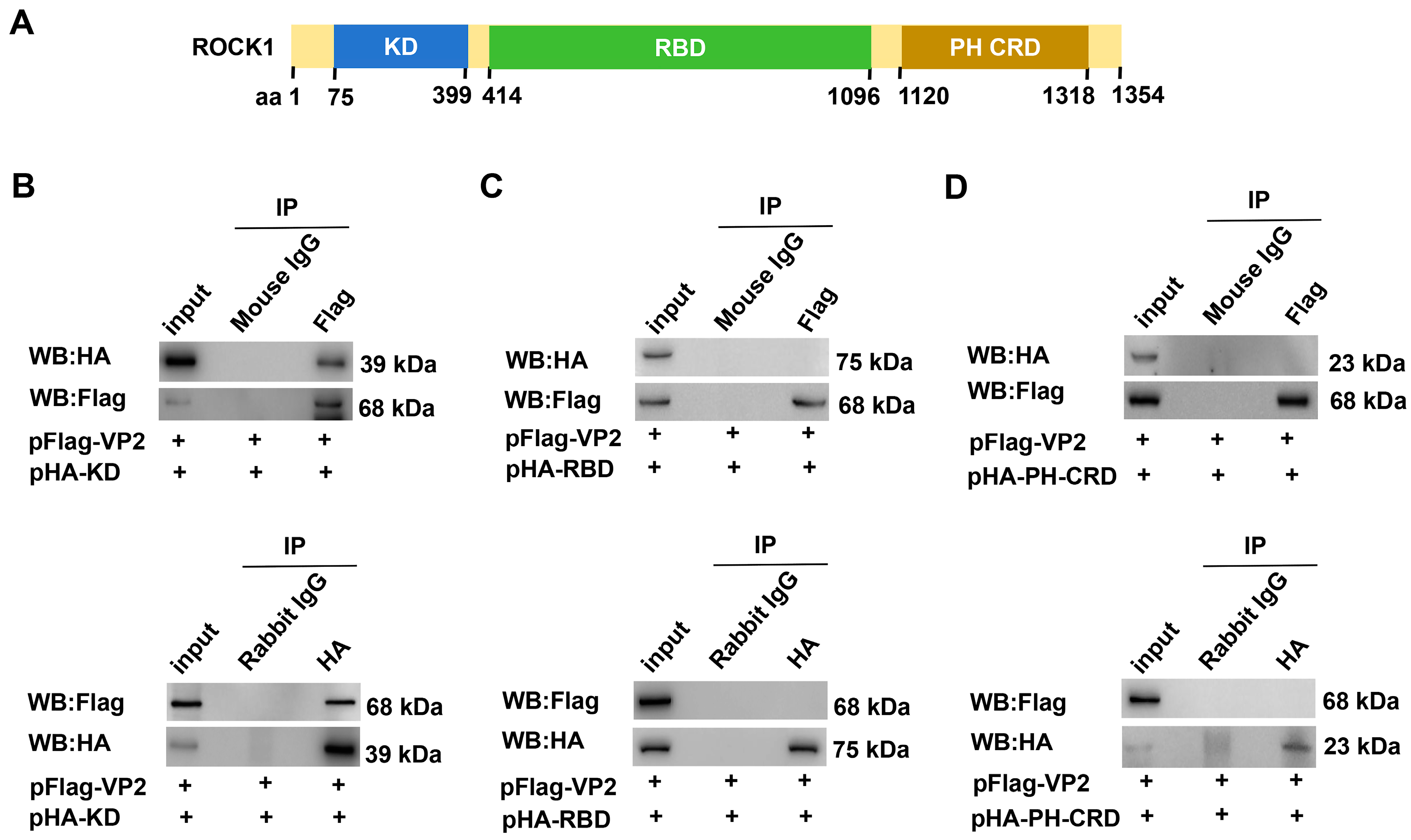
3.2. The Kinase Domain Mediates the Direct Interaction Between ROCK1 and VP2
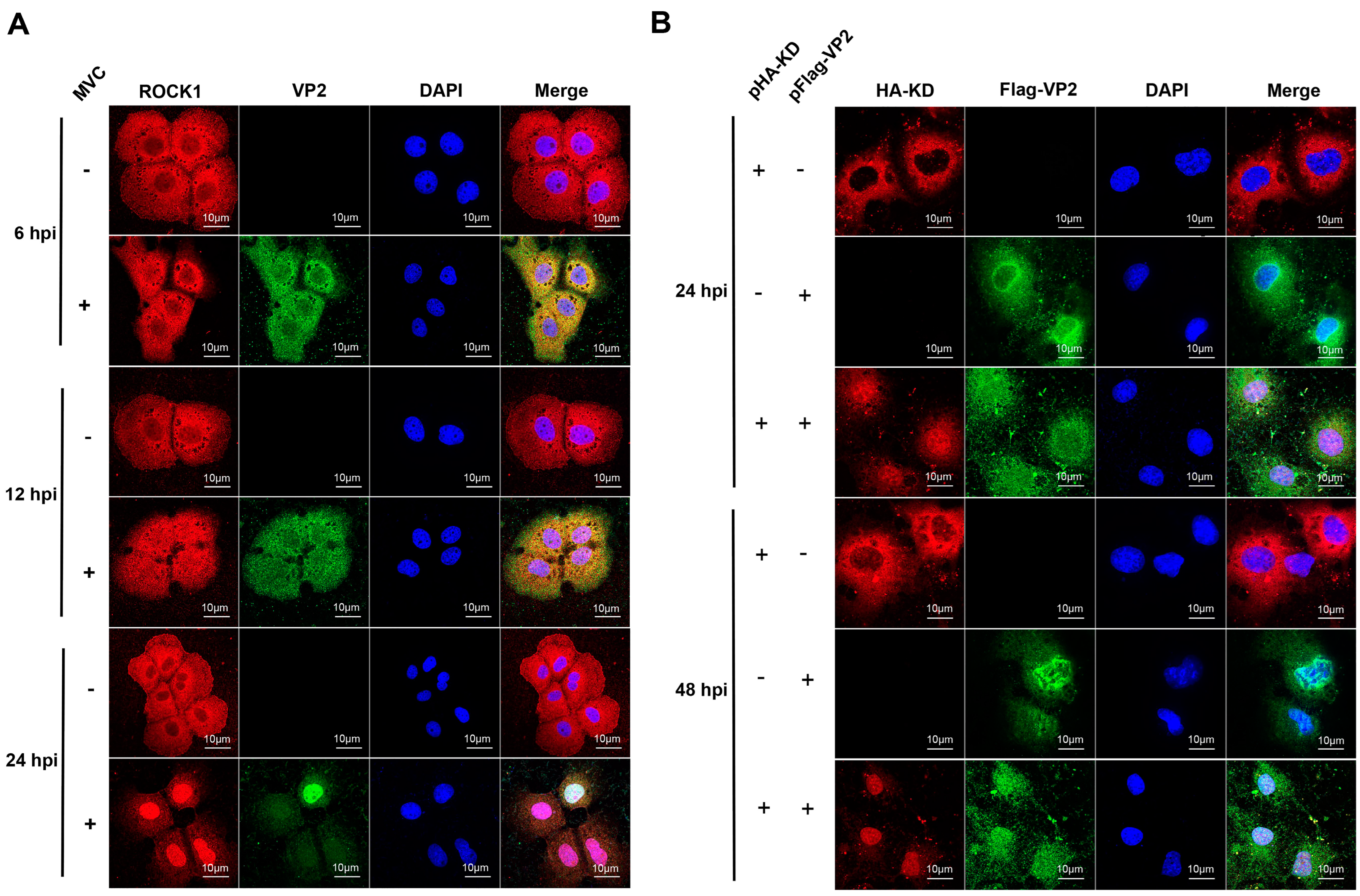
3.3. ROCK1 and VP2 Co-Localized in MVC-Infected WRD and Co-Transfected COS-1 Cells
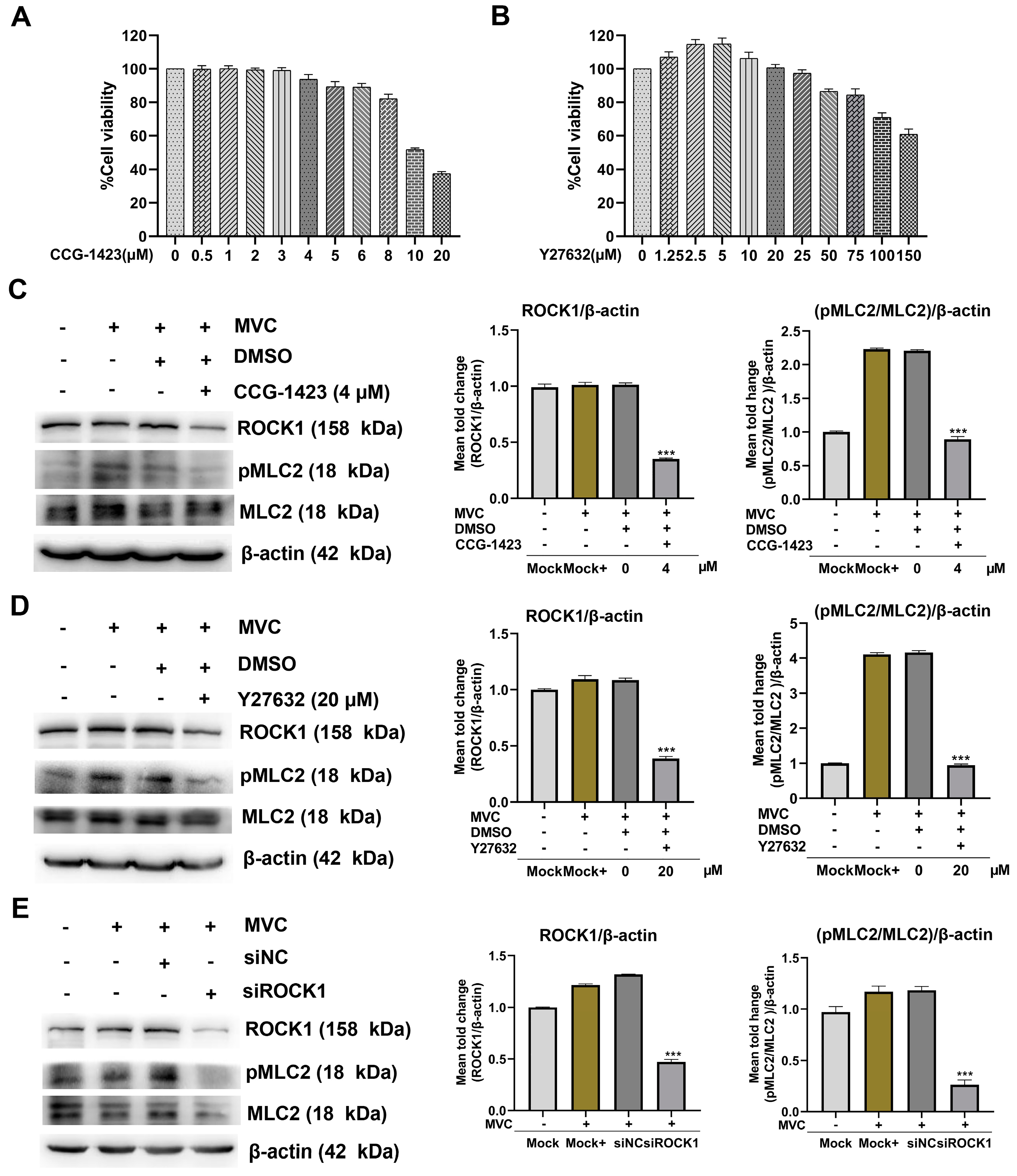
3.4. MVC Activates pMLC via the RhoA/ROCK1 Signaling Pathway in WRD Cells
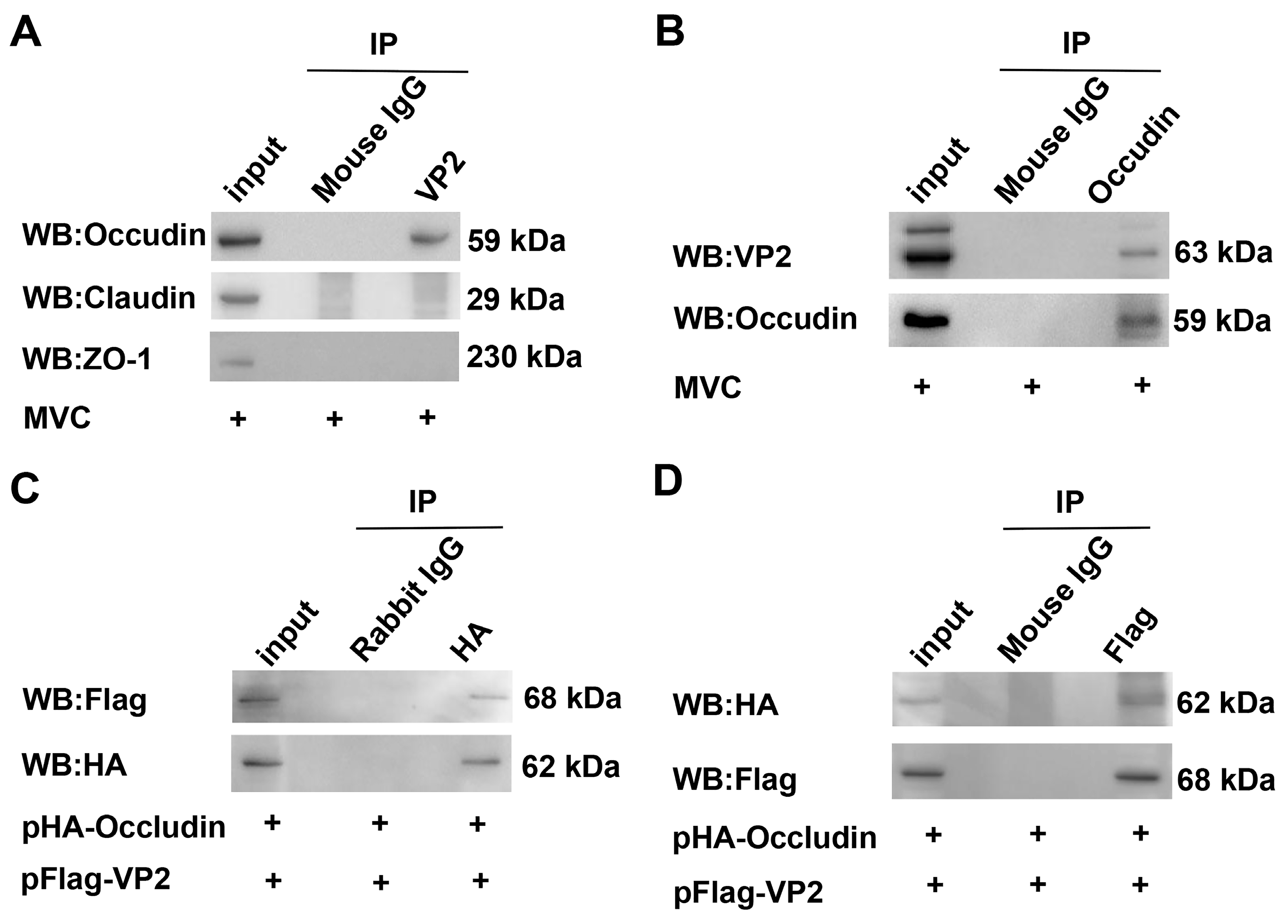
3.5. VP2 Interacts with Occludin but Not with Claudin or ZO-1
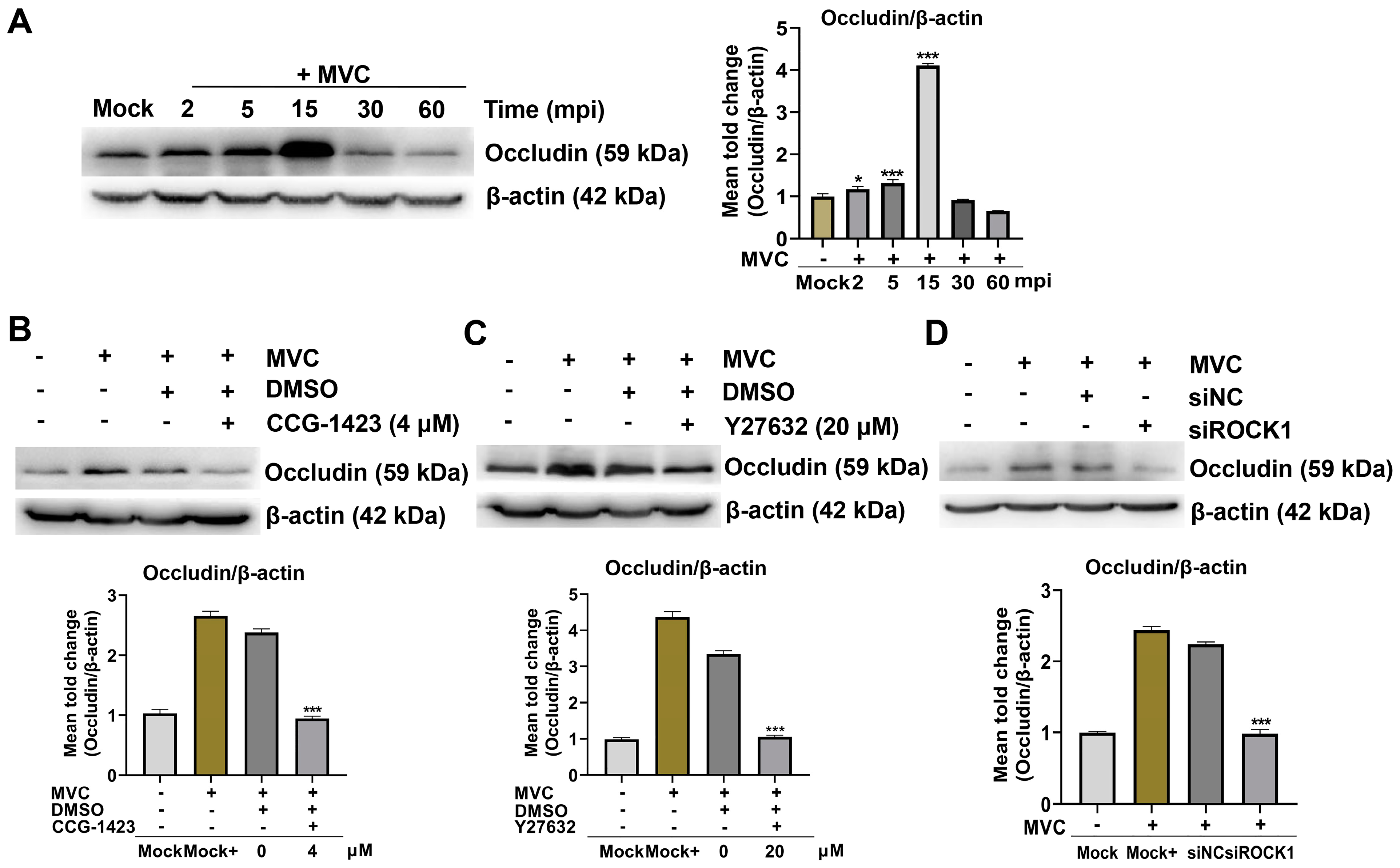
3.6. Inhibition of the RhoA/ROCK1/MLC2 Signaling Pathway Reduces MVC Viral Genome Copies and Viral Protein Expression
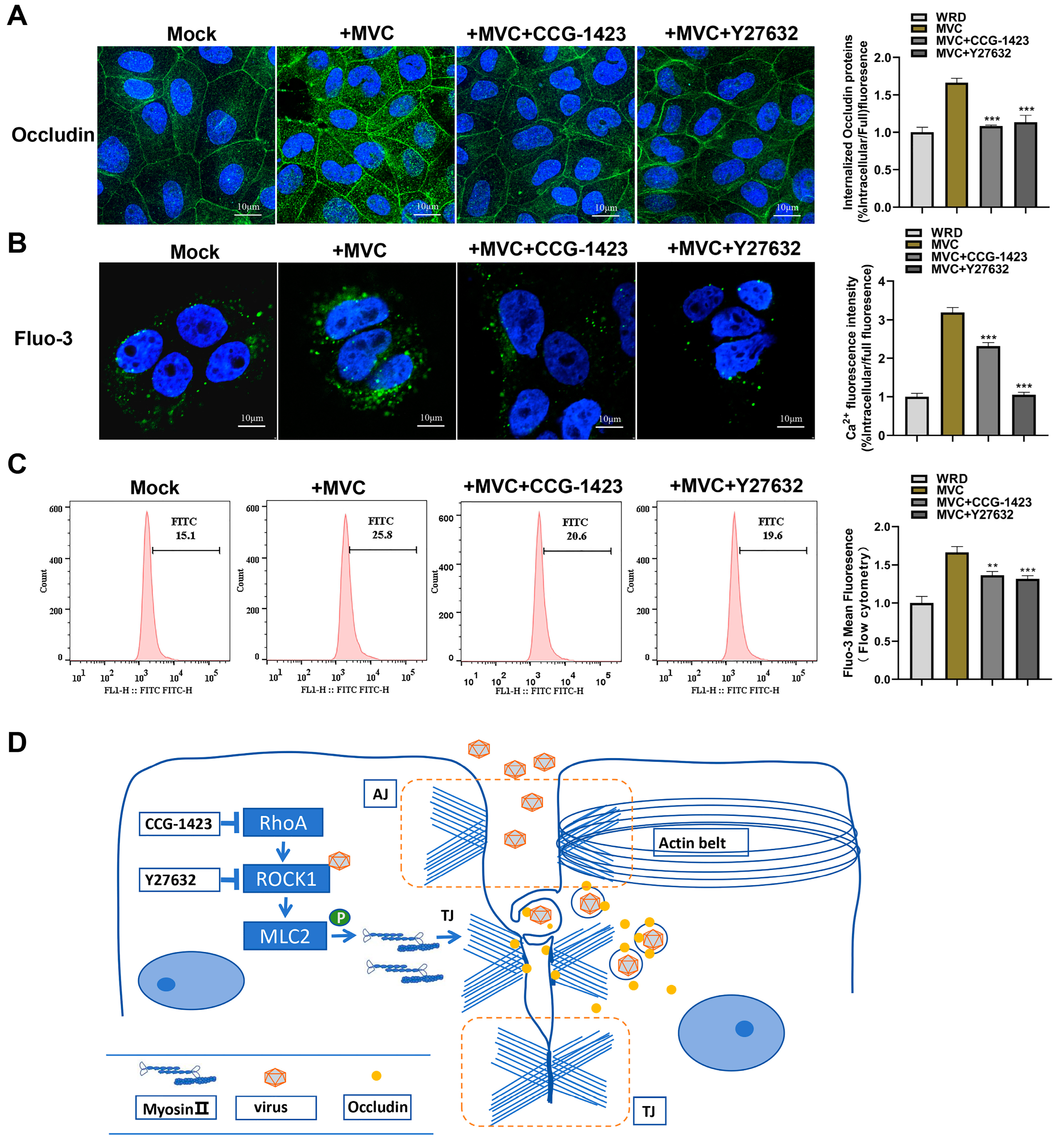
3.7. MVC-Induced Early Activation of the RhoA/ROCK1/MLC2 Pathway Modifies Occludin Protein Distribution and Cell Membrane Permeability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Piewbang, C.; Wardhani, S.W.; Phongroop, K.; Lohavicharn, P.; Sirivisoot, S.; Kasantikul, T.; Techangamsuwan, S. Naturally acquired feline bocavirus type 1 and 3 infections in cats with neurologic deficits. Transbound Emerg. Dis. 2022, 69, e3076–e3087. [Google Scholar] [CrossRef]
- Mohan Jacob, D.; Lee, C.Y.; Arshad, S.S.; Selvarajah, G.T.; Bande, F.; Ong, B.L.; Ooi, P.T. First molecular detection of porcine bocavirus in Malaysia. Trop. Anim. Health Prod. 2018, 50, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Azami, S.N.I.; Loong, S.K.; Khoo, J.J.; Sahimin, N.; Lim, F.S.; Husin, N.A.; Mahfodz, N.H.; Mohd-Taib, F.S.; Ishak, S.N.; Makepeace, B.L.; et al. Molecular evidence of rat bocavirus among rodents in Peninsular Malaysia. J. Vet. Med. Sci. 2022, 84, 938–941. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Li, W.; Fan, Z.; Jiang, L.; Lin, Y.; Fu, X.; Shen, Q.; Sun, Z.; Wang, X.; et al. A novel bocavirus from domestic mink, China. Virus Genes 2016, 52, 887–890. [Google Scholar] [CrossRef]
- Yan, N.; Yue, H.; Kan, R.; Feng, F.; Tang, C. A novel recombinant genome of minute virus of canines in China. Arch. Virol. 2019, 164, 861–865. [Google Scholar] [CrossRef]
- Campalto, M.; Carrino, M.; Tassoni, L.; Rizzo, G.; Rossmann, M.C.; Cocchi, M.; De Benedictis, P.; Beato, M.S. Divergent minute virus of canines strains identified in illegally imported puppies in Italy. Arch. Virol. 2020, 165, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.N.; Cibulski, S.P.; Olegario, J.C.; da Silva, M.S.; Puhl, D.E.; Mosena, A.C.S.; Alves, C.; Paim, W.P.; Baumbach, L.F.; Mayer, F.Q.; et al. Characterization of dog serum virome from Northeastern Brazil. Virology 2018, 525, 192–199. [Google Scholar] [CrossRef]
- Ohshima, T.; Kawakami, K.; Abe, T.; Mochizuki, M. A minute virus of canines (MVC: Canine bocavirus) isolated from an elderly dog with severe gastroenteritis, and phylogenetic analysis of MVC strains. Vet. Microbiol. 2010, 145, 334–338. [Google Scholar] [CrossRef]
- Decaro, N.; Altamura, M.; Pratelli, A.; Pepe, M.; Tinelli, A.; Casale, D.; Martella, V.; Tafaro, A.; Camero, M.; Elia, G.; et al. Evaluation of the innate immune response in pups during canine parvovirus type 1 infection. New Microbiol. 2002, 25, 291–298. [Google Scholar]
- Decaro, N.; Buonavoglia, C. Canine parvovirus—A review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef]
- Piewbang, C.; Wardhani, S.W.; Dankaona, W.; Lacharoje, S.; Chai-In, P.; Yostawonkul, J.; Chanseanroj, J.; Boonrungsiman, S.; Kasantikul, T.; Poovorawan, Y.; et al. Canine bocavirus-2 infection and its possible association with encephalopathy in domestic dogs. PLoS ONE 2021, 16, e0255425. [Google Scholar] [CrossRef]
- Choi, J.W.; Jung, J.Y.; Lee, J.I.; Lee, K.K.; Oem, J.K. Molecular characteristics of a novel strain of canine minute virus associated with hepatitis in a dog. Arch. Virol. 2016, 161, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, A.Y.; Cheng, F.; Guan, W.; Johnson, F.B.; Qiu, J. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J. Virol. 2009, 83, 3956–3967. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.; Green, B.; Carmichael, L.E.; Parrish, C.R. The canine minute virus (minute virus of canines) is a distinct parvovirus that is most similar to bovine parvovirus. Virology 2002, 302, 219–223. [Google Scholar] [CrossRef]
- Dong, Y.; Fasina, O.O.; Pintel, D.J. Minute Virus of Canines NP1 Protein Interacts with the Cellular Factor CPSF6 to Regulate Viral Alternative RNA Processing. J. Virol. 2019, 93, e01530-18. [Google Scholar] [CrossRef]
- Fasina, O.O.; Stupps, S.; Figueroa-Cuilan, W.; Pintel, D.J. Minute Virus of Canines NP1 Protein Governs the Expression of a Subset of Essential Nonstructural Proteins via Its Role in RNA Processing. J. Virol. 2017, 91, e00260-17. [Google Scholar] [CrossRef]
- Tu, M.; Liu, F.; Chen, S.; Wang, M.; Cheng, A. Role of capsid proteins in parvoviruses infection. Virol. J. 2015, 12, 114. [Google Scholar] [CrossRef]
- Dudleenamjil, E.; Lin, C.Y.; Dredge, D.; Murray, B.K.; Robison, R.A.; Johnson, F.B. Bovine parvovirus uses clathrin-mediated endocytosis for cell entry. J. Gen. Virol. 2010, 91 Pt 12, 3032–3041. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Cline, S.E.; Hemming, J.P.; Johnson, F.B. Attachment of bovine parvovirus to O-linked alpha 2,3 neuraminic acid on glycophorin A. Arch. Virol. 2005, 150, 1477–1484. [Google Scholar] [CrossRef]
- Binn, L.N.; Lazar, E.C.; Eddy, G.A.; Kajima, M. Recovery and characterization of a minute virus of canines. Infect. Immun. 1970, 1, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Q.; Yao, Q.; Chen, L.; Li, J.; Qiu, J.; Sun, Y. The DNA replication, virogenesis and infection of canine minute virus in non-permissive and permissive cells. Virus Res. 2014, 179, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Baek, Y.B.; Naveed, A.; Stalin, N.; Kang, M.I.; Park, S.I.; Soliman, M.; Cho, K.O. Porcine Sapovirus-Induced Tight Junction Dissociation via Activation of RhoA/ROCK/MLC Signaling Pathway. J. Virol. 2021, 95, e00051-21. [Google Scholar] [CrossRef]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskelet 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Torres-Flores, J.M.; Arias, C.F. Tight Junctions Go Viral! Viruses 2015, 7, 5145–5154. [Google Scholar] [CrossRef]
- Fukata, Y.; Kimura, K.; Oshiro, N.; Saya, H.; Matsuura, Y.; Kaibuchi, K. Association of the myosin-binding subunit of myosin phosphatase and moesin: Dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J. Cell Biol. 1998, 141, 409–418. [Google Scholar] [CrossRef]
- Duan, X.; Liu, J.; Dai, X.X.; Liu, H.L.; Cui, X.S.; Kim, N.H.; Wang, Z.B.; Wang, Q.; Sun, S.C. Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol. Reprod. 2014, 90, 37. [Google Scholar] [CrossRef]
- Soliman, M.; Cho, E.H.; Park, J.G.; Kim, J.Y.; Alfajaro, M.M.; Baek, Y.B.; Kim, D.S.; Kang, M.I.; Park, S.I.; Cho, K.O. Rotavirus-Induced Early Activation of the RhoA/ROCK/MLC Signaling Pathway Mediates the Disruption of Tight Junctions in Polarized MDCK Cells. Sci. Rep. 2018, 8, 13931. [Google Scholar] [CrossRef]
- Luo, X.; Guo, L.; Zhang, J.; Xu, Y.; Gu, W.; Feng, L.; Wang, Y. Tight Junction Protein Occludin Is a Porcine Epidemic Diarrhea Virus Entry Factor. J. Virol. 2017, 91, e00202-17. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, Y.; Yu, Y.; Wei, J.; Huang, W. Rho-kinase inhibitor hydroxyfasudil protects against HIV-1 Tat-induced dysfunction of tight junction and neprilysin/Abeta transfer receptor expression in mouse brain microvessels. Mol. Cell Biochem. 2021, 476, 2159–2170. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Xuan, Z.; Zhang, S.; Wang, X.; Hao, Y.; Wu, J.; Zhang, S. p38MAPK, Rho/ROCK and PKC pathways are involved in influenza-induced cytoskeletal rearrangement and hyperpermeability in PMVEC via phosphorylating ERM. Virus Res. 2014, 192, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, F.; Duan, L.; Wang, B.; Ye, Y.; Li, P.; Li, D.; Yang, S.; Zhou, L.; Chen, W. E-cadherin Plays a Role in Hepatitis B Virus Entry Through Affecting Glycosylated Sodium-Taurocholate Cotransporting Polypeptide Distribution. Front. Cell. Infect. Microbiol. 2020, 10, 74. [Google Scholar] [CrossRef]
- Guo, J.; Yan, Y.; Sun, J.; Ji, K.; Hei, Z.; Zeng, L.; Xu, H.; Ren, X.; Sun, Y. Chaperones Hsc70 and Hsp70 play distinct roles in the replication of bocaparvovirus minute virus of canines. Mol. Microbiol. 2024, 121, 1127–1147. [Google Scholar] [CrossRef]
- Tsai, B. Penetration of nonenveloped viruses into the cytoplasm. Annu. Rev. Cell Dev. Biol. 2007, 23, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 276. [Google Scholar] [CrossRef]
- Mohan, S.; Das, D.; Bauer, R.J.; Heroux, A.; Zalewski, J.K.; Heber, S.; Dosunmu-Ogunbi, A.M.; Trakselis, M.A.; Hildebrand, J.D.; Vandemark, A.P. Structure of a highly conserved domain of Rock1 required for Shroom-mediated regulation of cell morphology. PLoS ONE 2013, 8, e81075. [Google Scholar] [CrossRef]
- Ubersax, J.A.; Ferrell, J.E., Jr. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 530–541. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta. 2008, 1778, 729–756. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Parkos, C.A.; Nusrat, A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am. J. Pathol. 2010, 177, 512–524. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar] [CrossRef]
- Kaufmann, B.; Simpson, A.A.; Rossmann, M.G. The structure of human parvovirus B19. Proc. Natl. Acad. Sci. USA 2004, 101, 11628–11633. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Roy, P. Sialic Acid Binding Sites in VP2 of Bluetongue Virus and Their Use during Virus Entry. J. Virol. 2022, 96, e0167721. [Google Scholar] [CrossRef]
- Zhang, X.; Boyce, M.; Bhattacharya, B.; Zhang, X.; Schein, S.; Roy, P.; Zhou, Z.H. Bluetongue virus coat protein VP2 contains sialic acid-binding domains, and VP5 resembles enveloped virus fusion proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 6292–6297. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Coyne, C.B. The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses 2011, 3, 2462–2477. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Zhong, Z.; Grasso, L.; Sibilla, C.; Stevens, T.J.; Barry, N.; Bertolotti, A. Prion-like protein aggregates exploit the RHO GTPase to cofilin-1 signaling pathway to enter cells. EMBO J. 2018, 37, e97822. [Google Scholar] [CrossRef]
- Lucera, M.B.; Fleissner, Z.; Tabler, C.O.; Schlatzer, D.M.; Troyer, Z.; Tilton, J.C. HIV signaling through CD4 and CCR5 activates Rho family GTPases that are required for optimal infection of primary CD4+ T cells. Retrovirology 2017, 14, 4. [Google Scholar] [CrossRef]
- Evelyn, C.R.; Wade, S.M.; Wang, Q.; Wu, M.; Iniguez-Lluhi, J.A.; Merajver, S.D.; Neubig, R.R. CCG-1423: A small-molecule inhibitor of RhoA transcriptional signaling. Mol. Cancer Ther. 2007, 6, 2249–2260. [Google Scholar] [CrossRef]
- Ishizaki, T.; Uehata, M.; Tamechika, I.; Keel, J.; Nonomura, K.; Maekawa, M.; Narumiya, S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000, 57, 976–983. [Google Scholar] [PubMed]
- Xu, R.; Feng, X.; Xie, X.; Zhang, J.; Wu, D.; Xu, L. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res. 2012, 1436, 13–19. [Google Scholar] [CrossRef]
- Imai-Matsushima, A.; Martin-Sancho, L.; Karlas, A.; Imai, S.; Zoranovic, T.; Hocke, A.C.; Mollenkopf, H.J.; Berger, H.; Meyer, T.F. Long-Term Culture of Distal Airway Epithelial Cells Allows Differentiation Towards Alveolar Epithelial Cells Suited for Influenza Virus Studies. eBioMedicine 2018, 33, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, Y.; Lin, Z.; Shi, K.; Jiu, Y. Cytoskeleton-a crucial key in host cell for coronavirus infection. J. Mol. Cell Biol. 2020, 12, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Yumine, N.; Matsumoto, Y.; Ohta, K.; Fukasawa, M.; Nishio, M. Claudin-1 inhibits human parainfluenza virus type 2 dissemination. Virology 2019, 531, 93–99. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, W.; Chen, Y.; Zou, M.; Peng, D.; Chen, D. HIV-1 Transactivator Protein Induces ZO-1 and Neprilysin Dysfunction in Brain Endothelial Cells via the Ras Signaling Pathway. Oxid. Med. Cell Longev. 2017, 2017, 3160360. [Google Scholar] [CrossRef]
- Bellmann, C.; Schreivogel, S.; Gunther, R.; Dabrowski, S.; Schumann, M.; Wolburg, H.; Blasig, I.E. Highly conserved cysteines are involved in the oligomerization of occludin-redox dependency of the second extracellular loop. Antioxid. Redox Signal. 2014, 20, 855–867. [Google Scholar] [CrossRef]
- Lavie, M.; Linna, L.; Moustafa, R.I.; Belouzard, S.; Fukasawa, M.; Dubuisson, J. Role of the cytosolic domain of occludin in trafficking and hepatitis C virus infection. Traffic 2019, 20, 753–773. [Google Scholar] [CrossRef]
- Deffieu, M.S.; Clement, C.M.H.; Dorobantu, C.M.; Partiot, E.; Bare, Y.; Faklaris, O.; Riviere, B.; Ayala-Nunez, N.V.; Baumert, T.F.; Ronde, P.; et al. Occludin stalls HCV particle dynamics apart from hepatocyte tight junctions, promoting virion internalization. Hepatology 2022, 76, 1164–1179. [Google Scholar] [CrossRef]
- Liu, S.; Kuo, W.; Yang, W.; Liu, W.; Gibson, G.A.; Dorko, K.; Watkins, S.C.; Strom, S.C.; Wang, T. The second extracellular loop dictates Occludin-mediated HCV entry. Virology 2010, 407, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, W.; Roy, S.; Liu, H.; Roberts, R.M.; Wang, L.; Shi, L.; Ma, W. Tight junction protein occludin is an internalization factor for SARS-CoV-2 infection and mediates virus cell-to-cell transmission. Proc. Natl. Acad. Sci. USA 2023, 120, e2218623120. [Google Scholar] [CrossRef] [PubMed]




| Primers a | (bp) | Cloning Sites | Sequences (5′-3′) b |
|---|---|---|---|
| pCMV-HA-KD F | 972 | SalI | CGCGTCGACGATTATGAAGTAGTGAAGGTG |
| pCMV-HA-KD R | XhoI | TCCCTCGAGTGTAATTACTGTTCACCCTAC | |
| pCMV-HA-RBD F | 2046 | SalI | CGCGTCGACCGAAGTAGCCCCAATGTGGATA |
| pCMV-HA-RBD R | XhoI | TCCCTCGAGGTCCAAAAGTTTGGCACGCAGT | |
| pCMV-HA-PH-CRD F | 594 | SalI | CGCGTCGACAGAATTGAAGGTTGGCTTTCAA |
| pCMV-HA-PH-CRD R | XhoI | TCCCTCGAGTCTTCTTTACCAAAATGTGTTA | |
| pCMV-HA-Occludin F | 1566 | SalI | CGCGTCGACCAGGTTGGCTTATTTTGGGGA |
| pCMV-HA-Occludin R | XhoI | TCCCTCGAGTGCAAAGTTCACCGTGGGACC | |
| pGEX-4T-1-KD F | 972 | EcoRI | GCGGAATTCGATTATGAAGTAGTGAAGGTG |
| pGEX-4T-1-KD R | XhoI | GCGCTCGAGTGTAATTACTGTTCACCCTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Hei, Z.; Ji, K.; Yan, Y.; Tian, C.; Wei, Y.; Sun, Y. The Minute Virus of Canines (MVC) Activates the RhoA/ROCK1/MLC2 Signal Transduction Pathway Resulting in the Dissociation of Tight Junctions and Facilitating Occludin-Mediated Viral Infection. Microorganisms 2025, 13, 695. https://doi.org/10.3390/microorganisms13030695
Ren X, Hei Z, Ji K, Yan Y, Tian C, Wei Y, Sun Y. The Minute Virus of Canines (MVC) Activates the RhoA/ROCK1/MLC2 Signal Transduction Pathway Resulting in the Dissociation of Tight Junctions and Facilitating Occludin-Mediated Viral Infection. Microorganisms. 2025; 13(3):695. https://doi.org/10.3390/microorganisms13030695
Chicago/Turabian StyleRen, Xiang, Zhiping Hei, Kai Ji, Yan Yan, Chuchu Tian, Yin Wei, and Yuning Sun. 2025. "The Minute Virus of Canines (MVC) Activates the RhoA/ROCK1/MLC2 Signal Transduction Pathway Resulting in the Dissociation of Tight Junctions and Facilitating Occludin-Mediated Viral Infection" Microorganisms 13, no. 3: 695. https://doi.org/10.3390/microorganisms13030695
APA StyleRen, X., Hei, Z., Ji, K., Yan, Y., Tian, C., Wei, Y., & Sun, Y. (2025). The Minute Virus of Canines (MVC) Activates the RhoA/ROCK1/MLC2 Signal Transduction Pathway Resulting in the Dissociation of Tight Junctions and Facilitating Occludin-Mediated Viral Infection. Microorganisms, 13(3), 695. https://doi.org/10.3390/microorganisms13030695






