Candida Infections: The Role of Saliva in Oral Health—A Narrative Review
Abstract
1. Introduction
2. Candida Albicans and Non-albicans Candida Species
3. Oral Candidiasis
4. Saliva
4.1. Basic Proline-Rich Proteins (bPRPs)
4.2. Mucin
4.3. Histatin/Statherin
4.4. β-Defensin
4.5. Secretory Immunoglobulin A
4.6. Other Salivary Proteins
5. Virulence Factors of Candida Species
5.1. Secretory Aspartyl Proteinases (Saps)
5.2. Candidalysin
5.3. Agglutinin-Like Sequence (Als) Proteins
5.4. Ssa1/Ssa2
5.5. Other Adhesins
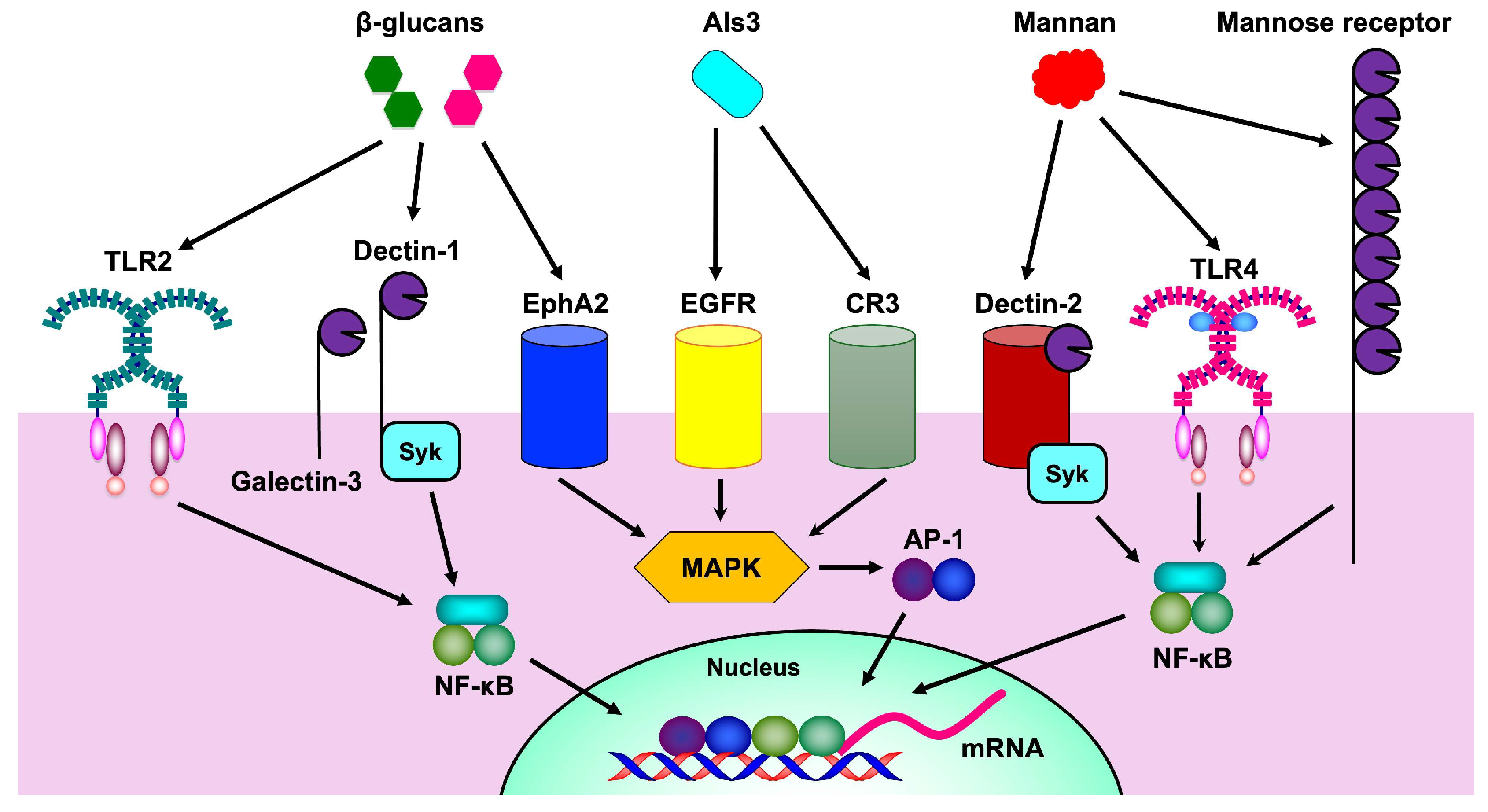
5.6. Mannan
5.7. β-Glucan
6. Candida Species and Oral Diseases
6.1. Candida Species and Dental Caries
6.2. Candida Species and Periodontitis
6.3. Other
7. Candida Species and Cancer
8. Candida Species and Virus Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Sap | Secretory asparaginyl proteinase |
| Als | Agglutinin-like sequence |
| COVID-19 | Coronavirus disease 2019 |
| Th17 | Helper T cell type 17 |
| IL | Interleukin |
| ROS | Reactive oxygen species |
| MAPK | Mitogen-activated protein kinase |
| PCR | Polymerase chain reaction |
| RFLP | Restriction fragment length polymorphism |
| bPRP | Basic proline-rich protein |
| HBD | Human β-defensin |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| sIgA | Secretory immunoglobulin A |
| EGFR | Epidermal growth factor receptor |
| Ece1 | Extent of cell elongation 1 |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 |
| HER2 | Human epidermal growth factor receptor 2 |
| EphA2 | Ephrin type-A receptor 2 |
| Hwp1 | Hyphal wall protein 1 |
| CFEM | Common in fungal extracellular membranes |
| CR3-RP | Complement receptor 3-related protein |
| Pra1 | pH-regulated antigen 1 |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| Gtf | Glucosyltransferase |
| PGE2 | Prostaglandin E2 |
| HIV | Human immunodeficiency virus |
| SARS-CoV | Severe acute respiratory syndrome-coronavirus |
| ICU | Intensive care unit |
| ECM | Extracellular matrix |
| Syk | Spleen tyrosine kinase |
| AP-1 | Activator protein-1 |
| AIDS | Acquired immunodeficiency syndrome |
| OSCC | Oral squamous cell carcinoma |
| OPMD | Oral potentially malignant disorder |
References
- Valand, N.; Gazioglu, O.; Yesilkaya, H.; Shivkumar, M.; Horley, N.; Arroo, R.; Wallis, R.; Kishore, U.; Venkatraman Girija, U. Interactions of Candida tropicalis pH-related antigen 1 with complement proteins C3, C3b, factor-H, C4BP and complement evasion. Immunobiology 2023, 228, 152303. [Google Scholar] [CrossRef]
- Launder, D.; Dillon, J.T.; Wuescher, L.M.; Glanz, T.; Abdul-Aziz, N.; Yi, E.M.; Naglik, J.R.; Worth, R.G.; Conti, H.R. Immunity to pathogenic mucosal C. albicans infections mediated by oral megakaryocytes activated by IL-17 and candidalysin. Mucosal Immunol. 2024, 17, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, A.A.; Wang, T.W.; Perez, P.; Sultan, A.S.; Meiller, T.F.; Rock, P.; Kleiner, D.E.; Chertow, D.S.; Hewitt, S.M.; Gasmi, B.; et al. SARS-CoV-2 infection of salivary glands compromises the production of a secreted antifungal peptide with potential implications for development of oral candidiasis. PLoS Pathog. 2024, 20, e1012375. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.V.G.; Nguyen, H.H.N.; Vo, T.H.; Le, M.T.; Tran-Nguyen, V.K.; Vu, T.T.; Nguyen, P.V. Prevalence and drug susceptibility of clinical Candida species in nasopharyngeal cancer patients in Vietnam. One Health 2024, 18, 100659. [Google Scholar] [CrossRef] [PubMed]
- Kibwana, U.O.; Manyahi, J.; Kamori, D.; Mushi, M.; Mwandigha, A.M.; Majigo, M. Predominance of non-Candida albicans species oral colonisation among patients on anticancer therapy: Findings from a cross-sectional study in Tanzania. BMJ Open 2023, 13, e070003. [Google Scholar] [CrossRef]
- Cavalcanti, Y.W.; Morse, D.J.; da Silva, W.J.; Del-Bel-Cury, A.A.; Wei, X.; Wilson, M.; Milward, P.; Lewis, M.; Bradshaw, D.; Williams, D.W. Virulence and pathogenicity of Candida albicans is enhanced in biofilms containing oral bacteria. Biofouling 2015, 31, 27–38. [Google Scholar] [CrossRef]
- Saijo, S.; Ikeda, S.; Yamabe, K.; Kakuta, S.; Ishigame, H.; Akitsu, A.; Fujikado, N.; Kusaka, T.; Kubo, S.; Chung, S.H.; et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 2010, 32, 681–691. [Google Scholar] [CrossRef]
- Pathirana, R.U.; Friedman, J.; Norris, H.L.; Salvatori, O.; McCall, A.D.; Kay, J.; Edgerton, M. Fluconazole-Resistant Candida auris Is Susceptible to Salivary Histatin 5 Killing and to Intrinsic Host Defenses. Antimicrob. Agents Chemother. 2018, 62, e01872-17. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Pinto-Almazan, R.; Saunte, D.M.L.; Hay, R.; Szepietowsk, J.; Moreno-Coutino, G.; Skerlev, M.; Prohic, A.; Martinez-Herrera, E. Virulence and resistance factors of Nakaseomyces glabratus (formerly known as Candida glabrata) in Europe: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2024, 39, 377–388. [Google Scholar] [CrossRef]
- Chybowska, A.D.; Childers, D.S.; Farrer, R.A. Nine Things Genomics Can Tell Us About Candida auris. Front. Genet. 2020, 11, 351. [Google Scholar] [CrossRef]
- Morace, G.; Perdoni, F.; Borghi, E. Antifungal drug resistance in Candida species. J. Glob. Antimicrob. Resist. 2014, 2, 254–259. [Google Scholar] [CrossRef]
- Kiyoura, Y.; Tamai, R. Innate immunity to Candida albicans. Jpn. Dent. Sci. Rev. 2015, 51, 59–64. [Google Scholar] [CrossRef]
- Shibata, N.; Suzuki, A.; Kobayashi, H.; Okawa, Y. Chemical structure of the cell-wall mannan of Candida albicans serotype A and its difference in yeast and hyphal forms. Biochem. J. 2007, 404, 365–372. [Google Scholar] [CrossRef]
- McKenzie, C.G.; Koser, U.; Lewis, L.E.; Bain, J.M.; Mora-Montes, H.M.; Barker, R.N.; Gow, N.A.; Erwig, L.P. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect. Immun. 2010, 78, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Gow, N.A.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Wellington, M.; Dolan, K.; Krysan, D.J. Live Candida albicans suppresses production of reactive oxygen species in phagocytes. Infect. Immun. 2009, 77, 405–413. [Google Scholar] [CrossRef]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef]
- Yang, W.; Yan, L.; Wu, C.; Zhao, X.; Tang, J. Fungal invasion of epithelial cells. Microbiol. Res. 2014, 169, 803–810. [Google Scholar] [CrossRef]
- Zhu, W.; Filler, S.G. Interactions of Candida albicans with epithelial cells. Cell Microbiol. 2010, 12, 273–282. [Google Scholar] [CrossRef]
- Wingard, J.R.; Dick, J.D.; Merz, W.G.; Sandford, G.R.; Saral, R.; Burns, W.H. Differences in virulence of clinical isolates of Candida tropicalis and Candida albicans in mice. Infect. Immun. 1982, 37, 833–836. [Google Scholar] [CrossRef] [PubMed]
- de Repentigny, L.; Phaneuf, M.; Mathieu, L.G. Gastrointestinal colonization and systemic dissemination by Candida albicans and Candida tropicalis in intact and immunocompromised mice. Infect. Immun. 1992, 60, 4907–4914. [Google Scholar] [CrossRef]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Vizuete, K.; Debut, A.; Tejera, E.; Machado, A. Evaluation of the biofilm life cycle between Candida albicans and Candida tropicalis. Front. Cell Infect. Microbiol. 2022, 12, 953168. [Google Scholar] [CrossRef]
- Ra, C.H.; Jung, J.H.; Sunwoo, I.Y.; Kang, C.H.; Jeong, G.T.; Kim, S.K. Detoxification of Eucheuma spinosum Hydrolysates with Activated Carbon for Ethanol Production by the Salt-Tolerant Yeast Candida tropicalis. J. Microbiol. Biotechnol. 2015, 25, 856–862. [Google Scholar] [CrossRef]
- Gong, Y.; Ding, P.; Xu, M.J.; Zhang, C.M.; Xing, K.; Qin, S. Biodegradation of phenol by a halotolerant versatile yeast Candida tropicalis SDP-1 in wastewater and soil under high salinity conditions. J. Environ. Manag. 2021, 289, 112525. [Google Scholar] [CrossRef]
- Chai, L.Y.; Denning, D.W.; Warn, P. Candida tropicalis in human disease. Crit. Rev. Microbiol. 2010, 36, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Comparison of adherence of Candida albicans and Candida parapsilosis to silicone catheters in vitro and in vivo. Clin. Microbiol. Infect. 2003, 9, 684–690. [Google Scholar] [CrossRef]
- Chapman, R.L. Prevention and treatment of Candida infections in neonates. Semin. Perinatol. 2007, 31, 39–46. [Google Scholar] [CrossRef]
- Holland, L.M.; Schroder, M.S.; Turner, S.A.; Taff, H.; Andes, D.; Grozer, Z.; Gacser, A.; Ames, L.; Haynes, K.; Higgins, D.G.; et al. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog. 2014, 10, e1004365. [Google Scholar] [CrossRef]
- Tóth, A.; Csonka, K.; Jacobs, C.; Vagvolgyi, C.; Nosanchuk, J.D.; Netea, M.G.; Gacser, A. Candida albicans and Candida parapsilosis induce different T-cell responses in human peripheral blood mononuclear cells. J. Infect. Dis. 2013, 208, 690–698. [Google Scholar] [CrossRef]
- Mirhendi, H.; Bruun, B.; Schonheyder, H.C.; Christensen, J.J.; Fuursted, K.; Gahrn-Hansen, B.; Johansen, H.K.; Nielsen, L.; Knudsen, J.D.; Arendrup, M.C. Molecular screening for Candida orthopsilosis and Candida metapsilosis among Danish Candida parapsilosis group blood culture isolates: Proposal of a new RFLP profile for differentiation. J. Med. Microbiol. 2010, 59, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Alastruey-Izquierdo, A.; Rodriguez, D.; Almirante, B.; Pahissa, A.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M.; Barcelona Candidemia Project Study, G. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: Results from population-based surveillance of candidemia in Spain. Antimicrob. Agents Chemother. 2008, 52, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Bertini, A.; De Bernardis, F.; Hensgens, L.A.; Sandini, S.; Senesi, S.; Tavanti, A. Comparison of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis adhesive properties and pathogenicity. Int. J. Med. Microbiol. 2013, 303, 98–103. [Google Scholar] [CrossRef] [PubMed]
- del Pilar Vercher, M.; Garcia Martinez, J.M.; Canton, E.; Peman, J.; Gomez Garcia, M.M.; Gomez, E.V.; del Castillo Agudo, L. Differentiation of Candida parapsilosis, C. orthopsilosis, and C. metapsilosis by specific PCR amplification of the RPS0 intron. Int. J. Med. Microbiol. 2011, 301, 531–535. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Messer, S.A.; Pfaller, M.A.; Diekema, D.J. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J. Clin. Microbiol. 2008, 46, 2659–2664. [Google Scholar] [CrossRef]
- Moran, G.P.; Coleman, D.C.; Sullivan, D.J. Candida albicans versus Candida dubliniensis: Why Is C. albicans More Pathogenic? Int. J. Microbiol. 2012, 2012, 205921. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Moran, G.P. Differential virulence of Candida albicans and C. dubliniensis: A role for Tor1 kinase? Virulence 2011, 2, 77–81. [Google Scholar] [CrossRef]
- Pasligh, J.; Radecke, C.; Fleischhacker, M.; Ruhnke, M. Comparison of phenotypic methods for the identification of Candida dubliniensis. J. Microbiol. Immunol. Infect. 2010, 43, 147–154. [Google Scholar] [CrossRef]
- Silveira-Gomes, F.; Sarmento, D.N.; Espirito-Santo, E.P.; Souza Nde, O.; Pinto, T.M.; Marques-da-Silva, S.H. Differentiation between Candida albicans and Candida dubliniensis using hypertonic Sabouraud broth and tobacco agar. Rev. Soc. Bras. Med. Trop. 2011, 44, 457–460. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef]
- Zamith-Miranda, D.; Heyman, H.M.; Cleare, L.G.; Couvillion, S.P.; Clair, G.C.; Bredeweg, E.L.; Gacser, A.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Multi-omics Signature of Candida auris, an Emerging and Multidrug-Resistant Pathogen. mSystems 2019, 4, e00257-19. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Fakhim, H.; Vaezi, A.; Dannaoui, E.; Chowdhary, A.; Nasiry, D.; Faeli, L.; Meis, J.F.; Badali, H. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses 2018, 61, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xia, K.; Yu, Y.; Miliakos, A.; Chaturvedi, S.; Zhang, F.; Chen, S.; Chaturvedi, V.; Linhardt, R.J. Unique Cell Surface Mannan of Yeast Pathogen Candida auris with Selective Binding to IgG. ACS Infect. Dis. 2020, 6, 1018–1031. [Google Scholar] [CrossRef]
- Ware, A.; Johnston, W.; Delaney, C.; Butcher, M.; Ramage, G.; Price, L.; Butcher, J.; Kean, R. Dry surface biofilm formation by Candida auris facilitates persistence and tolerance to sodium hypochlorite. bioRxiv 2023. [Google Scholar] [CrossRef]
- Areitio, M.; Antoran, A.; Rodriguez-Erenaga, O.; Aparicio-Fernandez, L.; Martin-Souto, L.; Buldain, I.; Zaldibar, B.; Ruiz-Gaitan, A.; Peman, J.; Rementeria, A.; et al. Identification of the Most Immunoreactive Antigens of Candida auris to IgGs from Systemic Infections in Mice. J. Proteome Res. 2024, 23, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, Y.; Chen, X.; Li, H.; Yin, Z.; Zhang, B.; Xu, Y.; Zhang, Y.; Zhang, R.; Huang, X.; et al. Innate immune responses against the fungal pathogen Candida auris. Nat. Commun. 2022, 13, 3553. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and White Manifestations in the Oral Cavity. Head Neck Pathol. 2019, 13, 25–32. [Google Scholar] [CrossRef]
- Lu, S.Y. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef]
- Reichart, P.A.; Samaranayake, L.P.; Philipsen, H.P. Pathology and clinical correlates in oral candidiasis and its variants: A review. Oral Dis. 2000, 6, 85–91. [Google Scholar]
- Acharya, S. Diagnosis and Management of Pseudomembranous Candidiasis. J. Otolaryngol. ENT Res. 2017, 8, 460–462. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef] [PubMed]
- Dodd, C.L.; Greenspan, D.; Katz, M.H.; Westenhouse, J.L.; Feigal, D.W.; Greenspan, J.S. Oral candidiasis in HIV infection: Pseudomembranous and erythematous candidiasis show similar rates of progression to AIDS. AIDS 1991, 5, 1339–1343. [Google Scholar]
- MacPhail, L.A.; Komaroff, E.; Alves, M.E.; Navazesh, M.; Phelan, J.A.; Redford, M. Differences in risk factors among clinical types of oral candidiasis in the Women’s Interagency HIV Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sitheeque, M.A.; Samaranayake, L.P. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit. Rev. Oral Biol. Med. 2003, 14, 253–267. [Google Scholar] [CrossRef]
- Pina, P.S.S.; Custodio, M.; Sugaya, N.N.; de Sousa, S. Histopathologic aspects of the so-called chronic hyperplastic candidiasis: An analysis of 36 cases. J. Cutan. Pathol. 2021, 48, 66–71. [Google Scholar] [CrossRef]
- Darling, M.R.; McCord, C.; Jackson-Boeters, L.; Copete, M. Markers of potential malignancy in chronic hyperplastic candidiasis. J. Investig. Clin. Dent. 2012, 3, 176–181. [Google Scholar] [CrossRef]
- Warnakulasuriya, K.A.; Samaranayake, L.P.; Peiris, J.S. Angular cheilitis in a group of Sri Lankan adults: A clinical and microbiologic study. J. Oral Pathol. Med. 1991, 20, 172–175. [Google Scholar] [CrossRef]
- Dias, A.P.; Samaranayake, L.P. Clinical, microbiological and ultrastructural features of angular cheilitis lesions in Southern Chinese. Oral Dis. 1995, 1, 43–48. [Google Scholar] [CrossRef]
- Jafari, A.A.; Lotfi-Kamran, M.H.; Falah-Tafti, A.; Shirzadi, S. Distribution Profile of Candida Species Involved in Angular Cheilitis Lesions Before and After Denture Replacement. Jundishapur J. Microbiol. 2013, 6, e10844. [Google Scholar] [CrossRef]
- Nelson, B.L.; Thompson, L. Median rhomboid glossitis. Ear Nose Throat J. 2007, 86, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Goregen, M.; Miloglu, O.; Buyukkurt, M.C.; Caglayan, F.; Aktas, A.E. Median rhomboid glossitis: A clinical and microbiological study. Eur. J. Dent. 2011, 5, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.B.; Bharucha, M.A. Median rhomboid glossitis: Secondary to colonisation of the tongue by Actinomyces (a case report). J. Postgrad. Med. 1991, 37, 238–240. [Google Scholar]
- Peric, M.; Milicic, B.; Kuzmanovic Pficer, J.; Zivkovic, R.; Arsic Arsenijevic, V. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida spp. Distribution. J Fungi 2024, 10, 328. [Google Scholar] [CrossRef]
- Gleiznys, A.; Zdanaviciene, E.; Zilinskas, J. Candida albicans importance to denture wearers. A literature review. Stomatologija 2015, 17, 54–66. [Google Scholar]
- Le Bars, P.; Kouadio, A.A.; Bandiaky, O.N.; Le Guehennec, L.; de La Cochetiere, M.F. Host’s Immunity and Candida Species Associated with Denture Stomatitis: A Narrative Review. Microorganisms 2022, 10, 1437. [Google Scholar] [CrossRef]
- Contaldo, M.; Romano, A.; Mascitti, M.; Fiori, F.; Della Vella, F.; Serpico, R.; Santarelli, A. Association between denture stomatitis, Candida species and diabetic status. J. Biol. Regu Homeost. Agents 2019, 33, 35–41. [Google Scholar]
- O’Sullivan, J.M.; Jenkinson, H.F.; Cannon, R.D. Adhesion of Candida albicans to oral streptococci is promoted by selective adsorption of salivary proteins to the streptococcal cell surface. Microbiology 2000, 146 Pt 1, 41–48. [Google Scholar] [CrossRef]
- Silao, F.G.S.; Jiang, T.; Bereczky-Veress, B.; Kuhbacher, A.; Ryman, K.; Uwamohoro, N.; Jenull, S.; Nogueira, F.; Ward, M.; Lion, T.; et al. Proline catabolism is a key factor facilitating Candida albicans pathogenicity. PLoS Pathog. 2023, 19, e1011677. [Google Scholar] [CrossRef]
- Jeng, H.W.; Holmes, A.R.; Cannon, R.D. Characterization of two Candida albicans surface mannoprotein adhesins that bind immobilized saliva components. Med. Mycol. 2005, 43, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Thanh Nguyen, H.; Zhang, R.; Inokawa, N.; Oura, T.; Chen, X.; Iwatani, S.; Niimi, K.; Niimi, M.; Holmes, A.R.; Cannon, R.D.; et al. Candida albicans Bgl2p, Ecm33p, and Als1p proteins are involved in adhesion to saliva-coated hydroxyapatite. J. Oral Microbiol. 2021, 13, 1879497. [Google Scholar] [CrossRef]
- Angiolella, L.; Vitali, A.; Stringaro, A.; Mignogna, G.; Maras, B.; Bonito, M.; Colone, M.; Palamara, A.T.; Cassone, A. Localisation of Bgl2p upon antifungal drug treatment in Candida albicans. Int. J. Antimicrob. Agents 2009, 33, 143–148. [Google Scholar] [CrossRef]
- de Repentigny, L.; Aumont, F.; Bernard, K.; Belhumeur, P. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect. Immun. 2000, 68, 3172–3179. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, N.L.; Zhang, A.Q.; Nobile, C.J.; Johnson, A.D.; Ribbeck, K. Mucins suppress virulence traits of Candida albicans. mBio 2014, 5, e01911. [Google Scholar] [CrossRef]
- Liu, B.; Rayment, S.A.; Gyurko, C.; Oppenheim, F.G.; Offner, G.D.; Troxler, R.F. The recombinant N-terminal region of human salivary mucin MG2 (MUC7) contains a binding domain for oral Streptococci and exhibits candidacidal activity. Biochem. J. 2000, 345 Pt 3, 557–564. [Google Scholar] [CrossRef]
- Takagi, J.; Aoki, K.; Turner, B.S.; Lamont, S.; Lehoux, S.; Kavanaugh, N.; Gulati, M.; Valle Arevalo, A.; Lawrence, T.J.; Kim, C.Y.; et al. Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity. Nat. Chem. Biol. 2022, 18, 762–773. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar]
- Tsai, H.; Bobek, L.A. Human salivary histatins: Promising anti-fungal therapeutic agents. Crit. Rev. Oral Biol. Med. 1998, 9, 480–497. [Google Scholar] [CrossRef]
- den Hertog, A.L.; van Marle, J.; van Veen, H.A.; Van’t Hof, W.; Bolscher, J.G.; Veerman, E.C.; Nieuw Amerongen, A.V. Candidacidal effects of two antimicrobial peptides: Histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 2005, 388, 689–695. [Google Scholar] [CrossRef]
- Rothstein, D.M.; Spacciapoli, P.; Tran, L.T.; Xu, T.; Roberts, F.D.; Dalla Serra, M.; Buxton, D.K.; Oppenheim, F.G.; Friden, P. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob. Agents Chemother. 2001, 45, 1367–1373. [Google Scholar] [CrossRef]
- Fitzgerald, D.H.; Coleman, D.C.; O’Connell, B.C. Susceptibility of Candida dubliniensis to salivary histatin 3. Antimicrob. Agents Chemother. 2003, 47, 70–76. [Google Scholar] [CrossRef]
- Li, X.S.; Sun, J.N.; Okamoto-Shibayama, K.; Edgerton, M. Candida albicans cell wall ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J. Biol. Chem. 2006, 281, 22453–22463. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.N.; Li, W.; Jang, W.S.; Nayyar, N.; Sutton, M.D.; Edgerton, M. Uptake of the antifungal cationic peptide Histatin 5 by Candida albicans Ssa2p requires binding to non-conventional sites within the ATPase domain. Mol. Microbiol. 2008, 70, 1246–1260. [Google Scholar] [CrossRef]
- Leito, J.T.; Ligtenberg, A.J.; Nazmi, K.; Veerman, E.C. Identification of salivary components that induce transition of hyphae to yeast in Candida albicans. FEMS Yeast Res. 2009, 9, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Makambi, W.K.; Chiu, V.L.; Kasper, L.; Hube, B.; Karlsson, A.J. Role of amino acid substitutions on proteolytic stability of histatin 5 in the presence of secreted aspartyl proteases and salivary proteases. Protein Sci. 2025, 34, e70011. [Google Scholar] [CrossRef]
- Jainkittivong, A.; Johnson, D.A.; Yeh, C.K. The relationship between salivary histatin levels and oral yeast carriage. Oral. Microbiol. Immunol. 1998, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luther, P.W.; Leng, Q.; Mixson, A.J. Synthetic histidine-rich peptides inhibit Candida species and other fungi in vitro: Role of endocytosis and treatment implications. Antimicrob. Agents Chemother. 2006, 50, 2797–2805. [Google Scholar] [CrossRef]
- Johansson, I.; Bratt, P.; Hay, D.I.; Schluckebier, S.; Stromberg, N. Adhesion of Candida albicans, but not Candida krusei, to salivary statherin and mimicking host molecules. Oral Microbiol. Immunol. 2000, 15, 112–118. [Google Scholar] [CrossRef]
- Niemi, L.D.; Johansson, I. Salivary statherin peptide-binding epitopes of commensal and potentially infectious Actinomyces spp. delineated by a hybrid peptide construct. Infect. Immun. 2004, 72, 782–787. [Google Scholar] [CrossRef]
- Pellissari, C.V.G.; Jorge, J.H.; Marin, L.M.; Sabino-Silva, R.; Siqueira, W.L. Statherin-derived peptides as antifungal strategy against Candida albicans. Arch. Oral Biol. 2021, 125, 105106. [Google Scholar] [CrossRef]
- Mathews, M.; Jia, H.P.; Guthmiller, J.M.; Losh, G.; Graham, S.; Johnson, G.K.; Tack, B.F.; McCray, P.B., Jr. Production of β-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect. Immun. 1999, 67, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Nayyar, N.; Li, W.; Edgerton, M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob. Agents Chemother. 2007, 51, 154–161. [Google Scholar] [CrossRef]
- Joly, S.; Maze, C.; McCray, P.B., Jr.; Guthmiller, J.M. Human β-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J. Clin. Microbiol. 2004, 42, 1024–1029. [Google Scholar] [CrossRef]
- Feng, Z.; Jiang, B.; Chandra, J.; Ghannoum, M.; Nelson, S.; Weinberg, A. Human beta-defensins: Differential activity against candidal species and regulation by Candida albicans. J. Dent. Res. 2005, 84, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Jarva, M.; Phan, T.K.; Lay, F.T.; Caria, S.; Kvansakul, M.; Hulett, M.D. Human β-defensin 2 kills Candida albicans through phosphatidylinositol 4,5-bisphosphate-mediated membrane permeabilization. Sci. Adv. 2018, 4, eaat0979. [Google Scholar] [CrossRef]
- Mizukawa, N.; Sugiyama, K.; Ueno, T.; Mishima, K.; Takagi, S.; Sugahara, T. Defensin-1, an antimicrobial peptide present in the saliva of patients with oral diseases. Oral. Dis. 1999, 5, 139–142. [Google Scholar] [CrossRef]
- Küçükkolbaşi, H.; Küçükkolbaşi, S.; Ayyildiz, H.F.; Dursun, R.; Kara, H. Evaluation of hβD-1 and hβD-2 Levels in Saliva of Patients with Oral Mucosal Diseases. West. Indian Med. J. 2018, 62, 230–238. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, Y.; Fang, M.; Chen, X.; Feng, D.; Liu, J.; Jiang, Q.; Tao, R. Dynamic changes of Th1/Th2/Th17 cytokines and hBD-2/3 in erosive oral lichen planus patients saliva before and after prednisone acetate treatment. Heliyon 2024, 10, e24043. [Google Scholar] [CrossRef]
- McKay, M.S.; Olson, E.; Hesla, M.A.; Panyutich, A.; Ganz, T.; Perkins, S.; Rossomando, E.F. Immunomagnetic recovery of human neutrophil defensins from the human gingival crevice. Oral Microbiol. Immunol. 1999, 14, 190–193. [Google Scholar] [CrossRef]
- Gardner, M.S.; Rowland, M.D.; Siu, A.Y.; Bundy, J.L.; Wagener, D.K.; Stephenson, J.L. Comprehensive defensin assay for saliva. Anal. Chem. 2009, 81, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Inzitari, R.; Cabras, T.; Pisano, E.; Castagnola, M.; Celletti, R.; Manni, A.; Messana, I. α-Defensin levels in whole saliva of totally edentulous subjects. Int. J. Immunopathol. Pharmacol. 2008, 21, 845–849. [Google Scholar] [CrossRef]
- Mizukawa, N.; Sugiyama, K.; Fukunaga, J.; Ueno, T.; Mishima, K.; Takagi, S.; Sugahara, T. Defensin-1, a peptide detected in the saliva of oral squamous cell carcinoma patients. Anticancer Res. 1998, 18, 4645–4649. [Google Scholar] [PubMed]
- Sawaki, K.; Mizukawa, N.; Yamaai, T.; Fukunaga, J.; Sugahara, T. Immunohistochemical study on expression of α-defensin and β-defensin-2 in human buccal epithelia with candidiasis. Oral Dis. 2002, 8, 37–41. [Google Scholar] [CrossRef]
- Puklo, M.; Guentsch, A.; Hiemstra, P.S.; Eick, S.; Potempa, J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol. Immunol. 2008, 23, 328–335. [Google Scholar] [CrossRef]
- Elguezabal, N.; Maza, J.L.; Moragues, M.D.; Ponton, J. Monoclonal antibody-mediated inhibition of adhesion of Candida albicans and Candida dubliniensis to human epithelial cells. Eur. J. Oral Sci. 2009, 117, 474–478. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Chen, D.; Liao, B.; Chen, X.; Zong, Y.; Wei, Y.; Shi, Y.; Liu, Y.; Gou, L.; et al. Secretory IgA reduced the ergosterol contents of Candida albicans to repress its hyphal growth and virulence. Appl. Microbiol. Biotechnol. 2024, 108, 244. [Google Scholar] [CrossRef] [PubMed]
- Goncalves e Silva, C.R.; Melo, K.E.; Leão, M.V.; Ruis, R.; Jorge, A.O. [Relationship between Candida in vaginal and oral mucosae and salivary IgA]. Rev. Bras. Ginecol. Obstet. 2008, 30, 300–305. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.Y.; Samaranayake, Y.H.; Samaranayake, L.P.; Nikawa, H. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 1999, 37, 35–41. [Google Scholar] [CrossRef]
- Ellepola, A.N.B.; Khan, Z.U. Impact of Brief Exposure to Lysozyme and Lactoferrin on Pathogenic Attributes of Oral Candida. Int. Dent. J. 2024, 74, 1161–1167. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kakeya, H.; Miyazaki, T.; Izumikawa, K.; Yanagihara, K.; Ohno, H.; Yamamoto, Y.; Tashiro, T.; Kohno, S. Synergistic antifungal effect of lactoferrin with azole antifungals against Candida albicans and a proposal for a new treatment method for invasive candidiasis. Jpn. J. Infect. Dis. 2011, 64, 292–296. [Google Scholar] [PubMed]
- Pinilla, G.; Coronado, Y.T.; Chaves, G.; Munoz, L.; Navarrete, J.; Salazar, L.M.; Taborda, C.P.; Munoz, J.E. In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp. J. Fungi 2022, 8, 1173. [Google Scholar] [CrossRef]
- Begum, N.; Lee, S.; Portlock, T.J.; Pellon, A.; Nasab, S.D.S.; Nielsen, J.; Uhlen, M.; Moyes, D.L.; Shoaie, S. Integrative functional analysis uncovers metabolic differences between Candida species. Commun. Biol. 2022, 5, 1013. [Google Scholar] [CrossRef]
- Gropp, K.; Schild, L.; Schindler, S.; Hube, B.; Zipfel, P.F.; Skerka, C. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol. Immunol. 2009, 47, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.; Nery, J.M.; Dias, A.L. Aspartic proteinases of Candida spp.: Role in pathogenicity and antifungal resistance. Mycoses 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Hornbach, A.; Heyken, A.; Schild, L.; Hube, B.; Loffler, J.; Kurzai, O. The glycosylphosphatidylinositol-anchored protease Sap9 modulates the interaction of Candida albicans with human neutrophils. Infect. Immun. 2009, 77, 5216–5224. [Google Scholar] [CrossRef]
- Copping, V.M.; Barelle, C.J.; Hube, B.; Gow, N.A.; Brown, A.J.; Odds, F.C. Exposure of Candida albicans to antifungal agents affects expression of SAP2 and SAP9 secreted proteinase genes. J. Antimicrob. Chemother. 2005, 55, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Triebel, T.; Grillhosl, B.; Kacani, L.; Lell, C.P.; Fuchs, A.; Speth, C.; Lass-Florl, C.; Steinmann, J.; Dierich, M.P.; Wurzner, R. Importance of the terminal complement components for immune defence against Candida. Int. J. Med. Microbiol. 2003, 292, 527–536. [Google Scholar] [CrossRef]
- Gabrielli, E.; Sabbatini, S.; Roselletti, E.; Kasper, L.; Perito, S.; Hube, B.; Cassone, A.; Vecchiarelli, A.; Pericolini, E. In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence 2016, 7, 819–825. [Google Scholar] [CrossRef]
- Kumar, R.; Rojas, I.G.; Edgerton, M. Candida albicans Sap6 Initiates Oral Mucosal Inflammation via the Protease Activated Receptor PAR2. Front. Immunol. 2022, 13, 912748. [Google Scholar] [CrossRef]
- Dutton, L.C.; Jenkinson, H.F.; Lamont, R.J.; Nobbs, A.H. Role of Candida albicans secreted aspartyl protease Sap9 in interkingdom biofilm formation. Pathog. Dis. 2016, 74, ftw005. [Google Scholar] [CrossRef]
- Mores, A.U.; Souza, R.D.; Cavalca, L.; de Paula e Carvalho, A.; Gursky, L.C.; Rosa, R.T.; Samaranayake, L.P.; Rosa, E.A. Enhancement of secretory aspartyl protease production in biofilms of Candida albicans exposed to sub-inhibitory concentrations of fluconazole. Mycoses 2011, 54, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Zainab, S.; Faryal, R.; Ali, N.; Sharafat, I. Inhibition of secreted aspartyl proteinase activity in biofilms of Candida species by mycogenic silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Hofs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Liang, S.H.; Sircaik, S.; Dainis, J.; Kakade, P.; Penumutchu, S.; McDonough, L.D.; Chen, Y.H.; Frazer, C.; Schille, T.B.; Allert, S.; et al. The hyphal-specific toxin candidalysin promotes fungal gut commensalism. Nature 2024, 627, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Kasper, L.; Konig, A.; Koenig, P.A.; Gresnigt, M.S.; Westman, J.; Drummond, R.A.; Lionakis, M.S.; Gross, O.; Ruland, J.; Naglik, J.R.; et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018, 9, 4260. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Z.; Guo, Y.; Song, L.; Weatherhead, J.E.; Huang, X.; Zeng, Y.; Bimler, L.; Chang, C.Y.; Knight, J.M.; et al. Candida albicans elicits protective allergic responses via platelet mediated T helper 2 and T helper 17 cell polarization. Immunity 2021, 54, 2595–2610. [Google Scholar] [CrossRef]
- Ho, J.; Yang, X.; Nikou, S.A.; Kichik, N.; Donkin, A.; Ponde, N.O.; Richardson, J.P.; Gratacap, R.L.; Archambault, L.S.; Zwirner, C.P.; et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat. Commun. 2019, 10, 2297. [Google Scholar] [CrossRef]
- Swidergall, M.; Khalaji, M.; Solis, N.V.; Moyes, D.L.; Drummond, R.A.; Hube, B.; Lionakis, M.S.; Murdoch, C.; Filler, S.G.; Naglik, J.R. Candidalysin Is Required for Neutrophil Recruitment and Virulence During Systemic Candida albicans Infection. J. Infect. Dis. 2019, 220, 1477–1488. [Google Scholar] [CrossRef]
- Richardson, J.P.; Brown, R.; Kichik, N.; Lee, S.; Priest, E.; Mogavero, S.; Maufrais, C.; Wickramasinghe, D.N.; Tsavou, A.; Kotowicz, N.K.; et al. Candidalysins Are a New Family of Cytolytic Fungal Peptide Toxins. mBio 2022, 13, e0351021. [Google Scholar] [CrossRef]
- Wickramasinghe, D.N.; Lyon, C.M.; Lee, S.; Hepworth, O.W.; Priest, E.L.; Maufrais, C.; Ryan, A.P.; Permal, E.; Sullivan, D.; McManus, B.A.; et al. Variations in candidalysin amino acid sequence influence toxicity and host responses. mBio 2024, 15, e0335123. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; Solis, N.V.; Millet, N.; Huang, M.Y.; Lin, J.; Phan, Q.T.; Lazarus, M.D.; Wang, Z.; Yeaman, M.R.; Mitchell, A.P.; et al. Activation of EphA2-EGFR signaling in oral epithelial cells by Candida albicans virulence factors. PLoS Pathog. 2021, 17, e1009221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willems, H.M.E.; Sansevere, E.A.; Allert, S.; Barker, K.S.; Lowes, D.J.; Dixson, A.C.; Xu, Z.; Miao, J.; DeJarnette, C.; et al. A variant ECE1 allele contributes to reduced pathogenicity of Candida albicans during vulvovaginal candidiasis. PLoS Pathog. 2021, 17, e1009884. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Renaming Candida glabrata-A case of taxonomic purity over clinical and public health pragmatism. PLoS Pathog. 2024, 20, e1012055. [Google Scholar] [CrossRef]
- Donohue, D.S.; Ielasi, F.S.; Goossens, K.V.; Willaert, R.G. The N-terminal part of Als1 protein from Candida albicans specifically binds fucose-containing glycans. Mol. Microbiol. 2011, 80, 1667–1679. [Google Scholar] [CrossRef]
- Kozik, A.; Karkowska-Kuleta, J.; Zajac, D.; Bochenska, O.; Kedracka-Krok, S.; Jankowska, U.; Rapala-Kozik, M. Fibronectin-, vitronectin- and laminin-binding proteins at the cell walls of Candida parapsilosis and Candida tropicalis pathogenic yeasts. BMC Microbiol. 2015, 15, 197. [Google Scholar] [CrossRef]
- Salgado, P.S.; Yan, R.; Taylor, J.D.; Burchell, L.; Jones, R.; Hoyer, L.L.; Matthews, S.J.; Simpson, P.J.; Cota, E. Structural basis for the broad specificity to host-cell ligands by the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. USA 2011, 108, 15775–15779. [Google Scholar] [CrossRef]
- Murciano, C.; Moyes, D.L.; Runglall, M.; Tobouti, P.; Islam, A.; Hoyer, L.L.; Naglik, J.R. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS ONE 2012, 7, e33362. [Google Scholar] [CrossRef]
- Nobbs, A.H.; Vickerman, M.M.; Jenkinson, H.F. Heterologous expression of Candida albicans cell wall-associated adhesins in Saccharomyces cerevisiae Reveals differential specificities in adherence and biofilm formation and in binding oral Streptococcus gordonii. Eukaryot. Cell 2010, 9, 1622–1634. [Google Scholar] [CrossRef]
- Liu, Y.; Filler, S.G. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell 2011, 10, 168–173. [Google Scholar] [CrossRef]
- Zhou, T.; Solis, N.V.; Marshall, M.; Yao, Q.; Garleb, R.; Yang, M.; Pearlman, E.; Filler, S.G.; Liu, H. Hyphal Als proteins act as CR3 ligands to promote immune responses against Candida albicans. Nat. Commun. 2024, 15, 3926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Solis, N.V.; Marshall, M.; Yao, Q.; Pearlman, E.; Filler, S.G.; Liu, H. Fungal Als proteins hijack host death effector domains to promote inflammasome signaling. Nat. Commun. 2025, 16, 1562. [Google Scholar] [CrossRef]
- Oh, S.H.; Smith, B.; Miller, A.N.; Staker, B.; Fields, C.; Hernandez, A.; Hoyer, L.L. Agglutinin-Like Sequence (ALS) Genes in the Candida parapsilosis Species Complex: Blurring the Boundaries Between Gene Families That Encode Cell-Wall Proteins. Front. Microbiol. 2019, 10, 781. [Google Scholar] [CrossRef]
- Zhu, W.; Phan, Q.T.; Boontheung, P.; Solis, N.V.; Loo, J.A.; Filler, S.G. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc. Natl. Acad. Sci. USA 2012, 109, 14194–14199. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.N.; Solis, N.V.; Phan, Q.T.; Bajwa, J.S.; Kashleva, H.; Thompson, A.; Liu, Y.; Dongari-Bagtzoglou, A.; Edgerton, M.; Filler, S.G. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010, 6, e1001181. [Google Scholar] [CrossRef]
- Teng, W.; Subsomwong, P.; Narita, K.; Nakane, A.; Asano, K. Heat Shock Protein SSA1 Enriched in Hypoxic Secretome of Candida albicans Exerts an Immunomodulatory Effect via Regulating Macrophage Function. Cells 2024, 13, 127. [Google Scholar] [CrossRef]
- Qiu, X.R.; Shen, C.R.; Jiang, L.W.; Ji, P.; Zhang, Y.; Hou, W.T.; Zhang, W.; Shen, H.; An, M.M. Ssa1-targeted antibody prevents host invasion by Candida albicans. Front. Microbiol. 2023, 14, 1182914. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef]
- Younes, S.; Bahnan, W.; Dimassi, H.I.; Khalaf, R.A. The Candida albicans Hwp2 is necessary for proper adhesion, biofilm formation and oxidative stress tolerance. Microbiol. Res. 2011, 166, 430–436. [Google Scholar] [CrossRef]
- Sundstrom, P.; Balish, E.; Allen, C.M. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J. Infect. Dis. 2002, 185, 521–530. [Google Scholar] [CrossRef]
- Abastabar, M.; Hosseinpoor, S.; Hedayati, M.T.; Shokohi, T.; Valadan, R.; Mirhendi, H.; Mohammadi, R.; Aghili, S.R.; Rahimi, N.; Aslani, N.; et al. Hyphal wall protein 1 gene: A potential marker for the identification of different Candida species and phylogenetic analysis. Curr. Med. Mycol. 2016, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Andes, D.R.; Nett, J.E.; Smith, F.J.; Yue, F.; Phan, Q.T.; Edwards, J.E.; Filler, S.G.; Mitchell, A.P. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006, 2, e63. [Google Scholar] [CrossRef]
- Fanning, S.; Xu, W.; Solis, N.; Woolford, C.A.; Filler, S.G.; Mitchell, A.P. Divergent targets of Candida albicans biofilm regulator Bcr1 in vitro and in vivo. Eukaryot. Cell 2012, 11, 896–904. [Google Scholar] [CrossRef]
- Ding, C.; Vidanes, G.M.; Maguire, S.L.; Guida, A.; Synnott, J.M.; Andes, D.R.; Butler, G. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS ONE 2011, 6, e28151. [Google Scholar] [CrossRef]
- Pannanusorn, S.; Ramírez-Zavala, B.; Lünsdorf, H.; Agerberth, B.; Morschhäuser, J.; Römling, U. Characterization of biofilm formation and the role of BCR1 in clinical isolates of Candida parapsilosis. Eukaryot. Cell 2014, 13, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Paulovičová, E.; Bujdáková, H.; Chupáčová, J.; Paulovičová, L.; Kertys, P.; Hrubiško, M. Humoral immune responses to Candida albicans complement receptor 3-related protein in the atopic subjects with vulvovaginal candidiasis. Novel sensitive marker for Candida infection. FEMS Yeast Res. 2015, 15, fou001. [Google Scholar] [CrossRef]
- Dekkerová, J.; Lopez-Ribot, J.L.; Bujdáková, H. Activity of anti-CR3-RP polyclonal antibody against biofilms formed by Candida auris, a multidrug-resistant emerging fungal pathogen. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 101–108. [Google Scholar] [CrossRef]
- Chupáčová, J.; Borghi, E.; Morace, G.; Los, A.; Bujdáková, H. Anti-biofilm activity of antibody directed against surface antigen complement receptor 3-related protein-comparison of Candida albicans and Candida dubliniensis. Pathog. Dis. 2018, 76, ftx127. [Google Scholar] [CrossRef]
- Losse, J.; Svobodova, E.; Heyken, A.; Hube, B.; Zipfel, P.F.; Jozsi, M. Role of pH-regulated antigen 1 of Candida albicans in the fungal recognition and antifungal response of human neutrophils. Mol. Immunol. 2011, 48, 2135–2143. [Google Scholar] [CrossRef]
- Luo, S.; Dasari, P.; Reiher, N.; Hartmann, A.; Jacksch, S.; Wende, E.; Barz, D.; Niemiec, M.J.; Jacobsen, I.; Beyersdorf, N.; et al. The secreted Candida albicans protein Pra1 disrupts host defense by broadly targeting and blocking complement C3 and C3 activation fragments. Mol. Immunol. 2018, 93, 266–277. [Google Scholar] [CrossRef]
- Bergfeld, A.; Dasari, P.; Werner, S.; Hughes, T.R.; Song, W.C.; Hortschansky, P.; Brakhage, A.A.; Hunig, T.; Zipfel, P.F.; Beyersdorf, N. Direct Binding of the pH-Regulated Protein 1 (Pra1) from Candida albicans Inhibits Cytokine Secretion by Mouse CD4(+) T Cells. Front. Microbiol. 2017, 8, 844. [Google Scholar] [CrossRef] [PubMed]
- Sentandreu, M.; Elorza, M.V.; Sentandreu, R.; Fonzi, W.A. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J. Bacteriol. 1998, 180, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Kobayashi, H.; Suzuki, S. Immunochemistry of pathogenic yeast, Candida species, focusing on mannan. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 250–265. [Google Scholar] [CrossRef]
- Miyakawa, Y.; Kuribayashi, T.; Kagaya, K.; Suzuki, M.; Nakase, T.; Fukazawa, Y. Role of specific determinants in mannan of Candida albicans serotype A in adherence to human buccal epithelial cells. Infect. Immun. 1992, 60, 2493–2499. [Google Scholar] [CrossRef]
- Nguyen, T.N.Y.; Matangkasombut, O.; Ritprajak, P. Differential dendritic cell responses to cell wall mannan of Candida albicans, Candida parapsilosis, and Candida dubliniensis. J. Oral. Sci. 2018, 60, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hakoyama, Y.; Yamada, S.I.; Nishimaki, F.; Hayashi, K.; Koizumi, T.; Kurita, H. Oral Candida Mannan Concentrations Correlate with Symptoms/Signs of Ill Health and the Immune Status. Mycopathologia 2020, 185, 629–637. [Google Scholar] [CrossRef]
- Marodi, L.; Schreiber, S.; Anderson, D.C.; MacDermott, R.P.; Korchak, H.M.; Johnston, R.B., Jr. Enhancement of macrophage candidacidal activity by interferon-γ. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J. Clin. Investig. 1993, 91, 2596–2601. [Google Scholar] [CrossRef]
- Porcaro, I.; Vidal, M.; Jouvert, S.; Stahl, P.D.; Giaimis, J. Mannose receptor contribution to Candida albicans phagocytosis by murine E-clone J774 macrophages. J. Leucoc. Biol. 2003, 74, 206–215. [Google Scholar] [CrossRef]
- Van der Graaf, C.A.; Netea, M.G.; Morré, S.A.; Den Heijer, M.; Verweij, P.E.; Van der Meer, J.W.; Kullberg, B.J. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur. Cytokine Netw. 2006, 17, 29–34. [Google Scholar]
- Tada, H.; Nemoto, E.; Shimauchi, H.; Watanabe, T.; Mikami, T.; Matsumoto, T.; Ohno, N.; Tamura, H.; Shibata, K.; Akashi, S.; et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 2002, 46, 503–512. [Google Scholar] [CrossRef]
- Honorato, L.; Bonilla, J.J.A.; Valdez, A.F.; Frases, S.; Araujo, G.; Sabino, A.; da Silva, N.M.; Ribeiro, L.; Ferreira Md, S.; Kornetz, J.; et al. Toll-like receptor 4 (TLR4) is the major pattern recognition receptor triggering the protective effect of a Candida albicans extracellular vesicle-based vaccine prototype in murine systemic candidiasis. mSphere 2024, 9, e0046724. [Google Scholar] [CrossRef] [PubMed]
- Luisa Gil, M.; Murciano, C.; Yanez, A.; Gozalbo, D. Role of Toll-like receptors in systemic Candida albicans infections. Front. Biosci. 2016, 21, 278–302. [Google Scholar] [CrossRef]
- Ifrim, D.C.; Quintin, J.; Courjol, F.; Verschueren, I.; van Krieken, J.H.; Koentgen, F.; Fradin, C.; Gow, N.A.; Joosten, L.A.; van der Meer, J.W.; et al. The Role of Dectin-2 for Host Defense Against Disseminated Candidiasis. J. Interferon Cytokine Res. 2016, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; da Fonseca, D.M.; Walker, L.; Griffiths, J.S.; Taylor, P.R.; Gow, N.A.R.; Orr, S.J. Dependence on Mincle and Dectin-2 Varies With Multiple Candida Species During Systemic Infection. Front. Microbiol. 2021, 12, 633229. [Google Scholar] [CrossRef] [PubMed]
- Jouault, T.; El Abed-El Behi, M.; Martinez-Esparza, M.; Breuilh, L.; Trinel, P.A.; Chamaillard, M.; Trottein, F.; Poulain, D. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J. Immunol. 2006, 177, 4679–4687. [Google Scholar] [CrossRef]
- Dongari-Bagtzoglou, A.; Kashleva, H.; Dwivedi, P.; Diaz, P.; Vasilakos, J. Characterization of mucosal Candida albicans biofilms. PLoS ONE 2009, 4, e7967. [Google Scholar] [CrossRef]
- Taher, J.M.; Raheem, N.N. Role of purified beta-glucanase from lactobacillus acidophilus in disruption of biofilm formation by Candida spp. causing of denture stomatitis. Mater. Today Proc. 2022, 60, 1507–1512. [Google Scholar] [CrossRef]
- Bonfim-Mendonça Pde, S.; Ratti, B.A.; Godoy Jda, S.; Negri, M.; Lima, N.C.; Fiorini, A.; Hatanaka, E.; Consolaro, M.E.; de Oliveira Silva, S.; Svidzinski, T.I. β-Glucan induces reactive oxygen species production in human neutrophils to improve the killing of Candida albicans and Candida glabrata isolates from vulvovaginal candidiasis. PLoS ONE 2014, 9, e107805. [Google Scholar] [CrossRef]
- Gow, N.A.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, S.; Mora-Montes, H.M.; Walker, L.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans β-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1571. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Li, X.; Utomo, A.; Cullere, X.; Choi, M.M.; Milner, D.A., Jr.; Venkatesh, D.; Yun, S.H.; Mayadas, T.N. The β-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell Host Microbe 2011, 10, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Sutmuller, R.; Hermann, C.; Van der Graaf, C.A.; Van der Meer, J.W.; van Krieken, J.H.; Hartung, T.; Adema, G.; Kullberg, B.J. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 2004, 172, 3712–3718. [Google Scholar] [CrossRef]
- Swidergall, M.; Solis, N.V.; Lionakis, M.S.; Filler, S.G. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat. Microbiol. 2018, 3, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; Solis, N.V.; Wang, Z.; Phan, Q.T.; Marshall, M.E.; Lionakis, M.S.; Pearlman, E.; Filler, S.G. EphA2 Is a Neutrophil Receptor for Candida albicans that Stimulates Antifungal Activity during Oropharyngeal Infection. Cell Rep. 2019, 28, 423–433. [Google Scholar] [CrossRef]
- Muzurovic, S.; Babajic, E.; Masic, T.; Smajic, R.; Selmanagic, A. The relationship between oral hygiene and oral colonisation with Candida species. Med. Arch. 2012, 66, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Moriyama, M.; Hayashida, J.N.; Tanaka, A.; Maehara, T.; Ieda, S.; Nakamura, S. Close association between oral Candida species and oral mucosal disorders in patients with xerostomia. Oral Dis. 2012, 18, 667–672. [Google Scholar] [CrossRef]
- Kim, D.; Sengupta, A.; Niepa, T.H.; Lee, B.H.; Weljie, A.; Freitas-Blanco, V.S.; Murata, R.M.; Stebe, K.J.; Lee, D.; Koo, H. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 2017, 7, 41332. [Google Scholar] [CrossRef]
- O’Connell, L.M.; Santos, R.; Springer, G.; Burne, R.A.; Nascimento, M.M.; Richards, V.P. Site-Specific Profiling of the Dental Mycobiome Reveals Strong Taxonomic Shifts during Progression of Early-Childhood Caries. Appl. Environ. Microbiol. 2020, 86, e02825-19. [Google Scholar] [CrossRef]
- Özgöçmen, E.; Yiğit, T.; Kutlu, H.H. Presence of Candida in the dental plaque and saliva of patients with severe early childhood caries and early childhood caries:a pilot study. Eur. Oral Res. 2024, 58, 102–107. [Google Scholar] [CrossRef]
- Ellepola, K.; Truong, T.; Liu, Y.; Lin, Q.; Lim, T.K.; Lee, Y.M.; Cao, T.; Koo, H.; Seneviratne, C.J. Multi-omics Analyses Reveal Synergistic Carbohydrate Metabolism in Streptococcus mutans-Candida albicans Mixed-Species Biofilms. Infect. Immun. 2019, 87, e00339-19. [Google Scholar] [CrossRef]
- Pereira, D.; Seneviratne, C.J.; Koga-Ito, C.Y.; Samaranayake, L.P. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2018, 24, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, L.M.; Deng, D.M.; van der Mei, H.C.; Crielaard, W.; Krom, B.P. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot. Cell 2009, 8, 1658–1664. [Google Scholar] [CrossRef]
- Joyner, P.M.; Liu, J.; Zhang, Z.; Merritt, J.; Qi, F.; Cichewicz, R.H. Mutanobactin A from the human oral pathogen Streptococcus mutans is a cross-kingdom regulator of the yeast-mycelium transition. Org. Biomol. Chem. 2010, 8, 5486–5489. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, R.; Lemme, A.; Ballhausen, B.; Thiel, V.; Schulz, S.; Jansen, R.; Sztajer, H.; Wagner-Dobler, I. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem 2010, 11, 1552–1562. [Google Scholar] [CrossRef]
- Khoury, Z.H.; Vila, T.; Puthran, T.R.; Sultan, A.S.; Montelongo-Jauregui, D.; Melo, M.A.S.; Jabra-Rizk, M.A. The Role of Candida albicans Secreted Polysaccharides in Augmenting Streptococcus mutans Adherence and Mixed Biofilm Formation: In vitro and in vivo Studies. Front. Microbiol. 2020, 11, 307. [Google Scholar] [CrossRef]
- Lobo, C.I.V.; Rinaldi, T.B.; Christiano, C.M.S.; De Sales Leite, L.; Barbugli, P.A.; Klein, M.I. Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J. Oral Microbiol. 2019, 11, 1581520. [Google Scholar] [CrossRef]
- Xiao, J.; Zeng, Y.; Rustchenko, E.; Huang, X.; Wu, T.T.; Falsetta, M.L. Dual transcriptome of Streptococcus mutans and Candida albicans interplay in biofilms. J. Oral Microbiol. 2023, 15, 2144047. [Google Scholar] [CrossRef]
- Martorano-Fernandes, L.; Goodwine, J.S.; Ricomini-Filho, A.P.; Nobile, C.J.; Del Bel Cury, A.A. Candida albicans Adhesins Als1 and Hwp1 Modulate Interactions with Streptococcus mutans. Microorganisms 2023, 11, 1391. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef]
- Yang, C.; Scoffield, J.; Wu, R.; Deivanayagam, C.; Zou, J.; Wu, H. Antigen I/II mediates interactions between Streptococcus mutans and Candida albicans. Mol. Oral Microbiol. 2018, 33, 283–291. [Google Scholar] [CrossRef]
- Ren, Z.; Jeckel, H.; Simon-Soro, A.; Xiang, Z.; Liu, Y.; Cavalcanti, I.M.; Xiao, J.; Tin, N.N.; Hara, A.; Drescher, K.; et al. Interkingdom assemblages in human saliva display group-level surface mobility and disease-promoting emergent functions. Proc. Natl. Acad. Sci. USA 2022, 119, e2209699119. [Google Scholar] [CrossRef] [PubMed]
- Lozano Moraga, C.P.; Rodriguez Martinez, G.A.; Lefimil Puente, C.A.; Morales Bozo, I.C.; Urzua Orellana, B.R. Prevalence of Candida albicans and carriage of Candida non-albicans in the saliva of preschool children, according to their caries status. Acta Odontol. Scand. 2017, 75, 30–35. [Google Scholar] [CrossRef]
- Udayalaxmi, J.; Shenoy, N. Comparison Between Biofilm Production, Phospholipase and Haemolytic Activity of Different Species of Candida Isolated from Dental Caries Lesions in Children. J. Clin. Diagn. Res. 2016, 10, DC21–DC23. [Google Scholar] [CrossRef] [PubMed]
- Pedro, N.A.; Mira, N.P. A molecular view on the interference established between vaginal Lactobacilli and pathogenic Candida species: Challenges and opportunities for the development of new therapies. Microbiol. Res. 2024, 281, 127628. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Front. Oral Health 2021, 2, 689382. [Google Scholar] [CrossRef]
- McKloud, E.; Delaney, C.; Sherry, L.; Kean, R.; Williams, S.; Metcalfe, R.; Thomas, R.; Richardson, R.; Gerasimidis, K.; Nile, C.J.; et al. Recurrent Vulvovaginal Candidiasis: A Dynamic Interkingdom Biofilm Disease of Candida and Lactobacillus. mSystems 2021, 6, e0062221. [Google Scholar] [CrossRef]
- De-La-Torre, J.; Quindos, G.; Marcos-Arias, C.; Marichalar-Mendia, X.; Gainza, M.L.; Eraso, E.; Acha-Sagredo, A.; Aguirre-Urizar, J.M. Oral Candida colonization in patients with chronic periodontitis. Is there any relationship? Rev. Iberoam. Micol. 2018, 35, 134–139. [Google Scholar] [CrossRef]
- Bartnicka, D.; Karkowska-Kuleta, J.; Zawrotniak, M.; Satala, D.; Michalik, K.; Zielinska, G.; Bochenska, O.; Kozik, A.; Ciaston, I.; Koziel, J.; et al. Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci. Rep. 2019, 9, 4376. [Google Scholar] [CrossRef]
- de Jongh, C.A.; Bikker, F.J.; de Vries, T.J.; Werner, A.; Gibbs, S.; Krom, B.P. Porphyromonas gingivalis interaction with Candida albicans allows for aerobic escape, virulence and adherence. Biofilm 2024, 7, 100172. [Google Scholar] [CrossRef]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community Development between Porphyromonas gingivalis and Candida albicans Mediated by InlJ and Als3. mBio 2018, 9, e00202-18. [Google Scholar] [CrossRef]
- Tamai, R.; Sugamata, M.; Kiyoura, Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb. Pathog. 2011, 51, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cen, L.; Kaplan, C.; Zhou, X.; Lux, R.; Shi, W.; He, X. Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum. J. Dent. Res. 2015, 94, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Bor, B.; Cen, L.; Agnello, M.; Shi, W.; He, X. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 2016, 6, 27956. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Falkler, W.A., Jr.; Merz, W.G.; Kelley, J.I.; Baqui, A.A.; Meiller, T.F. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J. Clin. Microbiol. 1999, 37, 1464–1468. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alshagroud, R.S.; Alsahhaf, A.; Almojaly, S.A.; Abduljabbar, T.; Javed, F. Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2019, 21, 781–785. [Google Scholar] [CrossRef]
- Lafuente-Ibanez-de-Mendoza, I.; Marichalar-Mendia, X.; Garcia-De-La-Fuente, A.M.; Quindos-Andres, G.; Eraso-Barrio, E.; Martinez-Conde-Llamosas, R.; Fernandez-Jimenez, A.; Aguirre-Urizar, J.M. Presence and implication of Candida spp. in patients with peri-implantitis enrolled in a supportive peri-implant therapy program of the Basque Country (Spain). A case-control study. Clin. Implant. Dent. Relat. Res. 2023, 25, 938–947. [Google Scholar] [CrossRef]
- Lafuente-Ibanez de Mendoza, I.; Cayero-Garay, A.; Quindos-Andres, G.; Aguirre-Urizar, J.M. A systematic review on the implication of Candida in peri-implantitis. Int. J. Implant. Dent. 2021, 7, 73. [Google Scholar] [CrossRef]
- Nørgaard, M.; Thomsen, R.W.; Farkas, D.K.; Mogensen, M.F.; Sorensen, H.T. Candida infection and cancer risk: A Danish nationwide cohort study. Eur. J. Intern. Med. 2013, 24, 451–455. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022, 185, 3807–3822.e3812. [Google Scholar] [CrossRef]
- Narunsky-Haziza, L.; Sepich-Poore, G.D.; Livyatan, I.; Asraf, O.; Martino, C.; Nejman, D.; Gavert, N.; Stajich, J.E.; Amit, G.; Gonzalez, A.; et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 2022, 185, 3789–3806. [Google Scholar] [CrossRef]
- Di Spirito, F.; Di Palo, M.P.; Folliero, V.; Cannata, D.; Franci, G.; Martina, S.; Amato, M. Oral Bacteria, Virus and Fungi in Saliva and Tissue Samples from Adult Subjects with Oral Squamous Cell Carcinoma: An Umbrella Review. Cancers 2023, 15, 5540. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, K.; Sankari, S. Oral Candidal Carriage Among Patients with Oral Squamous Cell Carcinoma: A Case-Control Study. J. Orofac. Sci. 2019, 11, 55–58. [Google Scholar] [CrossRef]
- Patil, S. Analyzing the Association between Candida Prevalence, Species Specificity, and Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis— Candida and OSCC. Appl. Sci. 2020, 10, 1099. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K. Oral Candidal Carriage Among Patients with Oral Potential Malignant Disorders: A Case-Control Study. Pesqui. Bras. Odontopediatria Clín Integr. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Arya, C.P.; Jaiswal, R.; Tandon, A.; Jain, A. Isolation and identification of oral Candida species in potentially malignant disorder and oral squamous cell carcinoma. Natl. J. Maxillofac. Surg. 2021, 12, 387–391. [Google Scholar] [CrossRef]
- Khangura, A.K.; Gupta, S.; Mehta, M.; Gulati, A. Isolation and Identification of Various Candida Species in Potentially Malignant Disorders of Oral Cavity: A Retrospective Study. Int. J. Trop. Dis. Health 2022, 43, 32–44. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K.; Kumar, V.N. A comparative study of Candida species diversity among patients with oral squamous cell carcinoma and oral potentially malignant disorders. BMC Res. Notes 2020, 13, 488. [Google Scholar] [CrossRef]
- Pradahan, M.; Srivastava, R. Cause and Effect Relationship between Candida Spp. and Oral Premalignant Conditions: A Review. Int. J. Contemp. Med. Res. 2019, 6, 28–32. [Google Scholar] [CrossRef]
- Tamai, R.; Kiyoura, Y. Candida albicans and Candida parapsilosis rapidly up-regulate galectin-3 secretion by human gingival epithelial cells. Mycopathologia 2014, 177, 75–79. [Google Scholar] [CrossRef]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, X.; Nash, G.B.; Stone, P.C.; Hilkens, J.; Rhodes, J.M.; Yu, L.G. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009, 69, 6799–6806. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Dong, X.W.; Guo, X.L. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Kohatsu, L.; Hsu, D.K.; Jegalian, A.G.; Liu, F.T.; Baum, L.G. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J. Immunol. 2006, 177, 4718–4726. [Google Scholar] [CrossRef]
- Linden, J.R.; De Paepe, M.E.; Laforce-Nesbitt, S.S.; Bliss, J.M. Galectin-3 plays an important role in protection against disseminated candidiasis. Med. Mycol. 2013, 51, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gunn, L.; Hansen, R.; Yan, J. Combined yeast-derived β-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp. Mol. Pathol. 2009, 86, 208–214. [Google Scholar] [CrossRef]
- He, X.; Ran, Q.; Li, X.; Xiong, A.; Zhang, L.; Jiang, M.; Bai, L.; Peng, D.; Wang, J.; Sun, B.; et al. Candida albicans-Derived β-Glucan as a Novel Modulator of Tumor Microenvironment: Targeting Macrophage Polarization and Inducing Ferroptosis in Lung Cancer. J. Inflamm. Res. 2024, 17, 10479–10494. [Google Scholar] [CrossRef]
- de Graaff, P.; Berrevoets, C.; Rsch, C.; Schols, H.A.; Verhoef, K.; Wichers, H.J.; Debets, R.; Govers, C. Curdlan, zymosan and a yeast-derived β-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunol. Immunother. 2020, 70, 547–561. [Google Scholar] [CrossRef]
- Qi, C.; Cai, Y.; Gunn, L.; Ding, C.; Li, B.; Kloecker, G.; Qian, K.; Vasilakos, J.; Saijo, S.; Iwakura, Y.; et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood 2011, 117, 6825–6836. [Google Scholar] [CrossRef]
- Siswanto, F.M.; Tamura, A.; Sakuma, R.; Imaoka, S. Yeast β-glucan Increases Etoposide Sensitivity in Lung Cancer Cell Line A549 by Suppressing Nuclear Factor Erythroid 2-Related Factor 2 via the Noncanonical Nuclear Factor Kappa B Pathway. Mol. Pharmacol. 2022, 101, 257–273. [Google Scholar] [CrossRef]
- Ascione, C.; Sala, A.; Mazaheri-Tehrani, E.; Paulone, S.; Palmieri, B.; Blasi, E.; Cermelli, C. Herpes simplex virus-1 entrapped in Candida albicans biofilm displays decreased sensitivity to antivirals and UVA1 laser treatment. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 72. [Google Scholar] [CrossRef]
- Erb-Downward, J.R.; Noverr, M.C. Characterization of prostaglandin E2 production by Candida albicans. Infect. Immun. 2007, 75, 3498–3505. [Google Scholar] [CrossRef]
- Coulombe, F.; Jaworska, J.; Verway, M.; Tzelepis, F.; Massoud, A.; Gillard, J.; Wong, G.; Kobinger, G.; Xing, Z.; Couture, C.; et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity 2014, 40, 554–568. [Google Scholar] [CrossRef]
- Plotkin, B.J.; Sigar, I.M.; Tiwari, V.; Halkyard, S. Herpes Simplex Virus (HSV) Modulation of Staphylococcus aureus and Candida albicans Initiation of HeLa 299 Cell-Associated Biofilm. Curr. Microbiol. 2016, 72, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Würzner, R.; Gruber, A.; Stoiber, H.; Spruth, M.; Chen, Y.H.; Lukasser-Vogl, E.; Schwendinger, M.G.; Dierich, M.P. Human immunodeficiency virus type 1 gp41 binds to Candida albicans via complement C3-like regions. J. Infect. Dis. 1997, 176, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.; Lell, C.P.; Speth, C.; Stoiber, H.; Lass-Flörl, C.; Sonneborn, A.; Ernst, J.F.; Dierich, M.P.; Würzner, R. Human immunodeficiency virus type 1 Tat binds to Candida albicans, inducing hyphae but augmenting phagocytosis in vitro. Immunology 2001, 104, 455–461. [Google Scholar]
- Alfaifi, A.; Sultan, A.S.; Montelongo-Jauregui, D.; Meiller, T.F.; Jabra-Rizk, M.A. Long-Term Post-COVID-19 Associated Oral Inflammatory Sequelae. Front. Cell Infect. Microbiol. 2022, 12, 831744. [Google Scholar] [CrossRef]
- Mudey, G.; Jha, A. Pathogenesis of Candida auris: A Threat Emerging during Corona Pandemic. J. Pharm. Res. Int. 2021, 33, 3018–3025. [Google Scholar] [CrossRef]
- Horton, M.V.; Johnson, C.J.; Kernien, J.F.; Patel, T.D.; Lam, B.C.; Cheong, J.Z.A.; Meudt, J.J.; Shanmuganayagam, D.; Kalan, L.R.; Nett, J.E. Candida auris Forms High-Burden Biofilms in Skin Niche Conditions and on Porcine Skin. mSphere 2020, 5, e00910-19. [Google Scholar] [CrossRef]

| Feature | Candida albicans | Candida parapsilosis |
|---|---|---|
| Morphology | Yeast, forms true hyphae and pseudohyphae | Yeast, forms pseudohyphae but lacks true hyphae |
| Germ Tube Test | Positive | Negative |
| Chlamydospore Formation | Present | Absent |
| Common Infections | Oral thrush, vaginal candidiasis, systemic infections | Bloodstream infections, catheter-related infections, wound infections |
| Virulence | High virulence, strong biofilm formation | Lower virulence, but strong biofilm formation on medical devices |
| Biofilm Formation | Strong on mucosal surfaces and devices | Strong on medical devices like catheters |
| Enzymatic Activity | Produces proteases, phospholipases, and lipases | Produces fewer proteases and phospholipases |
| Resistance to Antifungals | Generally susceptible to azoles, echinocandins, and polyenes, but resistance is emerging | More resistant to echinocandins than C. albicans |
| Natural Habitat | Human mucosal surfaces (oral cavity, gastrointestinal tract, and vagina) | Skin, hospital environments, and hands of healthcare workers |
| Epidemiology | Most common cause of candidiasis | Common in healthcare-associated infections, especially in neonates and ICU patients |
| Cytokines Induced | High levels of IL-1β, IL-6, IL-17, IL-22, and TNF-α (strong inflammatory response) | Lower levels of IL-1β, IL-6, IL-17, and TNF-α, but induces IL-10 (more immunotolerant response) |
| Candida Species [Reference] | Salivary Molecule | Binding Mechanism | Effect on Candida Species |
|---|---|---|---|
| C. albicans [69,71] | Proline-Rich Proteins (PRPs) | Adhesion via Bgl2p, Als1, and Hwp1 | Adherence, Biofilm formation |
| C. albicans [22,75,76,77] | Mucin (e.g., MUC5B, MUC7) | Interaction with Sap2 | Inhibition of hyphal formation and biofilm formation, Candidacidal activity |
| C. tropicalis C. parapsilosis C. dubliniensis [22] | |||
| C. albicans [78,79,80,81,82,83,84,85] | Histatin | Binding to Ssa1/2, interaction with cell membrane | Growth inhibition, Cell membrane damage, Antifungal activity |
| C. tropicalis C. parapsilosis [79,81] | |||
| C. dubliniensis [82] | |||
| C. auris [8] | |||
| C. albicans [85,89,90,91] | Statherin | Electrostatic forces | Adhesion to hydroxyapatite and epithelial cells, Transition from hyphae to yeast |
| C. albicans [92,93,94,95,96] | β-Defensin | Binding to PIP2 | Fungicidal effects, Membrane permeabilization, Cell death |
| C. tropicalis [94] | |||
| C. parapsilosis [94,95] | |||
| C. albicans [109,110] | Lactoferrin | Binding to lactoferrin receptors | Iron sequestration, Growth inhibition, Alteration of cell wall permeability |
| C. tropicalis C. parapsilosis [109] | |||
| C. dubliniensis [110] | |||
| C. albicans [80,112] | LL-37 | Binding to cell wall carbohydrates, mannan, glucan, and chitin | Transmembrane pore formation and intracellular damage |
| C. tropicalis C. parapsilosis [112] | |||
| Candida species [106,107] | Secretory IgA (sIgA) | Binding to epitopes | Agglutination and clearance, immune evasion, inhibition of hyphal growth and virulence |
| Molecule of Candida Species | Human Molecular Target | Function/Effect | Reference |
|---|---|---|---|
| Sap1, 2, 3, 9 | Histatin 5 | Inhibition of antifungal effect by degradation | [86] |
| Sap1, 2, 3 | Complement proteins (C3b, C4b, C5) | Degradation, Inhibition of terminal complement complex formation | [114] |
| Sap6 | Protease activated receptor (PAR) 2 | Production of chemokines, Induction of neutrophil chemotaxis | [119,120] |
| Candidalysin | GP1bα (von Willebrand factor receptor) | Release of Dickkopf-1, Upregulation of Th17 immunity | [127] |
| EGFR | Induction of immune responses | [128] | |
| Candidalysin, Als3 | EphA2/EGFR | Promotion of phagocytosis, Production of chemokines and ROS | [132] |
| Als3 | E-cadherin, N-cadherin | Induction of endocytosis | [140] |
| Als1, 3, 5 | Type IV collagen, fibronectin, laminin | Adhesins for invasion of hosts | [136,139] |
| Als proteins | CR3 | Induction of inflammasome activation | [141] |
| Als3 | Caspase-8, ASC | Activation of inflammasome | [142] |
| Als3, Ssa1 | EGFR/HER2, E-cadherin | Promotion of adhesion and colonization | [144] |
| Ssa1 | E-cadherin, N-cadherin | Promotion of epithelial barrier disruption | [145] |
| Hwp1 | Fibronectin | Adhesins for invasion of hosts | [136,139] |
| Pra1 | Complement proteins (C3, C3b, factor-H, C4BP) | Complement evasion, Enhancement of bloodstream dissemination | [1,160] |
| CR3 | Promotion of phagocytosis, Augmentation of tissue attachment | [159] | |
| Mannan | Mannose receptor | Initiation of innate immune response | [167,168] |
| TLR4 | Initiation of inflammatory response | [15,170] | |
| Dectin-2 | Initiation of inflammatory response, Th17 differentiation | [7,173,174] | |
| β-glucan | Dectin-1 | Initiation of inflammatory response | [15,179,180] |
| TLR2 | Initiation of inflammatory response | [15,182] | |
| EphA2 | Augmentation of ROS production | [183,184] |
| Oral Microorganism | Related Molecules (Bacteria/Candida) | Interaction Type | Reference |
|---|---|---|---|
| Streptococcus mutans | GtfB, GtfC/Farnesol | S. mutans growth, microcolony development, glucosyltransferase activity | [187] |
| ComC/- | Inhibition of germ tube formation | [192] | |
| Mutanobactin A/- | Inhibition of yeast–mycelium transition | [193] | |
| SDSF/Hwp1 | Inhibition of hyphal formation | [194] | |
| -/Als1, Hwp1 | Formation of dual-species biofilm | [198] | |
| GtfB/Mannan | Formation of mixed-species biofilm | [199] | |
| GtfB/Bcr1 | Promotion of C. albicans growth for biofilm formation | [190] | |
| Antigen I/II/- | Coaggregation, biofilm formation | [200] | |
| S. mutans, S. sanguinis, Actinomyces viscosus, A. odontolyticus | -/Hwp1, Sap4, Sap6 | Enhancement of tissue invasion and damage | [6] |
| Lactobacillaceae family | Bacteriocins/- | Competition, antifungal effects | [204] |
| Lactobacillus acidophilus | β-glucanase/β-glucan | Antifungal effects | [177] |
| Porphyromonas gingivalis | InlJ/Als3 | Binding, biofilm formation | [210] |
| RgpA/Als3, Mp65, enolase-1 | Binding, protection against anaerobes | [208] | |
| -/Als1, Als3 | Adherence, protection against anaerobes, gingipain activity | [209] | |
| Fusobacterium nucleatum | RadD/Flo9 | Binding, coaggregation | [212] |
| Inhibition of hyphal morphogenesis and growth | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamai, R.; Kiyoura, Y. Candida Infections: The Role of Saliva in Oral Health—A Narrative Review. Microorganisms 2025, 13, 717. https://doi.org/10.3390/microorganisms13040717
Tamai R, Kiyoura Y. Candida Infections: The Role of Saliva in Oral Health—A Narrative Review. Microorganisms. 2025; 13(4):717. https://doi.org/10.3390/microorganisms13040717
Chicago/Turabian StyleTamai, Riyoko, and Yusuke Kiyoura. 2025. "Candida Infections: The Role of Saliva in Oral Health—A Narrative Review" Microorganisms 13, no. 4: 717. https://doi.org/10.3390/microorganisms13040717
APA StyleTamai, R., & Kiyoura, Y. (2025). Candida Infections: The Role of Saliva in Oral Health—A Narrative Review. Microorganisms, 13(4), 717. https://doi.org/10.3390/microorganisms13040717






