Effect of Rare, Locally Isolated Entomopathogenic Fungi on the Survival of Bactrocera oleae Pupae in Laboratory Soil Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rearing of Bactrocera oleae
2.2. EPF Cultures
2.3. Preparation of Fungal Isolates
2.4. The Effect of the Fungal Isolates on the B. oleae Pupae
2.5. Data Analysis
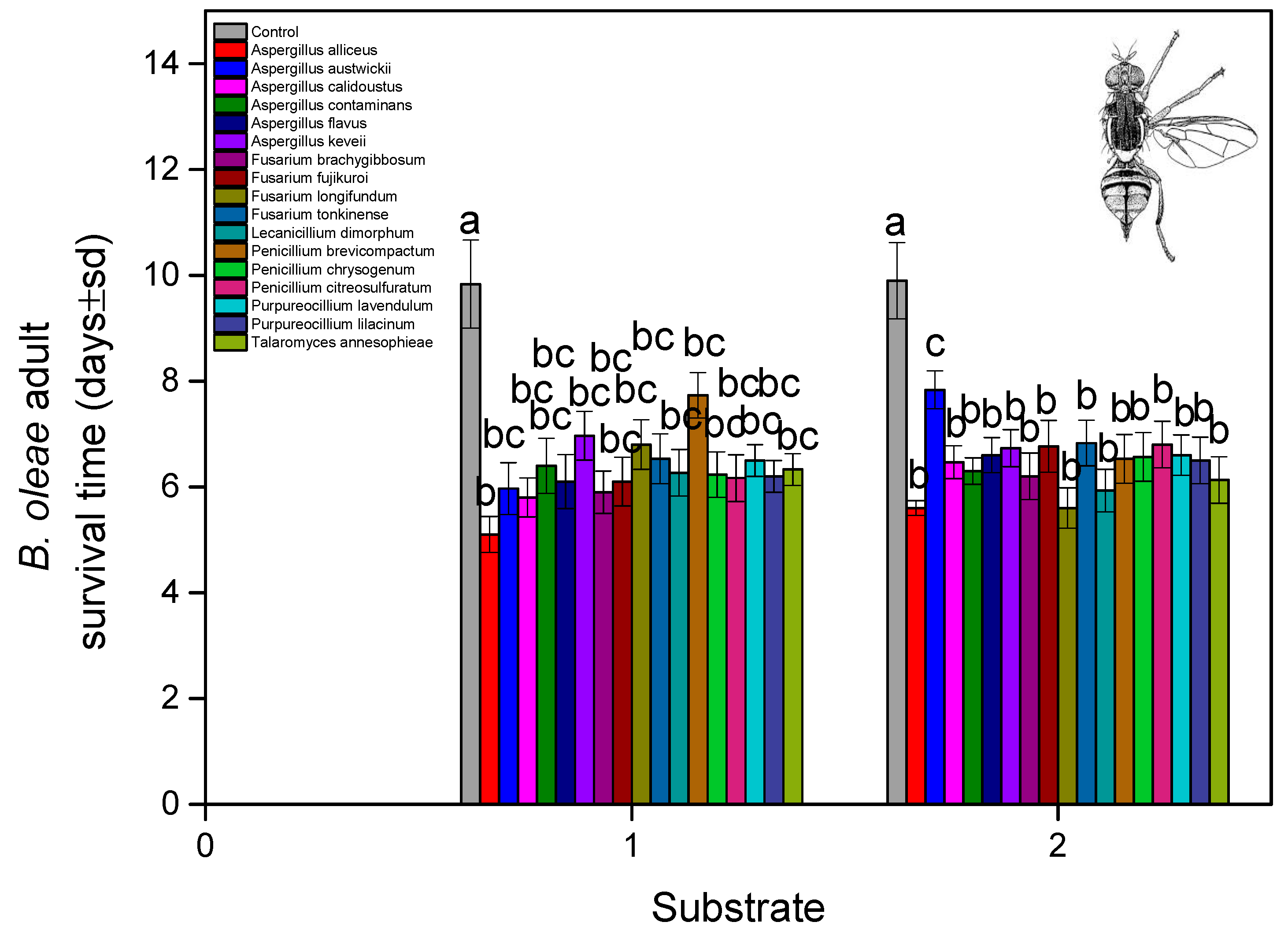
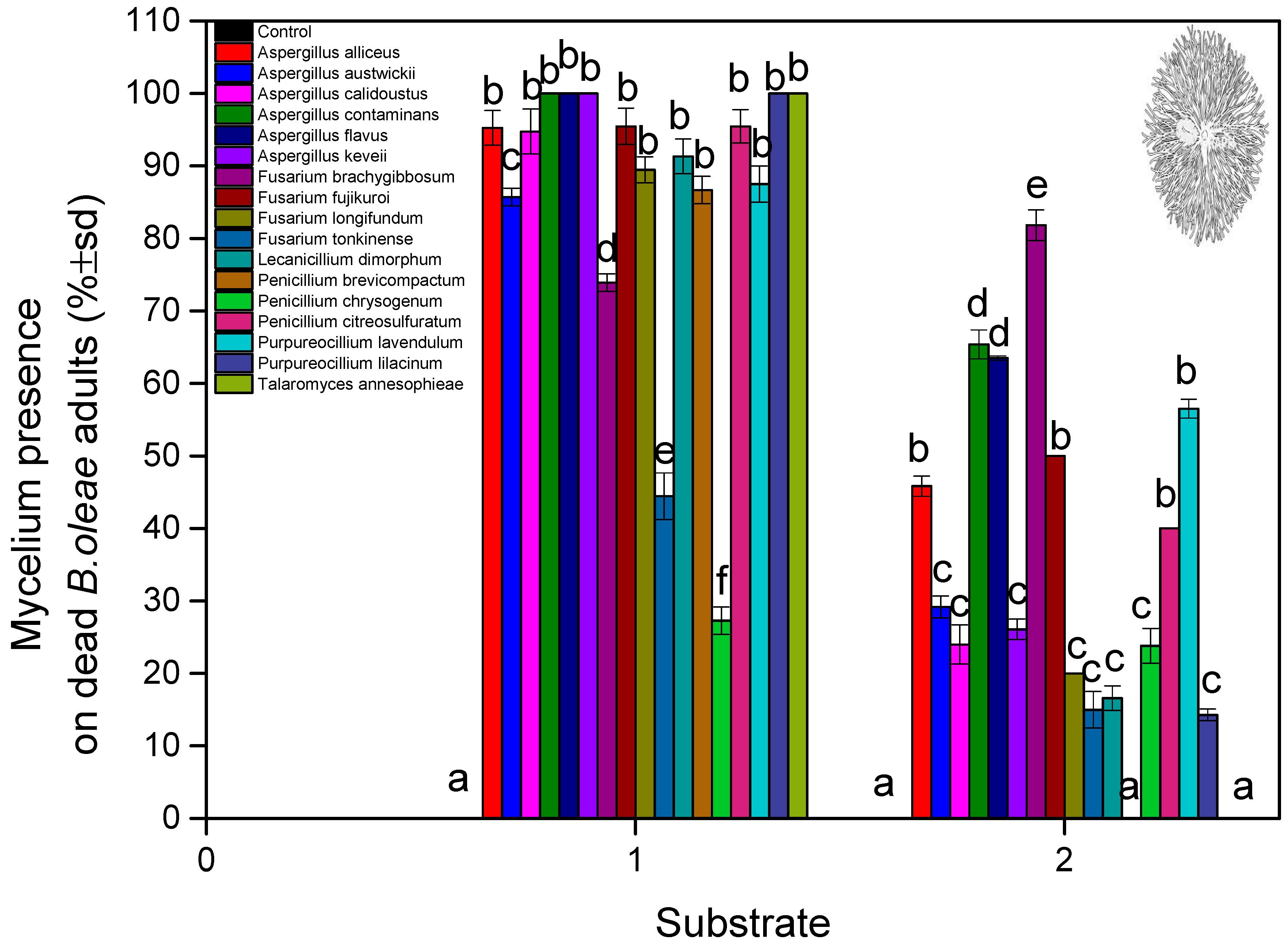
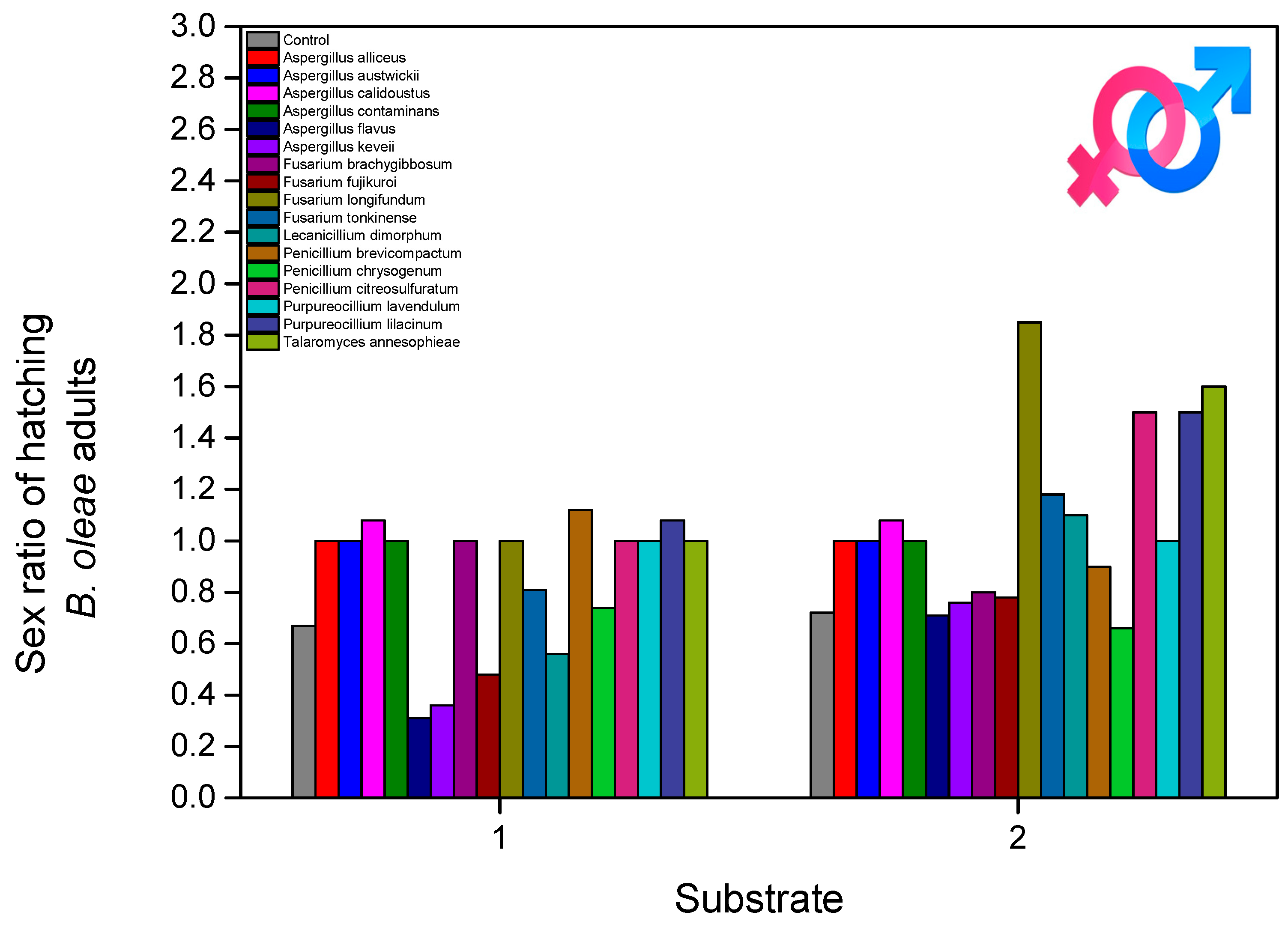
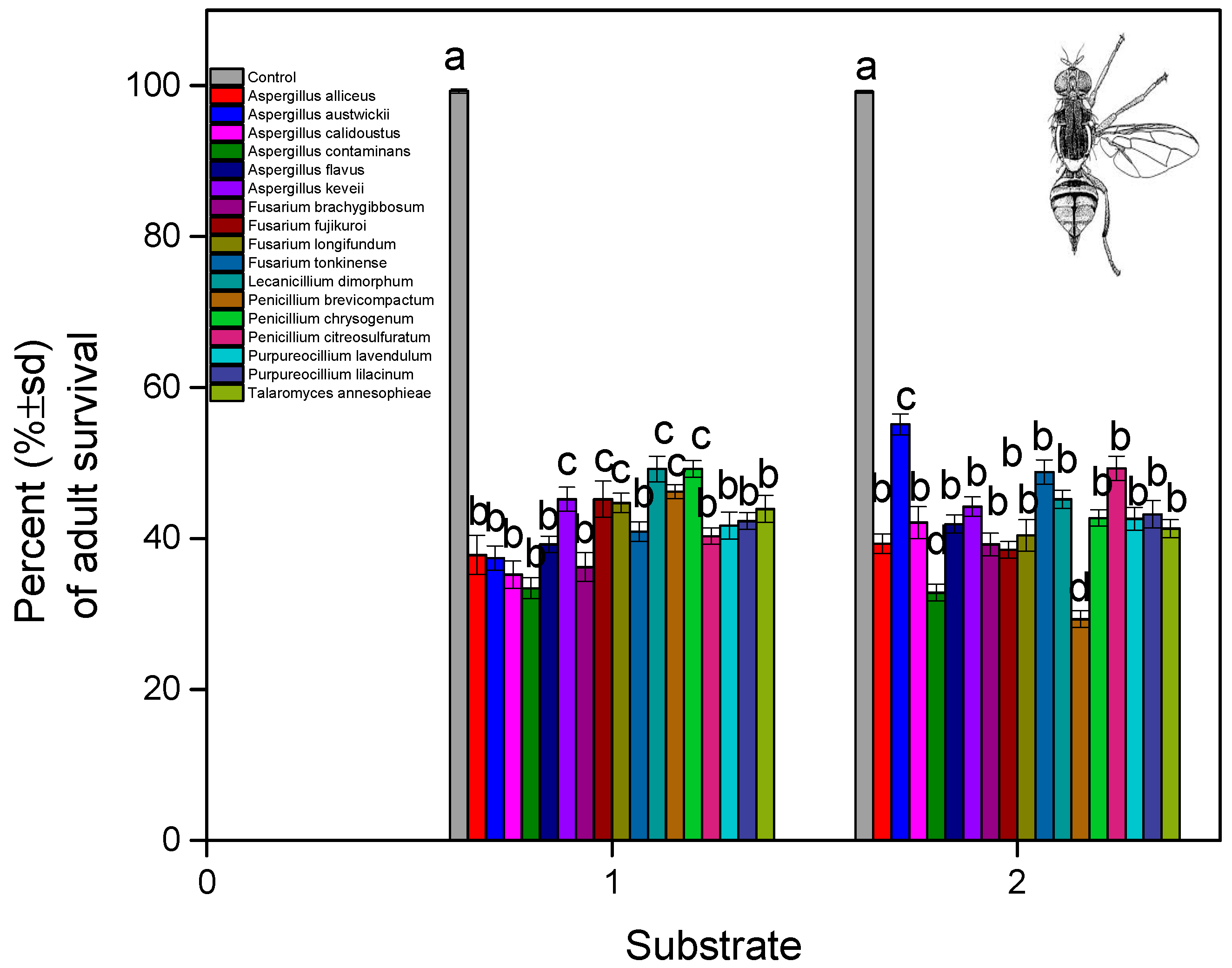
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, M.F.; Malheiro, R.; Casal, S.; Torres, L.; Pereira, J.A. Influence of Fruit Traits on Oviposition Preference of the Olive Fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), on Three Portuguese Olive Varieties (Cobrançosa, Madural and Verdeal Transmontana). Sci. Hortic. 2012, 145, 127–135. [Google Scholar] [CrossRef]
- Yousef, M.; Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E. Biocontrol of Bactrocera oleae (Diptera: Tephritidae) with Metarhizium brunneum and Its Extracts. J. Econ. Entomol. 2013, 106, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.A.; Amato, M.; Celano, G.; Koubouris, G.C. Organic Olive Orchards on Sloping Land: More than a Specialty Niche Production System? J. Environ. Manag. 2008, 89, 99–109. [Google Scholar] [CrossRef]
- Michalopoulos, G.; Kasapi, K.A.; Koubouris, G.; Psarras, G.; Arampatzis, G.; Hatzigiannakis, E.; Kavvadias, V.; Xiloyannis, C.; Montanaro, G.; Malliaraki, S.; et al. Adaptation of Mediterranean Olive Groves to Climate Change through Sustainable Cultivation Practices. Climate 2020, 8, 54. [Google Scholar] [CrossRef]
- Skiada, V.; Tsarouhas, P.; Varzakas, T. Preliminary Study and Observation of “Kalamata PDO” Extra Virgin Olive Oil, in the Messinia Region, Southwest of Peloponnese (Greece). Foods 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Athar, M. Infestation of Olive Fruit Fly, Bactrocera oleae, in California and Taxonomy of Its Host Trees. Agric. Conspec. Sci. 2005, 70, 135–138. [Google Scholar]
- Valenčič, V.; Butinar, B.; Podgornik, M.; Bučar-Miklavčič, M. The Effect of Olive Fruit Fly Bactrocera oleae (Rossi) Infestation on Certain Chemical Parameters of Produced Olive Oils. Molecules 2020, 26, 95. [Google Scholar] [CrossRef]
- Preu, M.; Frieß, J.L.; Breckling, B.; Schröder, W. Case Study 1: Olive Fruit Fly (Bactrocera oleae). In Gene Drives at Tipping Points: Precautionary Technology Assessment and Governance of New Approaches to Genetically Modify Animal and Plant Populations; Springer: Cham, Switzerland, 2020; pp. 79–101. [Google Scholar] [CrossRef]
- Pontikakos, C.M.; Tsiligiridis, T.A.; Yialouris, C.P.; Kontodimas, D.C. Pest Management Control of Olive Fruit Fly (Bactrocera oleae) Based on a Location-Aware Agro-Environmental System. Comput. Electron. Agric. 2012, 87, 39–50. [Google Scholar] [CrossRef]
- Balampekou, E.I.; Koutsos, T.M.; Menexes, G.C.; Koveos, D.S.; Kouloussis, N.A. Pest Management Pathways: Control Strategies for the Olive Fruit Fly (Bactrocera oleae)—A Systematic Map. Agronomy 2024, 14, 2929. [Google Scholar] [CrossRef]
- Katsikogiannis, G.; Kavroudakis, D.; Tscheulin, T.; Kizos, T. Population Dynamics of the Olive Fly, Bactrocera oleae (Diptera: Tephritidae), Are Influenced by Different Climates, Seasons, and Pest Management. Sustainability 2023, 15, 14466. [Google Scholar] [CrossRef]
- Khan, S.A.; Malik, S.H.; Lone, I.U.; Bhat, D.M. Eco-Friendly Pest Management Strategies for Conservation of Biodiversity. Insect Divers. Ecosyst. Serv. 2024, 2, 215–223. [Google Scholar] [CrossRef]
- Priyashantha, A.K.H.; Galappaththi, M.C.A.; Karunarathna, S.C.; Lumyong, S. Entomopathogenic Fungi: Bioweapons against Insect Pests. In The Role of Entomopathogenic Fungi in Agriculture; CRC Press: Boca Raton, FL, USA, 2024; pp. 91–116. [Google Scholar] [CrossRef]
- Gonçalves, R.B.; Aparecida, M.; Zawadneak, C.; Melissa De Azevedo Kuhn, T.; Fernando, T.; Gulinelli, M.; Pimentel, I.C.; Poltronieri, A.S.; Machado Da Rosa, J.; Mirás-Avalos, J.M.; et al. Occurrence and Behavior Analysis of Duponchelia fovealis on Strawberry Plants: Insights for Integrated Pest Management. Horticulturae 2025, 11, 86. [Google Scholar] [CrossRef]
- Hong, S.; Shang, J.; Sun, Y.; Tang, G.; Wang, C. Fungal Infection of Insects: Molecular Insights and Prospects. Trends Microbiol. 2024, 32, 302–316. [Google Scholar] [CrossRef]
- Brunner, M.; Zeisler, C.; Neu, D.; Rotondo, C.; Rubbmark, O.R.; Reinbacher, L.; Grabenweger, G.; Traugott, M. Trap Crops Enhance the Control Efficacy of Metarhizium brunneum against a Soil-Dwelling Pest. J. Pest Sci. 2024, 97, 1633–1645. [Google Scholar] [CrossRef]
- Marri, D.; Gomez, D.A.M.A.; Wilson, D.D.; Billah, M.; Yeboah, S.; Osae, M. Evaluation of the Efficacy of a Commercial Formulation of Beauveria bassiana for the Control of the Invasive Fruit Fly Bactrocera dorsalis (Diptera: Tephritidae). Biopestic. Int. 2016, 12, 9–19. [Google Scholar]
- Wang, D.; Liang, Q.; Chen, M.; Ye, H.; Liao, Y.; Yin, J.; Lü, L.; Lei, Y.; Cai, D.; Jaleel, W.; et al. Susceptibility of Oriental Fruit Fly, Bactrocera dorsalis (Diptera: Tephritidae) Pupae to Entomopathogenic Fungi. Appl. Entomol. Zool. 2021, 56, 269–275. [Google Scholar] [CrossRef]
- Abdellah, A.M.; Hassan, A.E.M.; Eisa, A.A.; Adam, Y.S.; Dafallah, A.B.; Abdellah, A.G.M.; Hassan, A.E.M.; Eisa, A.A.; Dafallah, A.B.; Adam, Y.S. Efficacy of a Sudanese Strain of Entomopathogenic Fungus, Metarhizium anisopliae Met. Sorokin on Puparia of Bactrocera dorsalis Hendel, Under Laboratory Conditions. Sustain. Manag. Invasive Pests Afr. 2020, 14, 73–78. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic Entomopathogenic Fungi: A Valuable Biological Control Tool against Plant Pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef]
- Sani, I.; Jamian, S.; Ismail, S.I.; Saad, N.; Abdullah, S.; Hata, E.M.; Kamarudin, M.A.; Jalinas, J. Identification of Entomopathogenic Fungi Metarhizium anisopliae and Purpureocillium lilacinum from Oil Palm Plantation Soils in Universiti Putra Malaysia. Malays. J. Microbiol. 2022, 18, 105. [Google Scholar] [CrossRef]
- Sani, I.; Jamian, S.; Saad, N.; Abdullah, S.; Hata, E.M.; Jalinas, J.; Ismail, S.I. Inoculation and Colonization of the Entomopathogenic Fungi, Isaria javanica and Purpureocillium lilacinum, in Tomato Plants, and Their Effect on Seedling Growth, Mortality and Adult Emergence of Bemisia Tabaci (Gennadius). PLoS ONE 2023, 18, e0285666. [Google Scholar] [CrossRef]
- Majchrowska-Safaryan, A.; Tkaczuk, C. Abundance of Entomopathogenic Fungi in Leaf Litter and Soil Layers in Forested Habitats in Poland. Insects 2021, 12, 134. [Google Scholar] [CrossRef]
- Murtaza, G.; Naeem, M.; Manzoor, S.; Khan, H.A.; Eed, E.M.; Majeed, W.; Makki, H.A.; Ramzan, U.; Ummara, U.E. Biological Control Potential of Entomopathogenic Fungal Strains against Peach Fruit Fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae). PeerJ 2022, 10, e13316. [Google Scholar] [CrossRef]
- Tahira Gul, H.; Freed, S.; Akmal, M.; Naeem Malik, M. Vulnerability of Different Life Stages of Bactrocera zonata (Tephritidae: Diptera) Against Entomogenous Fungi. Pak. J. Zool. 2015, 47, 307–317. [Google Scholar]
- Saminathan, N.; Subramanian, J.; Sankaran Pagalahalli, S.; Theerthagiri, A.; Mariappan, P. Entomopathogenic Fungi: Translating Research into Field Applications for Crop Protection. Arthropod-Plant Interact. 2024, 19, 8. [Google Scholar] [CrossRef]
- El Aalaoui, M.; Rammali, S.; Bencharki, B.; Sbaghi, M. Efficacy of Biorational Insecticides and Entomopathogenic Fungi for Controlling Cassida vittata Vill. (Coleoptera: Chrysomelidae) in Sugar Beet Crops. Neotrop. Entomol. 2025, 54, 2. [Google Scholar] [CrossRef] [PubMed]
- Intonti, M.; Mola, D.; De Leonardis, M.; Starace, G. Enhancing Circular Practices in Olive Oil Production: The Role of Green Finance. Sustainability 2025, 17, 294. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Vidali, E.; Zacharia, E.; Dris, S. Evaluation of Terminal Residues in Olives and Olive Oil Following a Targeted Large-Scale Plant Protection Application Strategy in Southern Greece. J. Food Compos. Anal. 2025, 140, 107210. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Margaritis, A.; Sourouni, T.; Georgopoulou, V.; Zarmakoupi, C.; Papantzikos, V.; Lagogiannis, I.; Eliopoulos, P.A.; Patakioutas, G. Insecticidal Action of Local Isolates of Entomopathogenic Fungi Against Bactrocera oleae Pupae. Biology 2024, 14, 5. [Google Scholar] [CrossRef]
- Rosengaus, R.B.; Traniello, J.F.A.; Chen, T.; Brown, J.J.; Karp, R.D.; Rosengaus, R.B.; Traniello, J.F.A.; Chen, T.; Brown, J.J.; Karp, R.D. Immunity in a Social Insect. Naturwissenschaften 1999, 86, 588–591. [Google Scholar] [CrossRef]
- Lamberty, M.; Zachary, D.; Lanot, R.; Bordereau, C.; Robert, A.; Hoffmann, J.A.; Bulet, P. Insect Immunity. Constitutive Expression of a Cysteine-Rich Antifungal and a Linear Antibacterial Peptide in a Termite Insect. J. Biol. Chem. 2001, 276, 4085–4092. [Google Scholar] [CrossRef]
- Cox, F.E.G. Concomitant Infections, Parasites and Immune Responses. Parasitology 2001, 122, S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.H.; Briggs, D.E.G.; Eisen, J.A.; Goldstein, D.B.; Patel, N.H. Evolution; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2007; ISBN 978-087969684-9. [Google Scholar]
- Graham, A.L. Ecological Rules Governing Helminth-Microparasite Coinfection. Proc. Natl. Acad. Sci. USA 2008, 105, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Tanada, Y.; Kaya, H.K. Insect Pathology; Academic Press: San Diego, CA, USA, 1993; ISBN 0-12-683255-2. [Google Scholar]
- Hajek, A.E.; St. Leger, R.J. Interactions between Fungal Pathogens and Insect Hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Bateman, R.; Carey, M.; Batt, D.; Prior, C.; Abraham, Y.; Moore, D.; Jenkins, N.; Fenlon, J. Screening for Virulent Isolates of Entomopathogenic Fungi Against the Desert Locust, Schistocerca gregaria (Forskål). Biocontrol Sci. Technol. 1996, 6, 549–560. [Google Scholar] [CrossRef]
- De la Rosa, W.; Lopez, F.L.; Liedo, P. Beauveria bassiana as a Pathogen of the Mexican Fruit Fly (Diptera: Tephritidae) under Laboratory Conditions. J. Econ. Entomol. 2002, 95, 36–43. [Google Scholar] [CrossRef]
- Lecuona, R. Microorganismos Patógenos Empleados En El Control Microbiano de Insectos Plaga; IMYZA–CICA–INTA Castelar Buenos Aires: Buenos Aires, Argentina, 1996; p. 338. [Google Scholar]
- Muñoz, J.A.; la Rosa, W.D.; Toledo, J. Mortalidad En Ceratitis Capitata (Wiedemann) (Diptera: Tephritidae) Por Diversas Cepas de Beauveria bassiana (Bals.) Vuillemin, En Condiciones de Laboratorio. Acta Zoológica Mex. (N.S.) 2009, 25, 609–624. [Google Scholar] [CrossRef]
- Lezama-Gutiérrez, R.; De La Luz, A.T.; Molina-Ochoa, J.; Rebolledo-Dominguez, O.; Pescador, A.R.; López-Edwards, M.; Aluja, M. Virulence of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) on Anastrepha Ludens (Diptera: Tephritidae): Laboratory and Field Trials. J. Econ. Entomol. 2000, 93, 1080–1084. [Google Scholar] [CrossRef]
- Díaz-Ordaz, N.H.; Pérez, N.; Toledo, J. Patogenicidad de Tres Cepas de Hongos Entomopatógenos a Adultos de Anastrepha Obliqua (Macquart) (Diptera: Tephritidae) En Condiciones de Laboratorio. Acta Zoológica Mex. (N.S.) 2010, 26, 481–494. [Google Scholar] [CrossRef]
- Osorio-Fajardo, A.; Canal, N.A. Selección de Cepas de Hongos Entomopatógenos Para El Manejo de Anastrepha Obliqua (Macquart, 1835) (Diptera: Tephritidae) En Colombia. Rev. Fac. Nac. Agron. Medellin 2011, 64, 6129–6139. [Google Scholar]
- Quesada-Moraga, E.; Ruiz-García, A.; Santiago-Álvarez, C. Laboratory Evaluation of Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae against Puparia and Adults of Ceratitis Capitata (Diptera: Tephritidae). J. Econ. Entomol. 2006, 99, 1955–1966. [Google Scholar] [CrossRef]
- Konstantopoulou, M.A.; Mazomenos, B.E. Evaluation of Beauveria bassiana and B. brongniartii Strains and Four Wild-Type Fungal Species against Adults of Bactrocera oleae and Ceratitis capitata. BioControl 2005, 50, 293–305. [Google Scholar] [CrossRef]
- Farag Mahmoud, M. Susceptibility of the Peach Fruit Fly, Bactrocera zonata (Saunders), (Diptera: Tephritidae) to Three Entomopathogenic Fungi. Egypt. J. Biol. Pest Control 2009, 19, 169–175. [Google Scholar]
- Hussein, M.A.; Khaled, A.S.; Ibrahim, A.A.; Soliman, N.A.; Attia, S.H. Evaluation of Entomopathogenic Fungi, Beauveriabassiana and Metarhizium anisopliae on Peach Fruit Fly, Bactrocera zonata (Saunders) (Diptera:Tephritidae). Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control 2018, 10, 59–68. [Google Scholar] [CrossRef]
- Beris, E.I.; Papachristos, D.P.; Fytrou, A.; Antonatos, S.A.; Kontodimas, D.C. Pathogenicity of Three Entomopathogenic Fungi on Pupae and Adults of the Mediterranean Fruit Fly, Ceratitis Capitata (Diptera: Tephritidae). J. Pest Sci. 2013, 86, 275–284. [Google Scholar] [CrossRef]
- Furlong, M.J.; Pell, J.K. Horizontal Transmission of Entomopathogenic Fungi by the Diamondback Moth. Biol. Control 2001, 22, 288–299. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Lux, S.A. Mortality in Three African Tephritid Fruit Fly Puparia and Adults Caused by the Entomopathogenic Fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci. Technol. 2002, 12, 7–17. [Google Scholar] [CrossRef]
- Poprawski, T.J.; Robert, P.H.; Majchrowicz, I.; Boivin, G. Susceptibility of Delia antiqua (Diptera: Anthomyiidae) to Eleven Isolates of Entomopathogenic Hyphomycetes. Environ. Entomol. 1985, 14, 557–561. [Google Scholar] [CrossRef]
- Goble, T.A.; Dames, J.F.; Hill, M.P.; Moore, S.D. Investigation of Native Isolates of Entomopathogenic Fungi for the Biological Control of Three Citrus Pests. Biocontrol Sci. Technol. 2011, 21, 1193–1211. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Mohamed, S.A.; Lux, S.A. Effect of Soil Application of Different Formulations of Metarhizium anisopliae on African Tephritid Fruit Flies and Their Associated Endoparasitoids. Biol. Control 2005, 35, 83–91. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological Factors in the Inundative Use of Fungal Entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Torrent, J.; Barrón, V.; Corpas, A.; Quesada-Moraga, E. Soil Properties Affect the Availability, Movement, and Virulence of Entomopathogenic Fungi Conidia against Puparia of Ceratitis capitata (Diptera: Tephritidae). Biol. Control 2011, 58, 277–285. [Google Scholar] [CrossRef]
- Ignoffo, C.M.; Garcia, C.; Hostetter, D.L.; Pinnell, R.E. Vertical Movement of Conidia of Nomuraea rileyi Through Sand and Loam Soils. J. Econ. Entomol. 1977, 70, 163–164. [Google Scholar] [CrossRef]
- Uchoa-Fernandes, M.A.; Della Lucia, T.M.C.; Vilela, E.F. Anais Da Sociedade Entomológica Do Brasil. An. Soc. Entomológica Bras. 1995, 24, 159–164. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Lux, S.A. Effect of Soil Temperature and Moisture on Survival and Infectivity of Metarhizium anisopliae to Four Tephritid Fruit Fly Puparia. J. Invertebr. Pathol. 2003, 83, 157–167. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Martin-Carballo, I.; Garrido-Jurado, I.; Santiago-Álvarez, C. Horizontal Transmission of Metarhizium anisopliae among Laboratory Populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Biol. Control 2008, 47, 115–124. [Google Scholar] [CrossRef]
- Sookar, P.; Bhagwant, S.; Allymamod, M.N. Effect of Metarhizium anisopliae on the Fertility and Fecundity of Two Species of Fruit Flies and Horizontal Transmission of Mycotic Infection. J. Insect Sci. 2014, 14, 100. [Google Scholar] [CrossRef]
- Scholte, E.J.; Knols, B.G.J.; Takken, W. Autodissemination of the Entomopathogenic Fungus Metarhizium anisopliae amongst Adults of the Malaria Vector Anopheles gambiae s.s. Malar. J. 2004, 3, 45. [Google Scholar] [CrossRef]
- Kaaya, G.P.; Okech, M.A. Horizontal Transmission of Mycotic Infection in Adult Tsetse, Glossina morsitans morsitans. Entomophaga 1990, 35, 589–600. [Google Scholar] [CrossRef]



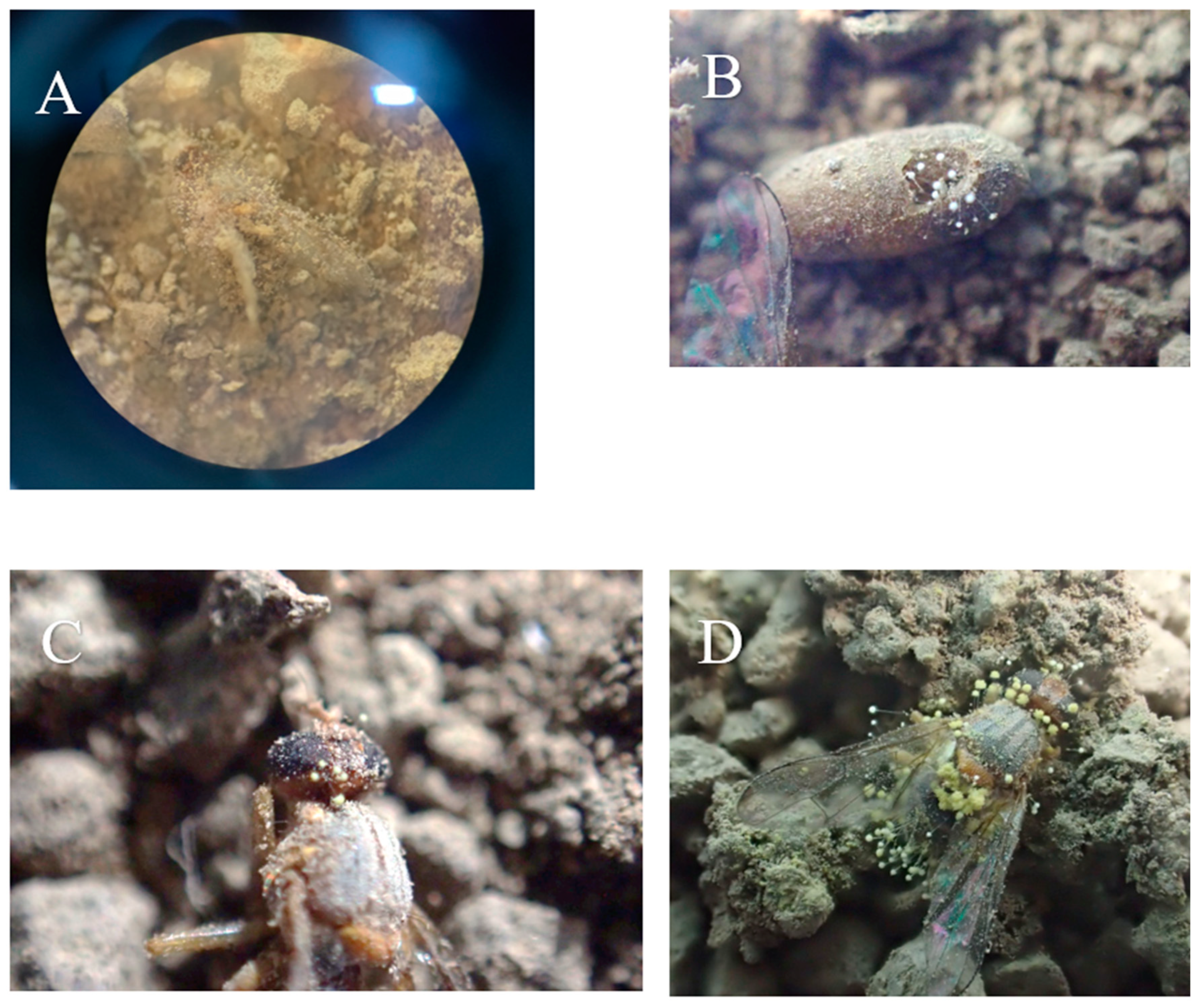

| Fungus Species | Isolate | Collection Site |
|---|---|---|
| Aspergillus alliaceus | 1 | Kastritsi Achaia |
| Aspergillus austwickii | 2 | Dasyllio Achaia |
| Aspergillus calidoustus | 3 | Dasyllio Achaia |
| Aspergillus contaminans | 4 | Dasyllio Achaia |
| Aspergillus flavus | 6 | Dasyllio Achaia |
| Aspergillus keveii | 10 | Elos Achaia |
| Fusarium brachygibbosum | 19 | Elos Achaia |
| Fusarium fujikuroi | 20 | Elos Achaia |
| Fusarium longifundum | 21 | Elos Achaia |
| Fusarium tonkinense | 22 | Dasyllio Achaia |
| Lecanicillium dimorphum | 23 | Dasyllio Achaia |
| Penicillium brevicompactum | 25 | Elos Achaia |
| Penicillium chrysogenum | 26 | Dasyllio Achaia |
| Penicillium citreosulfuratum | 27 | Dasyllio Achaia |
| Purpureocillium lavendulum | 37 | Elos Achaia |
| Purpureocillium lilacinum | 38 | Dasyllio Achaia |
| Talaromyces annesophieae | 40 | Elos Achaia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzoukas, S.; Margaritis, A.; Zarmakoupi, C.; Papantzikos, V.; Sourouni, T.; Georgopoulou, V.; Eliopoulos, P.A.; Lagogiannis, I.; Patakioutas, G. Effect of Rare, Locally Isolated Entomopathogenic Fungi on the Survival of Bactrocera oleae Pupae in Laboratory Soil Conditions. Microorganisms 2025, 13, 811. https://doi.org/10.3390/microorganisms13040811
Mantzoukas S, Margaritis A, Zarmakoupi C, Papantzikos V, Sourouni T, Georgopoulou V, Eliopoulos PA, Lagogiannis I, Patakioutas G. Effect of Rare, Locally Isolated Entomopathogenic Fungi on the Survival of Bactrocera oleae Pupae in Laboratory Soil Conditions. Microorganisms. 2025; 13(4):811. https://doi.org/10.3390/microorganisms13040811
Chicago/Turabian StyleMantzoukas, Spiridon, Alexandros Margaritis, Chrysanthi Zarmakoupi, Vasileios Papantzikos, Thomais Sourouni, Vasiliki Georgopoulou, Panagiotis A. Eliopoulos, Ioannis Lagogiannis, and George Patakioutas. 2025. "Effect of Rare, Locally Isolated Entomopathogenic Fungi on the Survival of Bactrocera oleae Pupae in Laboratory Soil Conditions" Microorganisms 13, no. 4: 811. https://doi.org/10.3390/microorganisms13040811
APA StyleMantzoukas, S., Margaritis, A., Zarmakoupi, C., Papantzikos, V., Sourouni, T., Georgopoulou, V., Eliopoulos, P. A., Lagogiannis, I., & Patakioutas, G. (2025). Effect of Rare, Locally Isolated Entomopathogenic Fungi on the Survival of Bactrocera oleae Pupae in Laboratory Soil Conditions. Microorganisms, 13(4), 811. https://doi.org/10.3390/microorganisms13040811









