Odoribacter splanchnicus—A Next-Generation Probiotic Candidate
Abstract
:1. Introduction
2. Isolation and Major Properties
3. Factors Influencing the Abundance of O. splanchnicus
4. The Multifaceted Roles of O. splanchnicus in Host Health
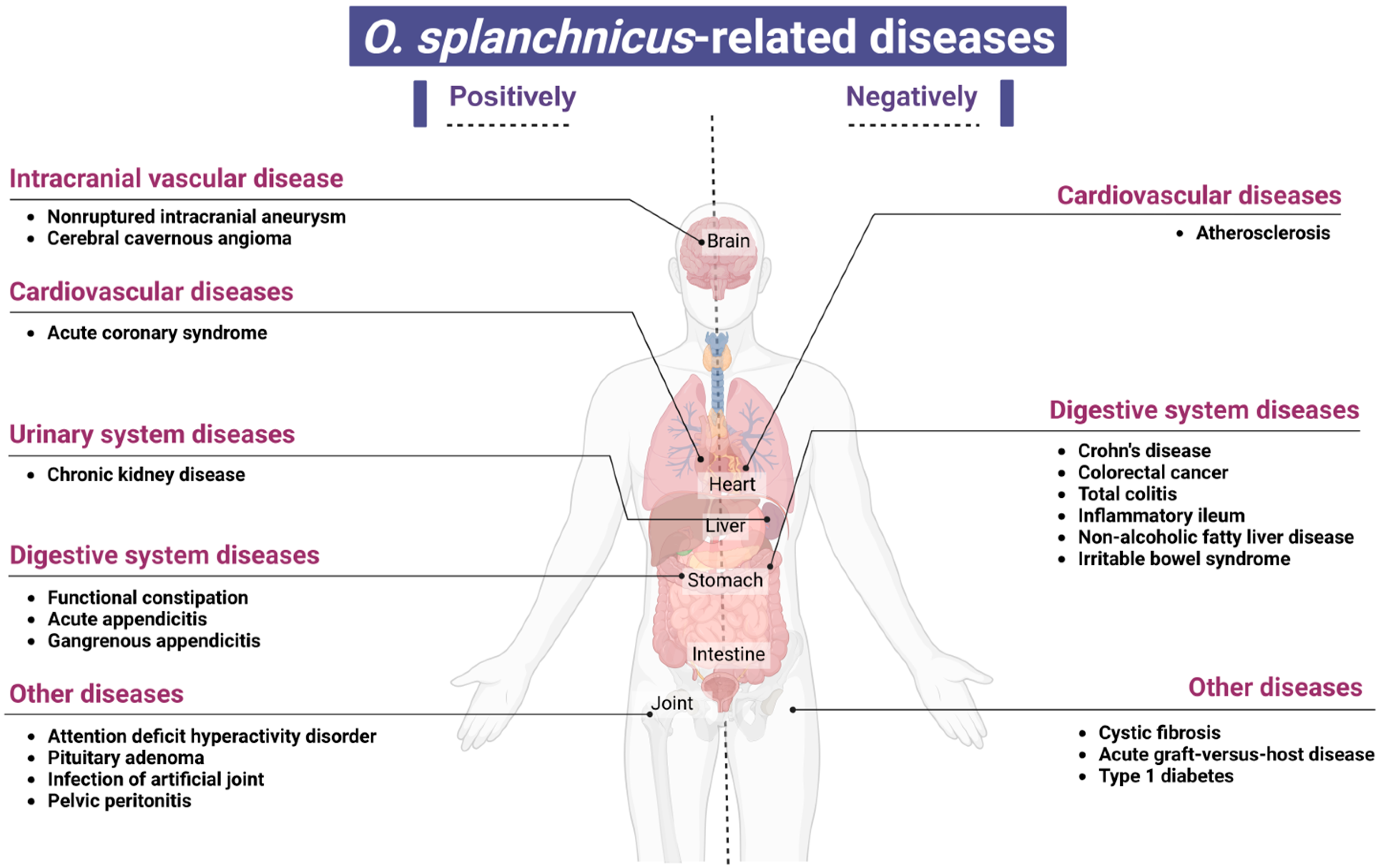
5. A Study on the Association Between O. splanchnicus and Various Disease States
6. Diseases Negatively Associated with the Presence of O. splanchnicus
7. Diseases Positively Associated with O. splanchnicus Presence
8. Biomarker
9. Potential Applications of O. splanchnicus
10. Challenges in Clinical Application
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NGPs | next-generation probiotics |
| SCFAs | short-chain fatty acids |
| CRC | colorectal cancer |
| T1D | type 1 diabetes |
| aGVHD | acute graft-versus-host disease |
| CF | cystic fibrosis |
| NAFLD | non-alcoholic fatty liver disease |
| IBS | irritable bowel syndrome |
| FMT | fecal microbiota transplantation |
| SLE | systemic lupus erythematosus |
| ADHD | attention-deficit hyperactivity disorder |
| ACS | acute coronary syndrome |
| HCC | hepatocellular carcinoma |
| KC | kidney cancer |
| PDAC | pancreatic ductal adenocarcinoma |
| AIP | autoimmune pancreatitis |
References
- Renwick, S.; Ganobis, C.M.; Elder, R.A.; Gianetto-Hill, C.; Higgins, G.; Robinson, A.V.; Vancuren, S.J.; Wilde, J.; Allen-Vercoe, E. Culturing Human Gut Microbiomes in the Laboratory. Annu. Rev. Microbiol. 2021, 75, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What defines a healthy gut microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.G.; Song, E.J.; Yoon, H.J.; Chung, W.-H.; Seo, H.Y.; Kim, D.; Lee, D.; Seo, J.-G.; Lee, H.; Kim, S.I.; et al. Clade-specific extracellular vesicles from Akkermansia muciniphila mediate competitive colonization via direct inhibition and immune stimulation. Nat. Commun. 2025, 16, 2708. [Google Scholar] [CrossRef]
- Murali, S.K.; Mansell, T.J. Next generation probiotics: Engineering live biotherapeutics. Biotechnol. Adv. 2024, 72, 108336. [Google Scholar] [CrossRef]
- Lin, X.; Hu, T.; Wu, Z.; Li, L.; Wang, Y.; Wen, D.; Liu, X.; Li, W.; Liang, H.; Jin, X.; et al. Isolation of potentially novel species expands the genomic and functional diversity of Lachnospiraceae. iMeta 2024, 3, e174. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes 2024, 16, 2304900. [Google Scholar] [CrossRef]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.-B.; Pires, S.; Guo, C.-J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable Immunoglobulin A–Coated Odoribacter splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology 2022, 162, 166–178. [Google Scholar] [CrossRef]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel Odoribacter splanchnicus Strain and Its Outer Membrane Vesicles Exert Immunoregulatory Effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef]
- Göker, M.; Gronow, S.; Zeytun, A.; Nolan, M.; Lucas, S.; Lapidus, A.; Hammon, N.; Deshpande, S.; Cheng, J.-F.; Pitluck, S.; et al. Complete genome sequence of Odoribacter splanchnicus type strain (1651/6T). Stand. Genom. Sci. 2011, 4, 200–209. [Google Scholar] [CrossRef]
- Werner, H.; Reichertz, C. Buttersäurebildende Bacteroides-Kulturen. Zentralbl. Bakteriol. Parasitenkd. Infekt. Hyg. Abt. 1 Orig. Reihe A 1971, 217, 206–216. [Google Scholar]
- Werner, H.; Rintelen, G.; Kunstek-Santos, H. A new butyric acid-producing Bacteroides species: B. splanchnicus n. sp. Zentralblatt Für Bakteriol. Parasitenkd. Infekt. Hyg. 1975, 231, 133–144. [Google Scholar]
- Johnson, J.L.; Harich, B. Ribosomal Ribonucleic Acid Homology among Species of the Genus Bacteroides. Int. J. Syst. Bacteriol. 1986, 36, 71–79. [Google Scholar] [CrossRef]
- Hardham, J.M.; King, K.W.; Dreier, K.; Wong, J.; Strietzel, C.; Eversole, R.R.; Sfintescu, C.; Evans, R.T. Transfer of Bacteroides splanchnicus to Odoribacter gen. nov. as Odoribacter splanchnicus comb. nov., and description of Odoribacter denticanis sp. nov., isolated from the crevicular spaces of canine periodontitis patients. Int. J. Syst. Evol. Microbiol. 2008, 58, 103–109. [Google Scholar] [CrossRef]
- Munoz, R.; Rosselló-Móra, R.; Amann, R. Revised phylogeny of Bacteroidetes and proposal of sixteen new taxa and two new combinations including Rhodothermaeota phyl. nov. Syst. Appl. Microbiol. 2016, 39, 281–296. [Google Scholar] [CrossRef]
- Nagai, F.; Morotomi, M.; Watanabe, Y.; Sakon, H.; Tanaka, R. Alistipes indistinctus sp. nov. and Odoribacter laneus sp. nov., common members of the human intestinal microbiota isolated from faeces. Int. J. Syst. Evol. Microbiol. 2010, 60, 1296–1302. [Google Scholar] [CrossRef]
- Labbe, M.; Mertens, A.; Schoutens, E. Pelviperitonitis and bacteremia due to Bacteroides splanchnicus. Report of a case. Zentralbl. Bakteriol. Orig. A 1977, 238, 251–254. [Google Scholar]
- Xing, C.; Wang, M.; Ajibade, A.A.; Tan, P.; Fu, C.; Chen, L.; Zhu, M.; Hao, Z.-Z.; Chu, J.; Yu, X.; et al. Microbiota regulate innate immune signaling and protective immunity against cancer. Cell Host Microbe 2021, 29, 959–974.e7. [Google Scholar] [CrossRef]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef]
- Shah, H.N.; Collins, M.D. Genus Bacteroides. A chemotaxonomical perspective. J. Appl. Bacteriol. 1983, 55, 403–416. [Google Scholar] [CrossRef]
- Lara-Taranchenko, Y.; Corona, P.S.; Rodríguez-Pardo, D.; Salmerón-Menéndez, P.; Vicente Ciurans, M.; García-Martínez, M.C.; Carrera Calderer, L. Prosthetic joint infection caused by an atypical gram-negative bacilli: Odoribacter splanchnicus. Anaerobe 2023, 82, 102740. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef] [PubMed]
- Jaagura, M.; Viiard, E.; Karu-Lavits, K.; Adamberg, K. Low-carbohydrate high-fat weight reduction diet induces changes in human gut microbiota. MicrobiologyOpen 2021, 10, e1194. [Google Scholar] [CrossRef]
- Wan, L.; Ge, W.R.; Zhang, S.; Sun, Y.L.; Wang, B.; Yang, G. Case-Control Study of the Effects of Gut Microbiota Composition on Neurotransmitter Metabolic Pathways in Children With Attention Deficit Hyperactivity Disorder. Front. Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takagaki, A.; Matsumoto, K.; Kato, Y.; Goto, K.; Benno, Y. Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family “Porphyromonadaceae” isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 2009, 59 Pt 7, 1748–1753. [Google Scholar] [CrossRef]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J. Dairy Sci. 2020, 103, 5816–5829. [Google Scholar] [CrossRef]
- Wei, W.; Wong, C.C.; Jia, Z.; Liu, W.; Liu, C.; Ji, F.; Pan, Y.; Wang, F.; Wang, G.; Zhao, L.; et al. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 2023, 8, 1534–1548. [Google Scholar] [CrossRef]
- Topping, D.L.; Fukushima, M.; Bird, A.R. Resistant starch as a prebiotic and synbiotic: State of the art. Proc. Nutr. Soc. 2003, 62, 171–176. [Google Scholar] [CrossRef]
- Wang, R.; Lin, F.; Ye, C.; Aihemaitijiang, S.; Halimulati, M.; Huang, X.; Jiang, Z.; Li, L.; Zhang, Z. Multi-omics analysis reveals therapeutic effects of Bacillus subtilis-fermented Astragalus membranaceus in hyperuricemia via modulation of gut microbiota. Food Chem. 2023, 399, 133993. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, W.; Zhao, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W.; Yang, B. Lactobacillus plantarum Ameliorates Colorectal Cancer by Ameliorating the Intestinal Barrier through the CLA-PPAR-γ Axis. J. Agric. Food Chem. 2024, 72, 19766–19785. [Google Scholar] [CrossRef]
- Etxeberria, U.; Hijona, E.; Aguirre, L.; Milagro, F.I.; Bujanda, L.; Rimando, A.M.; Martínez, J.A.; Portillo, M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2017, 61, 1500906. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Jiang, C.; Xing, X.; Tsai, M.-S.; Snyder, M.; Zhai, A.; Yao, G. Prevention of Severe Intestinal Barrier Dysfunction Through a Single-Species Probiotics is Associated with the Activation of Microbiome-Mediated Glutamate–Glutamine Biosynthesis. Shock 2021, 55, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, H.; Guo, X.; Wang, J.; Zhu, M.; Zhao, H.; Li, J.; Huang, H.; Zhou, Y. IDDF2023-ABS-0100 Odoribacter. splanchnicus alleviate colitis by regulating neutrophil extracellular traps formation. In Basic Gastroenterology; BMJ Publishing Group Ltd and British Society of Gastroenterology: London, UK, 2023; pp. A79–A81. [Google Scholar] [CrossRef]
- Oh, B.S.; Choi, W.J.; Kim, J.S.; Ryu, S.W.; Yu, S.Y.; Lee, J.-S.; Park, S.-H.; Kang, S.W.; Lee, J.; Jung, W.Y.; et al. Cell-Free Supernatant of Odoribacter splanchnicus Isolated from Human Feces Exhibits Anti-colorectal Cancer Activity. Front. Microbiol. 2021, 12, 736343. [Google Scholar] [CrossRef]
- Han, N.; Chang, H.J.; Yeo, H.Y.; Kim, B.C.; Kim, B.; Park, S.C.; Kim, J.; Park, J.W.; Oh, J.H. Association of gut microbiome with immune microenvironment in surgically treated colorectal cancer patients. Pathology 2024, 56, 528–539. [Google Scholar] [CrossRef]
- Png, C.W.; Chua, Y.K.; Law, J.H.; Zhang, Y.; Tan, K.K. Alterations in co-abundant bacteriome in colorectal cancer and its persistence after surgery: A pilot study. Sci. Rep. 2022, 12, 9829. [Google Scholar] [CrossRef]
- Cai, P.; Xiong, J.; Sha, H.; Dai, X.; Lu, J. Tumor bacterial markers diagnose the initiation and four stages of colorectal cancer. Front. Cell Infect. Microbiol. 2023, 13, 1123544. [Google Scholar] [CrossRef]
- Foegeding, N.J.; Byndloss, M.X. TAKing on cancer. Cell Host Microbe 2021, 29, 851–853. [Google Scholar] [CrossRef]
- Koelink, P.J.; Bloemendaal, F.M.; Li, B.; Westera, L.; Vogels, E.W.M.; van Roest, M.; Gloudemans, A.K.; van’t Wout, A.B.; Korf, H.; Vermeire, S.; et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut 2020, 69, 1053–1063. [Google Scholar] [CrossRef]
- Abdeljaoued, S.; Doussot, A.; Kroemer, M.; Laloy, E.; Pallandre, J.R.; El Kaddissi, A.; Spehner, L.; Ben Khelil, M.; Bouard, A.; Mougey, V.; et al. Liver metastases of colorectal cancer contain different subsets of tissue-resident memory CD8 T cells correlated with a distinct risk of relapse following surgery. OncoImmunology 2025, 14, 2455176. [Google Scholar] [CrossRef]
- Sun, R.; Lee, K.Y.; Mei, Y.; Nickles, E.; Le Lin, J.; Xia, R.; Liu, H.; Schwarz, H. Induction of cell death in malignant cells and regulatory T cells in the tumor microenvironment by targeting CD137. OncoImmunology 2025, 14, 2443265. [Google Scholar] [CrossRef]
- Galassi, C.; Esteller, M.; Vitale, I.; Galluzzi, L. Epigenetic control of immunoevasion in cancer stem cells. Trends Cancer 2024, 10, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Kilic, G.; Domínguez-Andrés, J. Immune Memory in Aging: A Wide Perspective Covering Microbiota, Brain, Metabolism, and Epigenetics. Clin. Rev. Allerg. Immunol. 2022, 63, 499–529. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, H.; Chen, H.; Liu, X.; Zhao, J.; Stanton, C.; Ross, R.P.; Chen, W.; Yang, B. Bifidobacterium inhibits the progression of colorectal tumorigenesis in mice through fatty acid isomerization and gut microbiota modulation. Gut Microbes 2025, 17, 2464945. [Google Scholar] [CrossRef]
- Murphy, C.L.; O’Toole, P.W.; Shanahan, F. The Gut Microbiota in Causation, Detection, and Treatment of Cancer. Am. J. Gastroenterol. 2019, 114, 1036–1042. [Google Scholar] [CrossRef]
- Roth-Schulze, A.J.; Penno, M.A.S.; Ngui, K.M.; Oakey, H.; Bandala-Sanchez, E.; Smith, A.D.; Allnutt, T.R.; Thomson, R.L.; Vuillermin, P.J.; Craig, M.E.; et al. Type 1 diabetes in pregnancy is associated with distinct changes in the composition and function of the gut microbiome. Microbiome 2021, 9, 167. [Google Scholar] [CrossRef]
- Solbach, P.; Chhatwal, P.; Woltemate, S.; Tacconelli, E.; Buhl, M.; Autenrieth, I.B.; Vehreschild, M.J.G.T.; Jazmati, N.; Gerhard, M.; Stein-Thoeringer, C.K.; et al. Microbiota-associated Risk Factors for Clostridioides difficile Acquisition in Hospitalized Patients: A Prospective, Multicentric Study. Clin. Infect. Dis. 2021, 73, e2625–e2634. [Google Scholar] [CrossRef]
- Rashidi, A.; Herman, A.; Gomes, A.L.C.; Peled, J.U.; Jenq, R.R.; Brereton, D.G.; Staley, C.; Blazar, B.R.; Weisdorf, D.J. An alpha-defensin gene single nucleotide polymorphism modulates the gut microbiota and may alter the risk of acute graft-versus-host disease. Br. J. Haematol. 2020, 189, 926–930. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2017, 22, 247. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Ghozlane, A.; Hu, H.; Li, X.; Xiao, Y.; Li, D.; Yu, G.; Zhang, T. Characteristics of Faecal Microbiota in Paediatric Crohn’s Disease and Their Dynamic Changes During Infliximab Therapy. J. Crohn’s Colitis 2018, 12, 337–346. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Wang, Z.; Peters, B.A.; Bryant, M.; Hanna, D.B.; Schwartz, T.; Wang, T.; Sollecito, C.C.; Usyk, M.; Grassi, E.; Wiek, F.; et al. Gut microbiota, circulating inflammatory markers and metabolites, and carotid artery atherosclerosis in HIV infection. Microbiome 2023, 11, 119. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Liu, X.; Cheng, L. Metagenomic analysis of the gut microbiome in atherosclerosis patients identify cross-cohort microbial signatures and potential therapeutic target. FASEB J. 2020, 34, 14166–14181. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.G.; Fouhy, F.; Harrison, M.J.; Rea, M.C.; Cotter, P.D.; O’Sullivan, O.; Stanton, C.; Hill, C.; Shanahan, F.; Plant, B.J.; et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017, 17, 58. [Google Scholar] [CrossRef]
- Li, F.; Sun, G.; Wang, Z.; Wu, W.; Guo, H.; Peng, L.; Wu, L.; Guo, X.; Yang, Y. Characteristics of fecal microbiota in non-alcoholic fatty liver disease patients. Sci. China Life Sci. 2018, 61, 770–778. [Google Scholar] [CrossRef]
- Han, L.; Zhao, L.; Zhou, Y.; Yang, C.; Xiong, T.; Lu, L.; Deng, Y.; Luo, W.; Chen, Y.; Qiu, Q.; et al. Altered metabolome and microbiome features provide clues in understanding irritable bowel syndrome and depression comorbidity. ISME J. 2022, 16, 983–996. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Q.; Zhao, Y.; Zou, Y.; Chen, M.; Zhou, S.; Wang, Z. The relationship of Megamonas species with nonalcoholic fatty liver disease in children and adolescents revealed by metagenomics of gut microbiota. Sci. Rep. 2022, 12, 22001. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, Y.; Zhang, B.; Yan, C.; Zhang, Q.; Ke, Y.; Chen, Z.; Wang, L.; Shi, H.; Hu, Y.; et al. Capsulized Fecal Microbiota Transplantation Induces Remission in Patients with Ulcerative Colitis by Gut Microbial Colonization and Metabolite Regulation. Microbiol. Spectr. 2023, 11, e0415222. [Google Scholar] [CrossRef]
- Ma, J.E.; Jiang, H.Y.; Li, L.M.; Zhang, X.-J.; Li, G.-Y.; Li, H.-M.; Jin, X.-J.; Chen, J.-P. The Fecal Metagenomics of Malayan Pangolins Identifies an Extensive Adaptation to Myrmecophagy. Front. Microbiol. 2018, 9, 2793. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia muciniphila in neuropsychiatric disorders: Friend or foe? Front. Cell Infect. Microbiol. 2023, 13, 1224155. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jia, X.; Xu, J.; Zhao, L.; Ji, J.; Wu, B.; Ma, Y.; Li, H.; Zuo, X.; Pan, W.; et al. An Autoimmunogenic and Proinflammatory Profile Defined by the Gut Microbiota of Patients With Untreated Systemic Lupus Erythematosus. Arthritis Rheumatol. 2021, 73, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H.; Li, Y.; Jiang, Y.; Hu, Y.; Liu, T.; Tian, X.; Zhao, X.; Zhu, Y.; Wang, S.; et al. Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat. Commun. 2020, 11, 3218. [Google Scholar] [CrossRef]
- Polster, S.P.; Sharma, A.; Tanes, C.; Tang, A.T.; Mericko, P.; Cao, Y.; Carrión-Penagos, J.; Girard, R.; Koskimäki, J.; Zhang, D.; et al. Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma. Nat. Commun. 2020, 11, 2659. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, L.; Sun, Y.L.; Yang, G. Characteristics of intestinal flora in children with attention deficit hyperactivity disorder. J. Clin. Pediatr. 2020, 38, 264–268. [Google Scholar]
- Gkougka, D.; Mitropoulos, K.; Tzanakaki, G.; Panagouli, E.; Psaltopoulou, T.; Thomaidis, L.; Tsolia, M.; Sergentanis, T.N.; Tsitsika, A. Gut microbiome and attention deficit/hyperactivity disorder: A systematic review. Pediatr. Res. 2022, 92, 1507–1519. [Google Scholar] [CrossRef]
- Talmor-Barkan, Y.; Bar, N.; Shaul, A.A.; Shahaf, N.; Godneva, A.; Bussi, Y.; Lotan-Pompan, M.; Weinberger, A.; Shechter, A.; Chezar-Azerrad, C.; et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med. 2022, 28, 295–302. [Google Scholar] [CrossRef]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef]
- Yang, C.; Hu, T.; Xue, X.; Su, X.; Zhang, X.; Fan, Y.; Shen, X.; Dong, X. Multi-omics analysis of fecal microbiota transplantation’s impact on functional constipation and comorbid depression and anxiety. BMC Microbiol. 2023, 23, 389. [Google Scholar] [CrossRef]
- Lin, B.; Wang, M.; Gao, R.; Ye, Z.; Yu, Y.; He, W.; Qiao, N.; Ma, Z.; Ji, C.; Shi, C.; et al. Characteristics of Gut Microbiota in Patients with GH-Secreting Pituitary Adenoma. Microbiol. Spectr. 2022, 10, e00425-21. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Ma, D.; Zhang, W.; Zhang, H. Lactobacillus casei Zhang exerts anti-obesity effect to obese glut1 and gut-specific-glut1 knockout mice via gut microbiota modulation mediated different metagenomic pathways. Eur. J. Nutr. 2022, 61, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Sasi, S.; Nair, A.P.; Doiphode, S.; Gutti, T.S.; Kolleri, J.; Al-Maslamani, M. Odoribacter splanchnicus bacteremia secondary to acute appendicitis: A case report with review of literature. J. Surg. Case Rep. 2024, 2024, rjae328. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, A.; Okamoto, K.; Nakagawa, N.; Sonoda, H.; Moriya, K. Identification of Odoribacter splanchnicus bacteremia using MALDI-TOF mass spectrometry and 16S rRNA sequencing: A case report. Anaerobe 2022, 78, 102663. [Google Scholar] [CrossRef]
- Bennion, R.S.; Baron, E.J.; Thompson, J.E.; Downes, J.; Summanen, P.; Talan, D.A.; Finegold, S.M. The Bacteriology of Gangrenous and Perforated Appendicitis—Revisited. Ann. Surg. 1990, 211, 165–171. [Google Scholar] [CrossRef]
- Li, X.; Yi, Y.; Wu, T.; Chen, N.; Gu, X.; Xiang, L.; Jiang, Z.; Li, J.; Jin, H. Integrated microbiome and metabolome analysis reveals the interaction between intestinal flora and serum metabolites as potential biomarkers in hepatocellular carcinoma patients. Front. Cell. Infect. Microbiol. 2023, 13, 1170748. [Google Scholar] [CrossRef]
- Liu, W.; Fang, X.; Zhou, Y.; Dou, L.; Dou, T. Machine learning-based investigation of the relationship between gut microbiome and obesity status. Microbes Infect. 2022, 24, 104892. [Google Scholar] [CrossRef]
- Lv, S.; Guo, Q.; He, Y.; Yu, Z.; Zhan, X.; Li, H.; Pan, Y. Exploring the gut microbiome and immunological landscape in kidney cancer: A Mendelian randomization analysis. Front. Immunol. 2024, 15, 1459967. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, D.; Li, Z.; Jiang, H.; Li, J.; Ren, R.; Gao, X.; Li, J.; Wang, X.; Wang, W.; et al. The fecal microbiota of patients with pancreatic ductal adenocarcinoma and autoimmune pancreatitis characterized by metagenomic sequencing. J. Transl. Med. 2021, 19, 215. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Dai, L.; Chen, H.; Yi, B.; Niu, J.; Wang, L.; Zhang, F.; Luo, J.; Wang, K.; et al. Ecological and network analyses identify four microbial species with potential significance for the diagnosis/treatment of ulcerative colitis (UC). BMC Microbiol. 2021, 21, 138. [Google Scholar] [CrossRef]
- Wang, J.; Qie, J.; Zhu, D.; Zhang, X.; Zhang, Q.; Xu, Y.; Wang, Y.; Mi, K.; Pei, Y.; Liu, Y.; et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging—Relevant neural and immune function. Gut Microbes 2022, 14, 2107288. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Hu, C.; Ding, J.; Zhou, Q.; Pang, G.; Wu, Z.; Yang, R.; Li, S.; Li, J.; et al. Age-dependent changes in the gut microbiota and serum metabolome correlate with renal function and human aging. Aging Cell. 2023, 22, e14028. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Chen, Z.; Zhang, X.; Wang, Z.; Yao, Y.; Wu, X.; Qiu, J.; Lin, H.; Yu, L.; Tu, H.; et al. 2,5-dimethylcelecoxib alleviated NK and T-cell exhaustion in hepatocellular carcinoma via the gastrointestinal microbiota-AMPK-mTOR axis. J. Immunother. Cancer 2023, 11, e006817. [Google Scholar] [CrossRef] [PubMed]
- Pötgens, S.A.; Lecop, S.; Havelange, V.; Li, F.; Neyrinck, A.M.; Neveux, N.; Maertens, J.; Walter, J.; Schoemans, H.; Delzenne, N.M.; et al. Gut microbiota alterations induced by intensive chemotherapy in acute myeloid leukaemia patients are associated with gut barrier dysfunction and body weight loss. Clin. Nutr. 2023, 42, 2214–2228. [Google Scholar] [CrossRef]
- Goudman, L.; Demuyser, T.; Pilitsis, J.G.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Gut dysbiosis in patients with chronic pain: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1342833. [Google Scholar] [CrossRef]
- Bosch, B.; Moutaharrik, S.; Gazzaniga, A.; Hiippala, K.; Santos, H.A.; Maroni, A.; Satokari, R. Development of a time-dependent oral colon delivery system of anaerobic Odoribacter splanchnicus for bacteriotherapy. Eur. J. Pharm. Biopharm. 2023, 190, 73–80. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhuang, Z.; Chen, B.; Yang, Y.; Chen, H.; Guan, G. Odoribacter splanchnicus-derived extracellular vesicles alleviate inflammatory bowel disease by modulating gastrointestinal inflammation and intestinal barrier function via the NLRP3 inflammasome suppression. Mol. Med. 2025, 31, 56. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wong, S.; Yu, J.; Lee, Y.Y.; Terauchi, J.; Lai, H.-C.; Luo, J.-C.; Kao, C.Y.; Yu, S.-L.; Liou, J.-M.; et al. Development of live biotherapeutic products: A position statement of Asia-Pacific Microbiota Consortium. Gut 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Jiang, J.N.; Kong, F.H.; Lei, Q.; Zhang, X.Z. Surface-functionalized bacteria: Frontier explorations in next-generation live biotherapeutics. Biomaterials 2025, 317, 123029. [Google Scholar] [CrossRef]
- Singh, T.P.; Natraj, B.H. Next-generation probiotics: A promising approach towards designing personalized medicine. Crit. Rev. Microbiol. 2021, 47, 479–498. [Google Scholar] [CrossRef]
- Alexander, L.M.; van Pijkeren, J.P. Modes of therapeutic delivery in synthetic microbiology. Trends Microbiol. 2023, 31, 197–211. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, J.; Guo, X.; Xu, H.; Huang, C.; Nie, Y.; Zhou, Y. Odoribacter splanchnicus—A Next-Generation Probiotic Candidate. Microorganisms 2025, 13, 815. https://doi.org/10.3390/microorganisms13040815
Li J, Xu J, Guo X, Xu H, Huang C, Nie Y, Zhou Y. Odoribacter splanchnicus—A Next-Generation Probiotic Candidate. Microorganisms. 2025; 13(4):815. https://doi.org/10.3390/microorganisms13040815
Chicago/Turabian StyleLi, Jianhong, Jing Xu, Xue Guo, Haoming Xu, Chen Huang, Yuqiang Nie, and Youlian Zhou. 2025. "Odoribacter splanchnicus—A Next-Generation Probiotic Candidate" Microorganisms 13, no. 4: 815. https://doi.org/10.3390/microorganisms13040815
APA StyleLi, J., Xu, J., Guo, X., Xu, H., Huang, C., Nie, Y., & Zhou, Y. (2025). Odoribacter splanchnicus—A Next-Generation Probiotic Candidate. Microorganisms, 13(4), 815. https://doi.org/10.3390/microorganisms13040815





