Abstract
Streptococcus pneumoniae (pneumococcus) is a significant human pathogen responsible for a range of diseases from mild infections to invasive pneumococcal diseases, particularly affecting children, the elderly, and immunocompromised individuals. Despite pneumococcal conjugate vaccines having reduced disease incidence, challenges persist due to serotype diversity, vaccine coverage gaps, and antibiotic resistance. This review highlights the role of LytA, a key autolysin (N-acetylmuramoyl-l-alanine amidase), in pneumococcal biology. LytA regulates autolysis, contributes to inflammation, and biofilm formation, and impairs bacterial clearance. It also modulates complement activation, aiding immune evasion. LytA expression is influenced by environmental signals and genetic regulation and is tied to competence for genetic transformation, which is an important virulence trait, particularly in meningitis. With the increase in antibiotic resistance, LytA has emerged as a potential therapeutic target. Current research explores its use in bacteriolytic therapies, vaccine development, and synergistic antibiotic strategies. Various compounds, including synthetic peptides, plant extracts, and small molecules, have been investigated for their ability to trigger LytA-mediated bacterial lysis. Future directions include the development of novel anti-pneumococcal interventions leveraging LytA’s properties while overcoming vaccine efficacy and resistance-related challenges. Human challenge models and animal studies continue to deepen our understanding of pneumococcal pathogenesis and potential treatment strategies.
1. Introduction
Streptococcus pneumoniae (pneumococcus) is a major human pathogen that typically colonizes the mucosal surfaces of the upper respiratory tract asymptomatically (carrier state). Carriage is a prerequisite for the development of pneumococcal disease [1,2]. Although traditionally regarded as a strictly extracellular bacterium, increasing evidence suggests that pneumococcus can also exist in an intracellular state [3,4,5,6,7,8]. Pneumococcus is a common cause of non-invasive conditions such as otitis, conjunctivitis, and pneumonia, as well as life-threatening invasive pneumococcal diseases (IPD), including sepsis, bacteremic pneumonia, and meningitis, particularly in children, the elderly, and immunocompromised individuals. Along with Neisseria meningitidis and Haemophilus influenzae type b, S. pneumoniae is responsible for over 70% of meningitis cases (>100,000 episodes) documented over an 80-year period, and its prevalence has increased in recent years [9]. Globally, the colonization rate of S. pneumoniae is estimated to average 1.9–5.8 billion individuals at any given time [10]. Additionally, in 2021, pneumococci accounted for more than 90 million pneumonia cases and 450,000 deaths worldwide [11].
Pneumococcal conjugate vaccines (PCV) have significantly reduced the burden of IPD [12]. However, the impact of PCV on pneumococcal carriage remains uncertain, with conflicting findings being reported [13]. The high diversity of pneumococcal serotypes (over 100 identified), limited serotype coverage, and serotype replacement by non-PCV13 strains present ongoing challenges [14]. Furthermore, regional disparities in vaccine coverage persist, ranging from 86% in the European region to only 26% in the Western Pacific region. Globally, the World Health Organization (WHO) estimates that 40% of children under 5 years old remain unprotected by PCV [15]. The severity of pneumococcal disease is largely attributed to a robust inflammatory response triggered by complement activation and cytokine release [16]. These responses are elicited by bacterial components such as capsular polysaccharides, surface proteins, or DNA released as bacterial byproducts.
Antibiotic resistance poses a significant global health threat and is projected to cause 10 million deaths annually by 2050 if current trends of inappropriate and excessive antibiotic use continue [17]. Of particular concern is the emergence of multidrug-resistant (MDR) S. pneumoniae strains, which are resistant to β-lactams, macrolides, fluoroquinolones, and sulfamethoxazole/trimethoprim [18]. In the 2024 Bacterial Priority Pathogens List, the WHO categorized S. pneumoniae as a medium-priority pathogen, emphasizing the urgent need to address its public health impact, particularly in vulnerable populations within resource-limited settings [19].
The extensive research on S. pneumoniae makes it challenging to cover all aspects of this pathogen comprehensively. Since 2011, approximately 14,000 articles mentioning “Streptococcus pneumoniae” have been added to the PubMed database (https://pubmed.ncbi.nlm.nih.gov/?term=Streptococcus+pneumoniae&sort=date (accessed on 25 March 2025)), reflecting the broad interest in studying various aspects of the biology of this microorganism. This review aims to summarize current knowledge, focusing on the major pneumococcal autolysin and addressing new, open questions such as the role of pneumococcal prophages in autolysin evolution. However, many other important topics and references in related fields will not be covered. For additional details, readers are encouraged to consult comprehensive reviews on the history of pneumococcal research, pathogenesis, virulence factors and host immunity, genomics and genetics, or vaccine development [5,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37].
- Streptococcus pneumoniae is a major pathogen that colonizes the upper respiratory tract and can cause serious invasive diseases. While vaccines have reduced disease burden, issues like serotype diversity, limited coverage, and antibiotic resistance remain. The WHO classifies it as a medium-priority pathogen. This review examines key biological aspects of the pneumococcal autolysin LytA, including its regulation and control, role in virulence, therapeutic potential, and evolutionary implications.
2. The “Suicidal Tendencies” of S. pneumoniae
In 1890, shortly after the isolation of S. pneumoniae by Pasteur et al. [38] and Sternberg [39], Welch noted that the resolution of pneumococcal exudate in empyema fluid following pneumonia was accompanied by the lysis of pneumococci within their capsules, a process observable microscopically [40]. In 1900, Neufeld [41] first reported the rapid lysis of pneumococci induced by bile or bile salts, which was attributed to their detergent action (for a thorough overview of early pneumococcal studies, refer to [42]). Sodium deoxycholate (Doc) is currently used as a replacement for bile [43,44,45,46]. Notably, Doc also kills pneumococci—but not other streptococci—by a, still unknown, non-autolytic mechanism [47]. The first detailed description of the autolytic process was likely provided by Rosenow in 1910 [48], who demonstrated that pneumococci disintegrate when suspended in physiological saline. This autolysis was neither due to the action of NaCl nor the solubility of pneumococci in water. Subsequent studies revealed that pneumococci possess bacteriolytic intracellular enzymes capable of lysing heat-killed pneumococci, with optimal activity at pH 6–8 [49,50].
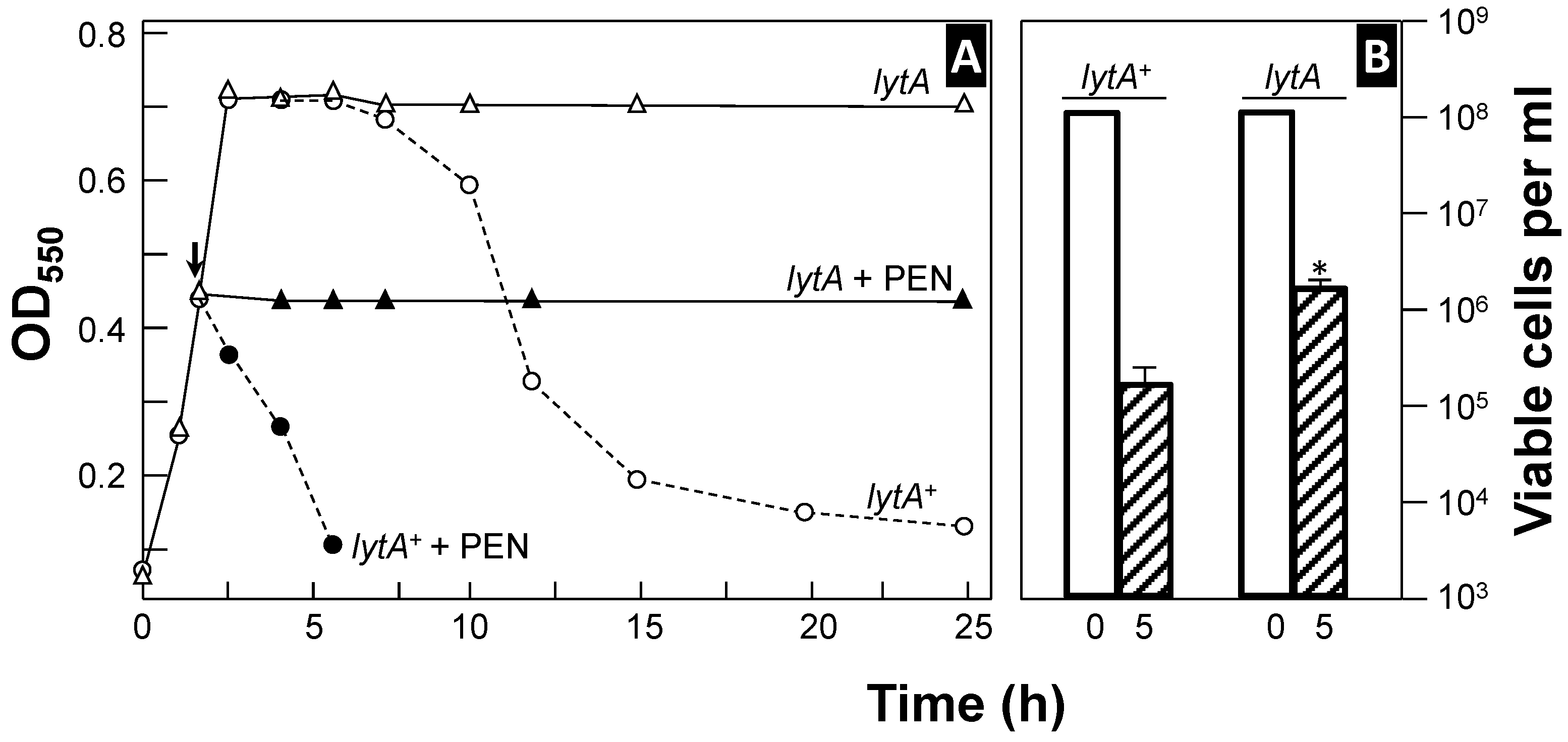
Autolysis during the stationary phase is more pronounced when pneumococci are incubated at 37 °C in a semi-synthetic or chemically defined medium (CDM) compared to rich media [51]. However, this phenomenon varies between strains; for example, strain TIGR4 readily undergoes autolysis in a rich medium but often does not in a CDM [52]. This distinctive autolytic behavior has been termed the “suicidal tendency” of pneumococci [53]. As expected, autolysis is concomitant with viability loss (Figure 1). However, spontaneous death at the stationary phase is not only due to autolysis but also the production of hydrogen peroxide (H2O2) [54]. Moreover, it should be underlined that false-positive blood cultures may result from the autolysis of S. pneumoniae in the culture medium [55,56]. Notably, antibiotics targeting cell wall synthesis are less lethal to pneumococcal strains deficient in autolytic activity [51,57,58,59,60]. Additionally, sitafloxacin, a fluoroquinolone with a high affinity for DNA gyrase and topoisomerase enzymes, exhibits strong bactericidal activity against S. pneumoniae by triggering the activity of LytA, the main pneumococcal autolysin [61]. Recent findings indicate that sitafloxacin treatment significantly increases the transcription and translation of the lytA gene encoding LytA [62] (for a complete list of the pneumococcal genes mentioned in this review, see Table S1). In addition, when grown under anaerobic conditions, pneumococcal autolysis is inhibited [63], and in contrast with microaerophilic conditions, the transcription of lytA under anaerobiosis was not altered upon entry into the stationary phase of growth [64].
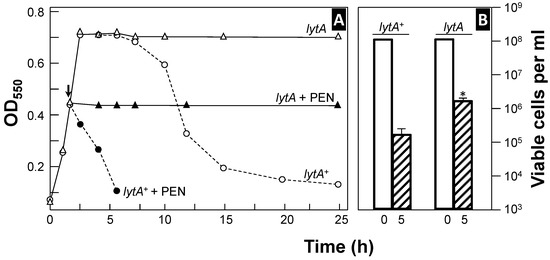
Figure 1.
Curves of growth and penicillin-induced lysis of two pneumococcal strains (panel (A)). Pneumococci were incubated in a semisynthetic medium at 37 °C, and their growth (and lysis) was monitored by measuring optical density at 550 nm (OD550). At the time indicated by the arrow, the samples from the same cultures were treated with penicillin (PEN, 100 × MIC), and incubation was continued at the same temperature (solid symbols). Panel (B) shows the viability of the cultures at the time of antibiotic addition (open bars) and after 5 h of incubation (hatched bars). The asterisk indicates statistically a significant difference (p < 0.001) compared to the results for the wild-type, lytA+ strain.
LytA, an N-acetylmuramoyl-l-alanine amidase (NAM-amidase; EC 3.5.1.28) [65,66], is the primary autolytic enzyme in S. pneumoniae and is responsible for both autolysis at the end of the exponential phase and Doc- or penicillin (PEN)-induced lysis [59,60,67,68,69]. LytA activity is optimal at 37 °C. To date, the lytA gene is universally employed for accurate qPCR-based identification of pneumococcal carriage [44,70].
Another autolytic enzyme, LytC lysozyme (EC 3.2.1.17), has also been identified, acting primarily at 30 °C [71,72]. Similarly to LytA, LytC contributes to the bactericidal effect of PEN, but only under conditions mimicking the temperature of the upper respiratory tract (approximately 30–34 °C) [71,73,74,75].
A unique case of pneumococcal autolysin is CbpD, a peptidoglycan (PG) hydrolase defined as an enzyme that induces self-lysis [76]. Unlike LytA and LytC, CbpD is secreted and plays a role in “fratricide” or “allolysis”. Allolysis is a killing mechanism that could be used by competent cells to acquire DNA from non-competent pneumococci; CbpD lyses non-competent sister cells in collaboration with LytA and LytC in liquid cultures [77,78,79,80]. Competence for genetic transformation is a physiological state that enables the uptake of exogenous DNA. Moreover, induction of competence for genetic transformation is a general response to stress in Gram-positive bacteria (for reviews see [22,35]). Allolysis also involves a previously undescribed bacteriocin system consisting of a two-peptide bacteriocin, CibAB, and its immunity factor, CibC [78]. CibAB alone cannot induce cell lysis but may function as a trigger factor for fratricide. Competent attacker cells are protected from CbpD-mediated lysis through the production of the immunity protein ComM, which also promotes a transient division delay [81,82]. While CbpD alone is insufficient to induce substantial lysis in mixed cultures, the addition of a chelating agent (e.g., EDTA) enhances fratricidal efficiency [83]. Although not confirmed yet, CbpD likely cleaves amide or peptide bonds in pneumococcal PG stem peptides in conjunction with LytA and LytC [80,84]. On the other hand, competent biofilm cells of S. pneumoniae undergo transformation more efficiently from neighboring cells than from DNA present in the growth medium. Effective lysis of target cells necessitates the cooperative action of CbpD and LytC, while LytA is not required for efficient gene exchange in the biofilm environment [85]. Of note, among the five genes involved in fratricide (lytA, lytC, cbpD, cibA, and cibB), genetic epistasis analyses indicated that LytA is the most dominant allolytic enzyme during pneumococcal pathogenesis in a mouse model of infection [86].
LytA, in addition to driving the suicidal tendencies of S. pneumoniae, has been attributed to three key roles in pneumococcal biology. First, it catalyzes the separation of the daughter cells at the end of the cell division to produce “diplo” cells [59,60]. Second, as a key virulence factor, LytA releases cell wall fragments and cytoplasmic proteins during infection, which serve as inflammatory mediators [87,88,89,90,91,92,93,94,95,96,97,98,99]. Notably, tripeptides were over 100-fold more potent than intact peptidoglycan. However, it is important to note that, on a weight-to-weight basis, the whole peptidoglycan from Gram-positive bacteria is approximately 1000-fold less active than the lipopolysaccharide of Gram-negative organisms [95]. Autolysis also contributes to the release of extracellular DNA (eDNA), which is considered important for in vitro biofilm formation [100,101,102]. Confocal micrographs also showed that the biofilms formed by a lytA mutant were consistently thinner (15–20 μm) than those formed by the parental lytA+_strain (≥30 μm). LytA plays a variable (but additive) role in biofilm formation on abiotic surfaces. Moreover, LytA, along with other choline-binding proteins (CBPs; see Section 4), has been shown to bind eDNA [103]. This DNA binding capacity of CBPs appears to be independent of their enzymatic activity and, at least in the case of LytA, does not require the choline-binding domain (CBD) characteristic of CBPs (see Section 4.3 below). These results have been independently confirmed using different experimental approaches [104,105].
Autolysis contributes not only to reducing in vitro growth but also to pneumococcal pathogenesis by shielding bacteria from the immune system and enhancing toxin release (see Section 6.2) [106,107]. These components may interfere with opsonization or induce localized inflammation that diverts immune surveillance. Moreover, the release of eDNA and other factors may contribute to a protective matrix, thereby hindering phagocytosis. In contrast, LytA-deficient mutants do not undergo autolysis and fail to release these modulatory factors, making them more susceptible to recognition and clearance by phagocytic cells. Furthermore, when LytA was inactivated, pneumococci stimulated significantly higher production of tumor necrosis factor, and pro-inflammatory cytokines like interferon-γ or IL-12 in human peripheral blood mononuclear cells, while levels of anti-inflammatory cytokines IL-6, IL-8, and IL-10 remained unchanged. Third, in cooperation with phage-encoded lytic enzymes, LytA facilitates phage progeny release, possibly by collapsing the proton motive force across the bacterial membrane [108,109,110,111,112].
- Streptococcus pneumoniae autolysis, first observed in the late 19th century, is primarily driven by LytA, a key enzyme responsible for cell lysis, virulence, biofilm formation, and immune evasion. LytA also facilitates the release of inflammatory molecules and extracellular DNA, aiding pathogenesis and genetic exchange. Additional autolysins, LytC and CbpD, contribute to antibiotic response and fratricide—a process enabling DNA uptake from sibling cells. LytA is essential for complete cell separation. Its inactivation increases immune detection and reduces inflammation.
3. Organization of the lytA Gene
The lytA gene is located immediately downstream of the cinA–recA–dinF gene cluster in the S. pneumoniae genome and forms part of a pathogenicity island (ply–lytA) which is flanked by a ~100 nt direct repeat (plREP), one copy located downstream of ply and the other overlapping the termination codon of dinF [113] (Figure 2). The ply gene codes for the pore-forming cytotoxin, pneumolysin (Ply), a well-known S. pneumoniae virulence factor [114,115]. The genes cinA, recA, dinF, and lytA form an operon and encode, respectively, a competence/damage-inducible protein A [116] that may have a role in facilitating the localization of RecA to the membrane [117], the RecA recombinase [118], a member of the multidrug and toxic compound extrusion (MATE) transporter family associated with quinolone susceptibility [119] that has been reported as essential for lung infection [120], and the LytA autolysin. Different studies have shown that the lytA gene is transcribed from four different promoters: (1) its own constitutive promoter [121], (2) the promoter associated with dinF, (3) the promoter corresponding to the recA gene, and (4) the competence-specific promoter located upstream of cinA. The latter promoter contains a conserved sequence, TACGAATA, designated as the Cin box (or Com box). The alternative sigma factor ComX (σX) allows the core RNA polymerase to recognize competence-specific promoters [122,123,124,125,126], which lack obvious σA (rpoD or sigA) promoter sites [127]. As ComW participates in the activation and stabilization of ComX and is required for full activity of σX in directing transcription of late competence genes, it can be assumed that ComW participates—in conjunction with ComX—in the stimulation of the transcription of lytA, as it happens in comW mutants where reduced expression of several late competence genes has been noted [128]. More recent results suggest that ComW functions as a novel σ factor activator during transformation in S. pneumoniae [129,130].
With respect to the constitutive promoter of lytA, Díaz and García [121] proposed the sequence TTGACt–17 nt–TAaAgT (consensus sequences appear in capital letters) located at a reasonable distance from the transcription start site (TSS). LytA levels remain constant before and at the onset of growth phase-dependent autolysis [121,131,132,133]. Alternatively, and as occurs for the recA promoter, where no obvious consensus −35 promoter signal was reported in the original publication [119], putative pneumococcal extended −10 sequences—sufficient for promoter activity in several documented cases [134]—can be found, i.e., TaTGaTATAAT for lytA and TtTGaTATAAT for recA. Recent data indicate that an additional −10 extended promoter (gcTGaTATAAT) may be located at 5′ of the initiation codon of dinF and that the corresponding transcript also includes lytA [135,136].
Notwithstanding an imperfect terminator located at 3′ of recA [135], only one high-confidence transcriptional terminator is located at 3′ of lytA on the whole cinA–recA–dinF–lytA operon [121]. The TSS of lytA was found 240 nt upstream of the initiation codon (ATG) of the gene (position 1,730,799 in the D39 genome). This long leader sequence, which potentially may encode two small proteins, was conjectured to be somehow involved in regulating the synthesis of the NAM-amidase [121] but, unfortunately, no experimental evidence for that assumption was available at that time. A recent study has reported that a small RNA (sRNA), SPD_sr95, is transcribed between positions 1,730,706 and 1,730,807 from the minus strand of the D39 chromosome [137]. Remarkably, polyribonucleotide nucleotidyltransferase (EC 2.7.7.8) (also named polynucleotide phosphorylase or PNPase) influences the levels of a large number of regulatory sRNAs. In particular, the transcript level of SPD_sr95 decreased by ~2.5-fold in a Δpnp mutant compared to the pnp+ parent strain during exponential growth in rich (BHI) broth [138]. Evidence indicating any defect on autolysis was not obtained since the Δpnp mutant did not show any detectable defect in vitro (although it was attenuated in vivo). To date, only a few small proteins have been characterized but, in those cases, they have shown many important and varied functions (for reviews, see [139,140]).
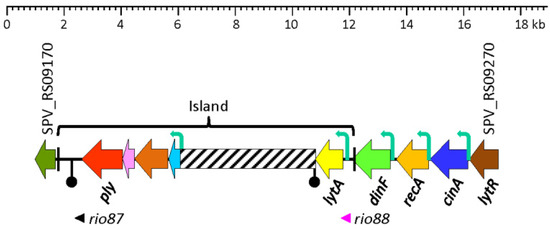
Figure 2.
Diagram showing the region of the S. pneumoniae D39V genome (NZ_CP027540.1) between SPV_RS09170 and SPV_RS09270, and containing the lytA gene. The pathogenicity island (ply–lytA) is flanked by a ~100 nt direct repeat (plREP; black bars). Genes are shown with arrows pointing in the direction of the transcription. The “inverted matchsticks” represent transcriptional terminators. Bent arrows show the location of functional promoters. A region which is highly variable among different S. pneumoniae strains is represented by a hatched bar. The sORF rio87 (SPV_2545) has been described elsewhere [141].
Figure 2.
Diagram showing the region of the S. pneumoniae D39V genome (NZ_CP027540.1) between SPV_RS09170 and SPV_RS09270, and containing the lytA gene. The pathogenicity island (ply–lytA) is flanked by a ~100 nt direct repeat (plREP; black bars). Genes are shown with arrows pointing in the direction of the transcription. The “inverted matchsticks” represent transcriptional terminators. Bent arrows show the location of functional promoters. A region which is highly variable among different S. pneumoniae strains is represented by a hatched bar. The sORF rio87 (SPV_2545) has been described elsewhere [141].
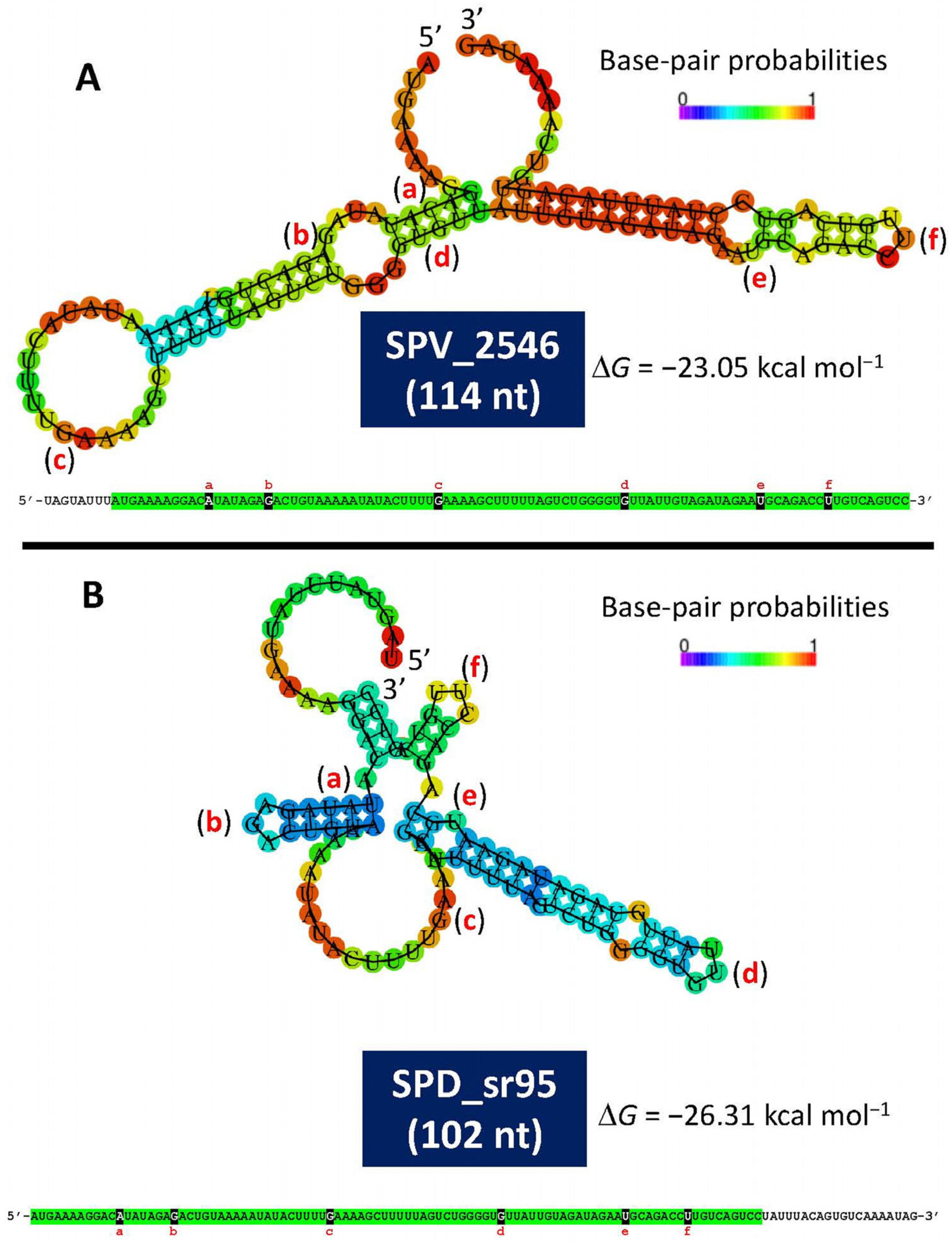
A recent study has provided experimental evidence of the existence of a small open reading frame (sORF) (locus tag SPV_2546; strain D39V) located in the lytA promoter-containing region [141]. This ORF encodes a 37-residues-long polypeptide, identical to one of the two small proteins predicted in 1990 [121]. This ORF, designated as rio88 (for Ribo-seq-identified ORFs), is likely expressed in every pneumococcal isolate. The location of SPV_2546/rio88 in the equivalent D39 genome is: 1,730,666–1,730,799 and is also encoded in the minus strand. It must be noted that SPV_2546 (114 bp) and SPD_sr95 (102 nt) do not completely overlap. Whether this is correct or simply represents an experimental artifact is not known yet. Notably, small proteins nearly identical to SPV_2546 are predicted to be encoded by any other S. pneumoniae genome analyzed (unpublished observations). In addition, a prediction of the secondary structures of single-stranded RNA complementary to SPV_2546 or to SPD_sr95 revealed noticeable free energies of the ensembles of −23.05 kcal mol−1 and −26.31 kcal mol−1, respectively (Figure 3). This finding raises the question of whether this ORF may represent a novel example of a dual-function or bifunctional (both coding and noncoding functions) sRNA (for reviews see [142,143] or another example of a small protein possibly involved in S. pneumoniae physiology [144]). Of note, with the remarkable exception of having ≥95% identity to predicted small genes located at an equivalent position in the genomes of other streptococci of the Mitis group (SMG) harboring lytA-like genes (lytASMG), SPV_2546 does not show any significant similarity to other previously reported proteins. Remarkably, no hits were found when SPV_2546 was searched against a global microbial sORFs catalog (GMSC) that contains more than 950 million non-redundant sORFs [145].
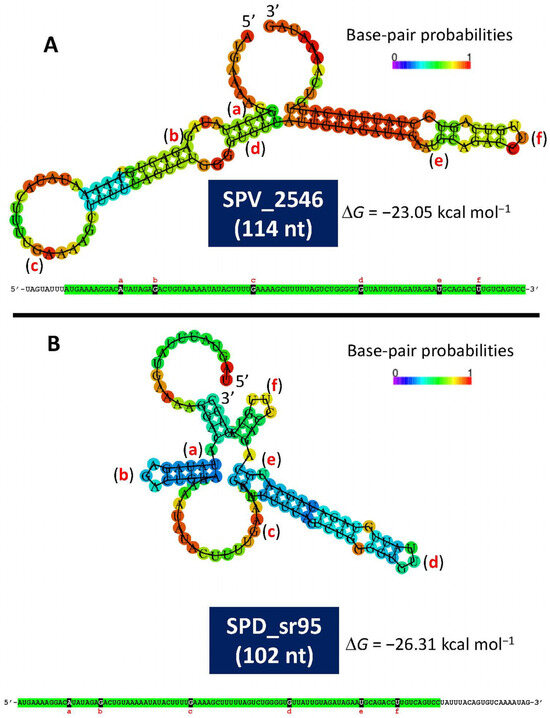
Figure 3.
Minimum free energy and secondary structure of the complementary sequence of SPV_2546 (A) and SPD_sr95 (B), as predicted by the RNAfold WebServer (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi (accessed on 25 March 2025)). The corresponding nucleotide sequences are also shown. Identical sequences are highlighted in green. Letters a to f indicate identical nucleotide positions in both RNA sequences.
- The lytA gene, encoding the major autolysin of S. pneumoniae, is part of a pathogenicity island alongside the ply gene and is co-transcribed with cinA, recA, and dinF. It is regulated by multiple promoters, including a competence-specific one activated by ComX. Transcription may also be influenced by upstream elements and small RNAs, such as SPD_sr95, and possibly by a small open reading frame (rio88/SPV_2546) located in the lytA promoter region. This sORF may encode a conserved 37-amino acid peptide found across pneumococcal strains and may have both coding and regulatory functions. Its role in LytA regulation and pneumococcal physiology remains to be clarified.
4. Structure and Function of the NAM-Amidase
4.1. LytA Requires Choline-Containing Cell Walls for Activity
The lytA gene encodes a 318-amino acid (aa) protein with a predicted relative molecular mass of approximately 36,500. The primary translation product of lytA is a monomeric, low-activity form (E-form) of the enzyme. This E-form can be activated, in vitro and in vivo, into the fully active form (C-form) through a process originally termed “conversion”. In vitro, this process requires incubation at 0 °C, either with phosphocholine (P-Cho)-containing pneumococcal cell walls [146,147], 2% choline chloride [148,149], or tertiary amines like diethylaminoethanol (DEAE) [150]. Streptococcus pneumoniae is an auxotroph for the aminoalcohol choline [151], which decorates the cell wall teichoic acid (WTA) and membrane-anchored lipoteichoic acid (LTA) as P-Cho moieties [152,153]. Both polymers possess identical chain structures within their repeating units, indicating they are synthesized through the same biosynthetic pathway [154,155]. The repeating units contain the rare amino sugar 2-acetamido-4-amino-2,4,6-trideoxygalactose, glucose, ribitol-phosphate, and two N-acetylgalactosamine residues, each carrying a P-Cho moiety. The number of P-Cho residues per repeat (1 or 2) is strain-specific. WTA is linked to PG through a phosphodiester bond to the O6 of some N-acetylmuramic acid (MurNAc) residues, while the LTA chain is β-1,3 glycosidically anchored to the cell membrane via a diacylglycerol-containing lipid anchor.
4.2. Peculiarities of Cell Wall Degradation In Vitro an In Vivo
Pneumococcal cell walls in which the normal P-Cho component of WTA is replaced with phosphoethanolamine are unable to bind LytA and are completely resistant to autolytic degradation [148]. However, soluble teichoic acids (TAs) containing either choline or ethanolamine, prepared by treating pneumococcal cell walls with the N-acetylmuramidase M-1 from Streptomyces globisporus or with hydrofluoric extraction, were hydrolyzed by LytA to the same extent. Additionally, free choline concentrations that completely inhibited the digestion of pneumococcal cell walls both in vivo and in vitro had no effect when soluble substrates were used [156,157]. This result suggests that the strict dependence of LytA on P-Cho residues for hydrolyzing insoluble substrates, such as cell walls, is lost when acting on soluble substrates. However, it has been reported that the addition of chloroform to an actively growing Escherichia coli strain expressing a cloned lytA gene led to rapid lysis of the culture. This finding was entirely unexpected, as it represents the only known instance in which LytA has been capable of hydrolyzing the E. coli murein, despite the absence of P-Cho residues or TAs [158].
Another unique feature of the enzymatic activity of LytA has been reported [159]. When pneumococci were labeled in vivo with radioactive choline and allowed to undergo autolysis following the addition of PEN, the soluble products released differed from those obtained by treating radioactively labeled S. pneumoniae cell walls with the purified NAM-amidase. It was proposed that the in vivo-triggered amidase activity initially targets amide bonds in strategically located (or unprotected) stem peptides, which hold together large segments of the cell wall. These findings suggest that the in vivo activity of the pneumococcal autolysin is influenced by topographic constraints [159].
4.3. Funtional Domains of LytA and Three-Dimensional Structure
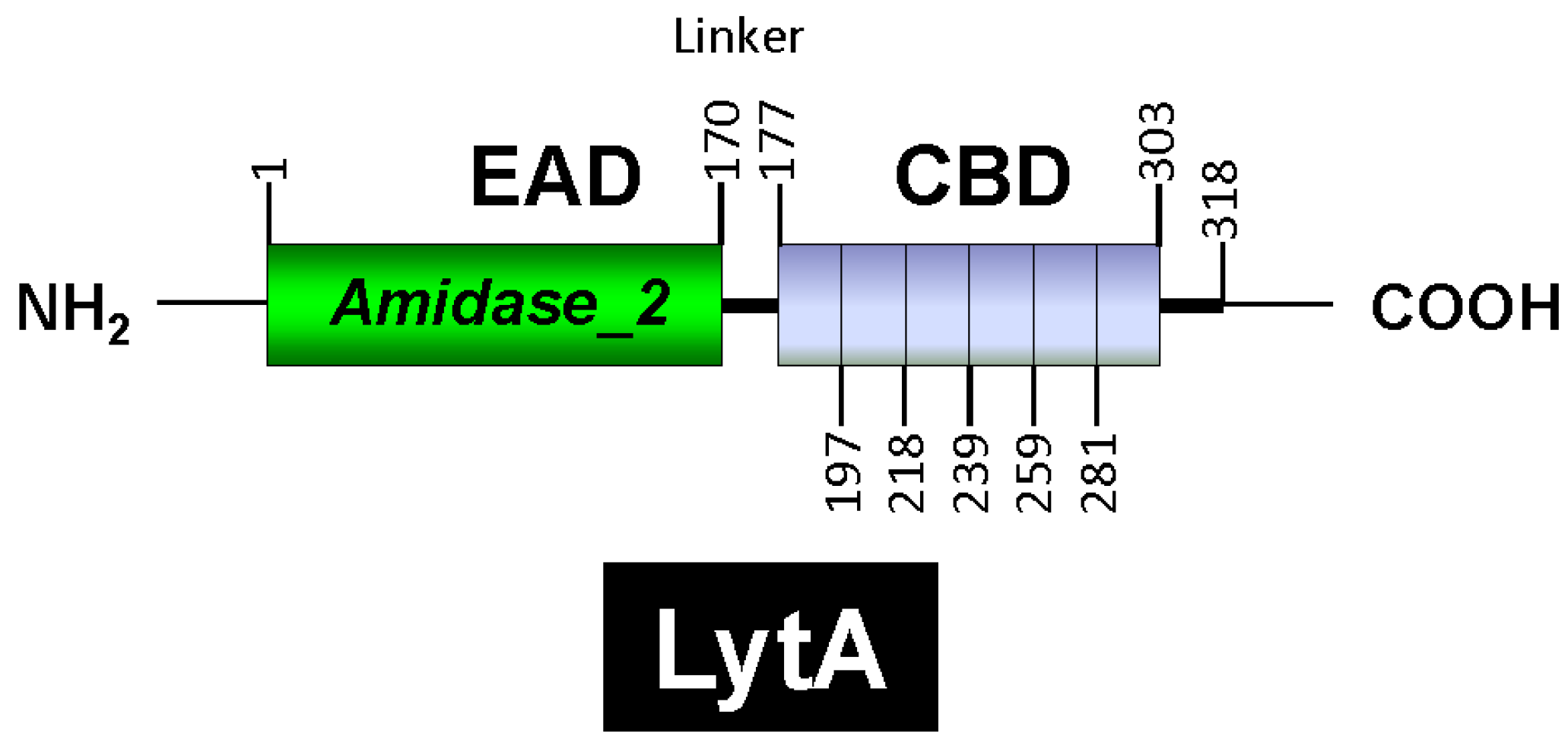
LytA is part of the amidase_2 family of proteins (which possess an Amidase_2 domain, PF01510) [160], including Zn-dependent NAM-amidases and PG recognition proteins (PGRPs), which are highly conserved pattern-recognition molecules of the innate immune system [161]. Notably, several PGRPs (e.g., PGRP-SB1, AgPGRP-S2, AmPGRP-S1, BmPGRP-S5, DmPGRP-LB, RAjPGRP-S, and PGRP-L) also exhibit NAM-amidase activity [162,163,164,165,166]. LytA was the first identified member of the CBP family. Other CBPs, such as the autolysins LytC and CbpD, are also characterized by a choline-binding domain (CBD) responsible for binding to choline residues in WTA and LTA (for reviews, see [153,167,168,169,170,171]). In LytA, the CBD comprises the C-terminal region of the enzyme and consists of six choline-binding repeats (CBRs), each ~20 aa long, and a tail (Figure 4). The C-terminal tail deviates structurally from the other repeats. The N-terminal domain of LytA contains the active center (enzymatically active domain or EAD) (see below). Since the EAD and CBD are found in other proteins, they are often also referred to as “modules” [22].
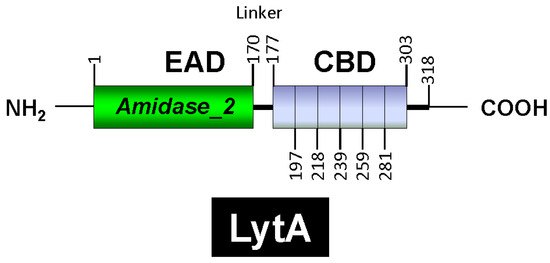
Figure 4.
Diagrammatic representation of the modular organization of the LytA NAM-amidase. The enzymatic active domain (EAD), the linker region, and the choline-binding domains (CBD) are shown. The six choline-binding repeats are also depicted. Residue positions are indicated.
Various approaches have demonstrated the existence and structure of the two LytA modules: sequence alignments [172,173], cloning and expression of the lytA gene [59,60], construction of enzymatically active chimeric proteins [174,175,176], independent expression of domains [177], and C-terminal truncations of LytA [178]. Moreover, physicochemical analyses have provided essential insights into the fine structure and organization of the LytA modules [179,180,181]. These studies revealed that at least four repeats are required for efficient autolysin anchoring to cell wall choline residues and that the active NAM-amidase forms a dimer in solution. It is known that most C-terminal residues play a crucial role in dimerization. Protein dimerization is a key factor in the regulation of proteins such as enzymes, ion channels, receptors, and transcription factors and plays a crucial role in regulating various biological processes, including enzymatic activation, signal transduction, and even pathogenic mechanisms [182,183].
An interesting characteristic of LytA is the discovery that an in-frame 6-bp deletion (ACAGGC), located between nucleotide positions 868 and 873 and encoding Thr290–Gly291, is responsible for the inability of pneumococci to undergo lysis upon the addition of Doc [184]. This deletion does not significantly compromise the enzymatic activity of the NAM-amidase under normal conditions but results in a 30% reduction in enzymatic activity in the presence of Doc. The 6-bp deletion is a distinguishing feature of certain SMG isolates, primarily Streptococcus pseudopneumoniae, and is responsible for the resistance of this species to lysis in the presence of Doc [184,185,186].
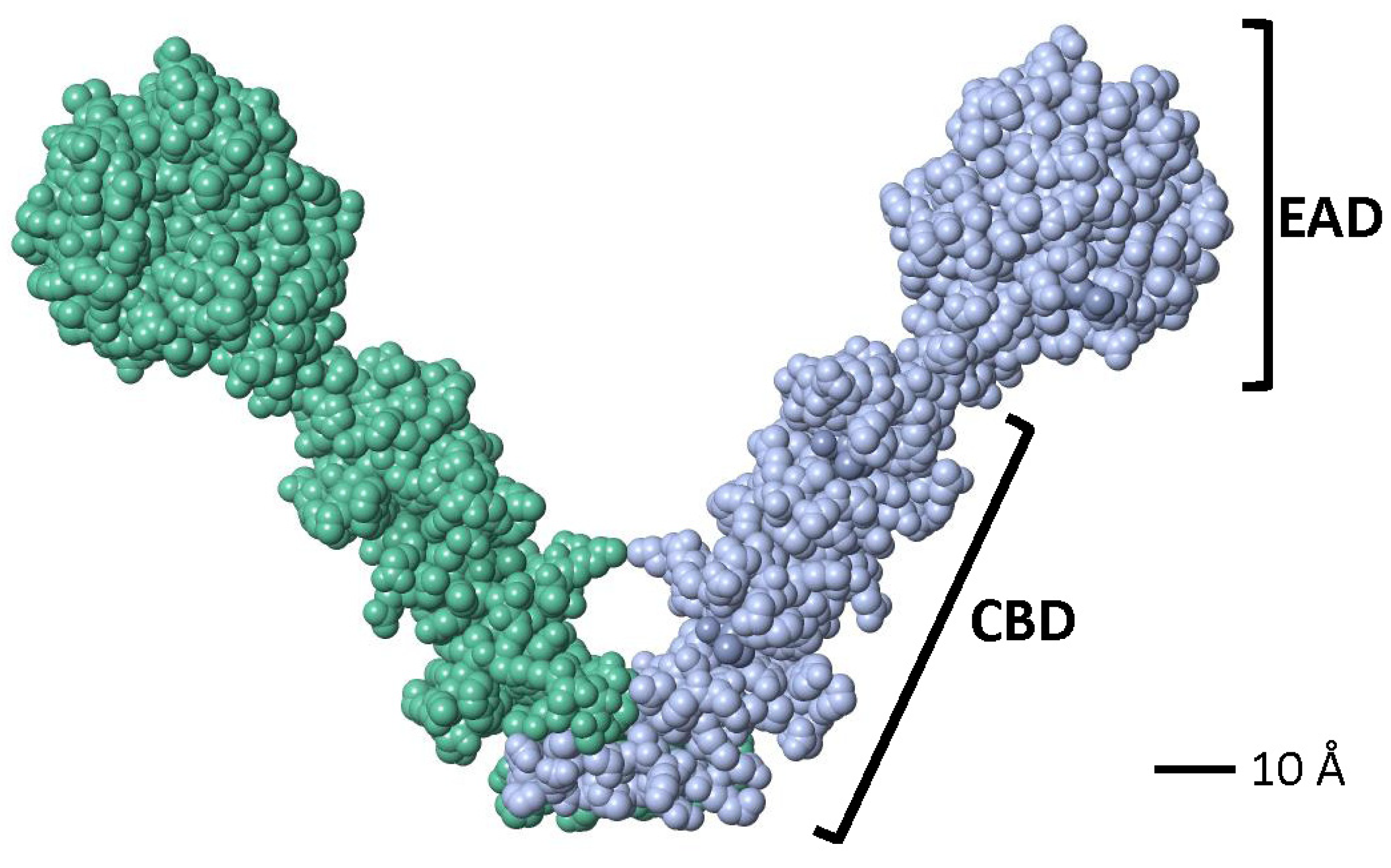
Crystallographic studies showed that a partial LytA CBD (residues 189–318) forms a boomerang-like homodimer. The tertiary structure of each monomer comprises independent β-hairpins and a connecting loop arranged in a left-handed superhelix, forming a solenoid fold [187,188]. Each pair of consecutive CBRs forms a canonical choline-binding site (CBS) containing two hydrophobic layers. Aromatic residues from the hairpins and a hydrophobic residue (Met or Leu) from the connecting loop create the CBS. The three-dimensional structure of a recombinant LytA EAD (residues 1–172, with Cys60 and Cys136 replaced by Ala) was later elucidated [189]. The EAD forms an elliptical globular domain with a Zn2+ ion coordinated by His26, His133, and Asp149. Site-directed mutagenesis revealed that Glu87 and His147 are key active site residues. Consistent with earlier findings [147], enzymatic activity is inhibited by 1–10 mM ZnCl2 in vitro [189]. Later studies reported the complete three-dimensional structure of the LytA NAM-amidase dimer [190], where the subunits form a boomerang-like structure with an internal angle of 85° and arms 106 Å long (Figure 5). Residues 1–170 constitute the EAD, and residues 177–318 (including the tail) form the CBD. Both domains are connected by a six-residue linker. The CBD contains six CBSs, including five canonical and one single-layered site. The consensus sequence for CBRs, GWXKX4–5WYYφX3–5GXMX2–3 (where φ represents a hydrophobic residue), aligns well with prior studies on other pneumococcal CBPs [191].
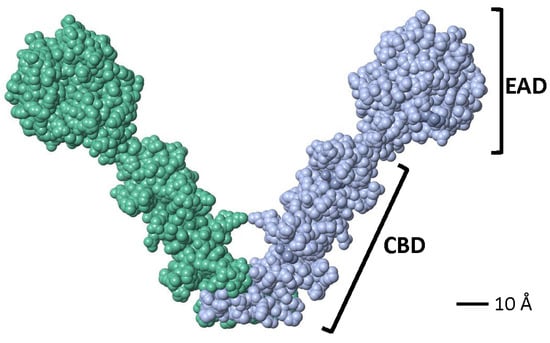
Figure 5.
Space-filling model of the active dimer of the LytA NAM-amidase of S. pneumoniae (Accession number 4X36) visualized using FISTGLANCE Version 4.31 in JMOL software (http://www.bioinformatics.org/firstglance/fgij/ (accessed on 5 March 2025)). Monomers are represented in different color. EAD, N-terminal, enzymatically active domain; CBD, C-terminal, choline-binding domain.
- LytA, the main autolysin of Streptococcus pneumoniae, is synthesized as an inactive form that transforms an active enzyme in the presence of choline-containing compounds. LytA specifically binds to P-Cho on WTAs and is essential for cell wall degradation. It has two domains: a Zn2+-dependent enzymatic domain (EAD) and a choline-binding domain (CBD) with six repeats, crucial for anchoring and dimerization. Structural studies show LytA forms a boomerang-shaped dimer.
5. Regulation and Control of LytA
Generally speaking, several mechanisms are employed to ensure that autolysins do not compromise the structural integrity of the cell [192,193]. These mechanisms include regulating PG hydrolase levels through (1) transcriptional or (2) post-transcriptional control, (3) direct activation or inhibition by regulatory proteins or small molecules, and (4) spatial regulation via proteins, surface polymers, or modifications of the PG substrate, allowing cell wall enzyme activity to be restricted to specific cellular sites. Remarkably, in virtually all cases, the regulation of autolytic enzymes involves a combination of control mechanisms, allowing for precise tuning of activity as well as spatial and temporal regulation.
In S. pneumoniae, the access of LytA and certain other CBPs to the cell wall requires interaction with and translocation across the cell membrane, because of the lack of a signal peptide [194]. Early results have shown that LytA is located in the cellular envelope of S. pneumoniae and E. coli through immunocytochemical labeling of ultrathin sections and whole-mounted cells [195]. In whole S. pneumoniae cells, it has been observed that the labeling is mainly found in the septal region. In addition to electron micrographs, cell fractionation studies in E. coli confirmed that the pneumococcal amidase is peripherally localized and weakly associated with the outer surface of the cytoplasmic membrane. This interaction is independent of choline and, notably, the NAM-amidase remains unprocessed during translocation [195]. Interestingly, recent studies have demonstrated that the LytA peptide 239-TGWKKIADKWYYFN-252, a segment of CBR4 [190], can reversibly change from a β-hairpin, in aqueous solution, to a well-defined, stable α-helix through its interaction with dodecylphosphocholine (DPC) micelles but not with individual phosphocholine molecules [196,197]. Additionally, it has been reported that the aromatic side chain of Y250 is involved in a stronger interaction with DPC micelles than Y249 [198]. This mechanism may represent a general strategy for sorting some proteins to the bacterial surface to perform their physiological functions. These proteins and peptides that change in folding have been referred to by various names, including chameleon/metamorphic proteins, proteins with two folds, switch peptides, and turncoat polypeptides (for reviews, see [199,200,201,202]).
5.1. Transcriptional Regulation
The vast majority of bacteria encounter physical and chemical changes in their environment that could be sensed as stress [203,204,205]. An obvious one is temperature. The surface temperature of the anterior nares is approximately 30 °C to 32 °C at the end of inspiration, increasing to about 34 °C in the posterior nasopharynx and tonsillar region [206,207]. These mucosal surface locations are notably cooler than the core body temperature of 37 °C, which is where bacteria replicate during IPD. Recent results indicate that transcriptional upregulation of dinF and cinA (but not lytA) occurs at 34 °C compared to higher incubation temperatures (37 °C or 40 °C) [208]. On the other hand, gene expression analysis of eleven targets demonstrated that lytA, lytC, comD, and pavA were the most highly expressed pneumococcal genes in the nasopharynx of healthy children, while the others (ply, codY, mgrA, nanA, nanB, pspA, and rrgB) exhibited only moderate to low expression levels [209]. An increase in temperature from 37 °C to 40 °C has been shown to significantly accelerate pneumococcal autolysis rates [210]. Additionally, heat stress—defined as transient exposure of pneumococci to 42 °C—induces both early and late competence genes (presumably including lytA) in a time- and dose-dependent manner [211]. This thermal regulation depends on the HtrA chaperone/protease and its proteolytic activity. HtrA (high-temperature requirement A) is a component of the CiaRH regulon and is recognized as an important virulence factor [212]. On the other hand, an in vitro study found no evidence of RNA thermosensors regulating the transcription of lytA [213]. Using a human middle ear epithelial cell line, it was observed that lytA transcription was significantly induced exclusively in pneumococci attached to the epithelium under simulated pathological middle ear mucosa conditions [214].
Interestingly, competence development is induced in S. pneumoniae under lethal stress conditions, including antibiotic treatment (for recent reviews, see [215,216]). Several hypotheses have been proposed regarding the role of competence in transformation, including serving as a source of nucleotide components, enabling DNA acquisition for genome repair, or facilitating the uptake of novel genetic material to drive evolution [217]. However, it is now widely accepted that competence, though not necessarily transformation, provides protection against both DNA-damaging and non-DNA-damaging stresses [218,219,220].
The fact that lytA is part of the same operon as cinA (see Section 3) strongly suggests that competence development in S. pneumoniae results in increased lytA transcription. However, it should be noted that transcript levels do not necessarily correlate with protein biosynthesis [221,222]. It has been well-documented that the incubation of pneumococci under conditions favorable for competence development leads to increased chromosomal DNA release into the medium and accelerated autolysis during the stationary phase, primarily due to LytA and LytC [77,223]. One study reported that lytA expression increases approximately four-fold in pneumococcal cells undergoing genetic transformation [224]. More recent data demonstrated that 10 min after the addition of the competence-stimulating peptide (CSP)—a 17-residue extracellular peptide that is ribosomally synthesized as a precursor peptide (ComC) [225,226]—lytA transcription increased up to 10-fold before rapidly returning to near-normal levels within another 10 min [135,227].
Aprianto and colleagues had observed that when S. pneumoniae D39 was incubated with human type II lung epithelial cell line A549, lytA transcription (along with that of many other competence-related genes) increased beginning 60 min after infection and remained elevated throughout the experiment [228]. Activation of lytA transcription during in vitro competence induction has been corroborated by recent independent studies [229,230]. Additionally, CSP1-E1A (a CSP1 analog) was able to competitively inhibit the development of competence and reduced the expression of pneumococcal virulence factors like CbpD and LytA in vitro [231]. In addition, overexpression of the S. pneumoniae spxA1 gene represses transcription of the early competence operon comCDE, thereby inhibiting the onset of competence [232]. Subsequently, it was noted that the deletion of spxA1 led to earlier autolysis in the stationary phase, although no significant impact was observed during logarithmic growth [233]. The spxA1 gene forms an operon with tenA, which slightly overlaps at its 3′ end. Together, these two genes represent a novel example of a type II toxin–antitoxin system in pneumococci [234].
Under in vitro conditions, the competence regulon in pneumococci governs both genetic transformation and virulence. However, detailed investigations of competence induction during host infection have only recently been undertaken [235,236]. Strain D39 and several other clinical isolates were used to study competence development in a mouse model of pneumonia-derived sepsis. Notably, in contrast to the characteristic short transient burst of competence observed in vitro, the competent state during pneumonia-derived sepsis was prolonged and persistent. Competence began approximately 20 h post-infection, facilitating systemic invasion, and sepsis development. Notably, the pneumococcal inoculum concentration did not significantly impact competence induction kinetics. Interestingly, exogenously added CSP failed to modulate the onset kinetics of competence development in vivo [235]. Proteomic analyses have shown that activation of competence is a key feature of pneumococcal meningitis progression. In a mouse model of infection, the absence of ComDE and the corresponding inhibition of competence development (see Section 6) resulted in diminished meningeal inflammation and milder disease symptoms compared to infections with wild-type pneumococci [237]. Using a zebrafish larval meningitis infection model, it was found that lytA transcription was lower when the larvae were infected with a Ply+ D39 strain compared to infection with a Ply− mutant; the reasons for this discrepancy remain unclear [238].
Furthermore, under in vivo conditions, lytA transcription was slightly (but significantly) upregulated in the heart compared to the nasopharynx, lungs, kidneys, or blood when the TIGR4 strain was used [239,240,241]. Across all these studies, strain-dependent variability was evident, indicating that different pneumococcal strains exhibit diverse transcriptomic profiles within the same organ and across different infection sites. Furthermore, analysis of RNA from pneumococci isolated from infected rabbit blood, cerebrospinal fluid (CSF), or bacteria attached to a pharyngeal epithelial cell line in vitro revealed decreased expression of lytA only in the CSF [242]. Autolysis dependent on LuxS was suppressed in a luxS mutant, indicating that LuxS (encoding S-ribosylhomocysteine lyase) is somehow involved in the control of LytA-dependent autolysis [243]. Adding 0.4% BSA to the medium further protected luxS mutants from autolysis. Surprisingly, these findings contrast with results showing that PEN (0.5 × minimum inhibitory concentration, MIC) treatment of strain D39 upregulated genes in both the CiaRH operon and luxS [244]. Notably, biofilms formed by a ΔluxS mutant showed unchanged lytA transcription levels when compared to the wild-type D39 strain, in a middle ear rat infection model [245]. This result contrasts with a previous report showing that LuxS regulates the transcript levels of lytA during in vitro biofilm formation [246]. The luxS gene was upregulated by 3.4-fold in the presence of sand dust—a common air pollutant of arid and semi-arid regions of many countries that is a risk factor for otitis media—and similarly, the lytA gene was upregulated by 2.3-fold in the presence of sand dust [247]. Upregulation of lytA transcription was also observed when S. pneumoniae TIGR4 strain was exposed for 2 h to nicotine-containing electronic cigarette vapor extract [248].
Remarkably, and as determined by Western blotting, it has been reported that some macrolide antibiotics, i.e., azithromycin and erythromycin, inhibit the release of LytA into the supernatant of cultures obtained until the stationary phase was reached [249]. Additionally, both macrolides significantly downregulate the transcription of the ply gene, while lytA transcription remains unaffected [249]. A follow-up study confirmed the dual effect of erythromycin on inhibiting both Ply synthesis and release, whereas clarithromycin significantly suppressed ply transcription but upregulated lytA transcription, leading to enhanced autolysis [250].
Additionally, pneumococcal autolysis and fratricide can be modulated by interactions with other bacterial species within a polymicrobial community. When S. pneumoniae TIGR4 was co-cultured with nontypeable H. influenzae, the expression of lytA (and cbpD) was downregulated, resulting in reduced LytA production [251,252].
5.2. Post-Transcriptional Regulation
5.2.1. Two-Component Systems and LytA
Bacteria adapt to environmental changes through a mechanism known as the two-component regulatory system (TCS) (also referred to as the “two-component signal transduction system” or “two-component system”) [253]. A TCS typically consists of at least two proteins: a response regulator and its corresponding sensor histidine kinase.
Streptococcus pneumoniae encodes 13 TCSs and a single orphan response regulator [254,255]. Among these, the CiaRH (competence induction and altered cefotaxime susceptibility) system (TCS05) regulates various processes, including autolysis. Increased autolysis of ciaR mutant cells has been observed under several conditions: upon addition of CSP (see above), during the stationary growth phase, when triggered by choline depletion, or following treatment with early or late inhibitors of cell wall biosynthesis [256]. Zähner et al. noted that the phenotype of a strain is influenced by individual ciaH mutations [257]. Mutants with an activated CiaRH system (designated cia ON), such as strain RCH1 with the ciaHC306 mutation (Thr230 to Pro) or strains harboring mosaic or point mutations in pbp2X combined with the ciaHC103 allele, are resistant to lysis induced by a variety of early and late cell wall inhibitors and are also less susceptible to drugs such as cycloserine, bacitracin, and vancomycin (VAN) [256]. In contrast, loss-of-function CiaRH mutants are hypersensitive to these drugs and lyse rapidly at the stationary growth phase. CiaR directly regulates 15 promoters that drive the transcription of 24 genes organized into five operons and ten monocistronic units [258,259]. Among these, five monocistronic units encode noncoding RNAs (csRNAs, or CiaR-dependent sRNAs). These csRNAs (csRNAs1–5), also known as CcnA–E (CiaR-controlled noncoding RNAs) [260], play a critical role. Specifically, csRNA4 and csRNA5 regulate autolysis during the stationary phase [258]. If both RNAs are absent, autolysis initiates significantly earlier and proceeds faster than in wild-type strains. These sRNAs lack any obvious complementarity to lytA mRNA, suggesting that their effects on autolysis are unlikely to involve direct interference with LytA biosynthesis [258].
Similarly to CiaRH, the LiaFSR system (TCS03) is activated during cell wall stress caused by the activity of the PG hydrolases CbpD, LytA, or LytC [254]. Like CiaRH, LiaFSR is not essential for survival. However, the LiaFSR system appears critical for protecting competent pneumococci from the potentially lethal effects of fratricide (see Section 2). In a ΔcomM mutant, CbpD activates the LiaFSR system in conjunction with LytA and LytC. Additionally, two members of the LiaFSR regulon, spr0810 and spr0351 (CbpF; formerly known as CbpC) [117,171], or PcpC [170,261,262]), are crucial for inhibiting fratricide-associated cell lysis [263]. Without a functional LiaFSR system, cell lysis doubles in both ComM-proficient and -deficient cells. CbpF blocks LytC-induced autolysis at 30 °C in vitro, potentially by preventing the access of the lysozyme to its substrate [264].
TCS09 remains poorly understood, with significant strain-specific functional differences [265]. When S. pneumoniae D39 was treated with Triton X-100, TCS09-deficient mutants exhibited higher autolysis rates than isogenic parental strains [266]. The strain-specific effects of TCS09 on cellular processes remain unclear, though TCS09 is known to regulate carbohydrate metabolism and, likely indirectly, influence the amount of capsular polysaccharide (see Section 5.2.3) [267].
5.2.2. Other Mechanisms of Regulation
Exposure to serum stimulated the expression of the pneumococcal lipase LipA at both the mRNA and protein levels. In the presence of serum, the ΔlipA mutant exhibited accelerated lysis rates and elevated LytA expression compared to the lipA+ parental strain, both in vitro and in vivo. Moreover, it was found that the expression of lytA in a sepsis model was inhibited in the D39 lipA+ strain, but not in the ΔlipA mutant and that the induction of lipA expression results in the inhibition of autolysis [268].
The incorporation of d-alanine in LTAs is accomplished in a two-step reaction involving d-alanine-d-alanyl carrier protein ligase (DltA) and d-alanyl carrier protein (DltC). During the stationary phase, autolysis began earlier in R6 ΔdltA and D39 ΔdltA, while it remained unaffected in strain Rx (another D39 derivative) [269]. In other bacteria, the absence of d-Ala in LTA increases the net negative charge of cell walls, inducing autolysis [270,271,272].
Although the polyamine spermidine is dispensable for growth in vitro, it plays a crucial role in regulating LytA activity, likely through interactions with negatively charged molecules such as TAs [273].
5.2.3. Antibiotic Tolerance
Antibiotic tolerance is defined as the ability of bacteria to survive transient exposure to bactericidal antibiotics, even at concentrations far exceeding the minimum inhibitory concentration (MIC) [274,275]. Unlike resistance, tolerance applies exclusively to bactericidal antibiotics and not to bacteriostatic ones, as all bacteria are expected to survive transient exposure to bacteriostatic antibiotics, which merely arrest growth rather than kill. In contrast to resistance and tolerance—attributes of entire bacterial populations—“persistence” refers to the ability of a subpopulation of bacteria to survive high antibiotic concentrations [276]. Confusion between the concepts of tolerance and persistence remains common [277].
While antimicrobial resistance has been extensively studied [278], the molecular mechanisms underlying antibiotic tolerance are less well understood, particularly in S. pneumoniae [279]. These mechanisms may also vary depending on the specific antibiotic used [280]. As previously noted, LytA-deficient S. pneumoniae strains exhibit tolerance when treated with antibiotics that inhibit cell wall synthesis [51,57]. Moreover, environmental factors, such as the pH of the growth medium, influence bacterial lysis [281,282]. These early studies demonstrated that the bacteriolytic effect of β-lactam antibiotics on S. pneumoniae depended on pH; lysis was inhibited when the pH of pneumococcal cultures remained below 6.0 during PEN treatment. Drug-treated cells merely ceased growth, with a significant reduction in cell death. Additionally, this effect was reversible, as lysis and loss of viability could be induced by post-incubating drug-treated bacteria at a lysis-permissive pH [281].
To date, only two studies from Iran have reported the isolation of VAN-resistant pneumococci (MIC 2–16 μg mL−1) [283,284]. However, the current MIC breakpoint for VAN in S. pneumoniae is 2 μg mL−1 [285] and it is generally accepted that S. pneumoniae is universally susceptible to VAN [286]. Nevertheless, a recent study [287] described an invasive serotype 4 strain with reduced VAN susceptibility (MIC 1 μg mL−1 compared to the typical 0.38–0.5 μg mL−1 for susceptible pneumococci), harboring a vanG-type resistance element [288,289]. Additionally, several studies have reported the isolation and characterization of VAN-tolerant pneumococci (for reviews on early studies, see [290,291]). This raises concerns as antibiotic tolerance may facilitate the evolution of resistance [292,293].
The simplest explanation for antibiotic tolerance is the failure of the bacterium to express an enzymatically active LytA autolysin. Although this feature is widely recognized, it is important to emphasize that, with the notable exception of a specific group of clonal pneumococci—naturally occurring only in horses—which possess a chromosomal deletion leading to a pneumolysin–autolysin fusion gene [113,294,295], to the best of my knowledge, only one clinical isolate of pneumococcus has been demonstrated to be a true lytA mutant [296,297]. Table 1 summarizes studies dealing with the molecular basis of antibiotic tolerance in S. pneumoniae.

Table 1.
The LytA autolysin and antibiotic tolerance factors.
Capsular polysaccharide (CPS), the primary virulence factor of S. pneumoniae, also negatively influences lysis efficiency. Encapsulated strains from different serotypes demonstrate reduced lysis upon PEN or VAN treatment compared to nonencapsulated mutants, though some serotype-specific differences in lysis have been noted [320]. It is conjectured that the capsule may inhibit LytA from accessing their target structure, PG, or may slow down the translocation of LytA to the cell wall, although the possibility that the capsule itself may protect from osmolysis cannot be completely discarded. This link between capsule presence and increased antibiotic tolerance was confirmed independently [297]. Furthermore, nonencapsulated D39 mutants underwent lysis more rapidly and exhibited increased susceptibility to Triton X-100-induced autolysis compared to their encapsulated counterparts [266]. More recently, additional evidence has confirmed that the capsule protects pneumococci from LytA-induced lysis [321].
Analysis of clinical VAN-tolerant isolates revealed additional insights. For example, strain S3 of serotype 23F exhibited VAN tolerance due to a LytA NAM-amidase deficiency caused by a frameshift mutation in the lytAS3 gene [297]. In addition, sequencing of the ciaRH genes in the Tupelo strain revealed a mixed population in the Tupelo stock, containing a mutation in the ciaH gene [297]. Only the mutants with a GCC-to-TCC mutation at position 592 from the start codon (ATG) of ciaH exhibited VAN tolerance. Additionally, exponentially growing Tupelo cells displayed a reduced LytA autolysin synthesis rate (~35% lower) compared to strains R6 and TIGR4.
Despite extensive research, misconceptions about VAN tolerance, persistence, and resistance continue. For instance, while the VncRS system is unrelated to VAN resistance, some studies have mistakenly linked it to this phenotype [322,323]. This underscores the ongoing challenges in fully understanding VAN tolerance in S. pneumoniae.
5.3. Regulatory Molecules
5.3.1. LTA
Although extensive studies have been conducted, it has been traditionally believed that the autolytic activity of LytA is regulated at the post-translational level by the membrane-anchored LTA and strictly requires the presence of P-Cho in WTA for enzymatic activity (see above). When pneumococcal LTA was added to growing pneumococci, it induced chain formation, prevented culture lysis during the stationary growth phase, and inhibited lysis caused by PEN or VAN. However, this inhibition could be reversed with low concentrations (0.2%) of Doc. Notably, WTA remained inactive even at concentrations several hundred-fold higher [324,325]. Furthermore, mere binding to LTA is unlikely to be responsible for the inhibitory effect; rather, the inhibition likely arises from the inaccessibility of the substrate to the NAM-amidase when bound to micellar LTA. Actually, LTAs that had its lipid moiety removed through lipase digestion lost its ability to inhibit the amidase, correlating with its reduced capacity to form micelles [149].
Pneumococci control LTA levels by modulating the abundance of the LTA synthase TacL [133,326] (previously referred to as RafX [327]). TacL depletion during growth in liquid media leads to premature LytA-dependent autolysis during the exponential growth phase. During this phase, S. pneumoniae primarily synthesizes LTAs that bind and sequester the major autolysin LytA. Additionally, the observed increase in WTAs when LTA synthesis is blocked suggests that the two pathways are antagonistic and likely compete for a shared precursor, i.e., a polymer that is linked to an undecaprenyl phosphate lipid carrier. By controlling the TacL levels, the cell can regulate the flux into either LTA or WTA synthesis, given their reliance on the same precursor [133,326]. Elevated LTA levels during exponential growth sequester the CBD of LytA away from the cell wall, thereby reducing its hydrolytic activity during this phase. However, during the stationary phase or in response to cell wall-targeting antibiotics (e.g., PEN), TacL levels decrease by the membrane protease FtsH leading to reduced LTAs and increased WTAs. Furthermore, consistent with early findings [328,329], LTAs are released from cells during autolysis. Coupled with the shift in TA synthesis favoring WTA over LTA, this release allows for rapid LTA depletion and the re-localization of LytA to WTAs, where it facilitates PG hydrolysis [133]. A recent report indicated that ComE, a transcription factor essential for competence development [330], negatively regulates the transcription of tacL hampering pneumococcal transformation [331]. This highlights a connection between competence development and the regulation of TA synthesis.
5.3.2. Enzymatic Activation of LytA
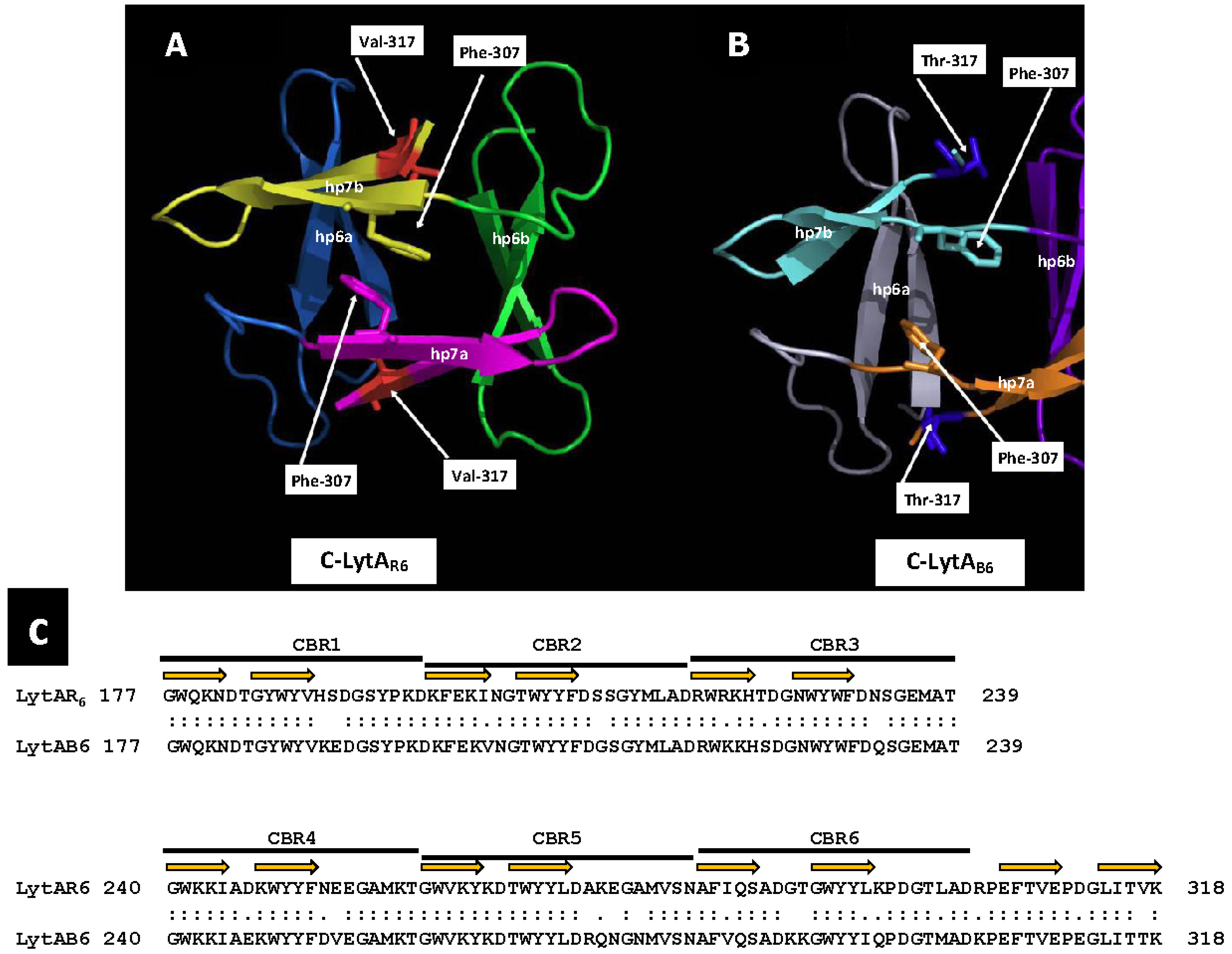
As already mentioned, the E-form (low activity) of LytA exists as a monomeric protein, whereas the active C-form is a dimer, shaped through the tail-to-tail association of two monomers in the presence of choline [182,332]. Once activated, LytA cannot revert to its low-activity form by dialysis against a choline-free buffer. In fact, the complete removal of choline leads to the irreversible denaturation of the enzyme. Interestingly, the temperate phages φB6 and φHER of Streptococcus mitis encode two LytA-like NAM-amidases, designated LytAB6 and LytAHER, respectively [333]. These enzymes are 318 residues long, sharing a global > 83% identity (>90% similarity) with the pneumococcal LytA (LytASpn). Similarly to LytASpn, the phage-derived lytic endolysins also require the activation for full enzymatic activity, which involves a transition from a monomeric to a dimeric state. However, unlike pneumococcal NAM-amidase, the active phage endolysins can reversibly deactivate when choline is removed from the solution, causing the proteins to adopt a predominantly monomeric structure [333]. Sequence alignment of LytA with the two phage NAM-amidases revealed a key distinction between the pneumococcal and S. mitis phage NAM-amidases: at position 317, LytASpn features a Val residue, whereas the phage enzymes possess a Thr residue. Structural studies of LytASpn indicated that Val317 interacts with Phe283 and Tyr294 within the same monomer to form a hydrophobic core critical for maintaining the dimeric structure [188]. Like the pneumococcal LytA, the isolated CBD moieties of the temperate phage enzymes described undergo a reversible dimer↔monomer transition triggered by the addition or removal of choline, respectively. The activation of the E-form to the C-form in these enzymes is invariably linked to these monomer–dimer transitions. However, it remains uncertain whether dimerization and activation are simply concurrent processes or if dimerization contributes, at least partially, to the activation effect.
In a preliminary study, our group determined the three-dimensional structures of the CBD of LytAB6 (C-LytAB6) (WP_000350519) using X-ray crystallography and 13C and 15N NMR spectroscopy to analyze the dimeric and monomeric states in the presence and absence of choline, respectively. Our findings revealed that the three-dimensional structure of the C-LytAB6 dimer closely resembles that of C-LytAR6, as expected, given their high sequence similarity (84% identity, 92% similarity over a 142 aa overlap) (Figure 6). However, in the absence of choline, part of the C-LytAB6 structure undergoes significant rearrangements, particularly in CBR5 (aa positions 260–281), resulting in notable architectural distortion. In addition, Thr317 does not integrate into the hydrophobic core due to its hydrophilic nature. In the absence of choline, the hydrophobic core in C-LytAB6 is expected to disassemble significantly faster than in C-LytAR6, allowing C-LytAB6 to more easily adopt its stable conformation without choline. Conversely, transitioning from the choline-free to the choline-bound form requires reassembly of the hydrophobic core. Temperatures near 273 K may facilitate this rearrangement by weakening hydrophobic interactions, though such conditions may not sufficiently lower the energy barrier for the disassembly of the tightly packed hydrophobic core in C-LytAR6. Furthermore, when LytA transitions from the low-activity to the active form, the catalytic unit shifts from having a single catalytic site to possessing two, substantially increasing the autolysin’s catalytic efficiency. More importantly, the transition from the E-form to the C-form exposes additional cell wall binding sites per monomer and forms a new catalytic dimer containing twelve CBSs. This significantly enhances the affinity of the catalytic domain for the cell wall, resulting in increased catalytic efficiency. This improved affinity arises from the additive free energy of binding provided by the linked CBSs and the reduced entropy cost due to their linkage. Such mechanisms suggest that linking binding fragments with millimolar affinities can lead to compounds exhibiting subnanomolar affinities [334]. In addition, among twenty tested LytAR6 mutants created by site-directed mutagenesis only LytAY294L, LytAV317W, and LytAL314T produced an enzyme that remained fully active but could, unlike the wild-type enzyme, reversibly return to its low-activity E-form upon dialysis [335]. However, despite these results, it is not clear if dimerization and activation are mere parallel phenomena or if dimerization can explain, at least in part, the activation effect. Additional insights from 1H NMR spectroscopy and analytical ultracentrifugation of the low-activity form of LytAB6 revealed that incubation with 140 mM choline chloride at 37 °C for 5 min induces self-association. However, full activation of LytAB6 was achieved only after incubation with its ligand at 0 °C [335].
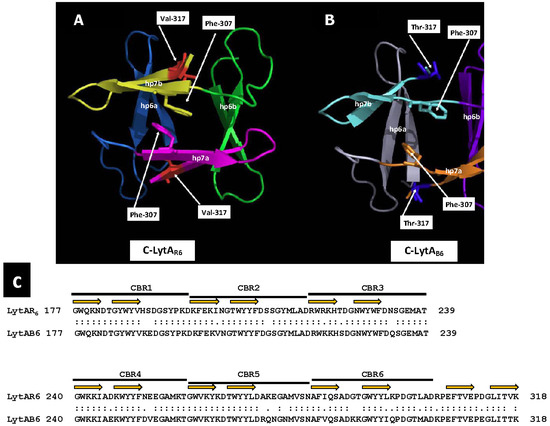
Figure 6.
Dimerization interface of C-LytAR6 and C-LytAB6. Ribbon diagrams showing the dimerization interface of the C-termini of LytAR6 (from LytASpn) (A) and C-LytAB6 (from LytAB6, a PPH endolysin) (B). Monomers are labeled a and b. Each hairpin (hp) is colored differently. The side chains of Phe307 and Val/Thr317 are shown. An alignment of the CBD of both enzymes is shown in panel (C). The portions of the sequences that form the first and second strands of the hairpin are indicated by orange arrows.
It is conceivable that beyond the self-association involving the CBD of LytA, structural modifications at the EAD may also contribute to its activation. Previous studies have shown that the NAM-amidases AmiB and AmiC from E. coli have their active sites blocked by an α-helix, with enzyme activation occurring upon displacement of this occluding helix. Further research demonstrated that EnvC specifically activates AmiB, while NlpD activates AmiC (reviewed in [192]). Additionally, AmpD from Citrobacter freundii, a member of the amidase_2 protein family like LytA, also adopts an inactive (“closed”) conformation that can transition to an active (“open”) state [336]. However, the triggering event for this conformational shift in AmpD remains unknown. In addition, a recent study revealed that LytB of S. pneumoniae, a CBP with N-acetylglucosaminidase activity (EC 3.2.1.96) responsible for the final step of daughter cell separation [337,338,339], also exhibits both inactive/closed and active/open conformations at its catalytic module [340]. In its closed conformation, access to the active site is blocked, whereas in the open conformation, the substrate-binding cavity is exposed. It has been suggested that this transition may occur through the accommodation of PG chains within the catalytic module [340]. A similar mechanism may underlie the enzymatic activation of LytA.
5.4. Spatial Regulation
The data outlined above suggest that LytA activity may be influenced by the structure of the PG network, including factors such as MurNAc O-acetylation, the presence or absence of branched muropeptides, and/or the spatial organization of WTAs. Multiple independent studies employing various experimental approaches have demonstrated that LytA localizes at the cell division septum [195,341,342,343], which corresponds to the site of the newly synthesized cell wall during cell division [344,345]. In the exponential phase, LytA is either diffusely distributed in the cytoplasm or attached to the membrane [149,343]. During the lytic phase, LytA binds to the surface of neighboring non-lysed cells specifically at the mid-cell position [343].
Capsular Polysaccharide, WTA and Autolysis Control
Recent experimental evidence indicates that several genes involved in capsule biosynthesis also contribute to regulating the NAM amidase LytA. In S. pneumoniae, capsular genes are organized into a single operon (cap/cps), with only the first four genes (cap/cpsABCD, now renamed wzg, wzh, wzd, and wze) being conserved across all pneumococcal serotypes, except for types 3 and 37 [194,346,347,348]. The first gene in the cps operon (capA/cpsA/wzg) encodes a protein from the LCP (LytR–CpsA–Psr) family, which typically facilitates the attachment of cell wall glycopolymers to the PG backbone of Gram-positive bacteria via a phosphodiester linkage [349,350]. Wzd and Wze co-localize at the division septum and bind Wzg [321]. These and other proteins form part of the pneumococcal divisome [351,352]. Compared to the wild type, pneumococci with mutations in wzd or wze lack capsular material at the midcell (although it remains on other parts of the cell), exhibit stronger binding to LytA, are more susceptible to LytA-induced lysis than encapsulated mutants, and show reduced virulence [321]. Notably, ΔlytR pneumococci exhibit reduced growth, premature autolysis [353], and produce the same amount of capsular polysaccharide as cpsA lytR double mutants [354]. Additionally, a psr mutant produces a reduced amount of capsule [355]. Recent evidence has also shown that LytR is the primary enzyme responsible for mediating the final step in WTA formation and, along with ComM, plays a critical role in providing immunity against CbpD [229]. In addition, depletion of FtsZ—the leader protein of the cell division machinery [356]—in S. pneumoniae is lethal and results in LytA-induced autolysis and loss of viability that is independent of LytA autolysis [357].
The WhyD protein is a membrane-anchored WTA hydrolase responsible for removing WTAs in S. pneumoniae [358]. WhyD regulates WTA levels to prevent LytA from being mistakenly activated and causing lysis during exponential growth. Crucially, WhyD activity reduces WTA content specifically at sites of PG synthesis. Interestingly, WhyD is essential not only for controlling the overall abundance of WTAs but also for restricting their localization to the midcell, where cell wall synthesis occurs. In cells lacking WhyD, WTA levels are significantly elevated, while LTA levels remain unaffected [358].
- LytA is tightly regulated to prevent premature lysis. Its activity is controlled at multiple levels, including transcriptional and post-transcriptional regulation, enzymatic activation via dimerization, spatial localization to the division septum, and modulation by two-component systems and surface structures like teichoic acids and capsule. Environmental cues, stress, and competence development also influence LytA expression. These complex regulatory networks ensure precise control of LytA during growth, autolysis, and pathogenesis.
6. The LytA Autolysin as a Virulence Factor
Streptococcus pneumoniae is primarily a human pathogen; however, it has also been isolated from pets [359], equine species [295,360,361], and great apes [362,363,364,365,366]. It has been realized that, in most cases, these infections likely originate from human carriers. To investigate the role of LytA (and other virulence factors) in pneumococcal pathogenesis, various animal models are utilized. Mice are by far the most commonly used laboratory animals, although other models—including rats, rabbits, chinchillas, gerbils, guinea pigs, swine, and nonhuman primates—have also been employed for pathogenicity studies and to assess antibiotic and/or vaccine efficacy [367,368]. Additionally, zebrafish models, both embryos and adults, are currently being explored [369,370]. Several studies have evaluated the pathogenic potential of LytA using various animal infection models. As documented in Table 2, 12 out of 15 studies revealed that the NAM-amidase LytA is indeed a virulence factor, this is, the lytA pneumococcal mutants exhibited lower virulence compared to their wild-type progenitors, regardless of the strain used for infection, the route of inoculation, or the animal model employed.

Table 2.
Virulence of lytA mutants vs. lytA+ strains of S. pneumoniae tested in animal models of infection.
6.1. Interactions Between LytA and Host Defenses
The complement system plays a crucial role in the immune defense against S. pneumoniae. To evade a complement attack, pneumococci have developed several mechanisms that inhibit complement-mediated opsonization and subsequent phagocytosis [385]. Previous studies have shown that the combined effects of β-lactam antibiotics and specific antibodies enhance bacterial clearance in cases of sepsis caused by antibiotic-resistant S. pneumoniae strains (for a review, see [386]). This phenomenon can be explained by the observation that the recognition of antibiotic-resistant S. pneumoniae strains by the complement component C3b is enhanced in the presence of specific anti-pneumococcal antibodies and subinhibitory concentrations of macrolides or β-lactams [378]. Notably, LytA has been identified as a key factor in bacterial recognition by the complement system, as phagocytosis by neutrophils and alveolar macrophages was found to be increased in lytA mutants [378]. Additionally, LytA has been shown to play a direct role in host immune evasion by preventing recognition by C3b [379]. LytA inhibits the activation of both the alternative and classical pathways of the complement system. The activation of complement cascades results in the formation of C3b allowing microbial opsonization and enhancing phagocytosis [387]. In addition, LytA recruits complement system down regulators (C4BP and FH) and, if enzymatically active, cleaves C3b and iC3b components bound to the pneumococcal surface [379]. These findings highlight the critical role of LytA in evading complement-mediated immunity and phagocytosis.
The P-selectin glycoprotein ligand-1 (PSGL-1) is a mucin-like transmembrane glycoprotein expressed on all leukocytes. It serves as the primary ligand for P-selectin and also interacts with E- and L-selectins, playing a crucial role in protecting against invasive bacterial infections ([388] and references therein). PSGL-1 has been shown to bind the LytA autolysin, promoting the phagocytosis of S. pneumoniae [389]. Studies using mouse models of pneumococcal disease have demonstrated significantly higher bacterial loads in the blood of PSGL-1−/− mice. During pneumonia, PSGL-1 regulates the extent of pneumococcal spread from the lungs to the bloodstream, while in systemic infections, it plays a key role in bacterial clearance by controlling replication in circulation. Although PSGL-1−/− mice exhibited increased neutrophil and macrophage counts in the blood during systemic infection, they were less effective in controlling the infection due to the absence of this functional receptor. These findings highlight the critical role of the LytA-PSGL-1 interaction in the innate immune response against S. pneumoniae [389].
6.2. LytA Cooperates in the Release of Additional Virulence Factors
In addition to the significant direct involvement of LytA in the pathogenesis of pneumococcal disease, the triggering of LytA facilitates the release of intracellularly located virulence factors. A main example is Ply, a cholesterol-dependent pore-forming toxin and one of the primary pneumococcal proteins contributing to virulence (for reviews, see [114,115,390,391,392,393]). Given that, as LytA, Ply lacks a canonical N-terminal signal peptide for export, its release has been debated, with some attributing it to LytA-dependent autolysis. While most studies emphasize the importance of autolysis in Ply release [249,250,394,395], others suggest that Ply may reside on the bacterial outer surface independently of LytA [375,396]. Additionally, Ply release has been reported to increase with spxB gene expression, encoding pyruvate oxidase, before the stationary phase [397]. More recently, it has been reported that inhibition of H2O2 production by three different mutants (ΔspxB, ΔlctO, and ΔspxBΔlctO) is accompanied by a reduction in the release of Ply [398]. The involvement of an accessory Sec system (SecY2A2) in Ply export has also been proposed [399,400,401]. Furthermore, the cell wall hydrolase activity of LytA and PG cleavage may play a key role in regulating toxin sorting during secretion, as observed in Staphylococcus aureus [402]. A recent study also suggests that the absence of a novel aquaporin (AqpC) reduces pneumococcal autolysis and, consequently, Ply release [403]. Remarkably, the deletion of aqpC does not alter the transcription of lytA. Thus, Ply secretion is likely regulated by multiple mechanisms that may work together to promote disease progression [115].
In addition to Ply, pneumococci produce several nonclassical cell surface proteins, including the metabolic enzymes triose phosphate isomerase (TpiA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). These proteins, which lack both the LPXTG motif and a signal peptide, are nonetheless surface-exposed and secreted by the bacteria [172,404]. The primary role of TpiA is the reversible isomerization of glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. However, it also functions as a moonlighting protein [405,406,407], exhibiting various additional roles [408]. TpiA is released extracellularly through LytA-dependent autolysis, where it binds to host plasminogen and promotes activation of plasmin, a plasma serine protease, potentially aiding bacterial invasion by degrading the extracellular matrix [409,410]. Similarly, GAPDH in S. pneumoniae also functions as a plasminogen-binding protein [411]. This protein plays a crucial role in the bacterium’s ability to cross endothelial and epithelial barriers [412] and is released during LytA-dependent autolysis in a subset of the bacterial population [413].
The PepO protease performs various important functions in pneumococcal virulence [414]. Similarly to TpiA, PepO lacks membrane-spanning domains, such as the LPXTG motif region—a hallmark of many surface-exposed proteins—as well as typical signal sequences. Nevertheless, PepO appears to be present on the cell surface and in culture supernatants, indicating it is a secreted protein [415]. While the precise mechanisms underlying the surface localization, secretion, or presence in the culture supernatant of PepO remain unclear, its localized concentration is anticipated to increase several-fold during pneumococcal autolysis. Comparable results have been published with another moonlighting protein, elongation factor Tu [416], which has been reported to bind human complement inhibitors Factor H, FHL-1, CFHR1, and also the proenzyme plasminogen [417,418].
6.3. Other Roles of LytA in Pneumococcal Pathogenesis
A new physiological function of LytA named capsular shedding has been described [419]. Rather than inducing autolysis, LytA, distributed circumferentially around the cell, enhances bacterial survival and facilitates rapid capsule shedding in response to cathelicidin LL-37, a cationic antimicrobial peptide (CAMP) present in the human epithelium [420]. Capsule shedding enhances bacterial resistance to this innate defense molecule and permits a close interaction of the bacteria with host cells, leading to the successful initiation of infection. CAMPs are among the relatively few epithelial surface molecules with direct microbicidal activity, with LL-37 being the only cathelicidin identified in humans (for a recent review, see [421]). Previous studies have independently demonstrated that pneumococci can shed their capsules during epithelial cell adherence and invasion [422] and that anionic bacterial capsules act as decoys to evade CAMPs [423]. The newly discovered role of LytA in facilitating capsule removal to counteract CAMPs may help explain why nearly all clinical isolates of pneumococci retain this enzyme, despite the strong selective pressure exerted by antibiotics.
In addition to cathelicidins, defensins represent another major group of mammalian CAMPs. These small, multifunctional cationic peptides play a crucial role in host defense [424,425]. It has been reported that the presence or absence of LytA does not affect encapsulated pneumococci. However, in nonencapsulated strains, lytA mutants were more susceptible to antimicrobial peptides than the lytA+ parental strain [426].
Streptococcus pneumoniae DNA released during LytA-dependent autolysis triggers the induction of Krüppel-like transcription factor 4 (KLF4) in human lung epithelial cells through a TLR-9-dependent mechanism [427,428]. As a member of the KLF family, KLF4 plays a crucial regulatory role in both physiological and pathological processes, including pneumonia [429,430]. Upon induction, KLF4 binds to the IL-10 promoter, fostering an anti-inflammatory response. In S. pneumoniae-infected polymorphonuclear neutrophils; however, KLF4 increases the expression of pro-inflammatory cytokines while decreasing the release of anti-inflammatory cytokines like IL-10 [431,432,433]. The release of potent pro-inflammatory mediators is vital for mounting a robust defense against infection; however, excessive inflammation can result in severe tissue injury. This balance is particularly critical in severe pneumococcal pneumonia, where the interplay between an effective inflammatory response to eliminate pneumococci and the preservation of organ function determines disease outcomes [434,435]. KLF4 functions as a counter-regulatory transcription factor in pneumococcal-induced pro-inflammatory activation of lung epithelial cells, potentially mitigating lung hyperinflammation and preventing subsequent organ failure.
Macrophages contain acidic phagolysosomes that play a crucial role in digesting and clearing invading bacteria. The low pH within these lysosomes facilitates bacterial degradation and ultimately leads to the death of the engulfed bacteria. Acidic stress can induce autolysis in S. pneumoniae when incubated at pH 5.9 [436]. While autolysis triggered by competence development (at pH 7.8) is regulated by the two-component system ComDE, no evidence of increased lytA transcription under acidic conditions was found [436], in agreement with a previous study using microarray analysis [437]. Additionally, assays using Doc-induced autolysis have demonstrated that LytA levels remained unchanged during acidic exposure [341]. It has been proposed that autolysis in acidic conditions may be driven by the translocation of LytA from the intracellular to the extracellular compartment after acidic pH stalled bacterial growth [436]. Apparently, under acidic conditions, the surface-expressed LytA is down-regulated by the CiaRH TCS via a CSP-independent ComE pathway [438]. Moreover, the F0F1-ATPase, a proton pump responsible for maintaining intracellular pH [28], is essential for the survival of S. pneumoniae within macrophages [436]. Additional research has also shown that, under acidic conditions, a StkP/ComE-independent pathway regulates the expression of over 100 genes involved in various cellular processes [439]. Mutants lacking StkP exhibit morphological abnormalities, impaired growth, defects in cell division, and enhanced LytA-dependent autolysis. Additionally, these mutants demonstrate reduced tolerance to stress, including acidic environments. In addition, it has been recently suggested that the SirRH (TCS01) is essential for the acidic stress response of S. pneumoniae [440].
- LytA is a critical virulence factor in S. pneumoniae, with its role validated across diverse animal infection models. It facilitates immune evasion by inhibiting complement activation, recruiting host regulatory proteins, and limiting phagocytosis. LytA also binds to PSGL-1, a host selectin ligand, promoting bacterial clearance during systemic infection. Functionally, LytA mediates the release of intracellular virulence determinants, including pneumolysin (Ply) and non-classically secreted surface-associated proteins which contribute to tissue invasion and host immune modulation. Moreover, LytA is involved in capsule shedding. Autolysis-dependent DNA release activates KLF4, a transcription factor involved in modulating host pro- and anti-inflammatory responses. Under acidic stress, such as within macrophage phagolysosomes, LytA activity supports bacterial survival through pH-dependent regulation—likely mediated by the CiaRH and SirRH two-component systems—and potentially via translocation rather than increased transcription. Altogether, LytA functions as a multifaceted effector of pneumococcal virulence, contributing to immune evasion, toxin release, host interaction, and environmental adaptation.
7. Therapeutic Perspectives
With the growing global challenge of antibiotic resistance, phage endolysins are being explored as potential alternatives or adjuncts to traditional antimicrobials [441]. Endolysins can be encoded by both virulent and temperate phages. To date, only a limited number of virulent phages capable of infecting S. pneumoniae have been identified. However, prophages (named PPH for Pneumococcal Prophage) are prevalent in pneumococcal genomes. Notably, including both full-length and partial PPH sequences, a substantial proportion (80–90%) of over 4000 putative pneumococcal isolates contain PPHs. The majority of these prophages encode LytA-like lysins of the amidase_2 family [442]. Interestingly, the phage genes coding for these lysins (lytAPPH) are identical in length and exhibit high sequence similarity (85–92% identity) to lytASpn. Surprisingly, only PPH endolysins unrelated to the host LytA NAM-amidase—such as Cpl-1/Cpl-7-like lysozymes or members of the Pal amidase_5 protein family—have been tested in vitro or in vivo so far [443,444]. One possible explanation for this condition is the widely accepted idea that LytASpn binds to the cell wall during the exponential growth phase without inducing lysis, while S. pneumoniae becomes susceptible to extracellular LytA only in the stationary phase or when cell wall synthesis is inhibited [341,445]. Only three studies have examined the potential therapeutic activity of LytASpn in comparison to various phage lysins. In vitro experiments using two serotype 3, PEN-susceptible strains and two PEN-resistant S. pneumoniae clinical isolates, were treated with different combinations of LytA, Cpl-1 (a lysozyme encoded by the virulent phage Cp-1), Pal (a phage-encoded lysin of the amidase_5 family), and/or antibiotics (cefotaxime and moxifloxacin). The results demonstrated that LytASpn exhibits greater bactericidal activity than Cpl-1 and Pal [446]. Furthermore, time-kill experiments in a mouse model of peritonitis-sepsis revealed that intraperitoneal therapy with LytASpn or high-dose Cpl-1 significantly reduced peritoneal bacterial counts (>5 log₁₀ colony-forming units/mL) compared to the controls. Notably, after intravenous administration, LytA proved to be the most effective treatment [447]. Additionally, S. pneumoniae LytA has also been shown to be the most potent enzyme in disrupting in vitro pneumococcal biofilms when compared to the phage lysins Cpl-1, Cpl-7, and Pal [448]. Notably, Ejl (NP_945312.1), a LytASpn homolog (86% identity/93% similarity) consisting of 316 aa residues and encoded by the S. mitis prophage EJ-1 [449,450], produced a biofilm disintegration of approximately 80%, a level comparable to that caused by the pneumococcal LytA [448].
Another complementary possibility resides in the use of molecules capable of triggering the destructive potential of LytA during IPD. A study demonstrated that various synthetic antimicrobial peptides exhibit significant therapeutic potential in murine models of septicemia and pneumonia [451]. Notably, the combination of one of such peptides, DM3, with PEN enhanced treatment outcomes through therapeutic synergism. Interestingly, in silico molecular docking analyses indicated that DM3 has a strong binding affinity for the LytA autolysin [451]. In addition, cationic ultrashort lipopeptides (USLPs) have recently emerged as promising antimicrobial agents for combating MDR bacteria. Notably, both anionic and neutral zwitterionic USLPs have demonstrated potent, LytA-dependent antimicrobial activity against S. pneumoniae [452]. Interestingly, a recent report has shown that extracts from Lawsonia inermis, a medicinal plant used in Indonesia, exhibited a potent antipneumococcal activity by producing bacterial lysis, antibiofilm activity, and PG disruption by the increase in the synthesis of lytA [453]. Moreover, Coptis rhizome, also known as Huang Lian in traditional Chinese medicine and commonly used for respiratory infections, is derived from the dried rhizome of Coptis chinensis Franch. Ethanolic (70%) extracts exhibited lytic activity against both planktonic and biofilm-grown S. pneumoniae cells, regardless of MDR. Notably, treatment with these extracts led to the upregulation of lytA, suggesting a potential mechanism involving autolysin activation [454].
Furthermore, 2CCA-1, an alkylated dicyclohexyl carboxylic acid and a polyunsaturated fatty acid mimetic has been identified as a novel inducer of autolysin-mediated lysis in S. pneumoniae [455]. It appears to be metabolized similarly to fatty acids and incorporated into phospholipid biosynthesis, leading to the accumulation of toxic phospholipid species and subsequent autolysis. This finding was not entirely unexpected, as the lytic effect of fatty acids on S. pneumoniae had been well documented in early studies [456,457].
Miltefosine (hexadecylphosphocholine), the first oral drug approved for the treatment of visceral leishmaniasis, has been found to induce pneumococcal autolysis by promoting the uncontrolled activation of LytA [458]. A similar effect was later observed in Bacillus subtilis [459]. Interestingly, miltefosine does not appear to affect membrane ordering or packing but instead alters the transport of small molecules across the membrane [460].
Ceragenins are a novel class of agents designed to mimic the function of endogenous antimicrobial peptides, making them promising candidates for the development of new antibacterial compounds [461]. In particular, ceragenin CSA-13, a cationic steroid, exhibits concentration-dependent bactericidal and bacteriolytic activity against pathogenic streptococci, including MDR S. pneumoniae. The lytic effect of CSA-13 is attributed to its activation of the major autolysin LytA [462]. Subsequently, CSA-13A demonstrated a bactericidal activity against various bacteria stronger than that produced by cathelicidin LL-37. Apparently, bacterial exposure to CSA-13 did not result in the emergence of resistance [463]. More recently, CSA-13 has been reported as a highly effective antimicrobial agent with activity against a broad range of bacterial species, including MDR Gram-negative rods [464,465].
- LytA shows strong therapeutic potential as an antimicrobial agent. Compared to phage lysins, LytA demonstrates superior bactericidal and antibiofilm activity in S. pneumoniae. Various compounds—including antimicrobial peptides, plant extracts, lipid mimetics, and ceragenins—exert LytA-dependent lytic effects, either by activating the enzyme or inducing its expression. These findings highlight LytA as a promising target for novel treatments against MDR pneumococcal isolates.
8. Evolutionary Considerations
Notably, lytAPPH genes exhibit a strong similarity to lytASpn [442]. Earlier studies reported that while most lytASpn genes could be categorized into two primary families (Fam_A and Fam_B), lytAPPH alleles displayed significantly greater diversity [466]. Sequence comparisons between lytASpn and lytAPPH alleles suggested that recombination events occurred between host DNA and prophages within the lytA gene [466,467]. Indeed, laboratory experiments documented evidence of recombination involving three distinct lytASpn alleles and hbl, the lytAPPH gene of prophage HB-3 [468]. Furthermore, recent sequence analyses of various PPH families have revealed that PPH090 represents a group of prophages capable of integrating into the lytASpn gene via recombination, leading to the formation of potentially chimeric (hybrid) lytA genes containing sequences of both phage and bacterial origin [442]. Additionally, recombination between prophage and host lytA genes has been identified as a significant driver of chromosomal rearrangements in S. pneumoniae though the consequences of such genome rearrangements on both bacterial and phage physiology warrants further investigation. Nevertheless, the recombination-to-mutation ratio is >1 in S. pneumoniae, indicating that recombination has played a more significant role in the diversification of this species than mutation [469].
- The lytA genes encoded by pneumococcal prophages (lytAPPH) show high similarity to the host lytASpn but exhibit greater sequence diversity. Studies have shown that recombination events between prophage and host DNA within the lytA locus can produce chimeric genes, contributing to chromosomal rearrangements and genetic diversity in S. pneumoniae. This highlights recombination, more than mutation, as a key driver of pneumococcal evolution.
9. Future Perspectives
LytA has demonstrated significant potential as a therapeutic agent due to its bactericidal and biofilm-disrupting properties. Its ability to hydrolyze pneumococcal cell walls and efficiently lyse bacterial cells makes it an attractive candidate for antimicrobial therapies, particularly against antibiotic-resistant strains. In this sense, LytA represents a promising alternative (or adjunct) to traditional antibiotics. However, further research is needed to investigate its safety in human models and explore potential synergies with existing antimicrobial therapies. With its strong bactericidal effects, biofilm-targeting capabilities, and potential for genetic optimization, LytA could play a key role in the development of novel anti-pneumococcal therapies.
As already mentioned (Section 1), the extensive diversity of pneumococcal serotypes, along with limited serotype coverage and the replacement of vaccine-covered strains by non-PCV13 variants, continue to present significant challenges. The novel 20-valent PCV, when used alone, is likely to be cost-effective or superior to other adult pneumococcal vaccination strategies, although further studies are needed to confirm its efficacy and impact [470]. In this context, alternatives to capsular polysaccharide-based vaccines are increasingly being explored (for recent reviews, see [471,472,473,474]). These alternatives include not only protein-based vaccines but also whole-cell pneumococcal ones, which may offer potential broad-spectrum protection against IPD. Among the various S. pneumoniae proteins that have been evaluated as immunogens—such as Ply, PspA, or PspC—LytA has also been tested, albeit in a limited number of studies and some promising results have been reported in animal models [475,476,477]. In the single instance where LytA was used as an immunogen in humans, a strong immunological response was observed [478]. However, as expected, variations were noted among different age groups. More studies must be performed to test the LytA autolysin for safety, tolerability, and immunogenicity of the future serotype-independent pneumococcal vaccines. In addition, two concerns have been raised regarding LytA as a vaccine candidate [471]. First, S. pseudopneumoniae (and some other SMG) possesses a LytA protein similar to that of pneumococcus (see above), potentially making it a target for LytA antibodies and disrupting the microbial balance. Second, secretory IgAs [479] may influence colonization dynamics and could create a favorable niche for other pathogens.
Animal experimentation has played a crucial role in developing treatments and vaccines that have saved countless lives. The use of animal models in pneumococcal vaccine research has both benefits and drawbacks [480,481]. On the positive side, they offer physiological similarity, allow for safety and efficacy assessments, and contribute to the development of effective treatments. However, challenges include inter-species differences, ethical concerns, and limitations in accurately replicating human diseases. Therefore, while animal models remain valuable tools in pneumococcal vaccine development, it is essential to acknowledge their limitations and explore complementary approaches to ensure vaccine safety and effectiveness in healthy humans since S. pneumoniae is primarily a human pathogen. Fortunately, human models of pneumococcal nasopharyngeal carriage are currently being tested, with the first experiments being conducted over two decades ago [482]. The Experimental Human Pneumococcal Challenge model enables the evaluation of vaccines by assessing their impact on experimental S. pneumoniae colonization [483]. In this model, human volunteers are intranasally inoculated with pneumococci, leading to a stable colonization episode lasting approximately 1–3 weeks at a density similar to natural colonization. Several of these studies are currently ongoing (see ref. [484,485] and references therein). Notably, a number of clinical trials are also testing protein-based vaccines [474,486], yielding promising results.
- LytA is a promising antimicrobial and vaccine candidate due to its strong bactericidal and biofilm-disrupting activity, especially against antibiotic-resistant S. pneumoniae. Though effective in animal models and immunogenic in humans, further studies are needed to confirm its safety and efficacy. As polysaccharide vaccines face challenges like serotype replacement, protein-based alternatives—including LytA—are under active investigation. Human challenge models and clinical trials are aiding progress toward serotype-independent pneumococcal vaccines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13040827/s1, Table S1: Accession numbers of genes discussed in this review across four S. pneumoniae strains.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The author is very grateful to Pedro García and Margarita Menéndez for helpful discussions and for critically reading the manuscript.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABC | ATP-binding cassette |
| CAMP | Cationic antimicrobial peptide |
| CBD | Choline-binding domain |
| CBP | Choline-binding protein |
| CBR | Choline-binding repeat |
| CBS | Choline-binding site |
| CDM | Chemically defined medium |
| CSF | Cerebrospinal fluid |
| CSP | Competence-stimulating peptide |
| csRNA | CiaR-dependent sRNA |
| Doc | Sodium deoxycholate |
| DPC | Dodecylphosphocholine |
| EAD | Enzymatically active domain |
| eDNA | Extracellular DNA |
| IPD | Invasive pneumococcal disease(s) |
| LTA | Lipoteichoic acid |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| NAM-amidase | N-acetylmuramoyl-l-alanine amidase |
| ORF | Open reading frame |
| P-Cho | Phosphocholine |
| PCV | Pneumococcal conjugate vaccine(s) |
| PEN | Penicillin |
| PG | Peptidoglycan |
| PGRP | PG recognition protein |
| PPH | Pneumococcal prophage |
| SMG | Streptococci of the Mitis group |
| sORF | Small ORF |
| sRNA | Small RNA |
| TA | Teichoic acid |
| TCS | Two-component regulatory system |
| TSS | Transcription start site |
| USLP | Ultrashort lipopeptides |
| VAN | Vancomycin |
| WHO | World Health Organization |
| WTA | Wall teichoic acid |
References
- Bogaert, D.; de Groot, R.; Hermans, P.W.M. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; for the Pneumococcal Carriage Group. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef]
- Ercoli, G.; Fernandes, V.E.; Chung, W.Y.; Wanford, J.J.; Thomson, S.; Bayliss, C.D.; Straatman, K.; Crocker, P.R.; Dennison, A.; Martinez-Pomares, L.; et al. Intracellular replication of Streptococcus pneumoniae inside splenic macrophages serves as a reservoir for septicaemia. Nat. Microbiol. 2018, 3, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Brissac, T.; Martínez, E.; Kruckow Katherine, L.; Riegler Ashleigh, N.; Ganaie, F.; Im, H.; Bakshi, S.; Arroyo-Diaz Nicole, M.; Spencer Brady, L.; Saad Jamil, S.; et al. Capsule promotes intracellular survival and vascular endothelial cell translocation during invasive pneumococcal disease. mBio 2021, 12, e02516-21. [Google Scholar] [CrossRef]
- Hernandez-Morfa, M.; Olivero, N.B.; Zappia, V.E.; Piñas, G.E.; Reinoso-Vizcaino, N.M.; Cian, M.B.; Nuñez-Fernandez, M.; Cortes, P.R.; Echenique, J. The oxidative stress response of Streptococcus pneumoniae: Its contribution to both extracellular and intracellular survival. Front. Microbiol. 2023, 14, 1269843. [Google Scholar] [CrossRef]
- Ogawa, M.; Shizukuishi, S.; Akeda, Y.; Ohnishi, M. Molecular mechanism of Streptococcus pneumoniae-targeting xenophagy recognition and evasion: Reinterpretation of pneumococci as intracellular bacteria. Microbiol. Immunol. 2023, 67, 224–227. [Google Scholar] [CrossRef]
- Santra, S.; Nayak, I.; Paladhi, A.; Das, D.; Banerjee, A. Estimates of differential toxin expression governing heterogeneous intracellular lifespans of Streptococcus pneumoniae. J. Cell Sci. 2024, 137, jcs260891. [Google Scholar] [CrossRef]
- Hernandez-Morfa, M.; Reinoso-Vizcaino, N.M.; Zappia, V.E.; Olivero, N.B.; Cortes, P.R.; Stempin, C.C.; Perez, D.R.; Echenique, J. Intracellular Streptococcus pneumoniae develops enhanced fluoroquinolone persistence during influenza A coinfection. Front. Microbiol. 2024, 15, 1423995. [Google Scholar] [CrossRef]
- van Ettekoven, C.N.; Liechti, F.D.; Brouwer, M.C.; Bijlsma, M.W.; van de Beek, D. Global case fatality of bacterial meningitis during an 80-year period: A systematic review and meta-analysis. JAMA Netw. Open 2024, 7, e2424802. [Google Scholar] [CrossRef]
- Short, K.R.; Diavatopoulos, D.A. Nasopharyngeal colonization with Streptococcus pneumoniae. In Streptococcus pneumoniae: Molecular Mechanisms of Host-Pathogen Interactions; Brown, J., Hammerschmidt, S., Orihuela, C., Eds.; Academic Press: New York, NY, USA, 2015; pp. 279–289. [Google Scholar]
- GBD 2021 Lower Respiratory Infections and Antimicrobial Resistance Collaborators. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [Google Scholar] [CrossRef]
- Bennett, J.C.; Knoll, M.D.; Kagucia, E.W.; Garcia Quesada, M.; Zeger, S.L.; Hetrich, M.K.; Yang, Y.; Herbert, C.; Ogyu, A.; Cohen, A.L.; et al. Global impact of ten-valent and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in all ages (the PSERENADE project): A global surveillance analysis. Lancet Infect. Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Jagne, I.; von Mollendorf, C.; Wee-Hee, A.; Ortika, B.; Satzke, C.; Russell, F.M. A systematic review of pneumococcal conjugate vaccine impact on pneumococcal nasopharyngeal colonisation density in children under 5 years of age. Vaccine 2023, 41, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Mungall, B.A.; Hoet, B.; Nieto Guevara, J.; Soumahoro, L. A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children. Expert Rev. Vaccines 2022, 21, 201–214. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Immunization Coverage. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage#:~:text=Pneumococcal%20vaccine%20had%20been%20introduced,the%20WHO%20Western%20Pacific%20Region (accessed on 26 February 2025).
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and inflammation. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Rajput, P.; Nahar, K.S.; Rahman, K.M. Evaluation of antibiotic resistance mechanisms in Gram-positive bacteria. Antibiotics 2024, 13, 1197. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Bacterial Priority Pathogens List. 2024. Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (accessed on 26 February 2025).
- Engholm, D.H.; Kilian, M.; Goodsell, D.S.; Andersen, E.S.; Kjaergaard, R.S. A visual review of the human pathogen Streptococcus pneumoniae. FEMS Microbiol. Rev. 2017, 41, 854–879. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.R.K.; Mias, G.I. Streptococcus pneumoniae’s virulence and host immunity: Aging, diagnostics, and prevention. Front. Immunol. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Santoro, F.; Iannelli, F.; Pozzi, G. Genomics and genetics of Streptococcus pneumoniae. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Subramanian, K.; Henriques-Normark, B.; Normark, S. Emerging concepts in the pathogenesis of the Streptococcus pneumoniae: From nasopharyngeal colonizer to intracellular pathogen. Cell. Microbiol. 2019, 21, e13077. [Google Scholar] [CrossRef]
- Morimura, A.; Hamaguchi, S.; Akeda, Y.; Tomono, K. Mechanisms underlying pneumococcal transmission and factors influencing host-pneumococcus interaction: A review. Front. Cell. Infect. Microbiol. 2021, 11, 639450. [Google Scholar] [CrossRef]
- Musher, D.M.; Anderson, R.; Feldman, C. The remarkable history of pneumococcal vaccination: An ongoing challenge. Pneumonia 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.F.G.; Chan, J.; Nguyen, C.D.; Russell, F.M. Factors associated with pneumococcal nasopharyngeal carriage: A systematic review. PLoS Glob. Public Health 2022, 2, e0000327. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A.; Avci, F.Y. Emerging vaccine strategies against the incessant pneumococcal disease. npj Vaccines 2023, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Martín-Galiano, A.J.; Ferrándiz, M.J.; de la Campa, A.G. The promoter of the operon encoding the F0F1 ATPase of Streptococcus pneumoniae is inducible by pH. Mol. Microbiol. 2001, 41, 1327–1338. [Google Scholar] [CrossRef]
- Li, L.; Ma, J.; Yu, Z.; Li, M.; Zhang, W.; Sun, H. Epidemiological characteristics and antibiotic resistance mechanisms of Streptococcus pneumoniae: An updated review. Microbiol. Res. 2023, 266, 127221. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.; Nunes, M.C.; Saadatian-Elahi, M. Epidemiology of community-acquired pneumonia caused by Streptococcus pneumoniae in older adults: A narrative review. Curr. Opin. Infect. Dis. 2024, 37, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ben Debba, L.; Derreumaux, D.; Lonnet, G.; Taddei, L.; Scherbakov, M. Clinical and economic burden of otitis media in children under 5 years of age in the United States: A retrospective study. Hum. Vaccines Immunother. 2024, 20, 2409510. [Google Scholar] [CrossRef]
- Johnson, C.N.; Wilde, S.; Tuomanen, E.; Rosch, J.W. Convergent impact of vaccination and antibiotic pressures on pneumococcal populations. Cell Chem. Biol. 2024, 31, 195–206. [Google Scholar] [CrossRef]
- Ricci Conesa, H.; Skroder, H.; Norton, N.; Bencina, G.; Tsoumani, E. Clinical and economic burden of acute otitis media caused by Streptococcus pneumoniae in European children, after widespread use of PCVs-A systematic literature review of published evidence. PLoS ONE 2024, 19, e0297098. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Dickson, R.P.; Horowitz, J.K.; Flanders, S.A. Community-acquired pneumonia: A review. JAMA 2024, 332, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Hiller, N.L.; Orihuela, C.J. Biological puzzles solved by using Streptococcus pneumoniae: A historical review of the pneumococcal studies that have impacted medicine and shaped molecular bacteriology. J. Bacteriol. 2024, 206, e0005924. [Google Scholar] [CrossRef] [PubMed]
- Narciso, A.R.; Dookie, R.; Nannapaneni, P.; Normark, S.; Henriques-Normark, B. Streptococcus pneumoniae epidemiology, pathogenesis and control. Nat. Rev. Microbiol. 2024, 23, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, F.A.; Beall, B.W.; Yu, J.; van der Linden, M.; McGee, L.; Satzke, C.; Manna, S.; Lo, S.W.; Bentley, S.D.; Ravenscroft, N.; et al. Update on the evolving landscape of pneumococcal capsule types: New discoveries and way forward. Clin. Microbiol. Rev. 2025, 38, e00175-24. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L.; Chamberland, C.; Roux, P.P.E. Sur une maladie nouvelle, provoquée par la salive d’un enfant mort de la rage. Compt. Rend. Acad. Sci. 1881, 92, 159–165. Available online: https://gallica.bnf.fr/ark:/12148/bpt6k7351t/f158.item (accessed on 1 April 2025).
- Sternberg, G.M. A fatal form of septicaemia in the rabbit, produced by the subcutaneous injection of human saliva. An experimental research. Natl. Board Health Bull. 1881, 2, 781–783. [Google Scholar]
- Anonymous. Report of Dr. Welch’s remarks on the Diplococcus pneumoniae at the meeting of the John Hopkins Hospital Medical Society, on February 17, 1890. Johns Hopkins Hosp. Bull. 1890, I, 73–74. Available online: https://babel.hathitrust.org/cgi/pt?id=iau.31858029237678&seq=80 (accessed on 1 April 2025).
- Neufeld, F. Ueber eine specifische bakteriolystische wirkung der galle. Z. Hyg. Infektionskrankh. 1900, 34, 454–464. [Google Scholar] [CrossRef]
- White, B.; Robinson, E.S.; Barnes, L.A. The Biology of Pneumococcus: The Bacteriological, Biochemical, and Immunological Characters and Activities of Diplococcus pneumoniae; Harvard University Press: Cambridge, MA, USA, 1979. [Google Scholar]
- Blaschke, A.J. Interpreting assays for the detection of Streptococcus pneumoniae. Clin. Infect. Dis. 2011, 52, S331–S337. [Google Scholar] [CrossRef]
- Satzke, C.; Turner, P.; Virolainen-Julkunen, A.; Adrian, P.V.; Antonio, M.; Hare, K.M.; Henao-Restrepo, A.M.; Leach, A.J.; Klugman, K.P.; Porter, B.D.; et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013, 32, 165–179. [Google Scholar] [CrossRef]
- Slotved, H.-C.; Facklam, R.R.; Fuursted, K. Assessment of a novel bile solubility test and MALDI-TOF for the differentiation of Streptococcus pneumoniae from other mitis group streptococci. Sci. Rep. 2017, 7, 7167. [Google Scholar] [CrossRef] [PubMed]
- Aupaix, A.; Verroken, A.; Rodriguez-Villalobos, H. Evaluation of a new protocol for rapid identification of Streptococcus pneumoniae in blood cultures using the modified bile solubility test: Gram staining is still standing. J. Clin. Microbiol. 2024, 63, e0122224. [Google Scholar] [CrossRef]
- Vidal, J.E.; Wier, M.N.; Angulo-Zamudio Uriel, A.; McDevitt, E.; Jop Vidal, A.G.; Alibayov, B.; Scasny, A.; Wong, S.M.; Akerley, B.J.; McDaniel, L.S. Prophylactic inhibition of colonization by Streptococcus pneumoniae with the secondary bile acid metabolite deoxycholic acid. Infect. Immun. 2021, 89, e0046321. [Google Scholar] [CrossRef]
- Rosenow, E.C. The autolysis of pneumococci and the effect of the injection of autolyzed pneumococci. JAMA 1910, 54, 1943. [Google Scholar] [CrossRef]
- Avery, O.T.; Cullen, G.E. Studies on the enzymes of pneumococcus. IV. Bacteriolytic enzyme. J. Exp. Med. 1923, 38, 199–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dubos, R.J. The autolytic system of pneumococci. J. Exp. Med. 1937, 65, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: Chain formation, loss of transformability, and loss of autolysis. Proc. Natl. Acad. Sci. USA 1968, 59, 86–93. [Google Scholar] [CrossRef]
- Warrier, I.; Perry, A.; Hubbell, S.M.; Eichelman, M.; van Opijnen, T.; Meyer, M.M. RNA cis-regulators are important for Streptococcus pneumoniae in vivo success. PLoS Genet. 2024, 20, e1011188. [Google Scholar] [CrossRef]
- McCarty, M. The Transforming Principle. In Discovering That Genes Are Made of DNA; W. W. Norton & Co.: New York, NY, USA, 1985. [Google Scholar]
- Regev-Yochay, G.; Trzcinski, K.; Thompson, C.M.; Lipsitch, M.; Malley, R. SpxB Is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 2007, 189, 6532–6539. [Google Scholar] [CrossRef]
- Vasallo, F.J.; López-Miragaya, I.; Rodríguez, A.; Torres, J. Apparently false-positive blood cultures due to autolyzed Streptococcus pneumoniae. Clin. Microbiol. Infect. 2000, 6, 688–689. [Google Scholar] [CrossRef][Green Version]
- Amano, M.; Matsumoto, M.; Sano, S.; Oyama, M.; Nagumo, H.; Watanabe-Okochi, N.; Tsuno Nelson, H.; Nakajima, K.; Muroi, K. Characteristics of false-positive alarms in the BacT/Alert 3D system. Microbiol. Spectr. 2022, 10, e00055-22. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, A.; Albino, A.; Zanati, E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 1970, 227, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, A. The mechanism of the irreversible antimicrobial effects of penicillins: How the beta-lactam antibiotics kill and lyse bacteria. Annu. Rev. Microbiol. 1979, 33, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Puelles, J.M.; Ronda, C.; García, J.L.; García, P.; López, R.; García, E. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur. J. Biochem. 1986, 158, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Ronda, C.; García, J.L.; García, E.; Sánchez-Puelles, J.M.; López, R. Biological role of the pneumococcal amidase. Cloning of the lytA gene in Streptococcus pneumoniae. Eur. J. Biochem. 1987, 164, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Hoshino, K.; Otani, T.; Yamamoto, T. Quinolones with enhanced bactericidal activity induce autolysis in Streptococcus pneumoniae. Chemotherapy 2009, 55, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Kanesaka, I.; Kanayama Katsuse, A.; Takahashi, H.; Okumura, R.; Nakanishi, Y.; Kaneko, A. Gene expression analysis in the potent bactericidal activity of sitafloxacin against Streptococcus pneumoniae. J. Infect. Chemother. 2019, 25, 322–324. [Google Scholar] [CrossRef]
- Brogan, O.; Garnett, P.A.; Fox, C.C.; McCabe, K.A. Evaluation of anaerobic culture and effect of culture medium supplementation with factor V on colonial morphology and efficacy of isolation of Streptococcus pneumoniae from sputum. J. Clin. Pathol. 1987, 40, 368. [Google Scholar] [CrossRef]
- Nagaoka, K.; Yamashita, Y.; Kimura, H.; Suzuki, M.; Konno, S.; Fukumoto, T.; Akizawa, K.; Morinaga, Y.; Yanagihara, K.; Nishimura, M. Effects of anaerobic culturing on pathogenicity and virulence-related gene-expression in pneumococcal pneumonia. J. Infect. Dis. 2018, 219, 1545–1553. [Google Scholar] [CrossRef]
- Mosser, J.L.; Tomasz, A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J. Biol. Chem. 1970, 245, 287–298. [Google Scholar] [CrossRef]
- Howard, L.V.; Gooder, H. Specificity of the autolysin of Streptococcus (Diplococcus) pneumoniae. J. Bacteriol. 1974, 117, 796–804. [Google Scholar] [CrossRef] [PubMed]
- García, E.; García, J.L.; Ronda, C.; García, P.; López, R. Cloning and expression of the pneumococcal autolysin gene in Escherichia coli. Mol. Gen. Genet. 1985, 201, 225–230. [Google Scholar] [CrossRef] [PubMed]
- García, P.; García, J.L.; García, E.; López, R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene 1986, 43, 265–272. [Google Scholar] [CrossRef] [PubMed]
- García, E.; Rojo, J.M.; García, P.; Ronda, C.; López, R.; Tomasz, A. Preparation of antiserum against the pneumococcal autolysin—Inhibition of autolysin activity and some autolytic processes by the antibody. FEMS Microbiol. Lett. 1982, 14, 133–136. [Google Scholar] [CrossRef]
- Miellet, W.R.; Almeida, S.T.; Trzciński, K.; Sá-Leão, R. Streptococcus pneumoniae carriage studies in adults: Importance, challenges, and key issues to consider when using quantitative PCR-based approaches. Front. Microbiol. 2023, 14, 1122276. [Google Scholar] [CrossRef]
- García, P.; González, M.P.; García, E.; García, J.L.; López, R. The molecular characterization of the first autolytic lysozyme of Streptococcus pneumoniae reveals evolutionary mobile domains. Mol. Microbiol. 1999, 33, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Monterroso, B.; Sáiz, J.L.; García, P.; García, J.L.; Menéndez, M. Insights into the structure-function relationships of pneumococcal cell wall lysozymes, LytC and Cpl-1. J. Biol. Chem. 2008, 283, 28618–28628. [Google Scholar] [CrossRef]
- Sánchez-Puelles, J.M.; Ronda, C.; García, E.; Méndez, E.; García, J.L.; López, R. A new peptidoglycan hydrolase in Streptococcus pneumoniae. FEMS Microbiol. Lett. 1986, 35, 163–166. [Google Scholar] [CrossRef][Green Version]
- López, R.; Ronda, C.; García, E. Autolysins are direct involved in the bactericidal effect caused by penicillin in wild type and in tolerant pneumococci. FEMS Microbiol. Lett. 1990, 66, 317–322. [Google Scholar] [CrossRef][Green Version]
- Lindemann, J.; Leiacker, R.; Rettinger, G.; Keck, T. Nasal mucosal temperature during respiration. Clin. Otolaryngol. Allied Sci. 2002, 27, 135–139. [Google Scholar] [CrossRef]
- Vázquez, R.; Briers, Y. What’s in a name? An overview of the proliferating nomenclature in the field of phage lysins. Cells 2023, 12, 2016. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, M.; Claverys, J.-P. Release of DNA into the medium by competent Streptococcus pneumoniae: Kinetics, mechanism and stability of the liberated DNA. Mol. Microbiol. 2004, 54, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Guiral, S.; Mitchell, T.J.; Martin, B.; Claverys, J.-P. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: Genetic requirements. Proc. Natl. Acad. Sci. USA 2005, 102, 8710–8715. [Google Scholar] [CrossRef] [PubMed]
- Kausmally, L.; Johnsborg, O.; Lunde, M.; Knutsen, E.; Håvarstein, L.S. Choline-binding protein D (CbpD) in Streptococcus pneumoniae is essential for competence-induced cell lysis. J. Bacteriol. 2005, 187, 4338–4345. [Google Scholar] [CrossRef] [PubMed]
- Eldholm, V.; Johnsborg, O.; Straume, D.; Ohnstad, H.S.; Berg, K.H.; Hermoso, J.A.; Håvarstein, L.S. Pneumococcal CbpD is a murein hydrolase that requires a dual cell envelope binding specificity to kill target cells during fratricide. Mol. Microbiol. 2010, 76, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Håvarstein, L.S.; Martin, B.; Johnsborg, O.; Granadel, C.; Claverys, J.-P. New insights into the pneumococcal fratricide: Relationship to clumping and identification of a novel immunity factor. Mol. Microbiol. 2006, 59, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Bergé, M.J.; Mercy, C.; Mortier-Barrière, I.; VanNieuwenhze, M.S.; Brun, Y.V.; Grangeasse, C.; Polard, P.; Campo, N. A programmed cell division delay preserves genome integrity during natural genetic transformation in Streptococcus pneumoniae. Nat. Commun. 2017, 8, 1621. [Google Scholar] [CrossRef]
- Eldholm, V.; Johnsborg, O.; Haugen, K.; Ohnstad, H.S.; Håvarstein, L.S. Fratricide in Streptococcus pneumoniae: Contributions and role of the cell wall hydrolases CbpD, LytA and LytC. Microbiology 2009, 155, 2223–2234. [Google Scholar] [CrossRef]
- Straume, D.; Stamsås, G.A.; Håvarstein, L.S. Natural transformation and genome evolution in Streptococcus pneumoniae. Infect. Genet. Evol. 2015, 33, 371–380. [Google Scholar] [CrossRef]
- Wei, H.; Håvarstein, L.S. Fratricide is essential for efficient gene transfer between pneumococci in biofilms. Appl. Environ. Microbiol. 2012, 78, 5897–5905. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, J.; Kuang, Z.; Vidal, J.E.; Lau, G.W. Deletion analysis of Streptococcus pneumoniae late competence genes distinguishes virulence determinants that are dependent or independent of competence induction. Mol. Microbiol. 2015, 97, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Tuomanen, E.; Liu, H.; Hengstler, B.; Zak, O.; Tomasz, A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 1985, 151, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Tuomanen, E.; Tomasz, A.; Hengstler, B.; Zak, O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J. Infect. Dis. 1985, 151, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Tuomanen, E.; Hengstler, B.; Zak, O.; Tomasz, A. Induction of meningeal inflammation by diverse bacterial cell walls. Eur. J. Clin. Microbiol. 1986, 5, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Giebink, G.S.; Ripley-Petzoldt, M.L.; Juhn, S.K.; Aeppli, D.; Tomasz, A.; Tuomanen, E. Contribution of pneumococcal cell wall to experimental otitis media pathogenesis. Ann. Otol. Rhinol. Laryngol. Suppl. 1988, 132, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Ripley-Petzoldt, M.L.; Giebink, G.S.; Juhn, S.K.; Aeppli, D.; Tomasz, A.; Tuomanen, E. The contribution of pneumococcal cell wall to the pathogenesis of experimental otitis media. J. Infect. Dis. 1988, 157, 245–255. [Google Scholar] [CrossRef]
- Sato, K.; Quartey, M.K.; Liebeler, C.L.; Le, C.T.; Giebink, G.S. Roles of autolysin and pneumolysin in middle ear inflammation caused by a type 3 Streptococcus pneumoniae strain in the chinchilla otitis media model. Infect. Immun. 1996, 64, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Majcherczyk, P.A.; Langen, H.; Heumann, D.; Fountoulakis, M.; Glauser, M.P.; Moreillon, P. Digestion of Streptococcus pneumoniae cell walls with its major peptidoglycan hydrolase releases branched stem peptides carrying proinflammatory activity. J. Biol. Chem. 1999, 274, 12537–12543. [Google Scholar] [CrossRef]
- Moore, L.J.; Pridmore, A.C.; Dower, S.K.; Read, R.C. Penicillin enhances the Toll-like receptor 2-mediated proinflammatory activity of Streptococcus pneumoniae. J. Infect. Dis. 2003, 188, 1040–1048. [Google Scholar] [CrossRef]
- Moreillon, P.; Majcherczyk, P.A. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis. 2003, 35, 632–641. [Google Scholar] [CrossRef]
- Steel, H.C.; Cockeran, R.; Anderson, R.; Feldman, C. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediat. Inflamm. 2013, 2013, 490346. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Domon, H.; Maekawa, T.; Oda, M.; Hiyoshi, T.; Tamura, H.; Yonezawa, D.; Arai, Y.; Yokoji, M.; Tabeta, K.; et al. Pneumococcal DNA-binding proteins released through autolysis induce the production of proinflammatory cytokines via toll-like receptor 4. Cell. Immunol. 2018, 325, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.C.; Brownridge, P.; Laing, G.; Terra, V.S.; Mlozowa, V.; Denis, B.; Nyirenda, M.; Allain, T.; Ramos-Sevillano, E.; Carrol, E.; et al. CSF levels of elongation factor Tu is associated with increased mortality in Malawian adults with Streptococcus pneumoniae meningitis. Front. Cell. Infect. Microbiol. 2020, 10, 603623. [Google Scholar] [CrossRef] [PubMed]
- Kruckow, K.L.; Zhao, K.; Bowdish, D.M.E.; Orihuela, C.J. Acute organ injury and long-term sequelae of severe pneumococcal infections. Pneumonia 2023, 15, 5. [Google Scholar] [CrossRef]
- Moscoso, M.; García, E.; López, R. Biofilm formation by Streptococcus pneumoniae: Role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J. Bacteriol. 2006, 188, 7785–7795. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; García, E.; Moscoso, M. Biofilm formation in Streptococcus pneumoniae. Microb. Biotechnol. 2012, 5, 455–465. [Google Scholar] [CrossRef]
- Domenech, M.; García, E.; Prieto, A.; Moscoso, M. Insight into the composition of the intercellular matrix of Streptococcus pneumoniae biofilms. Environ. Microbiol. 2013, 15, 502–516. [Google Scholar] [CrossRef]
- Domenech, M.; Ruiz, S.; Moscoso, M.; García, E. In vitro biofilm development of Streptococcus pneumoniae and formation of choline-binding protein–DNA complexes. Environ. Microbiol. Rep. 2015, 7, 715–727. [Google Scholar] [CrossRef]
- Brown, L.R.; Caulkins, R.C.; Schartel, T.E.; Rosch, J.W.; Honsa, E.S.; Schultz-Cherry, S.; Meliopoulos, V.A.; Cherry, S.; Thornton, J.A. Increased zinc availability enhances initial aggregation and biofilm formation of Streptococcus pneumoniae. Front. Cell. Infect. Microbiol. 2017, 7, 233. [Google Scholar] [CrossRef]
- Vilhena, C.; Du, S.; Battista, M.; Westermann, M.; Kohler, T.; Hammerschmidt, S.; Zipfel, P.F. The choline-binding proteins PspA, PspC, and LytA of Streptococcus pneumoniae and their interaction with human endothelial and red blood cells. Infect. Immun. 2023, 91, e00154-23. [Google Scholar] [CrossRef]
- Martner, A.; Skovbjerg, S.; Paton, J.C.; Wold, A.E. Streptococcus pneumoniae autolysis prevents phagocytosis and production of phagocyte-activating cytokines. Infect. Immun. 2009, 77, 3826–3837. [Google Scholar] [CrossRef] [PubMed]
- Skovbjerg, S.; Nordén, R.; Martner, A.; Samuelsson, E.; Hynsjö, L.; Wold, A.E. Intact pneumococci trigger transcription of interferon-related genes in human monocytes, while fragmented, autolyzed bacteria subvert this response. Infect. Immun. 2017, 85, e00960-16. [Google Scholar] [CrossRef] [PubMed]
- Ronda-Lain, C.; Lopez, R.; Tapia, A.; Tomasz, A. Role of the pneumococcal autolysin (murein hydrolase) in the release of phage progeny bacteriophage and in the bacteriophage-induced lysis of the host cells. J. Virol. 1977, 21, 366–374. [Google Scholar] [CrossRef]
- Garcia, P.; Lopez, R.; Ronda, C.; Garcia, E.; Tomasz, A. Mechanism of phage-induced lysis in pneumococci. J. Gen. Microbiol. 1983, 129, 479–487. [Google Scholar] [CrossRef]
- Frias, M.J.; Melo-Cristino, J.; Ramirez, M. The autolysin LytA contributes to efficient bacteriophage progeny release in Streptococcus pneumoniae. J. Bacteriol. 2009, 191, 5428–5440. [Google Scholar] [CrossRef]
- Frias, M.J.R. Lysis Strategy of Streptococcus pneumoniae Bacteriophages: Mechanisms and Host Implication. Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2011. Available online: https://repositorio.ul.pt/bitstream/10451/4644/3/ulsd061521_td_Maria_Frias.pdf (accessed on 5 March 2025).
- Frias, M.J.; Melo-Cristino, J.; Ramirez, M. Export of the pneumococcal phage SV1 lysin requires choline-containing teichoic acids and is holin-independent. Mol. Microbiol. 2013, 87, 430–445. [Google Scholar] [CrossRef]
- Morales, M.; Martín-Galiano, A.J.; Domenech, M.; García, E. Insights into the evolutionary relationships of LytA autolysin and Ply pneumolysin-like genes in Streptococcus pneumoniae and related streptococci. Genome Biol. Evol. 2015, 7, 2747–2761. [Google Scholar] [CrossRef]
- Nishimoto, A.T.; Rosch, J.W.; Tuomanen, E.I. Pneumolysin: Pathogenesis and therapeutic target. Front. Microbiol. 2020, 11, 1543. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Xu, S.; Leong, J.M.; Sousa, S. The Yin and Yang of pneumolysin during pneumococcal infection. Front. Immunol. 2022, 13, 878244. [Google Scholar] [CrossRef]
- Masure, H.R.; Pearce, B.J.; Shio, H.; Spellerberg, B. Membrane targeting of RecA during genetic transformation. Mol. Microbiol. 1998, 27, 845–852. [Google Scholar] [CrossRef]
- Martin, B.; García, P.; Castanié, M.P.; Claverys, J.-P. The recA gene of Streptococcus pneumoniae is part of a competence-induced operon and controls lysogenic induction. Mol. Microbiol. 1995, 15, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.J.; Naughton, A.M.; Campbell, E.A.; Masure, H.R. The rec locus, a competence-induced operon in Streptococcus pneumoniae. J. Bacteriol. 1995, 177, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tocci, N.; Iannelli, F.; Bidossi, A.; Ciusa, M.L.; Decorosi, F.; Viti, C.; Pozzi, G.; Ricci, S.; Oggioni, M.R. Functional analysis of pneumococcal drug efflux pumps associates the MATE DinF transporter with quinolone susceptibility. Antimicrob. Agents Chemother. 2013, 57, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Hava, D.L.; Camilli, A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 2002, 45, 1389–1406. [Google Scholar] [CrossRef]
- Díaz, E.; García, J.L. Characterization of the transcription unit encoding the major pneumococcal autolysin. Gene 1990, 90, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Mortier-Barrière, I.; de Saizieu, A.; Claverys, J.-P.; Martin, B. Competence-specific induction of recA is required for full recombination proficiency during transformation in Streptococcus pneumoniae. Mol. Microbiol. 1998, 27, 159–170. [Google Scholar] [CrossRef]
- Campbell, E.A.; Choi, S.Y.; Masure, H.R. A competence regulon in Streptococcus pneumoniae revealed by genomic analysis. Mol. Microbiol. 1998, 27, 929–939. [Google Scholar] [CrossRef]
- Pestova, E.V.; Morrison, D.A. Isolation and characterization of three Streptococcus pneumoniae transformation-specific loci by use of a lacZ reporter insertion vector. J. Bacteriol. 1998, 180, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Morrison, D.A. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 1999, 181, 5004–5016. [Google Scholar] [CrossRef]
- Luo, P.; Morrison, D.A. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 2003, 185, 349–358. [Google Scholar] [CrossRef]
- Solano-Collado, V.; Ruiz-Cruz, S.; Lorenzo-Díaz, F.; Pluta, R.; Espinosa, M.; Bravo, A. Recognition of streptococcal promoters by the pneumococcal SigA protein. Front. Mol. Biosci. 2021, 8, 666504. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.K.; Morrison, D.A. Two distinct functions of ComW in stabilization and activation of the alternative sigma factor ComX in Streptococcus pneumoniae. J. Bacteriol. 2005, 187, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Tovpeko, Y.; Bai, J.; Morrison, D.A. Competence for genetic transformation in Streptococcus pneumoniae: Mutations in σA bypass the ComW requirement for late gene expression. J. Bacteriol. 2016, 198, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Inniss, N.L.; Prehna, G.; Morrison, D.A. The pneumococcal σX activator, ComW, is a DNA-binding protein critical for natural transformation. J. Biol. Chem. 2019, 294, 11101–11118. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.; Henriques, B.; Charpentier, E.; Normark, S.; Tuomanen, E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 1999, 399, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.; Olivares Trejo, J.; Arellano-Galindo, J.; Zuñiga, G.; Escalona, G.; Vigueras, J.C.; Marín, P.; Xicohtencatl, J.; Valencia, P.; Velázquez-Guadarrama, N. pep27 and lytA in vancomycin-tolerant pneumococci. J. Microbiol. Biotechnol. 2011, 21, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Flores-Kim, J.; Dobihal, G.S.; Fenton, A.; Rudner, D.Z.; Bernhardt, T.G. A switch in surface polymer biogenesis triggers growth-phase-dependent and antibiotic-induced bacteriolysis. eLife 2019, 8, e44912. [Google Scholar] [CrossRef]
- Sabelnikov, A.G.; Greenberg, B.; Lacks, S.A. An extended –10 promoter alone directs transcription of the DpnII operon of Streptococcus pneumoniae. J. Mol. Biol. 1995, 250, 144–155. [Google Scholar] [CrossRef]
- Slager, J.; Aprianto, R.; Veening, J.-W. Refining the pneumococcal competence regulon by RNA sequencing. J. Bacteriol. 2019, 201, e00780-18. [Google Scholar] [CrossRef]
- Janssen, A.B.; Gibson, P.S.; Bravo, A.M.; de Bakker, V.; Slager, J.; Veening, J.-W. PneumoBrowse 2: An integrated visual platform for curated genome annotation and multiomics data analysis of Streptococcus pneumoniae. Nucleic Acids Res. 2024, 53, D839–D851. [Google Scholar] [CrossRef]
- Sinha, D.; Zimmer, K.; Cameron, T.A.; Rusch, D.B.; Winkler, M.E.; De Lay, N.R. Redefining the small regulatory RNA transcriptome in Streptococcus pneumoniae serotype 2 strain D39. J. Bacteriol. 2019, 201, e00764-18. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Frick Jacob, P.; Clemons, K.; Winkler, M.E.; De Lay, N.R. Pivotal roles for ribonucleases in Streptococcus pneumoniae pathogenesis. mBio 2021, 12, e02385-21. [Google Scholar] [CrossRef]
- Mohsen, J.J.; Martel, A.A.; Slavoff, S.A. Microproteins—Discovery, structure, and function. Proteomics 2023, 23, 2100211. [Google Scholar] [CrossRef]
- Simoens, L.; Fijalkowski, I.; Van Damme, P. Exposing the small protein load of bacterial life. FEMS Microbiol. Rev. 2023, 47, fuad063. [Google Scholar] [CrossRef] [PubMed]
- Laczkovich, I.; Mangano, K.; Shao, X.; Hockenberry Adam, J.; Gao, Y.; Mankin, A.; Vázquez-Laslop, N.; Federle Michael, J. Discovery of unannotated small open reading frames in Streptococcus pneumoniae D39 involved in quorum sensing and virulence using ribosome profiling. mBio 2022, 13, e01247-22. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, J.J.; Storz, G. Two for one: Regulatory RNAs that encode small proteins. Trends Biochem. Sci. 2023, 48, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, S.; Brignoli, T.; Bertoni, G. Little reason to call them small noncoding RNAs. Front. Microbiol. 2023, 14, 1191166. [Google Scholar] [CrossRef]
- Jordan, B.; Weidenbach, K.; Schmitz, R.A. The power of the small: The underestimated role of small proteins in bacterial and archaeal physiology. Curr. Opin. Microbiol. 2023, 76, 102384. [Google Scholar] [CrossRef]
- Duan, Y.; Santos-Júnior, C.D.; Schmidt, T.S.; Fullam, A.; de Almeida, B.L.S.; Zhu, C.; Kuhn, M.; Zhao, X.-M.; Bork, P.; Coelho, L.P. A catalog of small proteins from the global microbiome. Nat. Commun. 2024, 15, 7563. [Google Scholar] [CrossRef]
- Tomasz, A.; Westphal, M. Abnormal autolytic enzyme in a pneumococcus with altered teichoic acid composition. Proc. Natl. Acad. Sci. USA 1971, 68, 2627–2630. [Google Scholar] [CrossRef]
- Höltje, J.V.; Tomasz, A. Purification of the pneumococcal N-acetylmuramyl-l-alanine amidase to biochemical homogeneity. J. Biol. Chem. 1976, 251, 4199–4207. [Google Scholar] [CrossRef] [PubMed]
- Giudicelli, S.; Tomasz, A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J. Bacteriol. 1984, 158, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Briese, T.; Hakenbeck, R. Interaction of the pneumococcal amidase with lipoteichoic acid and choline. Eur. J. Biochem. 1985, 146, 417–427. [Google Scholar] [CrossRef]
- Sanz, J.M.; Lopez, R.; Garcia, J.L. Structural requirements of choline derivatives for ‘conversion’ of pneumococcal amidase. A new single-step procedure for purification of this autolysin. FEBS Lett. 1988, 232, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Rane, L.; Subbarow, Y. Choline, pantothenic acid, and nicotinic acid as essential growth factors for pneumococcus. J. Biol. Chem. 1940, 134, 455–456. [Google Scholar] [CrossRef]
- Denapaite, D.; Brückner, R.; Hakenbeck, R.; Vollmer, W. Biosynthesis of teichoic acids in Streptococcus pneumoniae and closely related species: Lessons from genomes. Microb. Drug Resist. 2012, 18, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Massidda, O.; Tomasz, A. The cell wall of Streptococcus pneumoniae. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef]
- Seo, H.S.; Cartee, R.T.; Pritchard, D.G.; Nahm, M.H. A new model of pneumococcal lipoteichoic acid structure resolves biochemical, biosynthetic, and serologic inconsistencies of the current model. J. Bacteriol. 2008, 190, 2379–2387. [Google Scholar] [CrossRef]
- Gisch, N.; Kohler, T.; Ulmer, A.J.; Müthing, J.; Pribyl, T.; Fischer, K.; Lindner, B.; Hammerschmidt, S.; Zähringer, U. Structural reevaluation of Streptococcus pneumoniae lipoteichoic acid and new insights into its immunostimulatory potency. J. Biol. Chem. 2013, 288, 15654–15667. [Google Scholar] [CrossRef]
- Garcia-Bustos, J.F.; Tomasz, A. Teichoic acid-containing muropeptides from Streptococcus pneumoniae as substrates for the pneumococcal autolysin. J. Bacteriol. 1987, 169, 447–453. [Google Scholar] [CrossRef]
- Severin, A.; Horne, D.; Tomasz, A. Autolysis and cell wall degradation in a choline-independent strain of Streptococcus pneumoniae. Microb. Drug Resist. 1997, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Munthali, M.; Lunsdorf, H.; Höltje, J.-V.; Timmis, K.N. The two-step lysis system of pneumococcal bacteriophage EJ-1 is functional in Gram-negative bacteria: Triggering of the major pneumococcal autolysin in Escherichia coli. Mol. Microbiol. 1996, 19, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, J.; Tomasz, A. Mechanisms of pneumococcal cell wall degradation in vitro and in vivo. J. Bacteriol. 1989, 171, 114–119. [Google Scholar] [CrossRef]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Lazaro Pinto, B.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The protein sequence classification resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Mellroth, P.; Steiner, H. PGRP-SB1: An N-acetylmuramoyl L-alanine amidase with antibacterial activity. Biochem. Biophys. Res. Commun. 2006, 350, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Gupta, D. Mammalian PGRPs: Novel antibacterial proteins. Cell. Microbiol. 2006, 8, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, M.; Liu, X.; Xia, H.; Chen, K. Peptidoglycan recognition proteins in insect immunity. Mol. Immunol. 2019, 106, 69–76. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, X.; Guo, M.; Li, C. Identification and characterization of a novel short-type peptidoglycan recognition protein in Apostichopus japonicus. Fish Shellfish Immunol. 2020, 99, 257–266. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, J.; Feng, D.; Wang, X.; Zhang, R. Immune functions of pattern recognition receptors in Lepidoptera. Front. Immunol. 2023, 14, 1203061. [Google Scholar] [CrossRef]
- Hakenbeck, R.; Madhour, A.; Denapaite, D.; Brückner, R. Versatility of choline metabolism and choline-binding proteins in Streptococcus pneumoniae and commensal streptococci. FEMS Microbiol. Rev. 2009, 33, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Frolet, C.; Beniazza, M.; Roux, L.; Gallet, B.; Noirclerc-Savoye, M.; Vernet, T.; Di Guilmi, A.M. New adhesin functions of surface-exposed pneumococcal proteins. BMC Microbiol. 2010, 10, 190. [Google Scholar] [CrossRef]
- Pérez-Dorado, I.; Galan-Bartual, S.; Hermoso, J.A. Pneumococcal surface proteins: When the whole is greater than the sum of its parts. Mol. Oral Microbiol. 2012, 27, 221–245. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Sanz, J.M. Choline binding proteins from Streptococcus pneumoniae: A dual role as enzybiotics and targets for the design of new antimicrobials. Antibiotics 2016, 5, 21. [Google Scholar] [CrossRef]
- Aceil, J.; Avci, F.Y. Pneumococcal surface proteins as virulence factors, immunogens, and conserved vaccine targets. Front. Cell. Infect. Microbiol. 2022, 12, 832254. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; García, E.; Arrarás, A.; García, P.; Ronda, C.; López, R. Cloning, purification, and biochemical characterization of the pneumococcal bacteriophage Cp-1 lysin. J. Virol. 1987, 61, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- García, P.; García, J.L.; García, E.; Sánchez-Puelles, J.M.; López, R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene 1990, 86, 81–88. [Google Scholar] [CrossRef]
- Díaz, E.; López, R.; García, J.L. Chimeric phage-bacterial enzymes: A clue to the modular evolution of genes. Proc. Natl. Acad. Sci. USA 1990, 87, 8125–8129. [Google Scholar] [CrossRef]
- Croux, C.; Ronda, C.; López, R.; García, J.L. Role of the C-terminal domain of the lysozyme of Clostridium acetobutylicum ATCC 824 in a chimeric pneumococcal-clostridial cell wall lytic enzyme. FEBS Lett. 1993, 336, 111–114. [Google Scholar] [CrossRef]
- Croux, C.; Ronda, C.; Lopez, R.; Garcia, J.L. Interchange of functional domains switches enzyme specificity: Construction of a chimeric pneumococcal-clostridial cell wall lytic enzyme. Mol. Microbiol. 1993, 9, 1019–1025. [Google Scholar] [CrossRef]
- Sánchez-Puelles, J.M.; Sanz, J.M.; García, J.L.; García, E. Cloning and expression of gene fragments encoding the choline-binding domain of pneumococcal murein hydrolases. Gene 1990, 89, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.L.; Diaz, E.; Romero, A.; Garcia, P. Carboxy-terminal deletion analysis of the major pneumococcal autolysin. J. Bacteriol. 1994, 176, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Usobiaga, P.; Medrano, F.J.; Gasset, M.; García, J.L.; Saiz, J.L.; Rivas, G.; Laynez, J.; Menéndez, M. Structural organization of the major autolysin from Streptococcus pneumoniae. J. Biol. Chem. 1996, 271, 6832–6838. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Santiveri, C.M.; Jiménez, M.A.; Sanz, J.M. Structural autonomy of a β-hairpin peptide derived from the pneumococcal choline-binding protein LytA. Protein Eng. Des. Sel. 2011, 24, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Zamora-Carreras, H.; Jiménez, M.Á.; Sanz, J.M. Inter-hairpin linker sequences determine the structure of the ββ-solenoid fold: A “bottom-up” study of pneumococcal LytA choline-binding module. Int. J. Biol. Macromol. 2021, 190, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Marianayagam, N.J.; Sunde, M.; Matthews, J.M. The power of two: Protein dimerization in biology. Trends Biochem. Sci. 2004, 29, 618–625. [Google Scholar] [CrossRef]
- Dang, D.T. Molecular approaches to protein dimerization: Opportunities for supramolecular chemistry. Front. Chem. 2022, 10, 829312. [Google Scholar] [CrossRef]
- Obregón, V.; García, P.; García, E.; Fenoll, A.; López, R.; García, J.L. Molecular peculiarities of the lytA gene isolated from clinical pneumococcal strains that are bile insoluble. J. Clin. Microbiol. 2002, 40, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Arbique, J.C.; Poyart, C.; Trieu-Cuot, P.; Quesne, G.; Carvalho, M.d.G.S.; Steigerwalt, A.G.; Morey, R.E.; Jackson, D.; Davidson, R.J.; Facklam, R.R. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J. Clin. Microbiol. 2004, 42, 4686–4696. [Google Scholar] [CrossRef]
- Llull, D.; López, R.; García, E. Characteristic signatures of the lytA gene provide a rapid and reliable diagnosis of Streptococcus pneumoniae infections. J. Clin. Microbiol. 2006, 44, 1250–1256. [Google Scholar] [CrossRef]
- Fernández-Tornero, C.; López, R.; García, E.; Giménez-Gallego, G.; Romero, A. A novel solenoid fold in the cell wall anchoring domain of the pneumococcal virulence factor LytA. Nat. Struct. Biol. 2001, 8, 1020–1024. [Google Scholar] [CrossRef]
- Fernández-Tornero, C.; García, E.; López, R.; Giménez-Gallego, G.; Romero, A. Two new crystal forms of the choline-binding domain of the major pneumococcal autolysin: Insights into the dynamics of the active homodimer. J. Mol. Biol. 2002, 321, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mellroth, P.; Sandalova, T.; Kikhney, A.; Vilaplana, F.; Hesek, D.; Lee, M.; Mobashery, S.; Normark, S.; Svergun, D.; Henriques-Normark, B.; et al. Structural and functional insights into peptidoglycan access for the lytic amidase LytA of Streptococcus pneumoniae. mBio 2014, 5, e01120-13. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, W.; Morlot, C.; Bai, X.-H.; Jiang, Y.-L.; Wang, W.; Roper, D.I.; Vernet, T.; Dong, Y.-H.; Chen, Y.; et al. Full-length structure of the major autolysin LytA. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1373–1381. [Google Scholar] [CrossRef]
- Hermoso, J.A.; Lagartera, L.; González, A.; Stelter, M.; García, P.; Martínez-Ripoll, M.; García, J.L.; Menéndez, M. Insights into pneumococcal pathogenesis from the crystal structure of the modular teichoic acid phosphorylcholine esterase Pce. Nat. Struct. Mol. Biol. 2005, 12, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Page, J.E.; Walker, S. Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes. J. Biol. Chem. 2020, 295, 3347–3361. [Google Scholar] [CrossRef]
- Brogan, A.P.; Rudner, D.Z. Regulation of peptidoglycan hydrolases: Localization, abundance, and activity. Curr. Opin. Microbiol. 2023, 72, 102279. [Google Scholar] [CrossRef]
- López, R.; García, E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. 2004, 28, 553–580. [Google Scholar] [CrossRef]
- Díaz, E.; García, E.; Ascaso, C.; Méndez, E.; López, R.; García, J.L. Subcellular localization of the major pneumococcal autolysin: A peculiar mechanism of secretion in Escherichia coli. J. Biol. Chem. 1989, 264, 1238–1244. [Google Scholar] [CrossRef]
- Zamora-Carreras, H.; Maestro, B.; Strandberg, E.; Ulrich, A.S.; Sanz, J.M.; Jiménez, M.Á. Micelle-triggered β-hairpin to α-helix transition in a 14-residue peptide from a choline-binding repeat of the pneumococcal autolysin LytA. Chemistry 2015, 21, 8076–8089. [Google Scholar] [CrossRef]
- Zamora-Carreras, H.; Maestro, B.; Strandberg, E.; Ulrich, A.S.; Sanz, J.M.; Jiménez, M.Á. Roles of amphipathicity and hydrophobicity in the micelle-driven structural switch of a 14-mer peptide core from a choline-binding repeat. Chem. Eur. J. 2018, 24, 5825–5839. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.V.; Ruiz-Castañeda, M.; Nitschke, P.; Gschwind, R.M.; Jiménez, M.A. Insights Into the micelle-induced β-hairpin-to-α-helix transition of a LytA-derived peptide by photo-CIDNP spectroscopy. Int. J. Mol. Sci. 2021, 22, 6666. [Google Scholar] [CrossRef]
- Lella, M.; Mahalakshmi, R. Metamorphic proteins: Emergence of dual protein folds from one primary sequence. Biochemistry 2017, 56, 2971–2984. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Carreras, H.; Maestro, B.; Sanz, J.M.; Jiménez, M.A. Turncoat polypeptides: We adapt to our environment. Chembiochem 2020, 21, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Roterman, I.; Slupina, M.; Stapor, K.; Konieczny, L.; Gądek, K.; Nowakowski, P. Chameleon sequences-Structural effects in proteins characterized by hydrophobicity disorder. ACS Omega 2024, 9, 38506–38522. [Google Scholar] [CrossRef]
- Porter, L.L.; Artsimovitch, I.; Ramírez-Sarmiento, C.A. Metamorphic proteins and how to find them. Curr. Opin. Struct. Biol. 2024, 86, 102807. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Park, S.S.; Rhee, D.K. Stress responses in Streptococcus species and their effects on the host. J. Microbiol. 2015, 53, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Mueller, E.A.; Levin, P.A. Bacterial cell wall quality control during environmental stress. mBio 2020, 11, e02456-20. [Google Scholar] [CrossRef]
- Keck, T.; Leiacker, R.; Schick, M.; Rettinger, G.; Kühnemann, S. Temperatur- und feuchteprofil der nasenwege vor und nach schleimhautabschwellung durch xylometazolin. Laryngorhinootologie 2000, 79, 749–752. [Google Scholar] [CrossRef]
- Abbas, A.K.; Heimann, K.; Jergus, K.; Orlikowsky, T.; Leonhardt, S. Neonatal non-contact respiratory monitoring based on real-time infrared thermography. Biomed. Eng. Online 2011, 10, 93. [Google Scholar] [CrossRef]
- Gazioglu, O.; Kareem, B.O.; Afzal, M.; Shafeeq, S.; Kuipers, O.P.; Ulijasz, A.T.; Andrew, P.W.; Yesilkaya, H. Glutamate dehydrogenase (GdhA) of Streptococcus pneumoniae is required for high temperature adaptation. Infect. Immun. 2021, 89, e0040021. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Talekar, S.J.; Lanata, C.F.; Grijalva, C.G.; Klugman, K.P.; Vidal, J.E.; for the RESPIRA PERU Group. Expression of virulence-related genes in the nasopharynx of healthy children. PLoS ONE 2013, 8, e67147. [Google Scholar] [CrossRef] [PubMed]
- Jusot, J.-F.; Neill, D.R.; Waters, E.M.; Bangert, M.; Collins, M.; Bricio Moreno, L.; Lawan, K.G.; Moussa, M.M.; Dearing, E.; Everett, D.B.; et al. Airborne dust and high temperatures are risk factors for invasive bacterial disease. J. Allergy Clin. Immunol. 2017, 139, 977–986.e972. [Google Scholar] [CrossRef] [PubMed]
- Maziero, M.; Lane, D.; Polard, P.; Bergé, M. Fever-like temperature bursts promote competence development via an HtrA-dependent pathway in Streptococcus pneumoniae. PLoS Genet. 2023, 19, e1010946. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Q.; Kohler, T.P.; Schulig, L.; Burchhardt, G.; Hammerschmidt, S. Pneumococcal extracellular serine proteases: Molecular analysis and impact on colonization and disease. Front. Cell. Infect. Microbiol. 2021, 11, 763152. [Google Scholar] [CrossRef] [PubMed]
- Eichner, H.; Karlsson, J.; Spelmink, L.; Pathak, A.; Sham, L.-T.; Henriques-Normark, B.; Loh, E. RNA thermosensors facilitate Streptococcus pneumoniae and Haemophilus influenzae immune evasion. PLoS Pathog. 2021, 17, e1009513. [Google Scholar] [CrossRef]
- Li-Korotky, H.-S.; Lo, C.-Y.; Zeng, F.-R.; Lo, D.; Banks, J.M. Interaction of phase variation, host and pressure/gas composition: Pneumococcal gene expression of PsaA, SpxB, Ply and LytA in simulated middle ear environments. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1417–1422. [Google Scholar] [CrossRef]
- Prudhomme, M.; Johnston, C.H.G.; Soulet, A.-L.; Boyeldieu, A.; De Lemos, D.; Campo, N.; Polard, P. Pneumococcal competence is a populational health sensor driving multilevel heterogeneity in response to antibiotics. Nat. Commun. 2024, 15, 5625. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Li, Y.; Zhou, L.; Wu, Y. Competence regulation in Streptococcus pneumoniae and the competence-targeted anti-pneumococcal strategies. Curr. Med. Chem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.W.; Blokesch, M. Interbacterial predation as a strategy for DNA acquisition in naturally competent bacteria. Nat. Rev. Microbiol. 2017, 15, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Claverys, J.-P.; Prudhomme, M.; Martin, B. Induction of competence regulons as a general response to stress in Gram-positive bacteria. Annu. Rev. Microbiol. 2006, 60, 451–475. [Google Scholar] [CrossRef]
- Engelmoer, D.J.; Rozen, D.E. Competence increases survival during stress in Streptococcus pneumoniae. Evolution 2011, 65, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, D.; Soulet, A.-L.; Morales, V.; Berge, M.; Polard, P.; Johnston, C. Competence induction of homologous recombination genes protects pneumococcal cells from genotoxic stress. mBio 2024, 16, e03142-24. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Choi, P.J.; Li, G.-W.; Chen, H.; Babu, M.; Hearn, J.; Emili, A.; Xie, X.S. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 2010, 329, 533–538. [Google Scholar] [CrossRef]
- Ning, K.; Fermin, D.; Nesvizhskii, A.I. Comparative analysis of different label-free mass spectrometry based protein abundance estimates and their correlation with RNA-Seq gene expression data. J. Proteome Res. 2012, 11, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Dagkessamanskaia, A.; Moscoso, M.; Hénard, V.; Guiral, S.; Overweg, K.; Reuter, M.; Martin, B.; Wells, J.; Claverys, J.-P. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: Competence triggers stationary phase autolysis of ciaR mutant cells. Mol. Microbiol. 2004, 51, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.N.; Sung, C.K.; Cline, R.; Desai, B.V.; Snesrud, E.C.; Luo, P.; Walling, J.; Li, H.; Mintz, M.; Tsegaye, G.; et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 2004, 51, 1051–1070. [Google Scholar] [CrossRef]
- Tomasz, A.; Mosser, J.L. On the nature of the pneumococcal activator substance. Proc. Natl. Acad. Sci. USA 1966, 55, 58–66. [Google Scholar] [CrossRef]
- Håvarstein, L.S.; Coomaraswamy, G.; Morrison, D.A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 1995, 92, 11140–11144. [Google Scholar] [CrossRef]
- Aprianto, R.; Slager, J.; Holsappel, S.; Veening, J.-W. High-resolution analysis of the pneumococcal transcriptome under a wide range of infection-relevant conditions. Nucleic Acids Res. 2018, 46, 9990–10006. [Google Scholar] [CrossRef] [PubMed]
- Aprianto, R.; Slager, J.; Holsappel, S.; Veening, J.-W. Time-resolved dual RNA-seq reveals extensive rewiring of lung epithelial and pneumococcal transcriptomes during early infection. Genome Biol. 2016, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Minhas, V.; Domenech, A.; Synefiaridou, D.; Straume, D.; Brendel, M.; Cebrero, G.; Liu, X.; Costa, C.; Baldry, M.; Sirard, J.-C.; et al. Competence remodels the pneumococcal cell wall exposing key surface virulence factors that mediate increased host adherence. PLoS Biol. 2023, 21, e3001990. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, J.-P.; Page, A.; Polard, P.; Campo, N.; Grangeasse, C. Quantitative phosphoproteomic reveals that the induction of competence modulates protein phosphorylation in Streptococcus pneumonaie. J. Proteom. 2025, 105399. [Google Scholar] [CrossRef]
- Zhu, L.; Lau, G.W. Inhibition of competence development, horizontal gene transfer and virulence in Streptococcus pneumoniae by a modified competence stimulating peptide. PLoS Pathog. 2011, 7, e1002241. [Google Scholar] [CrossRef]
- Turlan, C.; Prudhomme, M.; Fichant, G.; Martin, B.; Gutierrez, C. SpxA1, a novel transcriptional regulator involved in X-state (competence) development in Streptococcus pneumoniae. Mol. Microbiol. 2009, 73, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.-Y.; Zhu, M.; Deng, H.; Wang, J.; Zhang, J.-R. The SpxA1-TenA toxin-antitoxin system regulates epigenetic variations of Streptococcus pneumoniae by targeting protein synthesis. PLoS Pathog. 2024, 20, e1012801. [Google Scholar] [CrossRef]
- Chan, W.T.; Garcillán-Barcia, M.P.; Yeo, C.C.; Espinosa, M. Type II bacterial toxin–antitoxins: Hypotheses, facts, and the newfound plethora of the PezAT system. FEMS Microbiol. Rev. 2023, 47, fuad052. [Google Scholar] [CrossRef]
- Lin, J.; Park, P.; Li, H.; Oh, M.W.; Dobrucki, I.T.; Dobrucki, W.; Lau, G.W. Streptococcus pneumoniae elaborates persistent and prolonged competent state during pneumonia-derived sepsis. Infect. Immun. 2020, 88, e00919-19. [Google Scholar] [CrossRef]
- Chong, S.Y.; Lew, S.Q.; Alam, T.; Gaulke, C.A.; Lau, G.W. Comparative analysis of the Streptococcus pneumoniae competence development in vitro versus in vivo during pneumonia-derived sepsis. Front. Microbiol. 2025, 16, 1540511. [Google Scholar] [CrossRef]
- Schmidt, F.; Kakar, N.; Meyer, T.C.; Depke, M.; Masouris, I.; Burchhardt, G.; Gómez-Mejia, A.; Dhople, V.; Håvarstein, L.S.; Sun, Z.; et al. In vivo proteomics identifies the competence regulon and AliB oligopeptide transporter as pathogenic factors in pneumococcal meningitis. PLoS Pathog. 2019, 15, e1007987. [Google Scholar] [CrossRef]
- Jim, K.K.; Aprianto, R.; Koning, R.; Domenech, A.; Kurushima, J.; van de Beek, D.; Vandenbroucke-Grauls, C.M.J.E.; Bitter, W.; Veening, J.-W. Pneumolysin promotes host cell necroptosis and bacterial competence during pneumococcal meningitis as shown by whole-animal dual RNA-seq. Cell Rep. 2022, 41, 111851. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.T.; Brissac, T.; Gilley, R.P.; Kumar, N.; Wang, Y.; Gonzalez-Juarbe, N.; Hinkle, W.S.; Daugherty, S.C.; Shetty, A.C.; Ott, S.; et al. Streptococcus pneumoniae in the heart subvert the host response through biofilm-mediated resident macrophage killing. PLoS Pathog. 2017, 13, e1006582. [Google Scholar] [CrossRef]
- Shenoy, A.T.; Beno, S.M.; Brissac, T.; Bell, J.W.; Novak, L.; Orihuela, C.J. Severity and properties of cardiac damage caused by Streptococcus pneumoniae are strain dependent. PLoS ONE 2018, 13, e0204032. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, A.; Riegler, A.N.; Martínez, E.; Beno, S.M.; Ricketts, T.D.; Foxman, E.F.; Orihuela, C.J.; Tettelin, H. An in vivo atlas of host–pathogen transcriptomes during Streptococcus pneumoniae colonization and disease. Proc. Natl. Acad. Sci. USA 2020, 117, 33507–33518. [Google Scholar] [CrossRef]
- Orihuela, C.J.; Radin, J.N.; Sublett, J.E.; Gao, G.; Kaushal, D.; Tuomanen, E.I. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 2004, 72, 5582–5596. [Google Scholar] [CrossRef]
- Romao, S.; Memmi, G.; Oggioni, M.R.; Trombe, M.-C. LuxS impacts on LytA-dependent autolysis and on competence in Streptococcus pneumoniae. Microbiology 2006, 152, 333–341. [Google Scholar] [CrossRef]
- Rogers, P.D.; Liu, T.T.; Barker, K.S.; Hilliard, G.M.; English, B.K.; Thornton, J.; Swiatlo, E.; McDaniel, L.S. Gene expression profiling of the response of Streptococcus pneumoniae to penicillin. J. Antimicrob. Chemother. 2007, 59, 616–626. [Google Scholar] [CrossRef]
- Yadav, M.K.; Vidal, J.E.; Go, Y.Y.; Kim, S.H.; Chae, S.-W.; Song, J.-J. The LuxS/AI-2 quorum-sensing system of Streptococcus pneumoniae is required to cause disease, and to regulate virulence- and metabolism-related genes in a rat model of middle ear infection. Front. Cell. Infect. Microbiol. 2018, 8, 138. [Google Scholar] [CrossRef]
- Vidal, J.E.; Ludewick, H.P.; Kunkel, R.M.; Zähner, D.; Klugman, K.P. The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect. Immun. 2011, 79, 4050–4060. [Google Scholar] [CrossRef]
- Yadav, M.K.; Go, Y.Y.; Chae, S.W.; Park, M.K.; Song, J.J. Asian sand dust particles increased pneumococcal biofilm formation in vitro and colonization in human middle ear epithelial cells and rat middle ear mucosa. Front. Genet. 2020, 11, 323. [Google Scholar] [CrossRef]
- Bagale, K.; Paudel, S.; Cagle, H.; Sigel, E.; Kulkarni, R. Electronic Cigarette (E-cigarette) vapor exposure alters Streptococcus pneumoniae transcriptome in a nicotine-dependent manner without affecting pneumococcal virulence. Appl. Environ. Microbiol. 2019, 86, e02125-19. [Google Scholar] [CrossRef]
- Domon, H.; Maekawa, T.; Yonezawa, D.; Nagai, K.; Oda, M.; Yanagihara, K.; Terao, Y. Mechanism of macrolide-induced inhibition of pneumolysin release involves impairment of autolysin release in macrolide-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2018, 62, e00161-18. [Google Scholar] [CrossRef] [PubMed]
- Domon, H.; Isono, T.; Hiyoshi, T.; Tamura, H.; Sasagawa, K.; Maekawa, T.; Hirayama, S.; Yanagihara, K.; Terao, Y. Clarithromycin inhibits pneumolysin production via downregulation of ply gene transcription despite autolysis activation. Microbiol. Spectr. 2021, 9, e0031821. [Google Scholar] [CrossRef]
- Hong, W.; Khampang, P.; Erbe, C.; Kumar, S.; Taylor, S.R.; Kerschner, J.E. Nontypeable Haemophilus influenzae inhibits autolysis and fratricide of Streptococcus pneumoniae in vitro. Microbes Infect. 2014, 16, 203–213. [Google Scholar] [CrossRef]
- McMahon, F.; Ware, R.S.; Grimwood, K.; Atack, J.M. Haemophilus influenzae and pneumococci: Co-colonization, interactions, cooperation and competition. Pediatr. Pulmonol. 2024, 60, e27318. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.F.; Georgellis, D. Environmental adaptation and diversification of bacterial two-component systems. Curr. Opin. Microbiol. 2023, 76, 102399. [Google Scholar] [CrossRef]
- Gómez-Mejia, A.; Gámez, G.; Hammerschmidt, S. Streptococcus pneumoniae two-component regulatory systems: The interplay of the pneumococcus with its environment. Int. J. Med. Microbiol. 2018, 308, 722–737. [Google Scholar] [CrossRef]
- Pettersen, J.S.; Nielsen, F.D.; Andreassen, P.R.; Møller-Jensen, J.; Jørgensen, M.G. A comprehensive analysis of pneumococcal two-component system regulatory networks. NAR Genom. Bioinform. 2024, 6, lqae039. [Google Scholar] [CrossRef]
- Mascher, T.; Heintz, M.; Zähner, D.; Merai, M.; Hakenbeck, R. The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in β-lactam resistance. J. Bacteriol. 2006, 188, 1959–1968. [Google Scholar] [CrossRef]
- Zähner, D.; Kaminski, K.; van der Linden, M.; Mascher, T.; Meral, M.; Hakenbeck, R. The ciaR/ciaH regulatory network of Streptococcus pneumoniae. J. Mol. Microbiol. Biotechnol. 2002, 4, 211–216. [Google Scholar] [PubMed]
- Halfmann, A.; Kovács, M.; Hakenbeck, R.; Brückner, R. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: Five out of 15 promoters drive expression of small non-coding RNAs. Mol. Microbiol. 2007, 66, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, A.; Schnorpfeil, A.; Müller, M.; Marx, P.; Günzler, U.; Hakenbeck, R.; Brückner, R. Activity of the two-component regulatory system CiaRH in Streptococcus pneumoniae R6. J. Mol. Microbiol. Biotechnol. 2011, 20, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.C.; Mukherjee, D.; Ray, V.A.; Sham, L.T.; Feig, A.L.; Winkler, M.E. Identification and characterization of noncoding small RNAs in Streptococcus pneumoniae serotype 2 strain D39. J. Bacteriol. 2010, 192, 264–279. [Google Scholar] [CrossRef]
- García, J.L.; Sánchez-Beato, A.R.; Medrano, F.J.; López, R. Versatility of choline-binding domain. Microb. Drug Resist. 1998, 4, 25–36. [Google Scholar] [CrossRef]
- Rodríguez Sánchez-Beato, A.I. Caracterización Molecular de Genes de Clostridium acetobutylicum y Streptococcus pneumoniae que codifican Para Proteínas con Dominios de Unión a Colina. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2005. Available online: https://docta.ucm.es/bitstreams/274074c1-1c65-4064-a70c-d34c568eb827/download (accessed on 5 March 2025).
- Eldholm, V.; Gutt, B.; Johnsborg, O.; Brückner, R.; Maurer, P.; Hakenbeck, R.; Mascher, T.; Håvarstein, L.S. The pneumococcal cell envelope stress-sensing system LiaFSR is activated by murein hydrolases and lipid II-interacting antibiotics. J. Bacteriol. 2010, 192, 1761–1773. [Google Scholar] [CrossRef]
- Molina, R.; González, A.; Stelter, M.; Pérez-Dorado, I.; Kahn, R.; Morales, M.; Moscoso, M.; Campuzano, S.; Campillo, N.E.; Mobashery, S.; et al. Crystal structure of CbpF, a bifunctional choline-binding protein and autolysis regulator from Streptococcus pneumoniae. EMBO Rep. 2009, 10, 246–251. [Google Scholar] [CrossRef]
- Blue, C.E.; Mitchell, T.J. Contribution of a response regulator to the virulence of Streptococcus pneumoniae is strain dependent. Infect. Immun. 2003, 71, 4405–4413. [Google Scholar] [CrossRef]
- Hirschmann, S.; Gómez-Mejia, A.; Mäder, U.; Karsunke, J.; Driesch, D.; Rohde, M.; Häussler, S.; Burchhardt, G.; Hammerschmidt, S. The two-component system 09 regulates pneumococcal carbohydrate metabolism and capsule expression. Microorganisms 2021, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, S.; Gómez-Mejia, A.; Kohler, T.P.; Voß, F.; Rohde, M.; Brendel, M.; Hammerschmidt, S. The two-component system 09 of Streptococcus pneumoniae is important for metabolic fitness and resistance during dissemination in the host. Microorganisms 2021, 9, 1365. [Google Scholar] [CrossRef]
- Kim, G.-L.; Luong, T.T.; Park, S.-S.; Lee, S.; Ha, J.A.; Nguyen, C.T.; Ahn, J.H.; Park, K.-T.; Paik, M.-J.; Pyo, S.; et al. Inhibition of autolysis by lipase LipA in Streptococcus pneumoniae sepsis. Mol. Cells 2017, 40, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Kovács, M.; Halfmann, A.; Fedtke, I.; Heintz, M.; Peschel, A.; Vollmer, W.; Hakenbeck, R.; Brückner, R. A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in Gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 2006, 188, 5797–5805. [Google Scholar] [CrossRef] [PubMed]
- Wecke, J.; Perego, M.; Fischer, W. d-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 1996, 2, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Steen, A.; Palumbo, E.; Deghorain, M.; Cocconcelli, P.S.; Delcour, J.; Kuipers, O.P.; Kok, J.; Buist, G.; Hols, P. Autolysis of Lactococcus lactis is increased upon d-alanine depletion of peptidoglycan and lipoteichoic acids. J. Bacteriol. 2005, 187, 114–124. [Google Scholar] [CrossRef]
- Du, J.; Huang, S.; Wu, M.; Chen, S.; Zhou, W.; Zhan, L.; Huang, X. Dlt operon regulates physiological function and cariogenic virulence in Streptococcus mutans. Future Microbiol. 2023, 18, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.J.; Paton, J.C. Spermidine biosynthesis and transport modulate pneumococcal autolysis. J. Bacteriol. 2014, 196, 3556–3561. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Dörr, T. Understanding tolerance to cell wall-active antibiotics. Ann. N. Y. Acad. Sci. 2021, 1496, 35–58. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zheng, J.; Chan, E.W.; Chen, S. Proton motive force and antibiotic tolerance in bacteria. Microb. Biotechnol. 2024, 17, e70042. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.R.; Dörr, T. Bacterial metabolism and susceptibility to cell wall-active antibiotics. Adv. Microb. Physiol. 2023, 83, 181–219. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.H.; Echlin, H.; McKnight, A.; Marr, E.S.; Junker, J.; Jia, Q.; Hayden, R.; van Opijnen, T.; Isberg, R.R.; Cooper, V.S.; et al. Streptococcus pneumoniae favors tolerance via metabolic adaptation over resistance to circumvent fluoroquinolones. mBio 2024, 15, e02828-23. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Ronda-Lain, C.; Tapia, A.; Waks, S.B.; Tomasz, A. Suppression of the lytic and bactericidal effects of cell wall-inhibitory antibiotics. Antimicrob. Agents Chemother. 1976, 10, 697–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goodell, E.W.; Lopez, R.; Tomasz, A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc. Natl. Acad. Sci. USA 1976, 73, 3293–3297. [Google Scholar] [CrossRef]
- Ataee, R.A.; Habibian, S.; Mehrabi-Tavana, A.; Ahmadi, Z.; Jonaidi, N.; Salesi, M. Determination of vancomycin minimum inhibitory concentration for ceftazidime resistant Streptococcus pneumoniae in Iran. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 53. [Google Scholar] [CrossRef]
- Nazari Alam, A.; Rafiei Tabatabaii, S.; Hashemi, A.; Yousefi, M.; Hoseini Alfatemi, S.M. Characterization of 5 episodes of vancomycin nonsusceptible Streptococcus pneumoniae from clinical isolates in Tehran, Iran. Arch. Clin. Infect. Dis. 2017, 12, e57285. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf (accessed on 5 March 2025).
- Krčméry Jr, V.; Sefton, A. Vancomycin resistance in Gram-positive bacteria other than Enterococcus spp. Int. J. Antimicrob. Agents 2000, 14, 99–105. [Google Scholar] [CrossRef]
- Chochua, S.; Beall, B.; Lin, W.; Tran, T.; Rivers, J.; Li, Z.; Arvay, M.L.; Kobayashi, M.; Houston, J.; Arias, S.; et al. The emergent invasive serotype 4 ST10172 strain acquires vanG-type vancomycin-resistance element: A case of a 66-year-old with bacteremic pneumococcal pneumonia. J. Infect. Dis. 2024, 231, 746–750. [Google Scholar] [CrossRef]
- Moscoso, M.; Domenech, M.; García, E. Vancomycin tolerance in Gram-positive cocci. Environ. Microbiol. Rep. 2011, 3, 640–650. [Google Scholar] [CrossRef]
- Li, G.; Walker, M.J.; De Oliveira, D.M.P. Vancomycin resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Henriques Normark, B.; Normark, S. Antibiotic tolerance in pneumococci. Clin. Microbiol. Infect. 2002, 8, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.S.; Tuomanen, E.I. Molecular analysis of antibiotic tolerance in pneumococci. Int. J. Med. Microbiol. 2002, 292, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Whatmore, A.M.; King, S.J.; Doherty, N.C.; Sturgeon, D.; Chanter, N.; Dowson, C.G. Molecular characterization of equine isolates of Streptococcus pneumoniae: Natural disruption of genes encoding the virulence factors pneumolysin and autolysin. Infect. Immun. 1999, 67, 2776–2782. [Google Scholar] [CrossRef]
- Wong, H.E.; Tourlomousis, P.; Paterson, G.K.; Webster, S.; Bryant, C.E. Naturally-occurring serotype 3 Streptococcus pneumoniae strains that lack functional pneumolysin and autolysin have attenuated virulence but induce localized protective immune responses. PLoS ONE 2023, 18, e0282843. [Google Scholar] [CrossRef]
- Sung, H.; Shin, H.B.; Kim, M.-N.; Lee, K.; Kim, E.-C.; Song, W.; Jeong, S.H.; Lee, W.-G.; Park, Y.-J.; Eliopoulos, G.M. Vancomycin-tolerant Streptococcus pneumoniae in Korea. J. Clin. Microbiol. 2006, 44, 3524–3528. [Google Scholar] [CrossRef]
- Moscoso, M.; Domenech, M.; García, E. Vancomycin tolerance in clinical and laboratory Streptococcus pneumoniae isolates depends on reduced enzyme activity of the major LytA autolysin or cooperation between CiaH histidine kinase and capsular polysaccharide. Mol. Microbiol. 2010, 77, 1052–1064. [Google Scholar] [CrossRef]
- Novak, R.; Braun, J.S.; Charpentier, E.; Tuomanen, E. Penicillin tolerance genes of Streptococcus pneumoniae: The ABC-type manganese permease complex Psa. Mol. Microbiol. 1998, 29, 1285–1296. [Google Scholar] [CrossRef]
- Novak, R.; Charpentier, E.; Braun, J.S.; Park, E.; Murti, S.; Tuomanen, E.; Masure, R. Extracellular targeting of choline-binding proteins in Streptococcus pneumoniae by a zinc metalloprotease. Mol. Microbiol. 2000, 36, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Claverys, J.P.; Granadel, C.; Berry, A.M.; Paton, J.C. Penicillin tolerance in Streptococcus pneumoniae, autolysis and the Psa ATP-binding cassette (ABC) manganese permease. Mol. Microbiol. 1999, 32, 881–883. [Google Scholar] [CrossRef]
- Bergé, M.; García, P.; Iannelli, F.; Prère, M.F.; Granadel, C.; Polissi, A.; Claverys, J.P. The puzzle of zmpB and extensive chain formation, autolysis defect and non-translocation of choline-binding proteins in Streptococcus pneumoniae. Mol. Microbiol. 2001, 39, 1651–1660. [Google Scholar] [CrossRef]
- McAllister, L.J.; Tseng, H.-J.; Ogunniyi, A.D.; Jennings, M.P.; McEwan, A.G.; Paton, J.C. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 2004, 53, 889–901. [Google Scholar] [CrossRef]
- Charpentier, E.; Novak, R.; Tuomanen, E. Regulation of growth inhibition at high temperature, autolysis, transformation and adherence in Streptococcus pneumoniae by ClpC. Mol. Microbiol. 2000, 37, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.M.; Kerr, A.R.; Silva, N.A.; Mitchell, T.J. Contribution of the ATP-dependent protease ClpCP to the autolysis and virulence of Streptococcus pneumoniae. Infect. Immun. 2005, 73, 730–740. [Google Scholar] [CrossRef]
- Chastanet, A.; Prudhomme, M.; Claverys, J.-P.; Msadek, T. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 2001, 183, 7295–7307. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.T.; Ng, W.-L.; Foley, J.; Gilmour, R.; Winkler, M.E. Global transcriptional analysis of clpP mutations of type 2 Streptococcus pneumoniae and their effects on physiology and virulence. J. Bacteriol. 2002, 184, 3508–3520. [Google Scholar] [CrossRef]
- Novak, R.; Charpentier, E.; Braun, J.S.; Tuomanen, E. Signal transduction by a death signal peptide: Uncovering the mechanism of bacterial killing by penicillin. Mol. Cell 2000, 5, 49–57. [Google Scholar] [CrossRef]
- Robertson, G.T.; Zhao, J.; Desai, B.V.; Coleman, W.H.; Nicas, T.I.; Gilmour, R.; Grinius, L.; Morrison, D.A.; Winkler, M.E. Vancomycin tolerance induced by erythromycin but not by loss of vncRS, vex3, or pep27 function in Streptococcus pneumoniae. J. Bacteriol. 2002, 184, 6987–7000. [Google Scholar] [CrossRef] [PubMed]
- Haas, W.; Sublett, J.; Kaushal, D.; Tuomanen, E.I. Revising the role of the pneumococcal vex-vncRS locus in vancomycin tolerance. J. Bacteriol. 2004, 186, 8463–8471. [Google Scholar] [CrossRef] [PubMed]
- Haas, W.; Kaushal, D.; Sublett, J.; Obert, C.; Tuomanen, E.I. Vancomycin stress response in a sensitive and a tolerant strain of Streptococcus pneumoniae. J. Bacteriol. 2005, 187, 8205–8210. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Xu, J.-Y.; Wei, Q.-X.; Sun, X.; He, Q.-Y. Comparative proteomics of Streptococcus pneumoniae response to vancomycin treatment. OMICS 2017, 21, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.-W.; Feng, Z.; Luo, Y.; Veening, J.-W.; Zhang, J.-R. Transcriptional repressor PtvR regulates phenotypic tolerance to vancomycin in Streptococcus pneumoniae. J. Bacteriol. 2017, 199, e00054-17. [Google Scholar] [CrossRef]
- Moreillon, P.; Tomasz, A. Penicillin resistance and defective lysis in clinical isolates of pneumococci: Evidence for two kinds of antibiotic pressure operating in the clinical environment. J. Infect. Dis. 1988, 157, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, P.; Markiewicz, Z.; Nachman, S.; Tomasz, A. Two bactericidal targets for penicillin in pneumococci: Autolysis-dependent and autolysis-independent killing mechanisms. Antimicrob. Agents Chemother. 1990, 34, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Filipe, S.R.; Severina, E.; Tomasz, A. The role of murMN operon in penicillin resistance and antibiotic tolerance of Streptococcus pneumoniae. Microb. Drug Resist. 2001, 7, 303–316. [Google Scholar] [CrossRef]
- Filipe, S.R.; Severina, E.; Tomasz, A. The murMN operon: A functional link between antibiotic resistance and antibiotic tolerance in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 2002, 99, 1550–1555. [Google Scholar] [CrossRef]
- Aggarwal, S.D.; Lloyd, A.J.; Yerneni, S.S.; Narciso, A.R.; Shepherd, J.; Roper, D.I.; Dowson, C.G.; Filipe, S.R.; Hiller, N.L. A molecular link between cell wall biosynthesis, translation fidelity, and stringent response in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 2021, 118, e2018089118. [Google Scholar] [CrossRef]
- Crisóstomo, M.I.; Vollmer, W.; Kharat, A.S.; Inhülsen, S.; Gehre, F.; Buckenmaier, S.; Tomasz, A. Attenuation of penicillin resistance in a peptidoglycan O-acetyl transferase mutant of Streptococcus pneumoniae. Mol. Microbiol. 2006, 61, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.; Durmort, C.; Jacq, M.; Mortier-Barrière, I.; Campo, N.; VanNieuwenhze, M.S.; Brun, Y.V.; Arthaud, C.; Gallet, B.; Moriscot, C.; et al. Peptidoglycan O-acetylation is functionally related to cell wall biosynthesis and cell division in Streptococcus pneumoniae. Mol. Microbiol. 2017, 106, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Fernebro, J.; Andersson, I.; Sublett, J.; Morfeldt, E.; Novak, R.; Tuomanen, E.; Normark, S.; Henriques Normark, B. Capsular expression in Streptococcus pneumoniae negatively affects spontaneous and antibiotic-induced lysis and contributes to antibiotic tolerance. J. Infect. Dis. 2004, 189, 328–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figueiredo, J.; Henriques, M.X.; Catalão, M.J.; Pinheiro, S.; Narciso, A.R.; Mesquita, F.; Saraiva, B.M.; Carido, M.; Cabanes, D.; Pinho, M.G.; et al. Encapsulation of the septal cell wall protects Streptococcus pneumoniae from its major peptidoglycan hydrolase and host defenses. PLoS Pathog. 2022, 18, e1010516. [Google Scholar] [CrossRef]
- Ghosh, P.; Shah, M.; Ravichandran, S.; Park, S.-S.; Iqbal, H.; Choi, S.; Kim, K.K.; Rhee, D.K. Pneumococcal VncR strain-specifically regulates capsule polysaccharide synthesis. Front. Microbiol. 2019, 10, 2279. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-S.; Lee, S.; Rhee, D.-K. Crystal structure of the pneumococcal vancomycin-resistance response regulator DNA-binding domain. Mol. Cells 2021, 44, 179–185. [Google Scholar] [CrossRef]
- Holtje, J.V.; Tomasz, A. Biological effects of lipoteichoic acids. J. Bacteriol. 1975, 124, 1023–1027. [Google Scholar] [CrossRef]
- Höltje, J.-V.; Tomasz, A. Lipoteichoic acid: A specific inhibitor of autolysin activity in pneumococcus. Proc. Natl. Acad. Sci. USA 1975, 72, 1690–1694. [Google Scholar] [CrossRef]
- Heß, N.; Waldow, F.; Kohler, T.P.; Rohde, M.; Kreikemeyer, B.; Gómez-Mejia, A.; Hain, T.; Schwudke, D.; Vollmer, W.; Hammerschmidt, S.; et al. Lipoteichoic acid deficiency permits normal growth but impairs virulence of Streptococcus pneumoniae. Nat. Commun. 2017, 8, 2093. [Google Scholar] [CrossRef]
- Wu, K.; Huang, J.; Zhang, Y.; Xu, W.; Xu, H.; Wang, L.; Cao, J.; Zhang, X.; Yin, Y. A novel protein RafX is important for common cell wall polysaccharide biosynthesis in Streptococcus pneumoniae: Implications for bacterial virulence. J. Bacteriol. 2014, 196, 3324–3334. [Google Scholar] [CrossRef]
- Waks, S.; Tomasz, A. Secretion of cell wall polymers into the growth medium of lysis-defective pneumococci during treatment with penicillin and other inhibitors of cell wall synthesis. Antimicrob. Agents Chemother. 1978, 13, 293–301. [Google Scholar] [CrossRef]
- Hakenbeck, R.; Waks, S.; Tomasz, A. Characterization of cell wall polymers secreted into the growth medium of lysis-defective pneumococci during treatment with penicillin and other inhibitors of cell wall synthesis. Antimicrob. Agents Chemother. 1978, 13, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Granadel, C.; Campo, N.; Hénard, V.; Prudhomme, M.; Claverys, J.-P. Expression and maintenance of ComD–ComE, the two-component signal-transduction system that controls competence of Streptococcus pneumoniae. Mol. Microbiol. 2010, 75, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, K.; Song, G.; Hu, Y.; Chen, J.; Li, T.; Liang, L.; Wu, J.; Xu, H.; Wang, L.; et al. Transcriptional regulation of TacL-mediated lipoteichoic acids biosynthesis by ComE during competence impacts pneumococcal transformation. Front. Cell. Infect. Microbiol. 2024, 14, 1375312. [Google Scholar] [CrossRef] [PubMed]
- Varea, J.; Saiz, J.L.; López-Zumel, C.; Monterroso, B.; Medrano, F.J.; Arrondo, J.L.R.; Iloro, I.; Laynez, J.; García, J.L.; Menéndez, M. Do sequence repeats play an equivalent role in the choline-binding module of pneumococcal LytA amidase? J. Biol. Chem. 2000, 275, 26842–26855. [Google Scholar] [CrossRef]
- Romero, P.; López, R.; García, E. Characterization of LytA-like N-acetylmuramoyl-l-alanine amidases from two new Streptococcus mitis bacteriophages provides insights into the properties of the major pneumococcal autolysin. J. Bacteriol. 2004, 186, 8229–8239. [Google Scholar] [CrossRef] [PubMed]
- Erlendsson, S.; Teilum, K. Binding revisited-avidity in cellular function and signaling. Front. Mol. Biosci. 2020, 7, 615565. [Google Scholar] [CrossRef]
- Romero, P.; López, R.; García, E. Key role of amino acid residues in the dimerization and catalytic activation of the autolysin LytA, an important virulence factor in Streptococcus pneumoniae. J. Biol. Chem. 2007, 282, 17729–17737. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Rojas-Altuve, A.; Zhang, W.; Hesek, D.; Lee, M.; Barbe, S.; André, I.; Ferrer, P.; Silva-Martin, N.; Castro, G.R.; et al. Crystal structures of bacterial peptidoglycan amidase AmpD and an unprecedented activation mechanism. J. Biol. Chem. 2011, 286, 31714–31722. [Google Scholar] [CrossRef]
- García, P.; González, M.P.; García, E.; López, R.; García, J.L. LytB, a novel pneumococcal murein hydrolase essential for cell separation. Mol. Microbiol. 1999, 31, 1275–1277. [Google Scholar] [CrossRef]
- De las Rivas, B.; García, J.L.; López, R.; García, P. Purification and polar localization of pneumococcal LytB, a putative endo-β-N-acetylglucosaminidase: The chain-dispersing murein hydrolase. J. Bacteriol. 2002, 184, 4988–5000. [Google Scholar] [CrossRef]
- Rico-Lastres, P.; Díez-Martínez, R.; Iglesias-Bexiga, M.; Bustamante, N.; Aldridge, C.; Hesek, D.; Lee, M.; Mobashery, S.; Gray, J.; Vollmer, W.; et al. Substrate recognition and catalysis by LytB, a pneumococcal peptidoglycan hydrolase involved in virulence. Sci. Rep. 2015, 5, 16198. [Google Scholar] [CrossRef]
- Martínez-Caballero, S.; Freton, C.; Molina, R.; Bartual, S.G.; Gueguen-Chaignon, V.; Mercy, C.; Gago, F.; Mahasenan, K.V.; Muñoz, I.G.; Lee, M.; et al. Molecular basis of the final step of cell division in Streptococcus pneumoniae. Cell Rep. 2023, 42, 112756. [Google Scholar] [CrossRef]
- Mellroth, P.; Daniels, R.; Eberhardt, A.; Rönnlund, D.; Blom, H.; Widengren, J.; Normark, S.; Henriques-Normark, B. LytA, the major autolysin of Streptococcus pneumoniae, requires access to the nascent peptidoglycan. J. Biol. Chem. 2012, 287, 11018–11029. [Google Scholar] [CrossRef] [PubMed]
- Di Guilmi, A.M.; Bonnet, J.; Peiβert, S.; Durmort, C.; Gallet, B.; Vernet, T.; Gisch, N.; Wong, Y.-S. Specific and spatial labeling of choline-containing teichoic acids in Streptococcus pneumoniae by click chemistry. Chem. Commun. 2017, 53, 10572–10575. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.; Durmort, C.; Mortier-Barrière, I.; Campo, N.; Jacq, M.; Moriscot, C.; Straume, D.; Berg, K.H.; Håvarstein, L.; Wong, Y.S.; et al. Nascent teichoic acids insertion into the cell wall directs the localization and activity of the major pneumococcal autolysin LytA. Cell Surf. 2018, 2, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Briles, E.B.; Tomasz, A. Radioautographic evidence for equatorial wall growth in a Gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J. Cell Biol. 1970, 47, 786–790. [Google Scholar] [CrossRef]
- Lopez, R.; Garcia, E.; Garcia, P.; Ronda, C.; Tomasz, A. Choline-containing bacteriophage receptors in Streptococcus pneumoniae. J. Bacteriol. 1982, 151, 1581–1590. [Google Scholar] [CrossRef]
- Llull, D.; López, R.; García, E. Genetic bases and medical relevance of capsular polysaccharide biosynthesis in pathogenic streptococci. Curr. Mol. Med. 2001, 1, 475–491. [Google Scholar] [CrossRef]
- Yother, J. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu. Rev. Microbiol. 2011, 65, 563–581. [Google Scholar] [CrossRef]
- Paton, J.C.; Trappetti, C. Streptococcus pneumoniae capsular polysaccharide. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Kawai, Y.; Marles-Wright, J.; Cleverley, R.M.; Emmins, R.; Ishikawa, S.; Kuwano, M.; Heinz, N.; Bui, N.K.; Hoyland, C.N.; Ogasawara, N.; et al. A widespread family of bacterial cell wall assembly proteins. EMBO J. 2011, 30, 4931–4941. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, C.; Hager, F.F.; Schäffer, C. LytR-CpsA-Psr glycopolymer transferases: Essential bricks in Gram-positive bacterial cell wall assembly. Int. J. Mol. Sci. 2021, 22, 908. [Google Scholar] [CrossRef] [PubMed]
- Briggs, N.S.; Bruce, K.E.; Naskar, S.; Winkler, M.E.; Roper, D.I. The pneumococcal divisome: Dynamic control of Streptococcus pneumoniae cell division. Front. Microbiol. 2021, 12, 737396. [Google Scholar] [CrossRef]
- Nakamoto, R.; Bamyaci, S.; Blomqvist, K.; Normark, S.; Henriques-Normark, B.; Sham, L.-T. The divisome but not the elongasome organizes capsule synthesis in Streptococcus pneumoniae. Nat. Commun. 2023, 14, 3170. [Google Scholar] [CrossRef] [PubMed]
- Johnsborg, O.; Håvarstein, L.S. Pneumococcal LytR, a protein from the LytR-CpsA-Psr family, is essential for normal septum formation in Streptococcus pneumoniae. J. Bacteriol. 2009, 191, 5859–5864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, W.; Zhang, J.; Shu, Z.; Yin, Y.; Zhang, X.; Wu, K. Pneumococcal LytR protein is required for the surface attachment of both capsular polysaccharide and teichoic acids: Essential for pneumococcal virulence. Front. Microbiol. 2018, 9, 1199. [Google Scholar] [CrossRef]
- Eberhardt, A.; Hoyland, C.N.; Vollmer, D.; Bisle, S.; Cleverley, R.M.; Johnsborg, O.; Håvarstein, L.S.; Lewis, R.J.; Vollmer, W. Attachment of capsular polysaccharide to the cell wall in Streptococcus pneumoniae. Microb. Drug Resist. 2012, 18, 240–255. [Google Scholar] [CrossRef]
- Battaje, R.R.; Piyush, R.; Pratap, V.; Panda, D. Models versus pathogens: How conserved is the FtsZ in bacteria? Biosci. Rep. 2023, 43, BSR20221664. [Google Scholar] [CrossRef]
- Perez, A.J.; Villicana, J.B.; Tsui, H.-C.T.; Danforth, M.L.; Benedet, M.; Massidda, O.; Winkler, M.E. FtsZ-ring regulation and cell division are mediated by essential EzrA and accessory proteins ZapA and ZapJ in Streptococcus pneumoniae. Front. Microbiol. 2021, 12, 780864. [Google Scholar] [CrossRef]
- Flores-Kim, J.; Dobihal, G.S.; Bernhardt, T.G.; Rudner, D.Z. WhyD tailors surface polymers to prevent premature bacteriolysis and direct cell elongation in Streptococcus pneumoniae. eLife 2022, 11, e76392. [Google Scholar] [CrossRef]
- van der Linden, M.; Al-Lahham, A.; Nicklas, W.; Reinert, R.R. Molecular characterization of pneumococcal isolates from pets and laboratory animals. PLoS ONE 2009, 4, e8286. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.E.; Sweeney, C.R. Isolation of Streptococcus pneumoniae type 3 from equine species. J. Clin. Microbiol. 1984, 20, 1028–1030. [Google Scholar] [CrossRef]
- Timoney, J.F. The pathogenic equine streptococci. Vet. Res. 2004, 35, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Leider, M.; Leendertz, F.; Bergmann, C.; Boesch, C.; Schenk, S.; Pauli, G.; Ellerbrok, H.; Hakenbeck, R. New Streptococcus pneumoniae clones in deceased wild chimpanzees. J. Bacteriol. 2007, 189, 6085–6088. [Google Scholar] [CrossRef] [PubMed]
- Denapaite, D.; Hakenbeck, R. A new variant of the capsule 3 cluster occurs in Streptococcus pneumoniae from deceased wild chimpanzees. PLoS ONE 2011, 6, e25119. [Google Scholar] [CrossRef]
- Köndgen, S.; Calvignac-Spencer, S.; Grützmacher, K.; Keil, V.; Mätz-Rensing, K.; Nowak, K.; Metzger, S.; Kiyang, J.; Lübke-Becker, A.; Deschner, T.; et al. Evidence for human Streptococcus pneumoniae in wild and captive chimpanzees: A potential threat to wild populations. Sci. Rep. 2017, 7, 14581. [Google Scholar] [CrossRef]
- Dunay, E.; Apakupakul, K.; Leard, S.; Palmer, J.L.; Deem, S.L. Pathogen transmission from humans to great apes is a growing threat to primate conservation. Ecohealth 2018, 15, 148–162. [Google Scholar] [CrossRef]
- Grützmacher, K.S.; Keil, V.; Metzger, S.; Wittiger, L.; Herbinger, I.; Calvignac-Spencer, S.; Mätz-Rensing, K.; Haggis, O.; Savary, L.; Köndgen, S.; et al. Human respiratory syncytial virus and Streptococcus pneumoniae infection in wild bonobos. Ecohealth 2018, 15, 462–466. [Google Scholar] [CrossRef]
- Chiavolini, D.; Pozzi, G.; Ricci, S. Animal models of Streptococcus pneumoniae disease. Clin. Microbiol. Rev. 2008, 21, 666–685. [Google Scholar] [CrossRef]
- Borsa, N.; Di Pasquale, M.D.; Restrepo, M.I. Animal models of pneumococcal pneumonia. Int. J. Mol. Sci. 2019, 20, 4220. [Google Scholar] [CrossRef]
- Saralahti, A.K.; Harjula, S.-K.E.; Rantapero, T.; Uusi-Mäkelä, M.I.E.; Kaasinen, M.; Junno, M.; Piippo, H.; Nykter, M.; Lohi, O.; Rounioja, S.; et al. Characterization of the innate immune response to Streptococcus pneumoniae infection in zebrafish. PLoS Genet. 2023, 19, e1010586. [Google Scholar] [CrossRef] [PubMed]
- Balde, A.; Ramya, C.S.; Nazeer, R.A. A review on current advancement in zebrafish models to study chronic inflammatory diseases and their therapeutic targets. Heliyon 2024, 10, e31862. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.M.; Lock, R.A.; Hansman, D.; Paton, J.C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 1989, 57, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.M.; Paton, J.C.; Hansman, D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb. Pathog. 1992, 12, 87–93. [Google Scholar] [CrossRef]
- Berry, A.M.; Paton, J.C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 2000, 68, 133–140. [Google Scholar] [CrossRef]
- Canvin, J.R.; Marvin, A.P.; Sivakumaran, M.; Paton, J.C.; Boulnois, G.J.; Andrew, P.W.; Mitchell, T.J. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 1995, 172, 119–123. [Google Scholar] [CrossRef]
- Balachandran, P.; Hollingshead, S.K.; Paton, J.C.; Briles, D.E. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 2001, 183, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, A.; Moreillon, P.; Pozzi, G. Insertional inactivation of the major autolysin gene of Streptococcus pneumoniae. J. Bacteriol. 1988, 170, 5931–5934. [Google Scholar] [CrossRef]
- Benton, K.A.; Paton, J.C.; Briles, D.E. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect. Immun. 1997, 65, 1237–1244. [Google Scholar] [CrossRef]
- Ramos-Sevillano, E.; Rodríguez-Sosa, C.; Díez-Martínez, R.; Giménez, M.-J.; Olmedillas, E.; García, P.; García, E.; Aguilar, L.; Yuste, J. Macrolides and β-lactam antibiotics enhance C3b deposition on the surface of multidrug-resistant Streptococcus pneumoniae strains by a LytA autolysin-dependent mechanism. Antimicrob. Agents Chemother. 2012, 56, 5534–5540. [Google Scholar] [CrossRef]
- Ramos-Sevillano, E.; Urzainqui, A.; Campuzano, S.; Moscoso, M.; González-Camacho, F.; Domenech, M.; Rodríguez de Córdoba, S.; Sánchez-Madrid, F.; Brown, J.S.; García, E.; et al. Pleiotropic effects of the cell wall amidase LytA on Streptococcus pneumoniae sensitivity to the host immune response. Infect. Immun. 2015, 83, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, C.J.; Gao, G.; Francis, K.P.; Yu, J.; Tuomanen, E.I. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 2004, 190, 1661–1669. [Google Scholar] [CrossRef]
- Hirst, R.A.; Gosai, B.; Rutman, A.; Guerin, C.J.; Nicotera, P.; Andrew, P.W.; O’Callaghan, C. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J. Infect. Dis. 2008, 197, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Saralahti, A.; Piippo, H.; Parikka, M.; Henriques-Normark, B.; Rämet, M.; Rounioja, S. Adult zebrafish model for pneumococcal pathogenesis. Dev. Comp. Immunol. 2014, 42, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Austrian, R.; Sreenivasan, P.K.; Masure, H.R. Phase variation in pneumococcal opacity: Relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 1994, 62, 2582–2589. [Google Scholar] [CrossRef]
- Ng, E.W.M.; Costa, J.R.; Samiy, N.; Ruoff, K.L.; Connolly, E.; Cousins, F.V.; D’Amico, D.J. Contribution of pneumolysin and autolysin to the pathogenesis of experimental pneumococcal endophthalmitis. Retina 2002, 22, 622–632. [Google Scholar] [CrossRef]
- Andre, G.O.; Converso, T.R.; Politano, W.R.; Ferraz, L.F.C.; Ribeiro, M.L.; Leite, L.C.C.; Darrieux, M. Role of Streptococcus pneumoniae proteins in evasion of complement-mediated immunity. Front. Microbiol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Domenech, M.; Sempere, J.; de Miguel, S.; Yuste, J. Combination of antibodies and antibiotics as a promising strategy against multidrug-resistant pathogens of the respiratory tract. Front. Immunol. 2018, 9, 2700. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.; Noursadeghi, M.; Brown, J.S. Streptococcus pneumoniae interactions with the complement system. Front. Cell. Infect. Microbiol. 2022, 12, 929483. [Google Scholar] [CrossRef]
- Somers, W.S.; Tang, J.; Shaw, G.D.; Camphausen, R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLeX and PSGL-1. Cell 2000, 103, 467–479. [Google Scholar] [CrossRef]
- Ramos-Sevillano, E.; Urzainqui, A.; de Andrés, B.; González-Tajuelo, R.; Domenech, M.; González-Camacho, F.; Sánchez-Madrid, F.; Brown, J.S.; García, E.; Yuste, J. PSGL-1 on leukocytes is a critical component of the host immune response against invasive pneumococcal disease. PLoS Pathog. 2016, 12, e1005500. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; Dalziel, C.E. The biology of pneumolysin. Subcell. Biochem. 2014, 80, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Cima Cabal, M.D.; Molina, F.; López-Sánchez, J.I.; Pérez-Santín, E.; García-Suárez, M.d.M. Pneumolysin as a target for new therapies against pneumococcal infections: A systematic review. PLoS ONE 2023, 18, e0282970. [Google Scholar] [CrossRef]
- Anderson, R.; Feldman, C. The global burden of community-acquired pneumonia in adults, encompassing invasive pneumococcal disease and the prevalence of its associated cardiovascular events, with a focus on pneumolysin and macrolide antibiotics in pathogenesis and therapy. Int. J. Mol. Sci 2023, 24, 11038. [Google Scholar] [CrossRef] [PubMed]
- Sanford, T.C.; Tweten, R.K.; Abrahamsen, H.L. Bacterial cholesterol-dependent cytolysins and their interaction with the human immune response. Curr. Opin. Infect. Dis. 2024, 37, 164–169. [Google Scholar] [CrossRef]
- Martner, A.; Dahlgren, C.; Paton, J.C.; Wold, A.E. Pneumolysin released during Streptococcus pneumoniae autolysis is a potent activator of intracellular oxygen radical production in neutrophils. Infect. Immun. 2008, 76, 4079–4087. [Google Scholar] [CrossRef]
- Jacques, L.C.; Panagiotou, S.; Baltazar, M.; Senghore, M.; Khandaker, S.; Xu, R.; Bricio-Moreno, L.; Yang, M.; Dowson, C.G.; Everett, D.B.; et al. Increased pathogenicity of pneumococcal serotype 1 is driven by rapid autolysis and release of pneumolysin. Nat. Commun. 2020, 11, 1892. [Google Scholar] [CrossRef]
- Price, K.E.; Greene, N.G.; Camilli, A. Export requirements of pneumolysin in Streptococcus pneumoniae. J. Bacteriol. 2012, 194, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.C.; Dabbs, R.C.; Oswalt, K.L.; Brown, L.R.; Rosch, J.W.; Seo, K.S.; Donaldson, J.R.; McDaniel, L.S.; Thornton, J.A. Pyruvate oxidase of Streptococcus pneumoniae contributes to pneumolysin release. BMC Microbiol. 2016, 16, 271. [Google Scholar] [CrossRef]
- Bazant, J.; Ott, B.; Hudel, M.; Hain, T.; Lucas, R.; Mraheil, M.A. Impact of endogenous pneumococcal hydrogen peroxide on the activity and release of pneumolysin. Toxins 2023, 15, 593. [Google Scholar] [CrossRef]
- Rigel, N.W.; Braunstein, M. A new twist on an old pathway-accessory Sec systems. Mol. Microbiol. 2008, 69, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.; Bensing, B.; Sullam, P.M. The two distinct types of SecA2-dependent export systems. Microbiol. Spectr. 2019, 7, 3. [Google Scholar] [CrossRef]
- Bandara, M.; Skehel, J.M.; Kadioglu, A.; Collinson, I.; Nobbs, A.H.; Blocker, A.J.; Jenkinson, H.F. The accessory Sec system (SecY2A2) in Streptococcus pneumoniae is involved in export of pneumolysin toxin, adhesion and biofilm formation. Microbes Infect. 2017, 19, 402–412. [Google Scholar] [CrossRef]
- Zheng, X.; Sheya, X.M.; St. John, A.; Torres, V.J. The major autolysin Atl regulates the virulence of Staphylococcus aureus by controlling the sorting of LukAB. Infect. Immun. 2022, 90, e0005622. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Tong, H.; Wang, J.; Ge, P.; Zhu, L.; Liu, C.; Zhang, J.-r.; Dong, X. A novel aquaporin subfamily imports oxygen and contributes to pneumococcal virulence by controlling the production and release of virulence factors. mBio 2021, 12, e01309-21. [Google Scholar] [CrossRef] [PubMed]
- Satala, D.; Bednarek, A.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. The recruitment and activation of plasminogen by bacteria–The involvement in chronic infection development. Int. J. Mol. Sci. 2023, 24, 10436. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160523. [Google Scholar] [CrossRef]
- Gupta, M.N.; Uversky, V.N. Moonlighting enzymes: When cellular context defines specificity. Cell. Mol. Life Sci. 2023, 80, 130. [Google Scholar] [CrossRef]
- Liu, D.; Bhunia, A.K. Anchorless bacterial moonlighting metabolic enzymes modulate the immune system and contribute to pathogenesis. ACS Infect. Dis. 2024, 10, 2551–2566. [Google Scholar] [CrossRef]
- Rodríguez-Bolaños, M.; Perez-Montfort, R. Medical and veterinary importance of the moonlighting functions of triosephosphate isomerase. Curr. Protein Pept. Sci. 2019, 20, 304–315. [Google Scholar] [CrossRef]
- Hirayama, S.; Domon, H.; Hiyoshi, T.; Isono, T.; Tamura, H.; Sasagawa, K.; Takizawa, F.; Terao, Y. Triosephosphate isomerase of Streptococcus pneumoniae is released extracellularly by autolysis and binds to host plasminogen to promote its activation. FEBS Open Bio. 2022, 12, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Hiyoshi, T.; Yasui, Y.; Domon, H.; Terao, Y. C-terminal lysine residue of pneumococcal triosephosphate isomerase contributes to its binding to host plasminogen. Microorganisms 2023, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Rohde, M.; Hammerschmidt, S. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 2004, 72, 2416–2419. [Google Scholar] [CrossRef] [PubMed]
- Attali, C.; Durmort, C.; Vernet, T.; Di Guilmi, A.M. The interaction of Streptococcus pneumoniae with plasmin mediates transmigration across endothelial and epithelial monolayers by intercellular junction cleavage. Infect. Immun. 2008, 76, 5350–5356. [Google Scholar] [CrossRef]
- Terrasse, R.; Amoroso, A.; Vernet, T.; Di Guilmi, A.M. Streptococcus pneumoniae GAPDH is released by cell lysis and interacts with peptidoglycan. PLoS ONE 2015, 10, e0125377. [Google Scholar] [CrossRef] [PubMed]
- Marquart, M.E. Pathogenicity and virulence of Streptococcus pneumoniae: Cutting to the chase on proteases. Virulence 2021, 12, 766–787. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Sroka, M.; Fulde, M.; Bergmann, S.; Riesbeck, K.; Blom, A.M. Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J. Biol. Chem. 2014, 289, 15833–15844. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Jarocki, V.M.; Charles, I.G.; Djordjevic, S.P. The diverse functional roles of elongation factor Tu (EF-Tu) in microbial pathogenesis. Front. Microbiol. 2019, 10, 2351. [Google Scholar] [CrossRef]
- Wang, G.; Chen, H.; Xia, Y.; Cui, J.; Gu, Z.; Song, Y.; Chen, Y.Q.; Zhang, H.; Chen, W. How are the non-classically secreted bacterial proteins released into the extracellular milieu? Curr. Microbiol. 2013, 67, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Hertweck, C.; Dudda, A.; Hammerschmidt, S.; Skerka, C.; Hallstrom, T.; Zipfel, P.F. Tuf of Streptococcus pneumoniae is a surface displayed human complement regulator binding protein. Mol. Immunol. 2014, 62, 249–264. [Google Scholar] [CrossRef]
- Kietzman, C.C.; Gao, G.; Mann, B.; Myers, L.; Tuomanen, E.I. Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium. Nat. Commun. 2016, 7, 10859. [Google Scholar] [CrossRef] [PubMed]
- Ridyard, K.E.; Overhage, J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Souza Guerra, M.E.; Vieira, B.; Carvalho Thiers Calazans, A.P.; Destro, G.V.; Melo, K.; Rodrigues, E.; Waz, N.T.; Girardello, R.; Darrieux, M.; Converso, T.R. Recent advances in the therapeutic potential of cathelicidins. Front. Microbiol. 2024, 15, 1405760. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Wolff, S.; Hocke, A.; Rosseau, S.; Müller, E.; Rohde, M. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 2005, 73, 4653–4667. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E.; Tomás, J.M.; Bengoechea, J.A. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 2008, 154, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Lu, W. β-Defensins in human innate immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target Ther. 2023, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Beiter, K.; Wartha, F.; Hurwitz, R.; Normark, S.; Zychlinsky, A.; Henriques-Normark, B. The capsule sensitizes Streptococcus pneumoniae to α-defensins human neutrophil proteins 1 to 3. Infect. Immun. 2008, 76, 3710–3716. [Google Scholar] [CrossRef]
- Zahlten, J.; Herta, T.; Kabus, C.; Steinfeldt, M.; Kershaw, O.; García, P.; Hocke, A.C.; Gruber, A.D.; Hübner, R.-H.; Steinicke, R.; et al. Role of pneumococcal autolysin for KLF4 expression and chemokine secretion in lung epithelium. Am. J. Respir. Cell Mol. Biol. 2015, 53, 544–554. [Google Scholar] [CrossRef]
- Zahlten, J.; Kim, Y.-J.; Doehn, J.-M.; Pribyl, T.; Hocke, A.C.; García, P.; Hammerschmidt, S.; Suttorp, N.; Hippenstiel, S.; Hübner, R.-H. Streptococcus pneumoniae-induced oxidative stress in lung epithelial cells depends on pneumococcal autolysis and is reversible by resveratrol. J. Infect. Dis. 2015, 211, 1822–1830. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, J.; Dai, T.; Li, X.; Chen, L.; He, Z.; Guo, M.; Zhao, J.; Xu, L. A review of KLF4 and inflammatory disease: Current status and future perspective. Pharmacol. Res. 2024, 207, 107345. [Google Scholar] [CrossRef] [PubMed]
- Yuce, K.; Ozkan, A.I. The kruppel-like factor (KLF) family, diseases, and physiological events. Gene 2024, 895, 148027. [Google Scholar] [CrossRef] [PubMed]
- Herta, T.; Bhattacharyya, A.; Bollensdorf, C.; Kabus, C.; García, P.; Suttorp, N.; Hippenstiel, S.; Zahlten, J. DNA-release by Streptococcus pneumoniae autolysin LytA induced Krueppel-like factor 4 expression in macrophages. Sci. Rep. 2018, 8, 5723. [Google Scholar] [CrossRef] [PubMed]
- Herta, T.; Bhattacharyya, A.; Rosolowski, M.; Conrad, C.; Gurtner, C.; Gruber, A.D.; Ahnert, P.; Gutbier, B.; Frey, D.; Suttorp, N.; et al. Krueppel-like factor 4 expression in phagocytes regulates early inflammatory response and disease severity in pneumococcal pneumonia. Front. Immunol. 2021, 12, 726135. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Herta, T.; Conrad, C.; Frey, D.; García, P.; Suttorp, N.; Hippenstiel, S.; Zahlten, J. Induction of Krüppel-like factor 4 mediates polymorphonuclear neutrophil activation in Streptococcus pneumoniae infection. Front. Microbiol. 2021, 11, 582070. [Google Scholar] [CrossRef]
- Horn, K.J.; Fulte, S.; Yang, M.; Lorenz, B.P.; Clark, S.E. Neutrophil responsiveness to IL-10 impairs clearance of Streptococcus pneumoniae from the lungs. J. Leukoc. Biol. 2024, 115, 4–15. [Google Scholar] [CrossRef]
- McPeek, M.K.; Gomez, J.C.; Doerschuk, C.M. Neutrophils sing “IL[-10] be seeing you” in the lungs during pneumonia. J. Leukoc. Biol. 2024, 115, 1–3. [Google Scholar] [CrossRef]
- Cortes, P.R.; Piñas, G.E.; Cian, M.B.; Yandar, N.; Echenique, J. Stress-triggered signaling affecting survival or suicide of Streptococcus pneumoniae. Int. J. Med. Microbiol. 2015, 305, 157–169. [Google Scholar] [CrossRef]
- Martín-Galiano, A.J.; Overweg, K.; Ferrándiz, M.J.; Reuter, M.; Wells, J.M.; de la Campa, A.G. Transcriptional analysis of the acid tolerance response in Streptococcus pneumoniae. Microbiology 2005, 151, 3935–3946. [Google Scholar] [CrossRef]
- Piñas, G.E.; Cortes, P.R.; Albarracín Orio, A.G.; Echenique, J. Acidic stress induces autolysis by a CSP-independent ComE pathway in Streptococcus pneumoniae. Microbiology 2008, 154, 1300–1308. [Google Scholar] [CrossRef]
- Piñas, G.E.; Reinoso-Vizcaino, N.M.; Yandar Barahona, N.Y.; Cortes, P.R.; Duran, R.; Badapanda, C.; Rathore, A.; Bichara, D.R.; Cian, M.B.; Olivero, N.B.; et al. Crosstalk between the serine/threonine kinase StkP and the response regulator ComE controls the stress response and intracellular survival of Streptococcus pneumoniae. PLoS Pathog. 2018, 14, e1007118. [Google Scholar] [CrossRef] [PubMed]
- Reinoso-Vizcaíno, N.M.; Cian, M.B.; Cortes, P.R.; Olivero, N.B.; Hernandez-Morfa, M.; Piñas, G.E.; Badapanda, C.; Rathore, A.; Perez, D.R.; Echenique, J. The pneumococcal two-component system SirRH is linked to enhanced intracellular survival of Streptococcus pneumoniae in influenza-infected pulmonary cells. PLoS Pathog. 2020, 16, e1008761. [Google Scholar] [CrossRef]
- Cremelie, E.; Vázquez, R.; Briers, Y. A comparative guide to expression systems for phage lysin production. Essays Biochem. 2024, 68, 645–659. [Google Scholar] [CrossRef]
- Martín-Galiano, A.J.; García, E. Streptococcus pneumoniae: A plethora of temperate bacteriophages with a role in host genome rearrangement. Front. Cell. Infect. Microbiol. 2021, 11, 775402. [Google Scholar] [CrossRef]
- Vázquez, R.; García, E.; García, P. Phage lysins for fighting bacterial respiratory infections: A new generation of antimicrobials. Front. Immunol. 2018, 9, 2252. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Li, M.; Yang, Y.; Lu, S.; Rao, X. Therapeutic potential of bacteriophage endolysins for infections caused by Gram-positive bacteria. J. Biomed. Sci. 2023, 30, 29. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, A.; Waks, S. Mechanism of action of penicillin: Triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc. Natl Acad. Sci. USA 1975, 72, 4162–4166. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerrato, V.; García, P.; del Prado, G.; García, E.; Gracia, M.; Huelves, L.; Ponte, C.; López, R.; Soriano, F. In vitro interactions of LytA, the major pneumococcal autolysin, with two bacteriophage lytic enzymes (Cpl-1 and Pal), cefotaxime and moxifloxacin against antibiotic-susceptible and -resistant Streptococcus pneumoniae strains. J. Antimicrob. Chemother. 2007, 60, 1159–1162. [Google Scholar] [CrossRef]
- Rodríguez-Cerrato, V.; García, P.; Huelves, L.; García, E.; del Prado, G.; Gracia, M.; Ponte, C.; López, R.; Soriano, F. Pneumococcal LytA autolysin, a potent therapeutic agent in experimental peritonitis-sepsis caused by highly β-lactam-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2007, 51, 3371–3373. [Google Scholar] [CrossRef]
- Domenech, M.; García, E.; Moscoso, M. In vitro destruction of Streptococcus pneumoniae biofilms with bacterial and phage peptidoglycan hydrolases. Antimicrob. Agents Chemother. 2011, 55, 4144–4148. [Google Scholar] [CrossRef]
- Díaz, E.; López, R.; García, J.L. EJ-1, a temperate bacteriophage of Streptococcus pneumoniae with a Myoviridae morphotype. J. Bacteriol. 1992, 174, 5516–5525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romero, P.; López, R.; García, E. Genomic organization and molecular analysis of the inducible prophage EJ-1, a mosaic myovirus from an atypical pneumococcus. Virology 2004, 322, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Yusof, M.Y.M.; Hassan, M.A.A.; Lee, V.S.; Isa, D.M.; Sekaran, S.D. In vivo efficacy and molecular docking of designed peptide that exhibits potent antipneumococcal activity and synergises in combination with penicillin. Sci. Rep. 2015, 5, 11886. [Google Scholar] [CrossRef]
- Hernández-Ortiz, N.; Sánchez-Murcia, P.A.; Gil-Campillo, C.; Domenech, M.; Lucena-Agell, D.; Hortigüela, R.; Velázquez, S.; Camarasa, M.J.; Bustamante, N.; de Castro, S.; et al. Design, synthesis and structure-activity relationship (SAR) studies of an unusual class of non-cationic fatty amine-tripeptide conjugates as novel synthetic antimicrobial agents. Front. Pharmacol. 2024, 15, 1428409. [Google Scholar] [CrossRef]
- Tafroji, W.; Margyaningsih, N.I.; Khoeri, M.M.; Paramaiswari, W.T.; Winarti, Y.; Salsabila, K.; Putri, H.F.M.; Siregar, N.C.; Soebandrio, A.; Safari, D. Antibacterial activity of medicinal plants in Indonesia on Streptococcus pneumoniae. PLoS ONE 2022, 17, e0274174. [Google Scholar] [CrossRef]
- Lee, E.-B.; Lee, K. Coptis rhizome extract influence on Streptococcus pneumoniae through autolysin activation. AMB Express 2024, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Reithuber, E.; Nannapaneni, P.; Rzhepishevska, O.; Lindgren Anders, E.G.; Ilchenko, O.; Normark, S.; Almqvist, F.; Henriques-Normark, B.; Mellroth, P. The bactericidal fatty acid mimetic 2CCA-1 selectively targets pneumococcal extracellular polyunsaturated fatty acid metabolism. mBio 2020, 11, e03027-20. [Google Scholar] [CrossRef] [PubMed]
- Lamar, R.V. Chemo-immunological studies on localized infections. Fist paper: Action on the pneumococcus and its experimental infections of combined sodium oleate and antipneumococcal serum. J. Exp. Med. 1911, 13, 1–23. [Google Scholar] [CrossRef][Green Version]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Llull, D.; Rivas, L.; García, E. In vitro bactericidal activity of the antiprotozoal drug miltefosine against Streptococcus pneumoniae and other pathogenic streptococci. Antimicrob. Agents Chemother. 2007, 51, 1844–1848. [Google Scholar] [CrossRef]
- Falk, S.P.; Noah, J.W.; Weisblum, B. Screen for inducers of autolysis in Bacillus subtilis. Antimicrob. Agents Chemother. 2010, 54, 3723–3729. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.J.; Page, E.F.; Smith, M.E.; Calhoun, T.R. Miltefosine impacts small molecule transport in Gram-positive bacteria. RSC Chem. Biol. 2024, 5, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal activity of ceragenin CSA-13 in cell culture and in an animal model of peritoneal infection. Antimicrob. Agents Chemother. 2015, 59, 6274–6282. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, M.; Esteban-Torres, M.; Menéndez, M.; García, E. In vitro bactericidal and bacteriolytic activity of ceragenin CSA-13 against planktonic cultures and biofilms of Streptococcus pneumoniae and other pathogenic streptococci. PLoS ONE 2014, 9, e101037. [Google Scholar] [CrossRef]
- Leszczyńska, K.; Namiot, D.; Byfield, F.J.; Cruz, K.; Żendzian-Piotrowska, M.; Fein, D.E.; Savage, P.B.; Diamond, S.; McCulloch, C.A.; Janmey, P.A.; et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J. Antimicrob. Chemother. 2013, 68, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Oyardi, O.; Savage, P.B.; Guzel, C.B. Effects of ceragenins and antimicrobial peptides on the A549 cell line and an in vitro co-culture model of A549 cells and Pseudomonas aeruginosa. Pathogens 2022, 11, 1044. [Google Scholar] [CrossRef]
- Karasiński, M.; Wnorowska, U.; Durnaś, B.; Król, G.; Daniluk, T.; Skłodowski, K.; Głuszek, K.; Piktel, E.; Okła, S.; Bucki, R. Ceragenins and ceragenin-based core-shell nanosystems as new antibacterial agents against Gram-negative rods causing nosocomial infections. Pathogens 2023, 12, 1346. [Google Scholar] [CrossRef]
- Morales, M.; García, P.; de la Campa, A.G.; Liñares, J.; Ardanuy, C.; García, E. Evidence of localized prophage-host recombination in the lytA gene encoding the major pneumococcal autolysin. J. Bacteriol. 2010, 192, 2624–2632. [Google Scholar] [CrossRef]
- Whatmore, A.M.; Dowson, C.G. The autolysin-encoding gene (lytA) of Streptococcus pneumoniae displays restricted allelic variation despite localized recombination events with genes of pneumococcal bacteriophage encoding cell wall lytic enzymes. Infect. Immun. 1999, 67, 4551–4556. [Google Scholar] [CrossRef]
- Romero, A.; Lopez, R.; Garcia, P. Sequence of the Streptococcus pneumoniae bacteriophage HB-3 amidase reveals high homology with the major host autolysin. J. Bacteriol. 1990, 172, 5064–5070. [Google Scholar] [CrossRef]
- Didelot, X.; Davison, C.; Tallman, S.; de Ste-Croix, M.; Antonio, M.; Oggioni, M.R.; Kwambana-Adams, B.; Freund, F.; Beleza, S. Long-term evolution of Streptococcus mitis and Streptococcus pneumoniae leads to higher genetic diversity within rather than between human populations. PLoS Genet. 2024, 20, e1011317. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee, H.; Wannaadisai, W.; Vietri, J.; Chaiyakunapruk, N. Systematic literature review of cost-effectiveness analyses of adult 15- and 20-valent pneumococcal vaccines. Vaccine 2025, 46, 126656. [Google Scholar] [CrossRef] [PubMed]
- Sempere, J.; Llamosí, M.; del Río Menéndez, I.; López Ruiz, B.; Domenech, M.; González-Camacho, F. Pneumococcal choline-binding proteins involved in virulence as vaccine candidates. Vaccines 2021, 9, 181. [Google Scholar] [CrossRef]
- Silva, P.H.; Vázquez, Y.; Campusano, C.; Retamal-Díaz, A.; Lay, M.K.; Muñoz, C.A.; González, P.A.; Kalergis, A.M.; Bueno, S.M. Non-capsular based immunization approaches to prevent Streptococcus pneumoniae infection. Front. Cell. Infect. Microbiol. 2022, 12, 949469. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, H.; Zhao, S.H.; Yang, X.Y.; Guo, Z. Recent progress in pneumococcal protein vaccines. Front. Immunol. 2023, 14, 1278346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, G.; Wang, X.; Xie, Z.; Gou, J.; Huang, L.; Huang, H.; You, W.; Wang, R.; Yang, Y.; et al. Preliminary evaluation of the safety and immunogenicity of a novel protein-based pneumococcal vaccine in healthy adults aged 18–49: A phase Ia randomized, double blind, placebo-controlled clinical study. Vaccines 2024, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Lock, R.A.; Hansman, D.; Paton, J.C. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb. Pathog. 1992, 12, 137–143. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Lv, Z.Y.; Gan, H.Q.; Xian, M.; Zhang, K.X.; Mai, J.Y.; Yu, X.B.; Wu, Z.D. Intranasal immunization with autolysin (LytA) in mice model induced protection against five prevalent Streptococcus pneumoniae serotypes in China. Immunol. Res. 2011, 51, 108–115. [Google Scholar] [CrossRef]
- Corsini, B.; Aguinagalde, L.; Ruiz, S.; Domenech, M.; Yuste, J. Vaccination with LytA, LytC, or Pce of Streptococcus pneumoniae protects against sepsis by inducing IgGs that activate the complement system. Vaccines 2021, 9, 186. [Google Scholar] [CrossRef]
- Afshar, D.; Rafiee, F.; Kheirandish, M.; Ohadian Moghadam, S.; Azarsa, M. Autolysin (lytA) recombinant protein: A potential target for developing vaccines against pneumococcal infections. Clin. Exp. Vaccine Res. 2020, 9, 76–80. [Google Scholar] [CrossRef]
- van Dalen, R.; Elsherbini, A.M.A.; Harms, M.; Alber, S.; Stemmler, R.; Peschel, A. Secretory IgA impacts the microbiota density in the human nose. Microbiome 2023, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, C.J.; Maus, U.A.; Brown, J.S. Can animal models really teach us anything about pneumonia? Pro. Eur. Respir. J. 2020, 55, 1901539. [Google Scholar] [CrossRef] [PubMed]
- Metersky, M.; Waterer, G. Can animal models really teach us anything about pneumonia? Con. Eur. Respir. J. 2020, 55, 1901525. [Google Scholar] [CrossRef] [PubMed]
- McCool, T.L.; Cate, T.R.; Moy, G.; Weiser, J.N. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 2002, 195, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.; Jambo, K.C.; Gordon, S.B. Experimental human pneumococcal carriage models for vaccine research. Trends Microbiol. 2011, 19, 464–470. [Google Scholar] [CrossRef]
- Doherty, K.; Dula, D.; Chirwa, A.; Nsomba, E.; Nkhoma, V.S.; Toto, N.; Chikaonda, T.; Kamng’ona, R.; Phiri, J.; Reiné, J.; et al. Experimental pneumococcal carriage in people living with HIV in Malawi: The first controlled human infection model in a key at-risk population. Wellcome Open Res. 2024, 9, 2. [Google Scholar] [CrossRef]
- Hazenberg, P.; Robinson, R.E.; Farrar, M.; Solorzano, C.; Hyder-Wright, A.; Liatsikos, K.; Brunning, J.; Fleet, H.; Bettam, A.; Howard, A.; et al. Serotype 3 Experimental Human Pneumococcal Challenge (EHPC) study protocol: Dose ranging and reproducibility in a healthy volunteer population (challenge 3). BMJ Open 2024, 14, e075948. [Google Scholar] [CrossRef]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; Poh, C.L. Development of next generation Streptococcus pneumoniae vaccines conferring broad protection. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).