Role of opuB in Modulating Membrane Vesicle Composition and Function in Streptococcus mutans Under Neutral and Acidic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Preparation of S. mutans MVs
2.3. Characterization of MVs
2.4. Lipidomics Analysis
2.5. Proteomics Method
2.5.1. Sample Preparation and Protein Extraction
2.5.2. Protein Digestion
2.5.3. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.5.4. Data Processing and Protein Identification
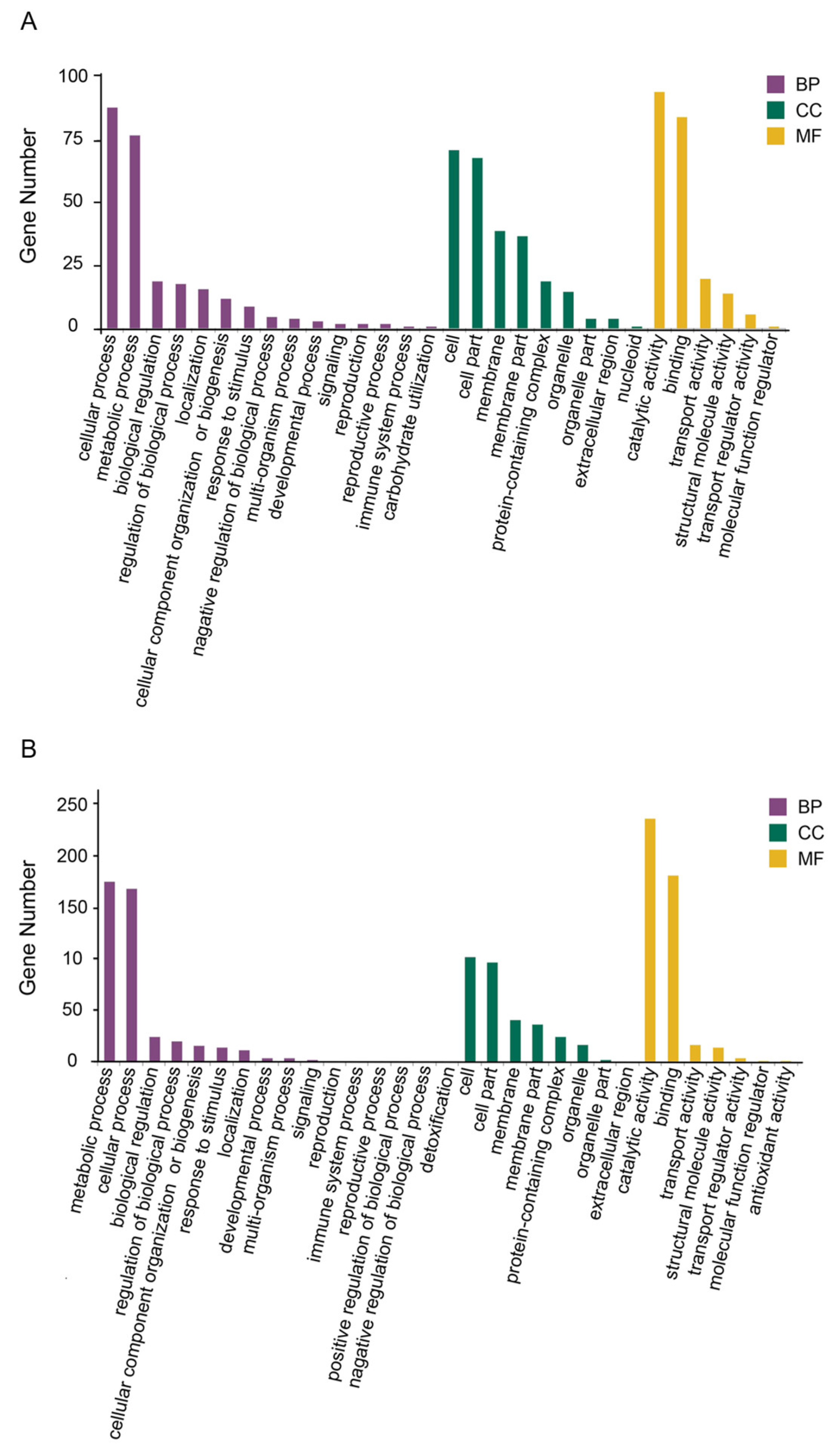
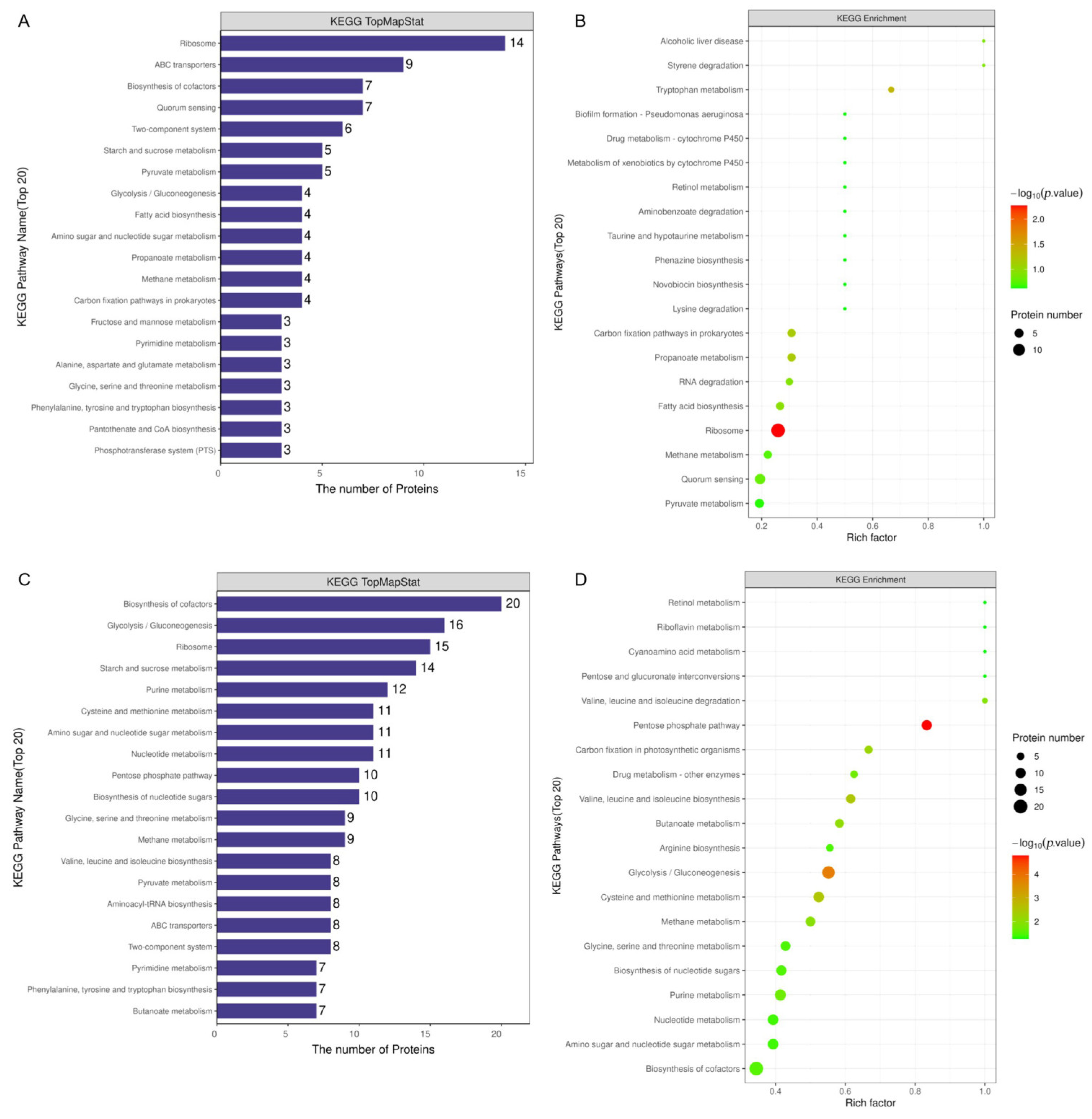
2.5.5. Functional Annotation and Enrichment Analysis
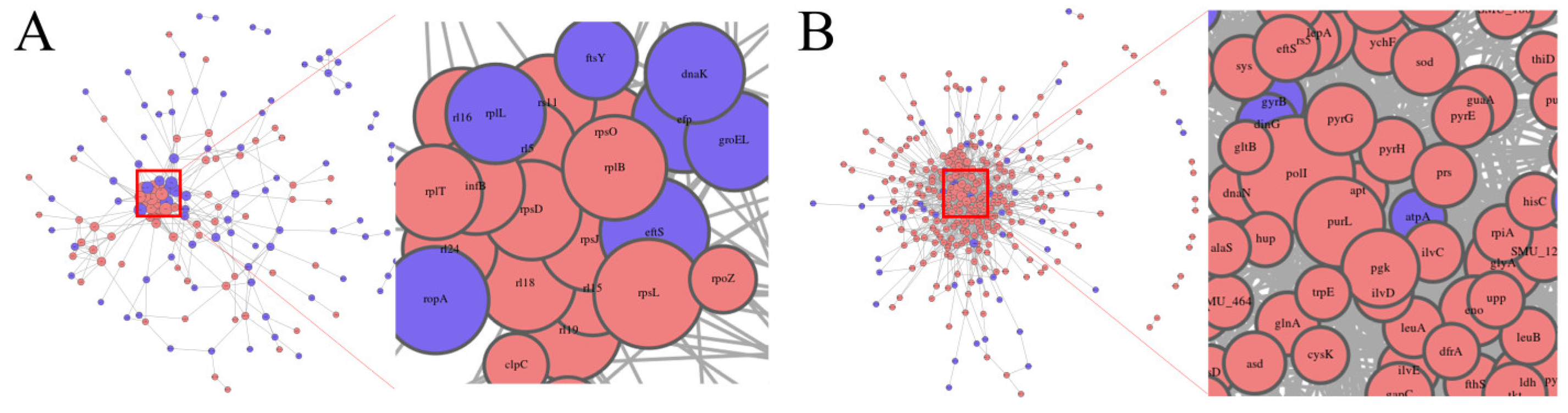
2.5.6. Protein-Protein Interaction Network Analysis
2.6. Statistical Analysis
3. Results
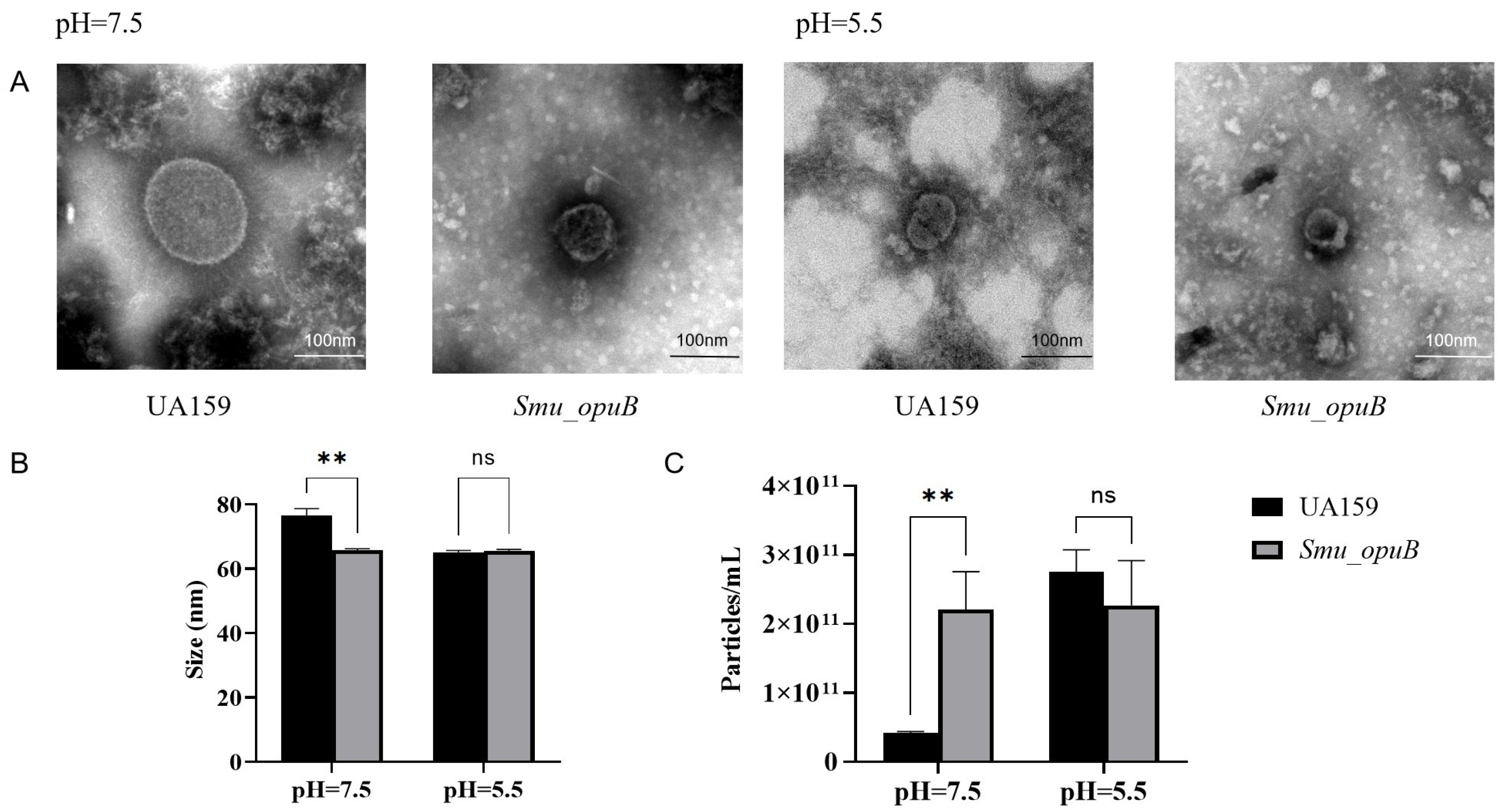
3.1. Characterization of MVs
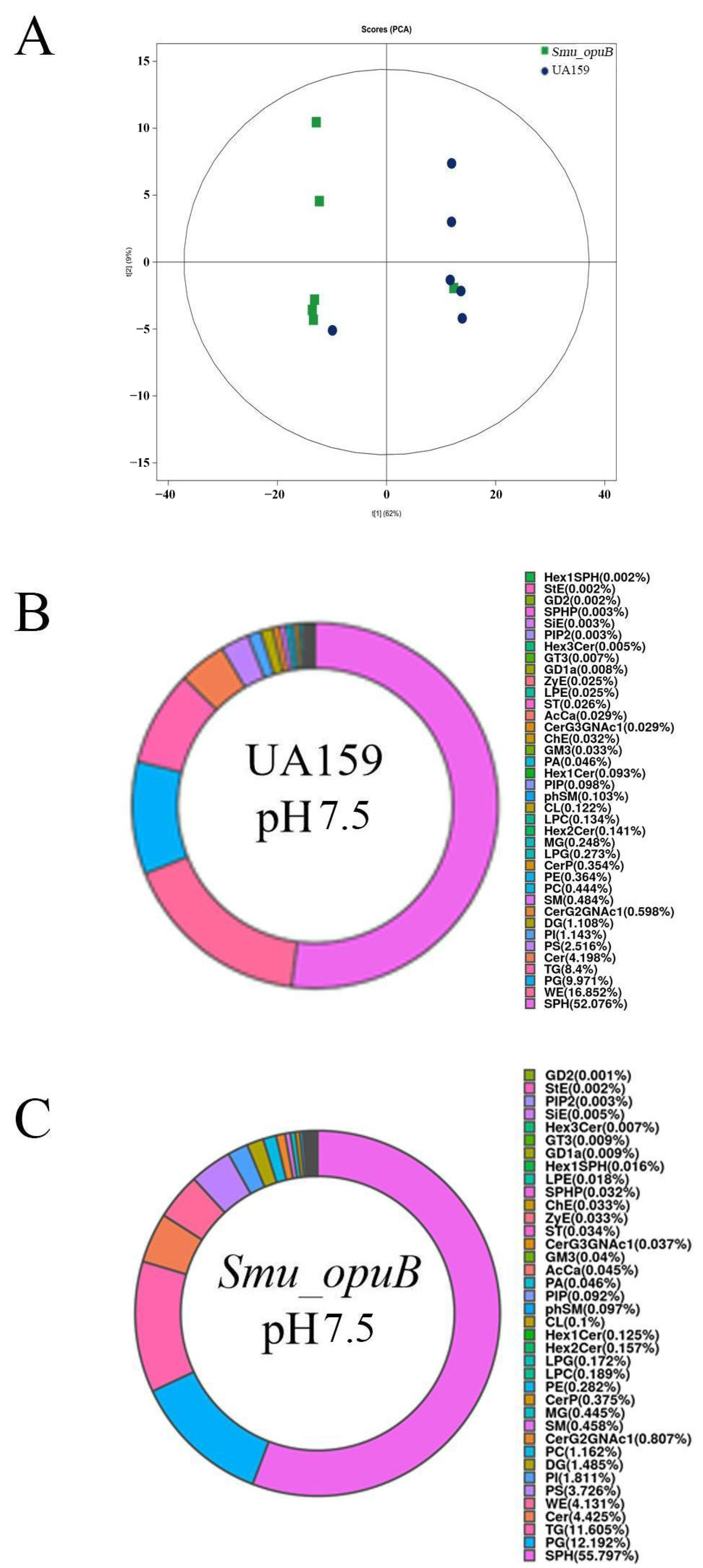
3.2. Lipidomics Analysis of MVs
3.2.1. Lipid Subclass Concentration at pH 7.5
3.2.2. Lipid Subclass Concentrations at pH 5.5
3.3. Proteomics of MVs
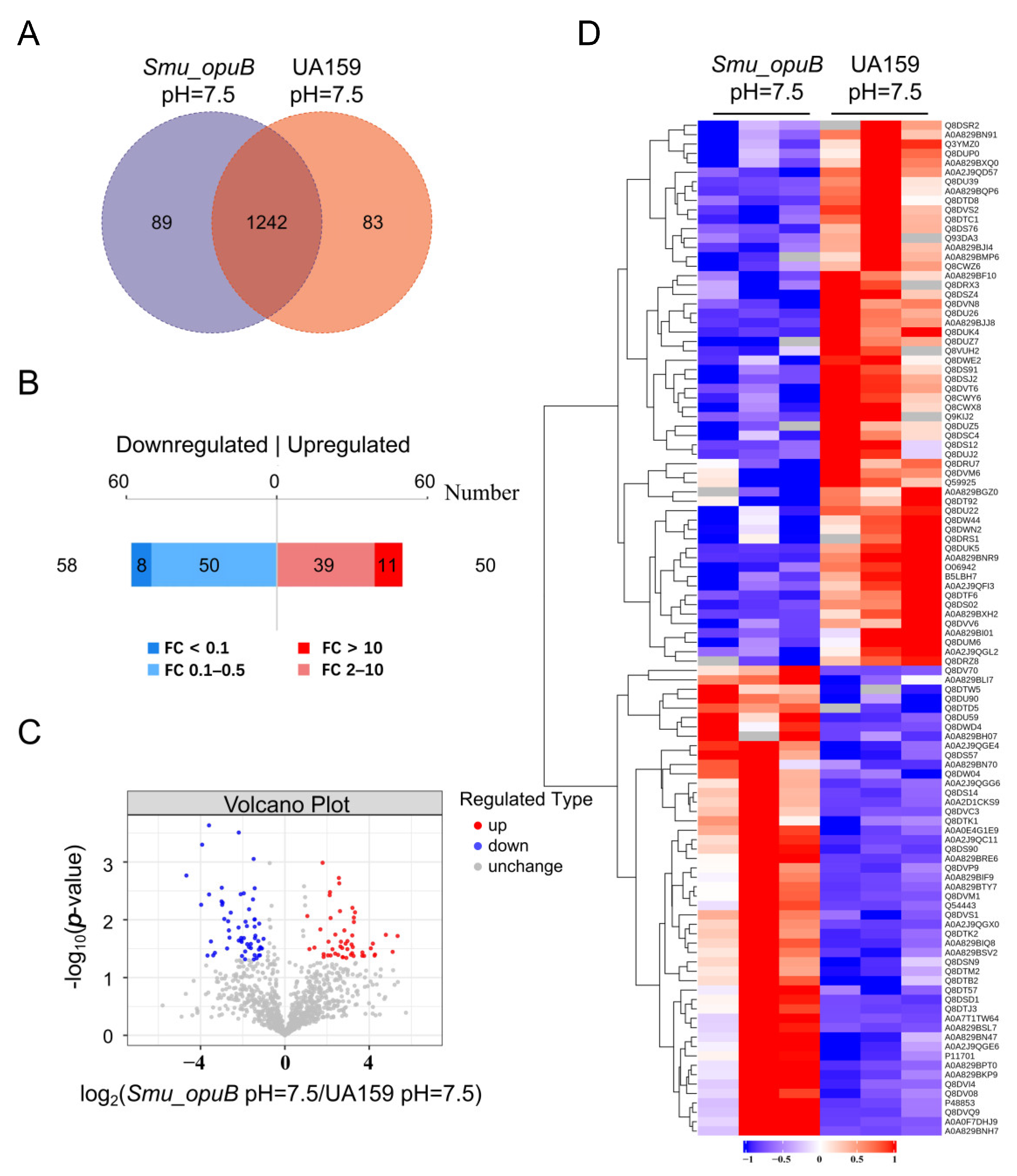
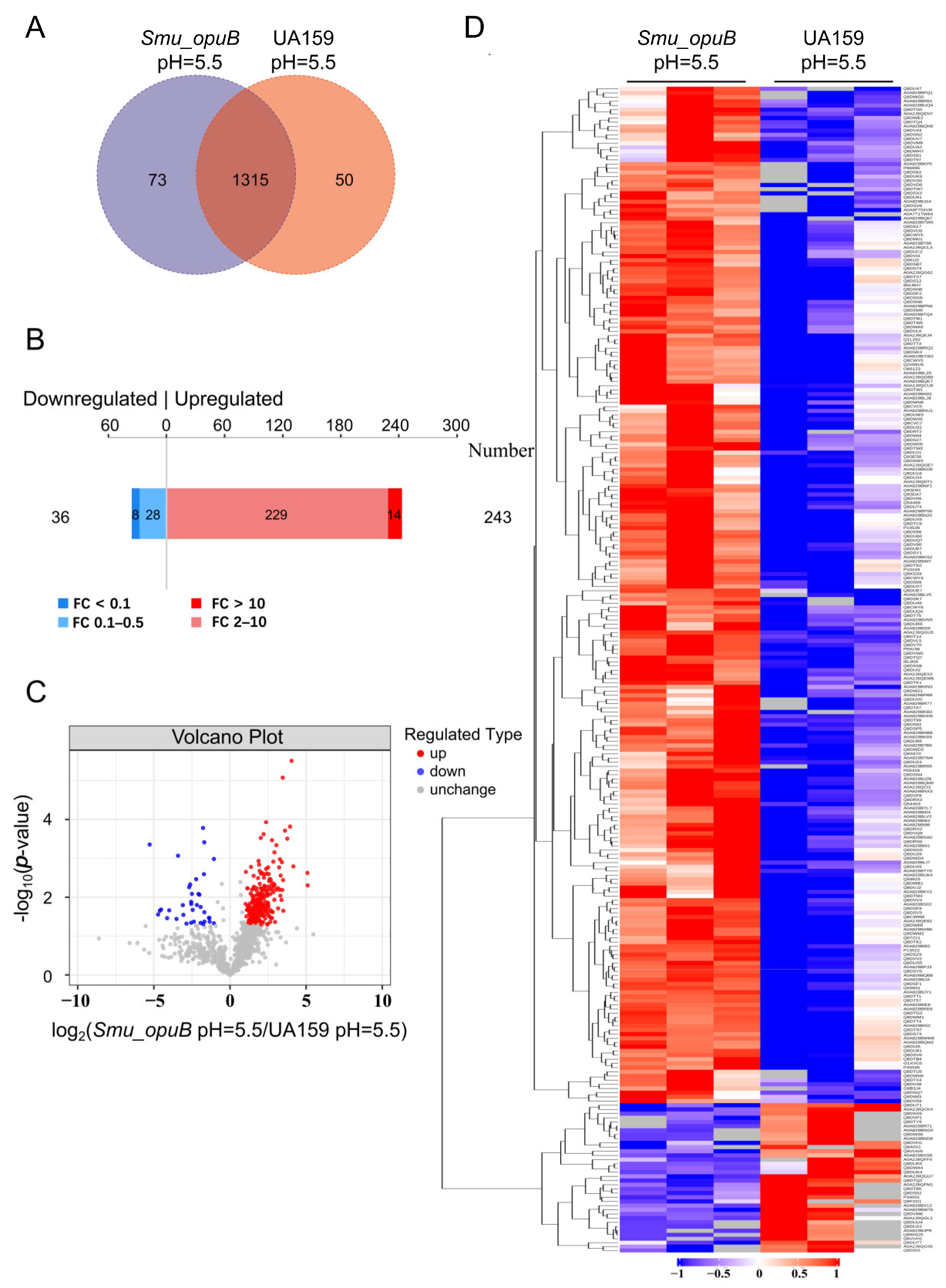
3.3.1. Proteomics Analysis of Differential Protein Expression
3.3.2. Functional Annotation and Pathway Analysis
3.3.3. Protein–Protein Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S. mutans | Streptococcus mutans |
| MVs | Membrane vesicles |
| Smu_opuB | opuB-deficient strain |
| BHI | brain heart infusion |
| TEM | Transmission Electron Microscopy |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| GO | gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | protein–protein interaction |
| PCA | principal component analysis |
| POLI | polymerase I |
| AcCa | acylcarnitine |
| CerG2GNAc1 | dihexosyl-N-acetylhexosyl-ceramideceramide |
| MG | monoglyceride |
| PC | phosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| LPG | Lysophosphatidylglycerol |
| PE | phosphatidylethanolamine |
| GT3 | glycosyltransferase 3 |
References
- Baker, J.L.; Faustoferri, R.C.; Quivey, R.G., Jr. Acid-adaptive mechanisms of Streptococcus mutans-the more we know, the more we don’t. Mol. Oral Microbiol. 2017, 32, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Burne, R.A. A model of efficiency: Stress tolerance by Streptococcus mutans. Microbiology 2008, 154, 3247–3255. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tao, Y.; Yu, L.; Zhuang, P.; Zhi, Q.; Zhou, Y.; Lin, H. Analysis of Small RNAs in Streptococcus mutans under Acid Stress-A New Insight for Caries Research. Int. J. Mol. Sci. 2016, 17, 1529. [Google Scholar] [CrossRef]
- Resch, U.; Tsatsaronis, J.A.; Le Rhun, A.; Stübiger, G.; Rohde, M.; Kasvandik, S.; Holzmeister, S.; Tinnefeld, P.; Wai, S.N.; Charpentier, E. A Two-Component Regulatory System Impacts Extracellular Membrane-Derived Vesicle Production in Group A Streptococcus. mBio 2016, 7, e00207-16. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, Y.; Chen, D.; Wu, R.; Guo, L.; Lin, H. Proteomic and metabolic characterization of membrane vesicles derived from Streptococcus mutans at different pH values. Appl. Microbiol. Biotechnol. 2020, 104, 9733–9748. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef]
- Wu, R.; Tao, Y.; Cao, Y.; Zhou, Y.; Lin, H. Streptococcus mutans Membrane Vesicles Harboring Glucosyltransferases Augment Candida albicans Biofilm Development. Front. Microbiol. 2020, 11, 581184. [Google Scholar] [CrossRef]
- Nakamura, T.; Iwabuchi, Y.; Hirayama, S.; Narisawa, N.; Takenaga, F.; Nakao, R.; Senpuku, H. Roles of membrane vesicles from Streptococcus mutans for the induction of antibodies to glucosyltransferase in mucosal immunity. Microb. Pathog. 2020, 149, 104260. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Wang, X.; Thompson, C.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Pittelkow, M.; Tschapek, B.; Smits, S.H.; Schmitt, L.; Bremer, E. The crystal structure of the substrate-binding protein OpuBC from Bacillus subtilis in complex with choline. J. Mol. Biol. 2011, 411, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P.; Mäder, U.; Dervyn, E.; Rochat, T.; Leduc, A.; Pigeonneau, N.; Bidnenko, E.; Marchadier, E.; Hoebeke, M.; Aymerich, S.; et al. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 2012, 335, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Bremer, E. Guardians in a stressful world: The Opu family of compatible solute transporters from Bacillus subtilis. Biol. Chem. 2017, 398, 193–214. [Google Scholar] [CrossRef]
- Rath, H.; Reder, A.; Hoffmann, T.; Hammer, E.; Seubert, A.; Bremer, E.; Völker, U.; Mäder, U. Management of Osmoprotectant Uptake Hierarchy in Bacillus subtilis via a SigB-Dependent Antisense RNA. Front. Microbiol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Xuan, S.; Xuan, G. Bacterial membrane vesicles: Formation, functions, and roles in bacterial-phage interactions. World J. Microbiol. Biotechnol. 2024, 40, 329. [Google Scholar] [CrossRef]
- Briaud, P.; Carroll, R.K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect. Immun. 2020, 88, e00433-20. [Google Scholar] [CrossRef]
- Mozaheb, N.; Mingeot-Leclercq, M.P. Membrane Vesicle Production as a Bacterial Defense Against Stress. Front. Microbiol. 2020, 11, 600221. [Google Scholar] [CrossRef]
- Wagner, T.; Joshi, B.; Janice, J.; Askarian, F.; Škalko-Basnet, N.; Hagestad, O.C.; Mekhlif, A.; Wai, S.N.; Hegstad, K.; Johannessen, M. Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins. J. Proteom. 2018, 187, 28–38. [Google Scholar] [CrossRef]
- Mladenović, D.; Brealey, J.; Peacock, B.; Koort, K.; Zarovni, N. Quantitative fluorescent nanoparticle tracking analysis and nano-flow cytometry enable advanced characterization of single extracellular vesicles. J. Extracell. Biol. 2025, 4, e70031. [Google Scholar] [CrossRef]
- Iannelli, F.; Lombardi, R.; Costantini, S.; Roca, M.S.; Addi, L.; Bruzzese, F.; Di Gennaro, E.; Budillon, A.; Pucci, B. Integrated proteomics and metabolomics analyses reveal new insights into the antitumor effects of valproic acid plus simvastatin combination in a prostate cancer xenograft model associated with downmodulation of YAP/TAZ signaling. Cancer Cell Int. 2024, 24, 381. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Deng, X.; Tan, M.; Wang, Y.; Hu, J.; Sun, Y.; Yu, C.; Zhang, M.; Jiang, N.; Jiang, R. Effect of resveratrol on the biofilm formation and physiological properties of avian pathogenic Escherichia coli. J. Proteom. 2021, 249, 104357. [Google Scholar] [CrossRef] [PubMed]
- Klimentova, J.; Pavkova, I.; Horcickova, L.; Bavlovic, J.; Kofronova, O.; Benada, O.; Stulik, J. Francisella tularensis subsp. holarctica Releases Differentially Loaded Outer Membrane Vesicles Under Various Stress Conditions. Front. Microbiol. 2019, 10, 2304. [Google Scholar] [CrossRef]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Li, S.; Zuo, X.; Zhou, H.; Gao, Z.; Xia, B. Golgi-derived extracellular vesicle production induced by SARS-CoV-2 envelope protein. Apoptosis 2025, 30, 197–209. [Google Scholar] [CrossRef]
- Yehuda, R.; Dromi, I.; Levin, Y.; Carell, T.; Geacintov, N.; Livneh, Z. Hypoxia-dependent recruitment of error-prone DNA polymerases to genome replication. Oncogene 2025, 44, 42–49. [Google Scholar] [CrossRef]
- Shilkin, E.S.; Petrova, D.V.; Novikova, A.A.; Boldinova, E.O.; Zharkov, D.O.; Makarova, A.V. Methylation and hydroxymethylation of cytosine alter activity and fidelity of translesion DNA polymerases. DNA Repair 2024, 141, 103712. [Google Scholar] [CrossRef]
- Das, A.; Gkoutos, G.V.; Acharjee, A. Analysis of translesion polymerases in colorectal cancer cells following cetuximab treatment: A network perspective. Cancer Med. 2024, 13, e6945. [Google Scholar] [CrossRef]
- Fedorowicz, M.; Halas, A.; Macias, M.; Sledziewska-Gojska, E.; Woodgate, R.; McIntyre, J. E3 ubiquitin ligase RNF2 protects polymerase ι from destabilization. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119743. [Google Scholar] [CrossRef]



| Strain or Plasmid | Description | Source of Reference |
|---|---|---|
| UA159 | S. mutans UA159 | ATCC 700610 |
| Smu_opuB | S. mutans UA159 with in-frame replacement with an erythromycin cassette | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Huang, Y.; Lin, H.; Cao, Y. Role of opuB in Modulating Membrane Vesicle Composition and Function in Streptococcus mutans Under Neutral and Acidic Conditions. Microorganisms 2025, 13, 884. https://doi.org/10.3390/microorganisms13040884
Wang W, Huang Y, Lin H, Cao Y. Role of opuB in Modulating Membrane Vesicle Composition and Function in Streptococcus mutans Under Neutral and Acidic Conditions. Microorganisms. 2025; 13(4):884. https://doi.org/10.3390/microorganisms13040884
Chicago/Turabian StyleWang, Wenyu, Yiyi Huang, Huancai Lin, and Yina Cao. 2025. "Role of opuB in Modulating Membrane Vesicle Composition and Function in Streptococcus mutans Under Neutral and Acidic Conditions" Microorganisms 13, no. 4: 884. https://doi.org/10.3390/microorganisms13040884
APA StyleWang, W., Huang, Y., Lin, H., & Cao, Y. (2025). Role of opuB in Modulating Membrane Vesicle Composition and Function in Streptococcus mutans Under Neutral and Acidic Conditions. Microorganisms, 13(4), 884. https://doi.org/10.3390/microorganisms13040884






