The Role of Vulture (Accipitriformes) Cutaneous Microbiota in Infectious Disease Protection
Abstract
1. Introduction
2. Vultures
2.1. Classification of Vultures
2.2. Morpho-Functional Adaptations of Vultures to Dietary Specialization
2.3. Adaptation to the “Extreme”: From Scavenging to Drastic Temperatures
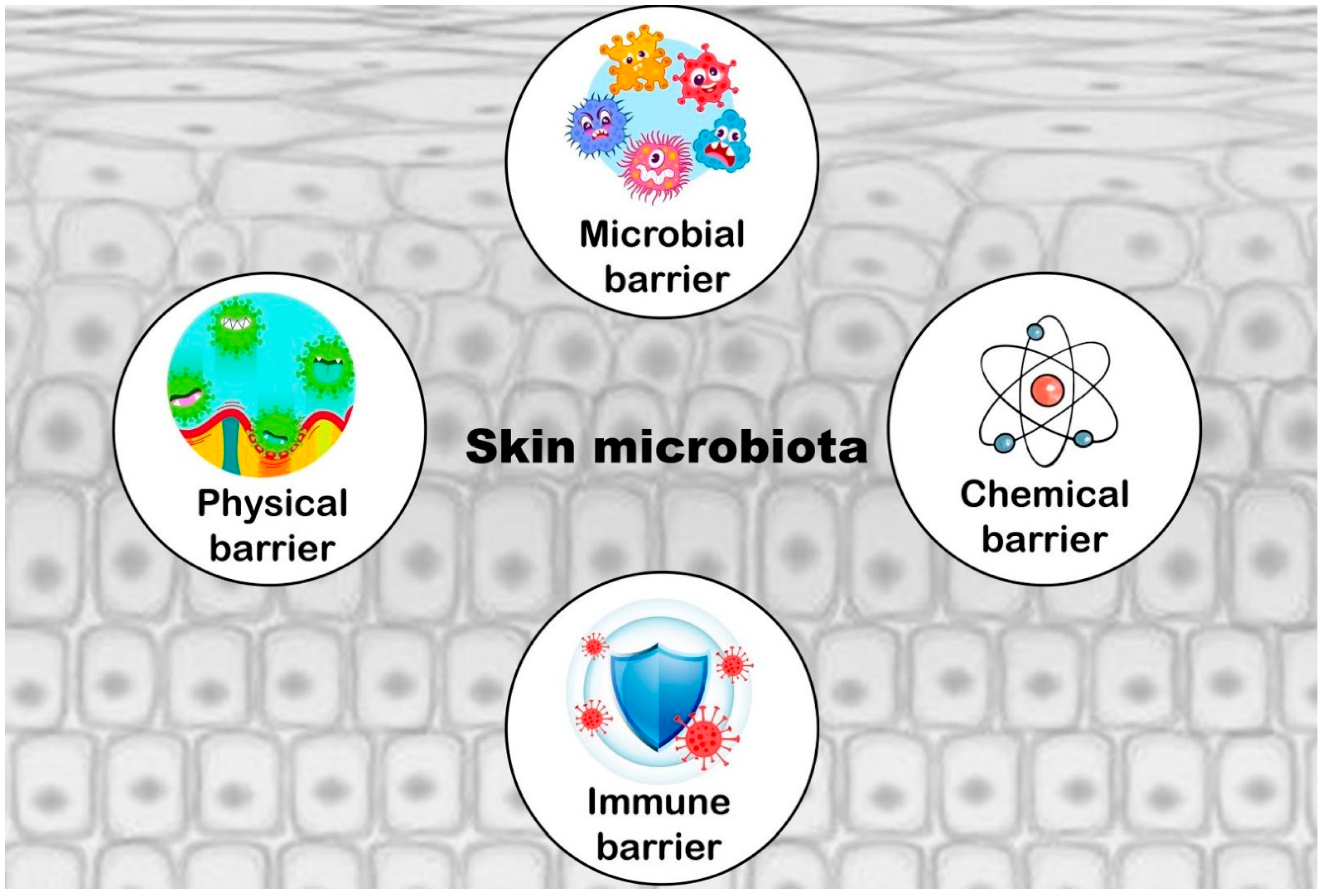
3. Cutaneous Microbiota in Necrophagous Animals
Composition of Cutaneous Microbiota and Main Interfering Variables
4. Role of Cutaneous Microbiota in Relation to Infectious Agents
5. The Role of Vultures in Preventing the Circulation of Infectious Diseases
6. The Role of Vultures in Antimicrobial Resistance Spread
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linde-Medina, M.; Guerra, C.; Alcover, J.A. A Revision of Vulture Feeding Classification. Zoology 2021, 148, 125946. [Google Scholar] [CrossRef] [PubMed]
- Sulzner, K.; Kelly, T.; Smith, W.; Johnson, C.K. Enteric Pathogens and Antimicrobial Resistance in Turkey Vultures (Cathartes Aura) Feeding at The Wildlife–Livestock Interface. J. Zoo Wildl. Med. 2014, 45, 931–934. [Google Scholar] [CrossRef]
- Roggenbuck, M.; Bærholm Schnell, I.; Blom, N.; Bælum, J.; Bertelsen, M.F.; Pontén, T.S.; Sørensen, S.J.; Gilbert, M.T.P.; Graves, G.R.; Hansen, L.H. The Microbiome of New World Vultures. Nat. Commun. 2014, 5, 5498. [Google Scholar] [CrossRef]
- Zepeda Mendoza, M.L.; Roggenbuck, M.; Manzano Vargas, K.; Hansen, L.H.; Brunak, S.; Gilbert, M.T.P.; Sicheritz-Pontén, T. Protective Role of the Vulture Facial Skin and Gut Microbiomes Aid Adaptation to Scavenging. Acta Vet. Scand. 2018, 60, 61. [Google Scholar] [CrossRef]
- Buechley, E.R.; Sekercioglu, C.H. Vultures. Curr. Biol. 2016, 26, R560–R561. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.C. The Ecology of Serengeti Vultures. Ph.D. Thesis, University of Oxford, Oxford, UK, 1972. [Google Scholar]
- Grigg, N.P.; Krilow, J.M.; Gutierrez-Ibanez, C.; Wylie, D.R.; Graves, G.R.; Iwaniuk, A.N. Anatomical Evidence for Scent Guided Foraging in the Turkey Vulture. Sci. Rep. 2017, 7, 17408. [Google Scholar] [CrossRef]
- Chung, O.; Jin, S.; Cho, Y.S.; Lim, J.; Kim, H.; Jho, S.; Kim, H.M.; Jun, J.H.; Lee, H.J.; Chon, A.; et al. The First Whole Genome and Transcriptome of the Cinereous Vulture Reveals Adaptation in the Gastric and Immune Defense Systems and Possible Convergent Evolution between the Old and New World Vultures. Genome Biol. 2015, 16, 215. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, C.; Prevoteau, J.; Duriez, O.; Abourachid, A. Gulper, Ripper and Scrapper: Anatomy of the Neck in Three Species of Vultures. Am. J. Anat. 2020, 236, 701–723. [Google Scholar] [CrossRef]
- Hertel, F. Diversity in Body Size and Feeding Morphology within Past and Present Vulture Assemblages. Ecology 1994, 75, 1074–1084. [Google Scholar] [CrossRef]
- Bildstein, K.L. Social Behavior. In Vultures of the World; Cornell University Press: New York, NY, USA, 2022; pp. 151–156. [Google Scholar] [CrossRef]
- Stepanova, I.; Andreychev, A.; Kulakhmetov, R.; Lobachev, E. Commensals of Underground Mammals: European Mole (Talpa Europaea, Eulipotyphla, Talpidae) and the Greater Mole-Rat (Spalax Microphthalmus, Rodentia, Spalacidae). Biodiversitas J. Biol. Divers. 2021, 22, 4665–4670. [Google Scholar] [CrossRef]
- Chiarore, A.; Bertocci, I.; Fioretti, S.; Meccariello, A.; Saccone, G.; Crocetta, F.; Patti, F.P. Syntopic Cystoseira Taxa Support Different Molluscan Assemblages in the Gulf of Naples (Southern Tyrrhenian Sea). Mar. Freshw. Res. 2019, 70, 1561–1575. [Google Scholar] [CrossRef]
- Lisney, T.J.; Stecyk, K.; Kolominsky, J.; Graves, G.R.; Wylie, D.R.; Iwaniuk, A.N. Comparison of Eye Morphology and Retinal Topography in Two Species of New World Vultures (Aves: Cathartidae). Anat. Rec. 2013, 296, 1954–1970. [Google Scholar] [CrossRef]
- Arad, Z.; Bernstein, M.H. Temperature Regulation in Turkey Vultures. Condor 1988, 90, 913–919. [Google Scholar] [CrossRef]
- Why Is It a Bad Idea to Scare a Vulture? How Stuff Works. Available online: https://animals.howstuffworks.com/birds/vulture-vomit.htm (accessed on 9 October 2024).
- Ward, J.; McCafferty, D.J.; Houston, D.C.; Ruxton, G.D. Why Do Vultures Have Bald Heads? The Role of Postural Adjustment and Bare Skin Areas in Thermoregulation. J. Therm. Biol. 2008, 33, 168–173. [Google Scholar] [CrossRef]
- Szép, T.; Dobránszky, J.; Møller, A.P.; Dyke, G.; Lendvai, Á.Z. Older Birds Have Better Feathers: A Longitudinal Study on the Long-Distance Migratory Sand Martin, Riparia Riparia. PLoS ONE 2019, 14, e0209737. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Geraldo, A.d.M.; Martínez-Burnes, J.; Gómez, J.; Hernández-ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and Function of Feathers, Hair, and Glabrous Skin in the Thermoregulation Strategies of Domestic Animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef]
- Lambertucci, S.A.; Margalida, A.; Speziale, K.L.; Amar, A.; Ballejo, F.; Bildstein, K.L.; Blanco, G.; Botha, A.J.; Bowden, C.G.R.; Cortés-Avizanda, A.; et al. Presumed Killers? Vultures, Stakeholders, Misperceptions, and Fake News. Conserv. Sci. Pract. 2021, 3, e415. [Google Scholar] [CrossRef]
- Ross, A.A.; Rodrigues Hoffmann, A.; Neufeld, J.D. The Skin Microbiome of Vertebrates. Microbiome 2019, 7, 79. [Google Scholar] [CrossRef]
- Graves, G.R.; Matterson, K.O.; Milensky, C.M.; Schmidt, B.K.; O’Mahoney, M.J.V.; Drovetski, S.V. Does Solar Irradiation Drive Community Assembly of Vulture Plumage Microbiotas? Anim. Microbiome 2020, 2, 24. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s Role in Health and Diseases. Environ. Sci. Pollut. Res. 2021, 28, 36967–36983. [Google Scholar] [CrossRef]
- Erin Chen, Y.; Fischbach, M.A.; Belkaid, Y. Skin Microbiota–Host Interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and Maintenance of Skin Barrier Function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Uberoi, A.; Bartow-McKenney, C.; Zheng, Q.; Flowers, L.; Campbell, A.; Knight, S.A.B.; Chan, N.; Wei, M.; Lovins, V.; Bugayev, J.; et al. Commensal Microbiota Regulates Skin Barrier Function and Repair via Signaling through the Aryl Hydrocarbon Receptor. Cell Host Microbe 2021, 29, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Costa, S.K.; Zaramela, L.S.; Khalil, S.; Todd, D.A.; Winter, H.L.; Sanford, J.A.; O’Neill, A.M.; Liggins, M.C.; Nakatsuji, T.; et al. Quorum Sensing between Bacterial Species on the Skin Protects against Epidermal Injury in Atopic Dermatitis. Sci. Transl. Med. 2019, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Paharik, A.E.; Parlet, C.P.; Chung, N.; Todd, D.A.; Rodriguez, E.I.; Van Dyke, M.J.; Cech, N.B.; Horswill, A.R. Coagulase-Negative Staphylococcal Strain Prevents Staphylococcus aureus Colonization and Skin Infection by Blocking Quorum Sensing. Cell Host Microbe 2017, 22, 746–756.e5. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from Human Skin Commensal Bacteria Protect against Staphylococcus aureus and Are Deficient in Atopic Dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Chen, Y.E.; Bouladoux, N.; Hurabielle, C.; Mattke, A.M.; Belkaid, Y.; Fischbach, M.A. Decoding Commensal-Host Communication through Genetic Engineering of Staphylococcus Epidermidis. BioRxiv 2019. [Google Scholar] [CrossRef]
- Latendorf, T.; Gerstel, U.; Wu, Z.; Bartels, J.; Becker, A.; Tholey, A.; Schröder, J.M. Cationic Intrinsically Disordered Antimicrobial Peptides (CIDAMPs) Represent a New Paradigm of Innate Defense with a Potential for Novel Anti-Infectives. Sci. Rep. 2019, 9, 3331. [Google Scholar] [CrossRef]
- Legoux, F.; Bellet, D.; Daviaud, C.; El Morr, Y.; Darbois, A.; Niort, K.; Procopio, E.; Salou, M.; Gilet, J.; Ryffel, B.; et al. Microbial Metabolites Control the Thymic Development of Mucosal-Associated Invariant T Cells. Science 2019, 366, 494–499. [Google Scholar] [CrossRef]
- Lombardo, M.; Feraco, A.; Armani, A.; Camajani, E.; Gorini, S.; Strollo, R.; Padua, E.; Caprio, M.; Bellia, A. Gender Differences in Body Composition, Dietary Patterns, and Physical Activity: Insights from a Cross-Sectional Study. Front. Nutr. 2024, 11, 1414217. [Google Scholar] [CrossRef]
- Bergman, G. Why Are the Wings of Larus f. Fuscus so Dark? Ornis Fenn. 1982, 59, 77–83. [Google Scholar]
- Burtt, E.H.; Ichida, J.M. Occurrence of Feather-Degrading Bacilli in the Plumage of Birds. Auk 1999, 116, 364–372. [Google Scholar] [CrossRef]
- Smith, V.S.; Ford, T.; Johnson, K.P.; Johnson, P.C.D.; Yoshizawa, K.; Light, J.E. Multiple Lineages of Lice Pass through the K–Pg Boundary. Biol. Lett. 2011, 7, 782–785. [Google Scholar] [CrossRef]
- Gupta, R.; Ramnani, P. Microbial Keratinases and Their Prospective Applications: An Overview. Appl. Microbiol. Biotechnol. 2006, 70, 21–33. [Google Scholar] [CrossRef]
- Madigan, M.; Martinko, J.; Parker, J. Brock Biology of Microorganisms; Prentice Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Moyer, B.R.; Wagenbach, G.E. Sunning by Black Noddies (Anous minutus) May Kill Chewing Lice (Quadraceps hopkinsi). Auk 1995, 112, 1073–1077. [Google Scholar] [CrossRef]
- Clark, R.G.; Ohmart, R.D. Spread-Winged Posture of Turkey Vultures: Single or Multiple Function? Condor 1985, 87, 350–355. [Google Scholar] [CrossRef]
- Gill, R.E.; Anderson, D.W.; Braun, C.; Bridge, E.S.; Clark, W.S.; Eichhorst, B.; Evens, J.; Evers, D.C.; Hayes, F.E.; Jaramillo, A.; et al. Identification Guide to North American Birds. Part II: Anatidae to Alcidae. Auk 2011, 128, 184–187. [Google Scholar] [CrossRef]
- Chandler, R.M.; Pyle, P.; Flannery, M.E.; Long, D.J.; Howell, S.N.G. Flight Feather Molt of Turkey Vultures. Wilson J. Ornithol. 2010, 122, 354–360. [Google Scholar] [CrossRef]
- Saranathan, V.; Burtt, E.H. Sunlight on Feathers Inhibits Feather-Degrading Bacteria. Wilson J. Ornithol. 2007, 119, 239–245. [Google Scholar] [CrossRef]
- Koenig, R. Vulture Research Soars as the Scavengers’ Numbers Decline. Science 2006, 312, 1591–1592. [Google Scholar] [CrossRef]
- Margalida, A.; Colomer, M.À. Modelling the Effects of Sanitary Policies on European Vulture Conservation. Sci. Rep. 2012, 2, 753. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, E.J.; Clasener, H.A.L. Colonization Resistance. Antimicrob. Agents Chemother. 1994, 38, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.L.; Xu, Z.Z.; Weiss, S.; Lax, S.; Van Treuren, W.; Hyde, E.R.; Song, S.J.; Amir, A.; Larsen, P.; Sangwan, N.; et al. Microbial Community Assembly and Metabolic Function during Mammalian Corpse Decomposition. Science 2016, 351, 158–162. [Google Scholar] [CrossRef]
- Pawlowski, D.R.; Raslawsky, A.; Siebert, G.; Metzger, D.J.; Koudelka, G.B.; Karalus, R.J. Identification of Hylemonella Gracilis as an Antagonist of Yersinia Pestis Persistence. J. Bioterr. Biodef. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of Biofilm Formation in Pseudomonas fluorescens WCS365 Proceeds via Multiple, Convergent Signalling Pathways: A Genetic Analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- El-Sayed, A.K.; Hothersall, J.; Cooper, S.M.; Stephens, E.; Simpson, T.J.; Thomas, C.M. Characterization of the Mupirocin Biosynthesis Gene Cluster from Pseudomonas fluorescens NCIMB 10586. Chem. Biol. 2003, 10, 419–430. [Google Scholar] [CrossRef]
- Kallimanis, A.; Frillingos, S.; Drainas, C.; Koukkou, A.I. Taxonomic Identification, Phenanthrene Uptake Activity, and Membrane Lipid Alterations of the PAH Degrading Arthrobacter sp. Strain Sphe3. Appl. Microbiol. Biotechnol. 2007, 76, 709–717. [Google Scholar] [CrossRef]
- Williams, J.D.; Jacobson, E.L.; Kim, H.; Kim, M.; Jacobson, M.K. Folate in Skin Cancer Prevention. Subcell. Biochem. 2012, 56, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Berry, C. The Bacterium, Lysinibacillus Sphaericus, as an Insect Pathogen. J. Invertebr. Pathol. 2012, 109, 1–10. [Google Scholar] [CrossRef]
- Vodovar, N.; Vinals, M.; Liehl, P.; Basset, A.; Degrouard, J.; Spellman, P.; Boccard, F.; Lemaitre, B. Drosophila Host Defense after Oral Infection by an Entomopathogenic Pseudomonas Species. Proc. Natl. Acad. Sci. USA 2005, 102, 11414–11419. [Google Scholar] [CrossRef]
- Vodovar, N.; Vallenet, D.; Cruveiller, S.; Rouy, Z.; Barbe, V.; Acosta, C.; Cattolico, L.; Jubin, C.; Lajus, A.; Segurens, B.; et al. Complete Genome Sequence of the Entomopathogenic and Metabolically Versatile Soil Bacterium Pseudomonas entomophila. Nat. Biotechnol. 2006, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Höltzel, A.; Kempter, C.; Metzger, J.W.; Jung, G.; Groth, I.; Fritz, T.; Fiedler, H.P. Spirofungin, a New Antifungal Antibiotic from Streptomyces violaceusniger Tü 4113. J. Antibiot. 1998, 51, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Strap, J.L.; Crawford, D.L. Isolation and Characterization of Potent Antifungal Strains of the Streptomyces violaceusniger Clade Active against Candida albicans. J. Ind. Microbiol. Biotechnol. 2010, 37, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, N.; Oguchi, A.; Ikeda, H.; Ishikawa, J.; Kitani, S.; Watanabe, Y.; Nakamura, S.; Katano, Y.; Kishi, E.; Sasagawa, M.; et al. Genome Sequence of Kitasatospora setae NBRC 14216T: An Evolutionary Snapshot of the Family Streptomycetaceae. DNA Res. 2010, 17, 393–406. [Google Scholar] [CrossRef]
- Wang, X.J.; Yan, Y.J.; Zhang, B.; An, J.; Wang, J.J.; Tian, J.; Jiang, L.; Chen, Y.H.; Huang, S.X.; Yin, M.; et al. Genome Sequence of the Milbemycin-Producing Bacterium Streptomyces bingchenggensis. J. Bacteriol. 2010, 192, 4526–4527. [Google Scholar] [CrossRef]
- Hoshino, T. Violacein and Related Tryptophan Metabolites Produced by Chromobacterium violaceum: Biosynthetic Mechanism and Pathway for Construction of Violacein Core. Appl. Microbiol. Biotechnol. 2011, 91, 1463–1475. [Google Scholar] [CrossRef]
- Hornung, C.; Poehlein, A.; Haack, F.S.; Schmidt, M.; Dierking, K.; Pohlen, A.; Schulenburg, H.; Blokesch, M.; Plener, L.; Jung, K.; et al. The Janthinobacterium sp. HH01 Genome Encodes a Homologue of the V. cholerae CqsA and L. pneumophila LqsA Autoinducer Synthases. PLoS ONE 2013, 8, e55045. [Google Scholar] [CrossRef]
- Jeon, C.O.; Park, W.; Ghiorse, W.C.; Madsen, E.L. Polaromonas naphthalenivorans sp. nov., a Naphthalene-Degrading Bacterium from Naphthalene-Contaminated Sediment. Int. J. Syst. Evol. Microbiol. 2004, 54, 93–97. [Google Scholar] [CrossRef]
- Fontes, G.C.; Ramos, N.M.; Amaral, P.F.F.; Nele, M.; Coelho, M.A.Z. Renewable Resources for Biosurfactant Production by Yarrowia Lipolytica. Braz. J. Chem. Eng. 2012, 29, 483–494. [Google Scholar] [CrossRef]
- Pacheco, G.J.; Ciapina, E.M.P.; de Barros Gomes, E.; Pereira Junior, N. Biosurfactant Production by Rhodococcus Erythropolis and Its Application to Oil Removal. Braz. J. Microbiol. 2010, 41, 685–693. [Google Scholar] [CrossRef]
- Rashel, M.; Uchiyama, J.; Ujihara, T.; Uehara, Y.; Kuramoto, S.; Sugihara, S.; Yagyu, K.I.; Muraoka, A.; Sugai, M.; Hiramatsu, K.; et al. Efficient Elimination of Multidrug-Resistant Staphylococcus aureus by Cloned Lysin Derived from Bacteriophage Phi MR11. J. Infect. Dis. 2007, 196, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Mumm, I.P.; Wood, T.L.; Chamakura, K.R.; Kuty Everett, G.F. Complete Genome of Acinetobacter baumannii Podophage Petty. Genome Announc. 2013, 1, e00850-13. [Google Scholar] [CrossRef]
- Merabishvili, M.; Vandenheuvel, D.; Kropinski, A.M.; Mast, J.; De Vos, D.; Verbeken, G.; Noben, J.P.; Lavigne, R.; Vaneechoutte, M.; Pirnay, J.P. Characterization of Newly Isolated Lytic Bacteriophages Active against Acinetobacter baumannii. PLoS ONE 2014, 9, e104853. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Deora, R.; Doulatov, S.R.; Gingery, M.; Eiserling, F.A.; Preston, A.; Maskell, D.J.; Simons, R.W.; Cotter, P.A.; Parkhill, J.; et al. Reverse Transcriptase-Mediated Tropism Switching in Bordetella Bacteriophage. Science 2002, 295, 2091–2094. [Google Scholar] [CrossRef]
- Straub, D.; Rothballer, M.; Hartmann, A.; Ludewig, U. The Genome of the Endophytic Bacterium, H. Frisingense GSF30T Identifies Diverse Strategies in the Herbaspirillum Genus to Interact with Plants. Front. Microbiol. 2013, 4, 49994. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Bhatnagar, N.B.; Bhatnagar, R. Bacterial Insecticidal Toxins. Crit. Rev. Microbiol. 2004, 30, 33–54. [Google Scholar] [CrossRef]
- Flot, J.F.; Hespeels, B.; Li, X.; Noel, B.; Arkhipova, I.; Danchin, E.G.J.; Hejnol, A.; Henrissat, B.; Koszul, R.; Aury, J.M.; et al. Genomic Evidence for Ameiotic Evolution in the Bdelloid Rotifer Adineta vaga. Nature 2013, 500, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, S.; Nesbakken, T.; Holck, A. Industrial Application of an Antilisterial Strain of Lactobacillus Sakei as a Protective Culture and Its Effect on the Sensory Acceptability of Cooked, Sliced, Vacuum-Packaged Meats. Int. J. Food Microbiol. 2001, 66, 191–196. [Google Scholar] [CrossRef]
- Balmford, A. Pollution, Politics, and Vultures. Science 2013, 339, 653–654. [Google Scholar] [CrossRef]
- Kanaujia, A.; Kushwaha, S. Vulnerable Vultures of India: Population, Ecology and Conservation. Rare Anim. India 2013, 1, 113–144. [Google Scholar] [CrossRef][Green Version]
- O’Bryan, C.J.; Braczkowski, A.R.; Beyer, H.L.; Carter, N.H.; Watson, J.E.M.; McDonald-Madden, E. The Contribution of Predators and Scavengers to Human Well-Being. Nat. Ecol. Evol. 2018, 2, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ogada, D.L.; Keesing, F.; Virani, M.Z. Dropping Dead: Causes and Consequences of Vulture Population Declines Worldwide. Ann. N. Y. Acad Sci. 2012, 1249, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.B.; Ghatak, S.; Banga, H.S.; Gill, J.P.S.; Singh, B. Veterinary Urban Hygiene: A Challenge for India. OIE Rev. Sci. Tech. 2013, 32, 645–656. [Google Scholar] [CrossRef]
- Jalihal, S.; Rana, S.; Sharma, S. Systematic Mapping on the Importance of Vultures in the Indian Public Health Discourse. Environ. Sustain. 2022, 5, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Movalli, P.; Krone, O.; Osborn, D.; Pain, D. Monitoring Contaminants, Emerging Infectious Diseases and Environmental Change with Raptors, and Links to Human Health. Bird. Study 2018, 65, S96–S109. [Google Scholar] [CrossRef]
- Vultures and Their People in India: Equity and Entanglement in a Time of Extinctions—AHR. Available online: https://australianhumanitiesreview.org/2011/05/01/vultures-and-their-people-in-india-equity-and-entanglement-in-a-time-of-extinctions/ (accessed on 12 October 2024).
- Sokolow, S.H.; Nova, N.; Pepin, K.M.; Peel, A.J.; Pulliam, J.R.C.; Manlove, K.; Cross, P.C.; Becker, D.J.; Plowright, R.K.; McCallum, H.; et al. Ecological Interventions to Prevent and Manage Zoonotic Pathogen Spillover. Philos. Trans. R. Soc. B 2019, 374, 20180342. [Google Scholar] [CrossRef]
- Swan, G.; Naidoo, V.; Cuthbert, R.; Green, R.E.; Pain, D.J.; Swarup, D.; Prakash, V.; Taggart, M.; Bekker, L.; Das, D.; et al. Removing the Threat of Diclofenac to Critically Endangered Asian Vultures. PLoS Biol. 2006, 4, e66. [Google Scholar] [CrossRef]
- Plaza, P.I.; Blanco, G.; Lambertucci, S.A. Implications of Bacterial, Viral and Mycotic Microorganisms in Vultures for Wildlife Conservation, Ecosystem Services and Public Health. Ibis 2020, 162, 1109–1124. [Google Scholar] [CrossRef]
- Brookes, V.J.; Gill, G.S.; Singh, B.B.; Sandhu, B.S.; Dhand, N.K.; Aulakh, R.S.; Ward, M.P. Challenges to Human Rabies Elimination Highlighted Following a Rabies Outbreak in Bovines and a Human in Punjab, India. Zoonoses Public Health 2019, 66, 325–336. [Google Scholar] [CrossRef]
- Hensel, M.E.; Negron, M.; Arenas-Gamboa, A.M. Brucellosis in Dogs and Public Health Risk. Emerg Infect Dis 2018, 24, 1401–1406. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the Wild: Antibiotic Resistance Genes in Natural Environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef]
- Chung, D.M.; Ferree, E.; Simon, D.M.; Yeh, P.J. Patterns of Bird-Bacteria Associations. Ecohealth 2018, 15, 627–641. [Google Scholar] [CrossRef]
- Blanco, G.; López-Hernández, I.; Morinha, F.; López-Cerero, L. Intensive Farming as a Source of Bacterial Resistance to Antimicrobial Agents in Sedentary and Migratory Vultures: Implications for Local and Transboundary Spread. Sci. Total Environ. 2020, 739, 140356. [Google Scholar] [CrossRef]
- The Ecology of Carrion Decomposition|Learn Science at Scitable. Available online: https://www.nature.com/scitable/knowledge/library/the-ecology-of-carrion-decomposition-84118259 (accessed on 12 October 2024).
- Phan, N.T.; Kim, K.H.; Jeon, E.C.; Kim, U.H.; Sohn, J.R.; Pandey, S.K. Analysis of Volatile Organic Compounds Released during Food Decaying Processes. Environ. Monit. Assess. 2011, 184, 1683–1692. [Google Scholar] [CrossRef]
- Chen, S.J.; Hsieh, L.T.; Chiu, S.C. Emission of Polycyclic Aromatic Hydrocarbons from Animal Carcass Incinerators. Sci. Total Environ. 2003, 313, 61–76. [Google Scholar] [CrossRef]
- Dhananjayan, V.; Muralidharan, S. Levels of Polycyclic Aromatic Hydrocarbons, Polychlorinated Biphenyls, and Organochlorine Pesticides in Various Tissues of White-Backed Vulture in India. Biomed. Res. Int. 2013, 2013, 190353. [Google Scholar] [CrossRef]
- Paller, G.; Hommel, R.K.; Kleber, H.P. Phenol Degradation by Acinetobacter Calcoaceticus NCIB 8250. J. Basic Microbiol. 1995, 35, 325–335. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Rossetti, C.A.; Arenas-Gamboa, A.M.; Maurizio, E. Caprine Brucellosis: A Historically Neglected Disease with Significant Impact on Public Health. PLoS Negl. Trop. Dis. 2017, 11, e0005692. [Google Scholar] [CrossRef]
- Singh, B.B.; Khatkar, M.S.; Aulakh, R.S.; Gill, J.P.S.; Dhand, N.K. Estimation of the Health and Economic Burden of Human Brucellosis in India. Prev. Vet. Med. 2018, 154, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Markandya, A.; Taylor, T.; Longo, A.; Murty, M.N.; Murty, S.; Dhavala, K. Counting the Cost of Vulture Decline—An Appraisal of the Human Health and Other Benefits of Vultures in India. Ecol. Econ. 2008, 67, 194–204. [Google Scholar] [CrossRef]
- Haagsma, J. Pathogenic Anaerobic Bacteria and the Environment. Rev. Sci. Tech. 1991, 10, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Speer, B.L. Current Therapy in Avian Medicine and Surgery; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; pp. 1–794. [Google Scholar] [CrossRef]
- Blanco, G.; Junza, A.; Segarra, D.; Barbosa, J.; Barrón, D. Wildlife Contamination with Fluoroquinolones from Livestock: Widespread Occurrence of Enrofloxacin and Marbofloxacin in Vultures. Chemosphere 2016, 144, 1536–1543. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial Food Animal Production, Antimicrobial Resistance, and Human Health. Annu. Rev. Public. Health 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.-B.; Zeng, Z.-L.; Yang, X.-W.; Huang, Y.; Liu, J.-H.; Wang, J.; Ma, Z.-B.; Zeng, Z.-L.; Yang, X.-W.; et al. The Role of Wildlife (Wild Birds) in the Global Transmission of Antimicrobial Resistance Genes. Zool. Res. 2017, 38, 55–80. [Google Scholar] [CrossRef]
- Dolejska, M.; Papagiannitsis, C.C. Plasmid-Mediated Resistance Is Going Wild. Plasmid 2018, 99, 99–111. [Google Scholar] [CrossRef]


| Microorganisms | Health-Promoting Activity | Protective Mechanism (s) | Reference |
|---|---|---|---|
| Hylemonella gracilis | Yersinia pestis control | Prevent colonization | [48] |
| Pseudomonas fluorescens | Control of multiple pathogenic bacteria | Production of antibiotic (mupirocin), formation of protective biofilm | [49,50] |
| Arthrobacter phenanthrenivorans | Irritating substances (phenanthrene) | Degradation of phenanthrene (skin-irritating polycyclic aromatic hydrocarbon) | [51] |
| Acinetobacter sp. NIPH 899 | Folate biosynthesis (potential role in skin cancer prevention) | - | [52] |
| Lysinibacillus sphaericus | Mosquito larvae | Production of insecticidal toxins (sphaericolysin) | [53] |
| Pseudomonas entomophila | Insects (fly larvae and adults) | Infection and lethality in insects, production of insecticidal toxin (SepC/Tcc class) | [54,55] |
| Streptomyces violaceusniger | Control of fungal pathogens | Antifungal activity | [56,57] |
| Kitasatospora setae | Trichomonas spp. control | Production of setamycin (antitrichomonal) | [58] |
| Streptomyces bingchenggensis | Control of helminths | Production of milbemycin (anthelmintic) | [59] |
| Chromobacterium violaceum | Control of multiple pathogens, tumor protection | Production of violacein (anticancer, antibacterial, antifungal, antiviral) | [60] |
| Janthinobacterium sp. HH01 | Control of multiple pathogens, tumor protection | Production of violacein (anticancer, antibacterial, antifungal, antiviral) | [61] |
| Polaromonas naphthalenivorans | Naphthalene | Degradation of naphthalene (potential carcinogen) | [62] |
| Yarrowia lipolytica | Control of multiple pathogens | Production of biosurfactants (broad-spectrum antimicrobial activity) | [63] |
| Rhodococcus erythropolis | Control of multiple pathogens | Production of biosurfactants (broad-spectrum antimicrobial activity) | [64] |
| Phage phi MR11 | Multidrug resistant Staphylococcus aureus | Lysis and clearance | [65] |
| Acinetobacter phage Petty | Acinetobacter baumanii control | Infection and lysis | [66] |
| Acibel004 | Acinetobacter baumanii control | Infection and lysis | [67] |
| Phage BPP-1 | Bordetella spp. | Infection and lysis | [68] |
| Herbaspirillum frisingense | - | Production of naphthocyclinones antibiotics | [69] |
| Heterorhabditis bacteriophora | Fleas, ants, and flies | Releasing Photorhabdus luminescens bacteria from their digestive tract | [70] |
| Adineta vaga | Scavenge dead bacteria and protozoans | Feeds on dead organic matter | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobello, M.; Bava, R.; Castagna, F.; Sotgiu, F.D.; Berlinguer, F.; Tilocca, B. The Role of Vulture (Accipitriformes) Cutaneous Microbiota in Infectious Disease Protection. Microorganisms 2025, 13, 898. https://doi.org/10.3390/microorganisms13040898
Lobello M, Bava R, Castagna F, Sotgiu FD, Berlinguer F, Tilocca B. The Role of Vulture (Accipitriformes) Cutaneous Microbiota in Infectious Disease Protection. Microorganisms. 2025; 13(4):898. https://doi.org/10.3390/microorganisms13040898
Chicago/Turabian StyleLobello, Miriam, Roberto Bava, Fabio Castagna, Francesca Daniela Sotgiu, Fiammetta Berlinguer, and Bruno Tilocca. 2025. "The Role of Vulture (Accipitriformes) Cutaneous Microbiota in Infectious Disease Protection" Microorganisms 13, no. 4: 898. https://doi.org/10.3390/microorganisms13040898
APA StyleLobello, M., Bava, R., Castagna, F., Sotgiu, F. D., Berlinguer, F., & Tilocca, B. (2025). The Role of Vulture (Accipitriformes) Cutaneous Microbiota in Infectious Disease Protection. Microorganisms, 13(4), 898. https://doi.org/10.3390/microorganisms13040898










