A Comparison Between High- and Low-Performing Lambs and Their Impact on the Meat Quality and Development Level Using a Multi-Omics Analysis of Rumen Microbe–Muscle–Liver Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lamb and Experimental Design
2.2. Sample Collection and Processing
2.3. Analyzing Slaughtering Performance and Meat Quality Characteristics
2.4. Morphometric Analysis of the Longissimus Dorsi and Rumen Tissue
2.5. Analysis of Rumen VFAs and Digestive Enzymes
2.6. DNA Extraction and Analysis of Bacterial Community in Rumen
2.7. Transcriptome Sequencing and Bioinformatics Analysis
2.8. Metabolome Sequencing and Bioinformatics Analysis
2.9. Data Statistics and Analysis
3. Results
3.1. Analysis of Carcass and Meat Physical Traits in Lambs of Different Growth and Development
3.2. Analysis of the Nutritional Components in Lambs of Different Growth and Development
3.3. Analysis of Rumen Fermentation Parameters and Histomorphology in Lambs
3.3.1. Analysis of the Rumen VFAs in Lambs of Different Growth and Development
3.3.2. Analysis of the Rumen Digestive Enzyme and Histomorphology in Lambs
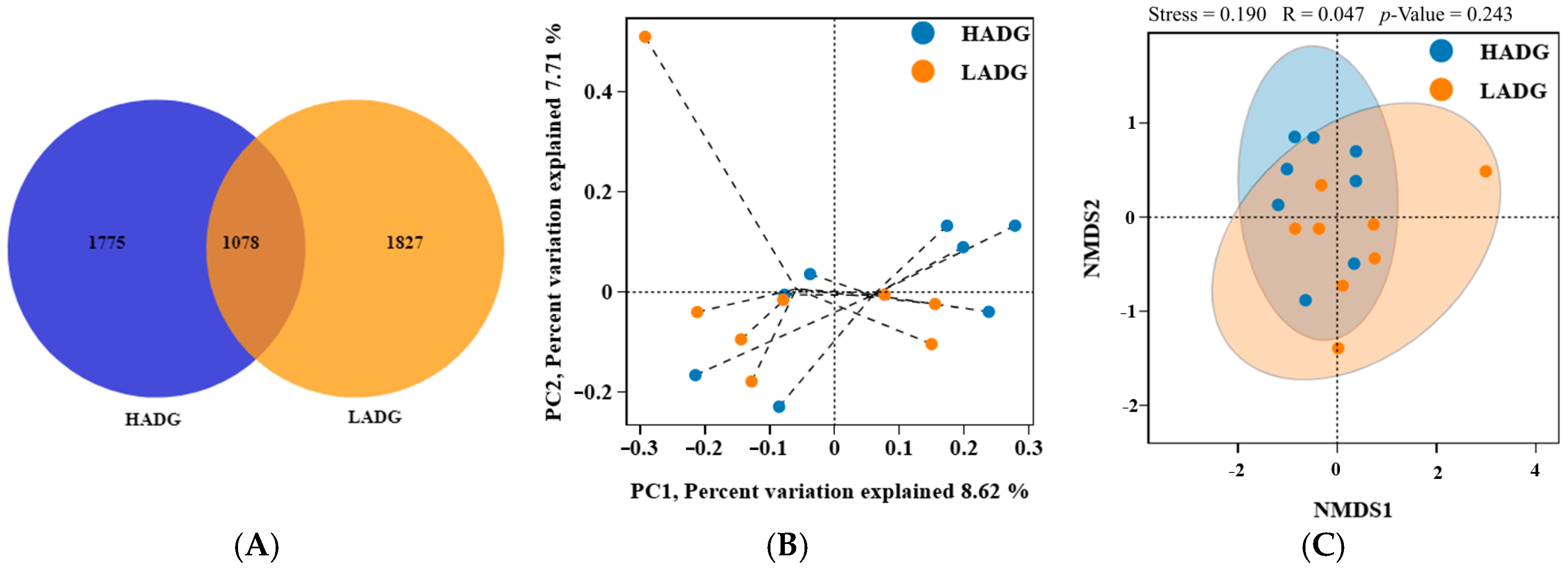
3.4. Analysis of the Rumen Microbiota of HADG and LADG Lambs
3.4.1. Analysis of the Microbiota Diversity of the Rumen
3.4.2. Analysis of the Rumen Microbiota Composition of the Rumen
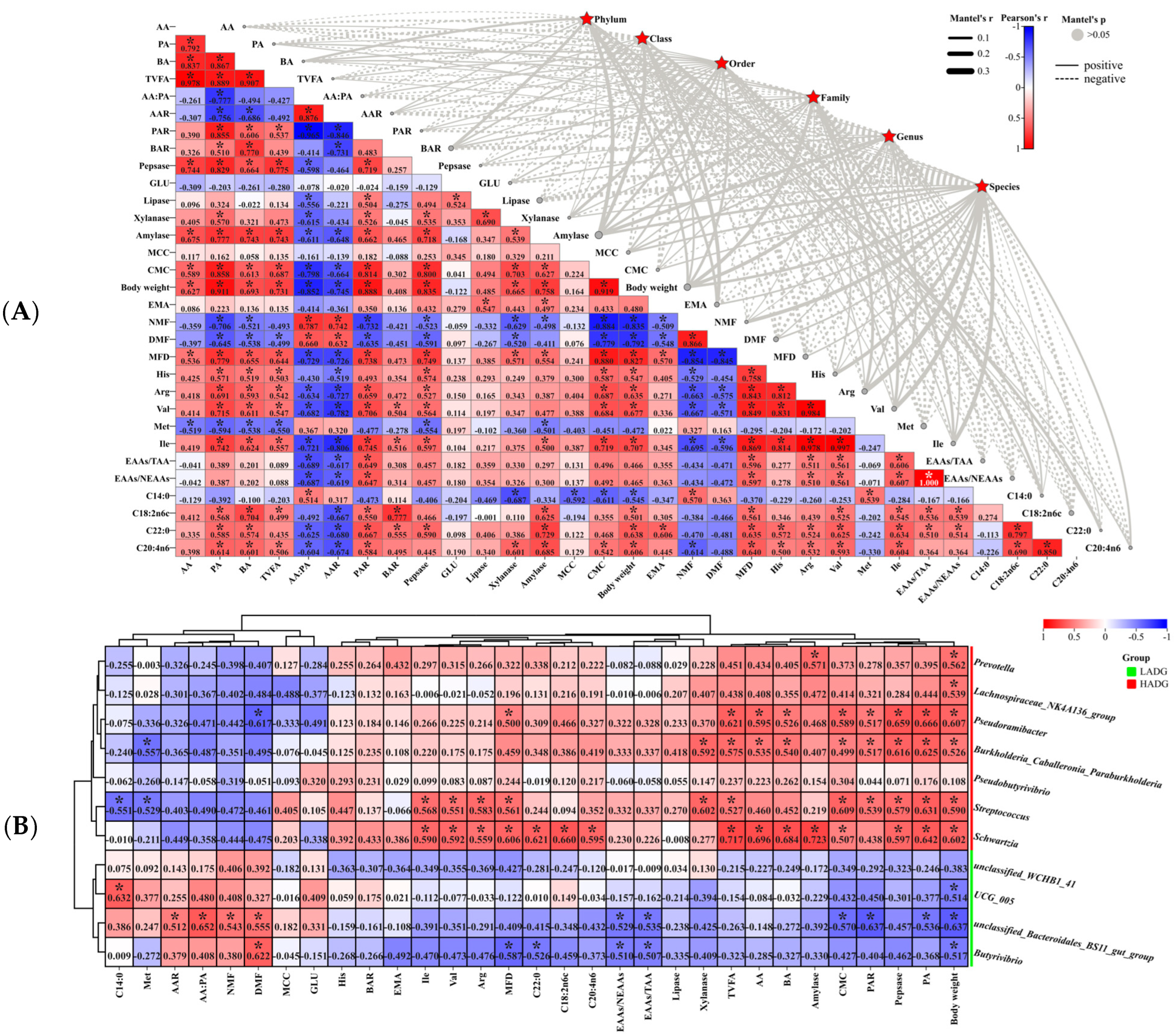
3.4.3. Analysis of the Rumen Microbiome in Correlation with Host Phenotype
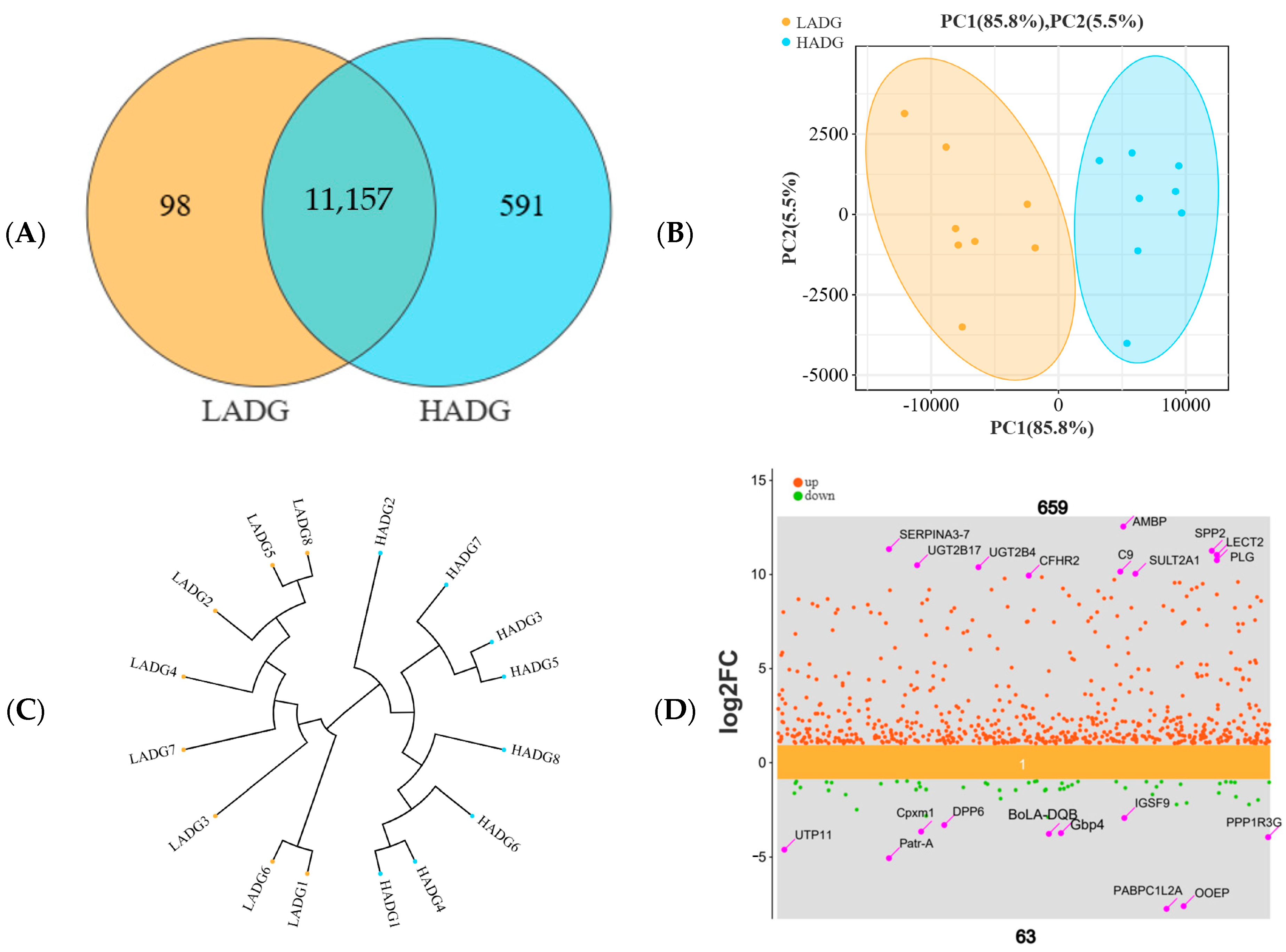
3.5. Analysis of Transcriptome Profiling of Longissimus Dorsi
3.5.1. Analysis of Transcriptome Differences in the Longissimus Dorsi of Lambs
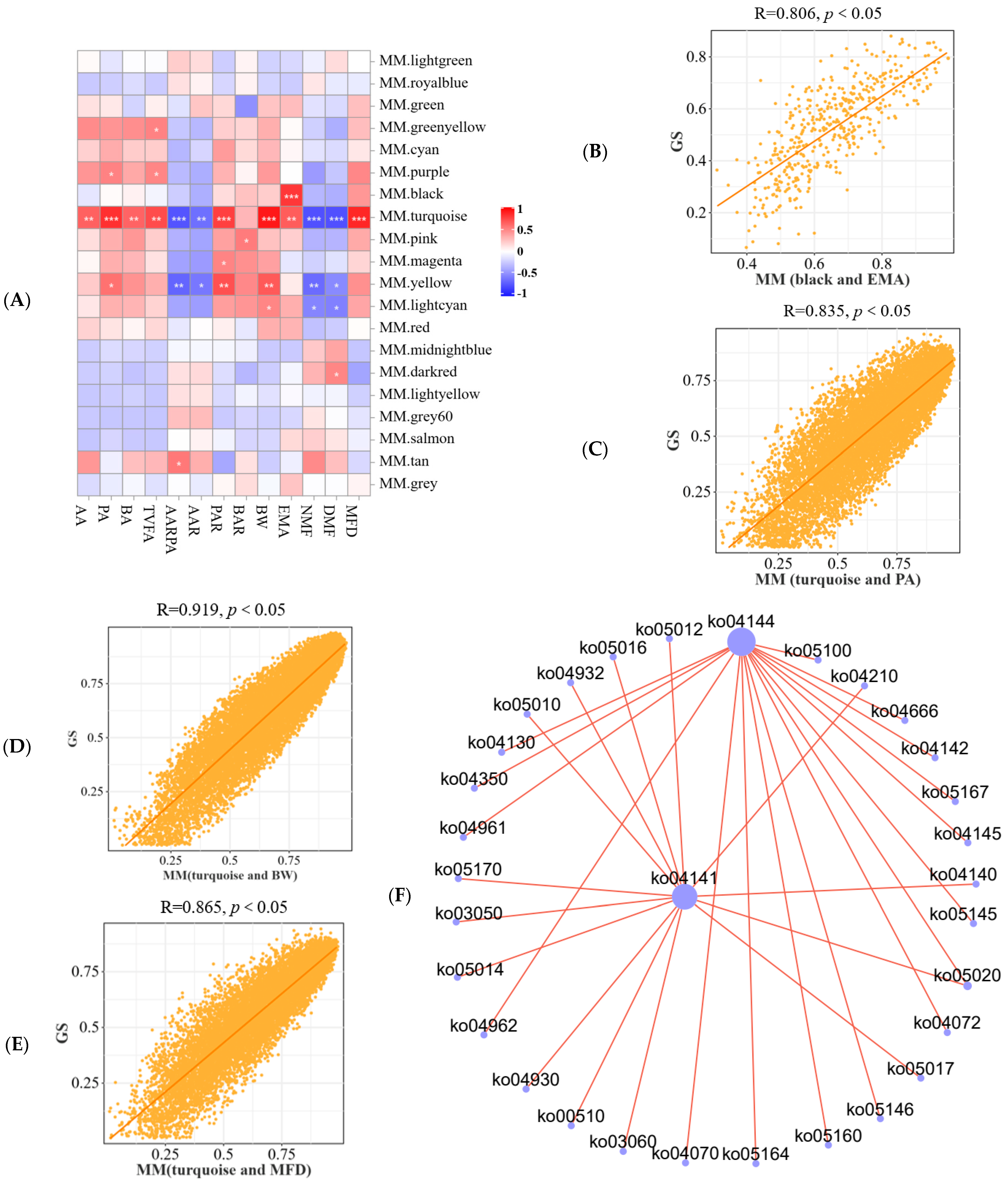
3.5.2. Host Phenotype and Longissimus Dorsi Gene Module Association Analysis
3.6. Analysis of Metabolism Profiling of Longissimus Dorsi
3.7. Liver Metabolism Profiling and Rumen Microbe–Muscle–Liver Interactions Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA (AAR) | acetic acid (acetic acid ratio) |
| BA (BAR) | butyric acid (butyric acid ratio) |
| CMC | carboxymethyl cellulose |
| DMF | Density of muscle fibers |
| EAAs | Essential amino acids |
| EMA | Eye muscle area |
| GLU | beta glucosidase |
| IBA (IBAR) | isobutyric acid (isobutyric acid ratio) |
| IVA (IVAR) | isovaleric acid (isovaleric acid ratio) |
| MCC | microcrystalline cellulose |
| MFD | Muscle fiber diameter |
| NEAAs | Nonessential amino acids |
| NMF | Number of muscle fibers |
| PA (PAR) | propionic acid (acetic acid ratio) |
| SFA | Saturated fatty acid |
| TAA | Total amino acid |
| TVFAs | total volatile fatty acids |
| UFA | Unsaturated fatty acid |
| VA (VAR) | valeric acid (valeric acid ratio) |
| VFAs | volatile fatty acids |
References
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef]
- Kost, C.; Patil, K.R.; Friedman, J.; Garcia, S.L.; Ralser, M. Metabolic exchanges are ubiquitous in natural microbial communities. Nat. Microbiol. 2023, 8, 2244–2252. [Google Scholar] [CrossRef]
- Mancin, L.; Wu, G.D.; Paoli, A. Gut microbiota–bile acid–skeletal muscle axis. Trends Microbiol. 2023, 31, 254–269. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short–chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Xu, L.; Mao, T.; Xia, M.; Wu, W.; Chen, J.; Jiang, C.; Zeng, T.; Tian, Y.; Lu, L.; Cai, Z. New evidence for gut–muscle axis: Lactic acid bacteria–induced gut microbiota regulates duck meat flavor. Food Chem. 2024, 450, 139354. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Zhang, X.; Li, C.; Yuan, L.; Zhang, D.; Zhao, Y.; Li, X.; Cheng, J.; Lin, C.; et al. Heritability and recursive influence of host genetics on the rumen microbiota drive body weight variance in male Hu sheep lambs. Microbiome 2023, 11, 197. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, H.; Song, Y.; Sun, C.; Zhang, Z.; Chen, S. Community composition of cecal microbiota in commercial yellow broilers with high and low feed efficiencies. Poult. Sci. 2021, 100, 100996. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Liu, C.; Chen, X.; Yang, Z.; Hu, G.; Zhang, M.; Sun, L.; Su, L.; Zhao, L.; Jin, Y. Supplemental Clostridium butyricum modulates skeletal muscle development and meat quality by shaping the gut microbiota of lambs. Meat Sci. 2023, 204, 109235. [Google Scholar] [CrossRef]
- Wen, C.; Wang, Q.; Gu, S.; Jin, J.; Yang, N. Emerging perspectives in the gut–muscle axis: The gut microbiota and its metabolites as important modulators of meat quality. Microb. Biotechnol. 2024, 17, e14361. [Google Scholar] [CrossRef]
- Furman, O.; Shenhav, L.; Sasson, G.; Kokou, F.; Honig, H.; Jacoby, S.; Hertz, T.; Cordero, O.X.; Halperin, E.; Mizrahi, I. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat. Commun. 2020, 11, 1904. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal microbiota–host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, B.; Wang, Y.; Bai, J.; Gao, Y.; Ru, X.; Bi, C.; Li, J.; Shan, A. Effects of sex on fat deposition through gut microbiota and short–chain fatty acids in weaned pigs. Anim. Nutr. 2024, 17, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhan, J.; Jiang, H.; Jia, H.; Pan, Y.; Zhong, X.; Huo, J.; Zhao, S. Metagenomics–metabolomics exploration of three–way–crossbreeding effects on rumen to provide basis for crossbreeding improvement of sheep microbiome and metabolome of sheep. Animals 2024, 14, 2256. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.E.; Visscher, P.M.; Goddard, M.E. Genetic architecture of body size in mammals. Genome Biol. 2012, 13, 244. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Shen, M.; Xie, X.L.; Liu, G.J.; Xu, Y.X.; Lv, F.H.; Yang, H.; Yang, Y.L.; Liu, C.B.; et al. Whole–genome resequencing of wild and domestic sheep identifies genes associated with morphological and agronomic traits. Nat. Commun. 2020, 11, 2815. [Google Scholar] [CrossRef]
- McHugh, N.; Pabiou, T.; McDermott, K.; Wall, E.; Berry, D.P. A novel measure of ewe efficiency for breeding and benchmarking purposes. J. Anim. Sci. 2018, 96, 2051–2059. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Guan, L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019, 20, 172. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Tong, A.; Li, Z.; Liu, X.; Ge, X.; Zhao, R.; Liu, B.; Zhao, L.; Zhao, C. Laminaria japonica polysaccharide alleviates type 2 diabetes by regulating the microbiota–gut–liver axis: A multi–omics mechanistic analysis. Int. J. Biol. Macromol. 2024, 258, 128853. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhan, J.; Zhao, S.; Jiang, H.; Jia, H.; Pan, Y.; Huo, J. Interaction between liver metabolism and gut short–chain fatty acids via liver–gut axis affects body weight in lambs. Int. J. Mol. Sci. 2024, 25, 13386. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial regulation of host physiology by short–chain fatty acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sichler, A.; Ecker, J.; Laschinger, M.; Liebisch, G.; Höring, M.; Basic, M.; Bleich, A.; Zhang, X.J.; Kübelsbeck, L.; et al. Gut microbiota promote liver regeneration through hepatic membrane phospholipid biosynthesis. J. Hepatol. 2023, 78, 820–835. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Chen, L.; Wang, W.; Zhuang, X.; Liu, Y.; Zhao, R. Parental betaine supplementation promotes gosling growth with epigenetic modulation of IGF gene family in the liver. J. Anim. Sci. 2024, 102, skae065. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Zhang, J.; Sun, Y.; Wang, J. Dietary effects of succinic acid on the growth, digestive enzymes, immune response and resistance to ammonia stress of Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 78, 10–17. [Google Scholar] [CrossRef]
- Chen, Z.T.; Weng, Z.X.; Lin, J.D.; Meng, Z.X. Myokines: Metabolic regulation in obesity and type 2 diabetes. Life Metab. 2024, 3, loae006. [Google Scholar] [CrossRef]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose tissue–liver cross talk in the control of whole–body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef]
- Yoo, E.S.; Yu, J.; Sohn, J.W. Neuroendocrine control of appetite and metabolism. Exp. Mol. Med. 2021, 53, 505–516. [Google Scholar] [CrossRef]
- López-Bermudo, L.; Luque-Sierra, A.; Maya-Miles, D.; Gallego-Durán, R.; Ampuero, J.; Romero-Gómez, M.; Berná, G.; Martín, F. Contribution of liver and pancreatic islet crosstalk to β–cell function/dysfunction in the presence of fatty liver. Front. Endocrinol. 2022, 13, 892672. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Jensen-Cody, S.O.; Potthoff, M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021, 44, 101138. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Gessner, D.K.; Eder, K. The gut–liver axis in the control of energy metabolism and food intake in animals. Annu. Rev. Anim. Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef]
- Ghafouri-Kesbi, F.; Abbasi, M.A.; Afraz, F.; Babaei, M.; Baneh, H.; Abdollahi Arpanahi, R. Genetic analysis of growth rate and Kleiber ratio in Zandi sheep. Trop. Anim. Health Prod. 2011, 43, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Mehrban, H.; Naserkheil, M.; Lee, D.H.; Ibáñez-Escriche, N. Genetic parameters and correlations of related feed efficiency, growth, and carcass traits in Hanwoo beef cattle. Anim. Biosci. 2021, 34, 824–832. [Google Scholar] [CrossRef] [PubMed]
- NY/T 3469–2019; Operating Procedures of Livestock and Poultry Slaughtering Sheep and Goat. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2019.
- Zhan, J.; Gu, Z.; Wang, H.; Liu, Y.; Wang, L.; Huang, L.; Huo, J.; Wu, Y. Effects of rutin supplementation on growth performance, slaughter performance, serum parameters, and meat quality of Nubian goats. Anim. Sci. J. 2023, 94, e13819. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhan, J.S.; Huo, J.H.; Zhong, X.J.; Liu, Y.H.; Zhao, S.G. Effects of dietary rutin on serum immune and antioxidant indices and muscle composition of Hu sheep. Chin. J. Anim. Nutr. 2022, 34, 2504–2513. [Google Scholar]
- Wang, H.; Zhan, J.; Jia, H.; Jiang, H.; Pan, Y.; Zhong, X.; Zhao, S.; Huo, J. Relationship between rumen microbial differences and phenotype traits among Hu sheep and crossbred offspring sheep. Animals 2024, 14, 1509. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High–resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra–fast all–in–one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA–seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript–level expression analysis of RNA–seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Argaw-Denboba, A.; Schmidt, T.S.B.; Di Giacomo, M.; Ranjan, B.; Devendran, S.; Mastrorilli, E.; Lloyd, C.T.; Pugliese, D.; Paribeni, V.; Dabin, J.; et al. Paternal microbiome perturbations impact offspring fitness. Nature 2024, 629, 652–659. [Google Scholar] [CrossRef]
- Li, G.Z.; Zhang, X.X.; Li, F.D.; La, Y.F.; Zhang, D.Y.; Li, X.L.; Zhang, Y.K.; Song, Q.Z.; Zhao, Y.; Wang, W.M. Growth and development characteristics and growth model of Hu sheep in the fattening period. Pratacultural Sci. 2020, 37, 1880–1890. [Google Scholar]
- Su, L.; Zhao, C.; Sun, B.; Dou, L.; Wang, C.; Yang, Z.; Li, T.; Jin, Y. Effects of exercise on muscle fiber conversion, muscle development and meat quality of Sunit sheep. Meat Sci. 2024, 211, 109440. [Google Scholar] [CrossRef]
- Kong, L.; Yue, Y.; Li, J.; Yang, B.; Chen, B.; Liu, J.; Lu, Z. Transcriptomics and metabolomics reveal improved performance of Hu sheep on hybridization with Southdown sheep. Food Res. Int. 2023, 173, 113240. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zhuang, Z.; Qiu, Y.; Ruan, D.; Wu, J.; Ye, J.; Cao, L.; Zhou, S.; Zheng, E.; Huang, W.; et al. Identify known and novel candidate genes associated with backfat thickness in duroc pigs by large–scale genome–wide association analysis. J. Anim. Sci. 2022, 100, skac012. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Li, F.; Zhang, D.; Zhang, Y.; Li, X.; Song, Q.; Zhou, B.; Zhao, L.; Wang, J.; et al. Whole genome sequencing analysis to identify candidate genes associated with the rib eye muscle area in Hu sheep. Front. Genet. 2022, 13, 824742. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS–GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Xu, J.; Yang, Q.; Sha, Y.; Jiao, T.; Zhao, S. Effects of yeast cultures on meat quality, flavor composition and rumen microbiota in lambs. Curr. Res. Food. Sci. 2024, 9, 100845. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Ma, X.Y.; Gong, X.X.; Peng, C.; Huang, J.; Zhao, G.Q.; Zhan, K. Effects of different protein levels on gluconeogenesis of Holstein bull calves. J. China Agric. Univ. 2022, 27, 187–194. [Google Scholar]
- Wang, Z.; An, X.; Yang, Y.; Zhang, L.; Jiao, T.; Zhao, S. Comprehensive analysis of the longissimus dorsi transcriptome and metabolome reveals the regulatory mechanism of different varieties of meat quality. J. Agric. Food Chem. 2023, 71, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, H.; Wang, D.; Liu, H.; Liu, J. Constraints on the utilization of cereal straw in lactating dairy cows: A review from the perspective of systems biology. Anim. Nutr. 2022, 9, 240–248. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva–Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota–generated metabolites promote metabolic benefits via gut–brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef]
- Madruga, M.; Dantas, I.; Queiroz, A.; Brasil, L.; Ishihara, Y. Volatiles and water–and fat–soluble precursors of Saanen goat and cross Suffolk lamb flavour. Molecules 2013, 18, 2150–2165. [Google Scholar] [CrossRef]
- Salter, A.M. Dietary fatty acids and cardiovascular disease. Animal 2013, 7, 163–171. [Google Scholar] [CrossRef]
- Nava Lauson, C.B.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650.e9. [Google Scholar] [CrossRef]
- Fei, S.; Chen, Z.; Xia, Y.; Liu, H.; Han, D.; Jin, J.; Xie, S. Effects of dietary arachidonic acid on reproduction performance, tissue fatty acid profile and gonadal steroidogenesis in female yellow catfish pelteobagrus fulvidraco. Aquac. Nutr. 2021, 27, 700–711. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Tang, G.; Yu, J.; Chen, J.; Li, Z.; Cao, Y.; Lei, X.; Deng, L.; Wu, S.; et al. Multi–omics revealed the long–term effect of ruminal keystone bacteria and the microbial metabolome on lactation performance in adult dairy goats. Microbiome 2023, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Shabat, S.K.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome–dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef]
- Wang, K.; Song, D.; Zhang, X.; Datsomor, O.; Jiang, M.; Zhao, G. Effects of high–grain diet on performance, ruminal fermentation, and rumen microbial flora of lactating holstein dairy cows. Animals 2024, 14, 2522. [Google Scholar] [CrossRef]
- Wang, Z.B.; Xin, H.S.; Bao, J.; Duan, C.Y.; Chen, Y.; Qu, Y.L. Effects of hainanmycin or monensin supplementation on ruminal protein metabolism and populations of proteolytic bacteria in Holstein heifers. Anim. Feed Sci. Technol. 2015, 201, 99–103. [Google Scholar] [CrossRef]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; McSweeney, C.S.; Elliott, R. Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay. Anim. Feed Sci. Technol. 2015, 200, 25–34. [Google Scholar] [CrossRef]
- Keogh, K.; Kenny, D.A.; Alexandre, P.A.; Waters, S.M.; McGovern, E.; McGee, M.; Reverter, A. Relationship between the rumen microbiome and liver transcriptome in beef cattle divergent for feed efficiency. Anim. Microbiome 2024, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhan, J.; Zhao, S.; Jiang, H.; Jia, H.; Pan, Y.; Zhong, X.; Huo, J. Microbial–metabolomic exploration of tea polyphenols in the regulation of serum indicators, liver metabolism, rumen microorganisms, and metabolism in Hu sheep. Animals 2024, 14, 2661. [Google Scholar] [CrossRef]
- Gálvez, E.J.C.; Iljazovic, A.; Amend, L.; Lesker, T.R.; Renault, T.; Thiemann, S.; Hao, L.; Roy, U.; Gronow, A.; Charpentier, E.; et al. Distinct polysaccharide utilization determines interspecies competition between intestinal Prevotella spp. Cell Host Microbe. 2020, 28, 838–852.e6. [Google Scholar] [CrossRef]
- Betancur-Murillo, C.L.; Aguilar-Marín, S.B.; Jovel, J. Prevotella: A key player in ruminal metabolism. Microorganisms 2022, 11, 1. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Insights into the role of erysipelotrichaceae in the human host. Front. Cell Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T. Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef]
- Culp, E.J.; Goodman, A.L. Cross–feeding in the gut microbiome: Ecology and mechanisms. Cell Host Microbe 2023, 31, 485–499. [Google Scholar] [CrossRef]
- Wahlström, A.; Brumbaugh, A.; Sjöland, W.; Olsson, L.; Wu, H.; Henricsson, M.; Lundqvist, A.; Makki, K.; Hazen, S.L.; Bergström, G.; et al. Production of deoxycholic acid by low–abundant microbial species is associated with impaired glucose metabolism. Nat. Commun. 2024, 15, 4276. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yao, X.; Pei, J.; Wang, X.; Guo, S.; Cao, M.; Bao, P.; Wang, H.; Yan, P.; Guo, X. Do microbial–gut–muscle mediated by SCFAs, microbial–gut–brain axis mediated by insulin simultaneously regulate yak IMF deposition? Int. J. Biol. Macromol. 2024, 257, 128632. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, S.; Wang, C.; Wang, Y.; Qin, Y.; Wang, Y.; Zhang, J.; Song, H.; Mi, S.; Yu, F.; et al. A genome–wide association study of growth and fatness traits in two pig populations with different genetic backgrounds. J. Anim. Sci. 2018, 96, 806–816. [Google Scholar] [CrossRef]
- La, Y.; Zhang, X.; Li, F.; Zhang, D.; Li, C.; Mo, F.; Wang, W. Molecular characterization and expression of SPP1, LAP3 and LCORL and their association with growth traits in sheep. Genes 2019, 10, 616. [Google Scholar] [CrossRef]
- Soto-Pedre, E.; Siddiqui, M.K.; Mordi, I.; Maroteau, C.; Soto-Hernaez, J.; Palmer, C.N.A.; Pearson, E.R.; Leese, G.P. Evidence of a causal relationship between serum thyroid–stimulating hormone and osteoporotic bone fractures. Eur. Thyroid. J. 2021, 10, 439–446. [Google Scholar] [CrossRef]
- Wu, X.F.; Liu, Y.; Wang, Y.G.; Zhang, F.; Li, W.Y. A novel 22–bp InDel within FGF7 gene is significantly associated with growth traits in goat. Anim. Biotechnol. 2024, 35, 2262537. [Google Scholar] [CrossRef]
- Pan, X.; Liu, B.; Chen, S.; Ding, H.; Yao, X.; Cheng, Y.; Xu, D.; Yin, Y.; Dai, X.; Sun, J.; et al. Nr4a1 as a myogenic factor is upregulated in satellite cells/myoblast under proliferation and differentiation state. Biochem. Biophys. Res. Commun. 2019, 513, 573–581. [Google Scholar] [CrossRef]
- Cortez-Toledo, O.; Schnair, C.; Sangngern, P.; Metzger, D.; Chao, L.C. Nur77 deletion impairs muscle growth during developmental myogenesis and muscle regeneration in mice. PLoS ONE 2017, 12, e0171268. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Kizilaslan, M.; Arzik, Y.; Behrem, S.; Ata, N.; Karaca, O.; Elmaci, C.; Cemal, I. Genome–wide association studies of preweaning growth and in vivo carcass composition traits in Esme sheep. J. Anim. Breed. Genet. 2022, 139, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Fan, Y.; Liang, Y.; Cai, Y.; Zhang, G.; Deng, M.; Wang, Z.; Lu, J.; Shi, J.; Wang, F.; et al. FTO–mediated demethylation of GADD45B promotes myogenesis through the activation of p38 MAPK pathway. Mol. Ther. Nucleic Acids 2021, 26, 34–48. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kohara, R.; Sugi, M.; Usui, A.; Oyama, K.; Mannen, H.; Sasazaki, S. The non–synonymous mutation in bovine SPP1 gene influences carcass weight. Heliyon 2019, 5, e03006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, H.; Liu, X.; Yang, Q.; Pan, C.; Lei, C.; Dang, R.; Chen, H.; Lan, X. The muscle development transcriptome landscape of ovariectomized goat. R. Soc. Open. Sci. 2017, 4, 171415. [Google Scholar] [CrossRef]
- Smith, J.A.B.; Murach, K.A.; Dyar, K.A.; Zierath, J.R. Exercise metabolism and adaptation in skeletal muscle. Nat. Rev. Mol. Cell Biol. 2023, 24, 607–632. [Google Scholar] [CrossRef]
- Guimarães-Ferreira, L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein 2014, 12, 126–131. [Google Scholar] [CrossRef]
- Tabe, Y.; Lorenzi, P.L.; Konopleva, M. Amino acid metabolism in hematologic malignancies and the era of targeted therapy. Blood 2019, 134, 1014–1023. [Google Scholar] [CrossRef]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hörmannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C.; et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun. 2018, 9, 3760. [Google Scholar] [CrossRef]







| Items | LADG | HADG | p-Value | |
|---|---|---|---|---|
| Body weight, Kg | 25.80 ± 0.46 b | 40.91 ± 0.78 a | <0.001 | |
| Back fat thickness, mm | 2.46 ± 0.26 | 2.77 ± 0.26 | 0.406 | |
| Rib thickness (GR), mm | 4.70 ± 0.44 | 6.15 ± 0.72 | 0.108 | |
| Eye muscle area (EMA), mm2 | 950.61 ± 78.82 b | 1456 ± 175.73 a | 0.020 | |
| Meat color (45 min) | a* | 75.07 ± 0.57 | 73.17 ± 0.95 | 0.113 |
| b* | 24.91 ± 0.60 | 23.71 ± 0.41 | 0.118 | |
| L* | 44.44 ± 1.34 | 42.19 ± 2.09 | 0.379 | |
| pH (45 min) | 6.58 ± 0.07 | 6.51 ± 0.07 | 0.479 | |
| Shear force, N | 64.15 ± 4.09 | 72.54 ± 2.87 | 0.115 | |
| Water loss rate, % | 5.37 ± 0.58 | 6.90 ± 1.43 | 0.347 | |
| Cooking loss, % | 63.53 ± 1.08 | 59.11 ± 2.36 | 0.111 | |
| Muscle fiber diameter (MFD), mm | 0.03 ± 0.00 b | 0.04 ± 0.00 a | <0.001 | |
| Number of muscle fibers (NMF), n | 213.25 ± 9.90 a | 154.88 ± 6.43 b | <0.001 | |
| Density of muscle fibers (DMF), n/mm2 | 1250.6 ± 75.72 a | 868.49 ± 34.34 b | <0.001 | |
| Items | LADG | HADG | p-Value | |
|---|---|---|---|---|
| Base nutritional components | Crude protein, % | 20.03 ± 0.42 | 20.57 ± 0.36 | 0.349 |
| Moisture, % | 73.06 ± 0.32 | 72.60 ± 0.47 | 0.432 | |
| Crude fat, % | 4.81 ± 0.43 | 3.21 ± 0.65 | 0.058 | |
| Amino acids | Aspartate (Asp), g/100 g | 1.53 ± 0.09 | 1.80 ± 0.15 | 0.153 |
| Glutamic acid (Glu), g/100 g | 2.54 ± 0.13 | 2.98 ± 0.25 | 0.141 | |
| Serine (Ser), g/100 g | 0.56 ± 0.03 | 0.60 ± 0.05 | 0.407 | |
| Glycine (Gly), g/100 g | 0.42 ± 0.03 | 0.46 ± 0.06 | 0.538 | |
| Histidine (His), g/100 g | 0.37 ± 0.05 b | 0.53 ± 0.06 a | 0.043 | |
| Arginine (Arg), g/100 g | 0.95 ± 0.06 b | 1.31 ± 0.13 a | 0.021 | |
| Alanine (Ala), g/100 g | 0.68 ± 0.03 | 0.76 ± 0.07 | 0.292 | |
| Proline (Pro), g/100 g | 0.26 ± 0.04 | 0.32 ± 0.03 | 0.279 | |
| Tyrosine (Tyr), g/100 g | 0.45 ± 0.02 | 0.54 ± 0.05 | 0.081 | |
| Valine (Val), g/100 g | 0.41 ± 0.02 b | 0.60 ± 0.06 a | 0.016 | |
| Methionine (Met), g/100 g | 0.14 ± 0.03 a | 0.07 ± 0.02 b | 0.047 | |
| Isoleucine (Ile), g/100 g | 0.38 ± 0.02 b | 0.58 ± 0.06 a | 0.010 | |
| Leucine (Leu), g/100 g | 0.87 ± 0.05 | 1.06 ± 0.09 | 0.089 | |
| Phenylalanine (Phe), g/100 g | 0.33 ± 0.02 | 0.42 ± 0.04 | 0.070 | |
| Lysine (Lys), g/100 g | 1.31 ± 0.07 | 1.66 ± 0.15 | 0.063 | |
| Threonine (Thr), g/100 g | 0.34 ± 0.02 | 0.41 ± 0.04 | 0.106 | |
| TAA, g/100 g | 11.55 ± 0.59 | 14.11 ± 1.26 | 0.086 | |
| NEAAs, g/100 g | 7.75 ± 0.38 | 9.31 ± 0.83 | 0.111 | |
| EAAs, g/100 g | 3.79 ± 0.21 | 4.81 ± 0.44 | 0.055 | |
| EAAs/TAA,% | 32.79 ± 0.41 b | 34.01 ± 0.32 a | 0.034 | |
| EAAs/NEAAs,% | 48.82 ± 0.92 b | 51.57 ± 0.73 a | 0.034 | |
| Fatty acid | Caproic acid (C6:0), mg/100 g | 5.33 ± 0.34 | 7.08 ± 1.20 | 0.199 |
| Caprylic acid (C8:0), mg/100 g | 5.01 ± 0.46 | 5.51 ± 0.42 | 0.435 | |
| Undecanoic acid (C11:0), mg/100 g | 15.95 ± 0.51 | 14.84 ± 0.40 | 0.109 | |
| Myristic acid (C14:0), mg/100 g | 57.38 ± 11.66 a | 28.44 ± 4.07 b | 0.034 | |
| Pentadecanoic acid (C15:0), mg/100 g | 7.97 ± 1.46 | 11.98 ± 3.82 | 0.344 | |
| Palmitic acid (C16:0), mg/100 g | 441.10 ± 71.39 | 286.80 ± 37.27 | 0.076 | |
| Palmitoleic acid (C16:1), mg/100 g, mg/100 g | 32.52 ± 6.22 | 29.13 ± 4.07 | 0.655 | |
| Margaric acid (C17:0), mg/100 g | 20.58 ± 2.97 | 14.73 ± 2.47 | 0.152 | |
| Margaroleic acid (C17:1), mg/100 g | 14.81 ± 1.99 | 16.75 ± 2.54 | 0.558 | |
| Stearic acid (C18:0), mg/100 g | 435.71 ± 65.31 | 368.03 ± 55.30 | 0.442 | |
| Oleic acid (C18:1n9c), mg/100 g | 1017.05 ± 166.84 | 682.8 ± 97.38 | 0.106 | |
| linoleic acid (C18:2n6c), mg/100 g | 64.29 ± 6.38 b | 86.04 ± 6.01 a | 0.026 | |
| γ–linolenic acid (C18:3n6), mg/100 g | 22.57 ± 3.22 | 30.73 ± 4.55 | 0.165 | |
| ɑ–Linolenic acid (C18:3n3), mg/100 g | 8.78 ± 0.82 | 10.40 ± 0.67 | 0.150 | |
| Heneicosanoic acid (C21:0), mg/100 g | 6.52 ± 1.11 | 5.37 ± 0.62 | 0.379 | |
| Behenic acid (C22:0), mg/100 g | 11.16 ± 0.91 b | 16.20 ± 1.06 a | 0.003 | |
| Arachidonic acid (C20:4n6), mg/100 g | 45.19 ± 2.95 b | 64.45 ± 5.41 a | 0.007 | |
| Tricosanoic acid (C23:0), mg/100 g | 15.98 ± 0.70 | 16.91 ± 1.08 | 0.481 | |
| SFA, mg/100 g | 1022.70 ± 151.96 | 775.90 ± 95.19 | 0.190 | |
| UFA, mg/100 g | 1205.22 ± 173.18 | 920.31 ± 98.91 | 0.175 | |
| Items | LADG | HADG | p-Value | |
|---|---|---|---|---|
| VFA molar concentration, mmol/L | AA | 33.44 ± 3.90 b | 48.17 ± 3.16 a | 0.011 |
| PA | 5.48 ± 0.33 b | 13.40 ± 1.13 a | <0.001 | |
| IBA | 0.76 ± 0.04 b | 1.05 ± 0.08 a | 0.011 | |
| BA | 5.72 ± 0.84 b | 10.33 ± 1.10 a | 0.005 | |
| IVA | 1.10 ± 0.06 | 1.35 ± 0.14 | 0.123 | |
| VA | 0.46 ± 0.05 b | 0.92 ± 0.17 a | 0.032 | |
| TVFA | 46.92 ± 4.98 b | 75.22 ± 5.22 a | 0.002 | |
| VFA molar proportion, % | AA:PA | 6.03 ± 0.37 a | 3.68 ± 0.21 b | <0.001 |
| AAR | 70.92 ± 0.75 a | 64.29 ± 1.61 b | 0.002 | |
| PAR | 12.00 ± 0.56 b | 17.71 ± 0.62 a | <0.001 | |
| IBAR | 1.72 ± 0.17 | 1.39 ± 0.07 | 0.088 | |
| BAR | 11.94 ± 0.54 | 13.63 ± 1.08 | 0.184 | |
| IVAR | 2.50 ± 0.25 a | 1.79 ± 0.11 b | 0.022 | |
| VAR | 1.04 ± 0.14 | 1.19 ± 0.16 | 0.468 | |
| Items | LADG | HADG | p-Value | |
|---|---|---|---|---|
| Digestive enzyme | Pepsase, ug/L | 15.42 ± 0.46 b | 20.90 ± 0.62 a | <0.001 |
| GLU, ng/L | 921.04 ± 51.12 | 897.04 ± 59.29 | 0.764 | |
| Lipase, ng/mL | 237.98 ± 13.77 b | 298.72 ± 20.85 a | 0.029 | |
| Xylanase, pg/mL | 130.77 ± 12.45 b | 184.29 ± 8.01 a | 0.003 | |
| Amylase, umol/L | 141.35 ± 3.87 b | 172.99 ± 5.09 a | <0.001 | |
| MCC, pg/mL | 114.58 ± 4.77 | 119.95 ± 5.72 | 0.483 | |
| CMC, pg/mL | 249.06 ± 5.10 b | 323.02 ± 7.82 a | <0.001 | |
| Histomorphology | Papilla height, mm | 2.15 ± 0.24 | 1.71 ± 0.05 | 0.112 |
| Papilla width, mm | 0.47 ± 0.05 | 0.41 ± 0.02 | 0.361 | |
| Muscle layer, mm | 1.46 ± 0.14 | 1.65 ± 0.17 | 0.402 | |
| Stratum corneum, mm | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.399 | |
| Basal layer thickness, mm | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.452 | |
| Stratum granular, mm | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.181 | |
| Stratum spinosum, mm | 0.08 ± 0.01 | 0.06 ± 0.00 | 0.062 | |
| Items | LADG | HADG | p-Value |
|---|---|---|---|
| ACE | 577.37 | 582.64 | 0.88 |
| Chao1 | 576.16 | 581.69 | 0.88 |
| Simpson | 0.99 | 0.98 | 0.13 |
| Shannon | 8.00 | 7.61 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhan, J.; Zhao, S.; Jiang, H.; Jia, H.; Pan, Y.; Zhong, X.; Huo, J. A Comparison Between High- and Low-Performing Lambs and Their Impact on the Meat Quality and Development Level Using a Multi-Omics Analysis of Rumen Microbe–Muscle–Liver Interactions. Microorganisms 2025, 13, 943. https://doi.org/10.3390/microorganisms13040943
Wang H, Zhan J, Zhao S, Jiang H, Jia H, Pan Y, Zhong X, Huo J. A Comparison Between High- and Low-Performing Lambs and Their Impact on the Meat Quality and Development Level Using a Multi-Omics Analysis of Rumen Microbe–Muscle–Liver Interactions. Microorganisms. 2025; 13(4):943. https://doi.org/10.3390/microorganisms13040943
Chicago/Turabian StyleWang, Haibo, Jinshun Zhan, Shengguo Zhao, Haoyun Jiang, Haobin Jia, Yue Pan, Xiaojun Zhong, and Junhong Huo. 2025. "A Comparison Between High- and Low-Performing Lambs and Their Impact on the Meat Quality and Development Level Using a Multi-Omics Analysis of Rumen Microbe–Muscle–Liver Interactions" Microorganisms 13, no. 4: 943. https://doi.org/10.3390/microorganisms13040943
APA StyleWang, H., Zhan, J., Zhao, S., Jiang, H., Jia, H., Pan, Y., Zhong, X., & Huo, J. (2025). A Comparison Between High- and Low-Performing Lambs and Their Impact on the Meat Quality and Development Level Using a Multi-Omics Analysis of Rumen Microbe–Muscle–Liver Interactions. Microorganisms, 13(4), 943. https://doi.org/10.3390/microorganisms13040943






