Bio-Organic Fertilizer Application Enhances Silage Maize Yield by Regulating Soil Physicochemical and Microbial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Soil Sampling
2.2. Analysis of Soil Physicochemical Properties
2.3. DNA Extraction and High-Throughput Sequencing
2.4. Determination of Ecosystem Multifunctionality
2.5. Statistical Analysis
3. Results
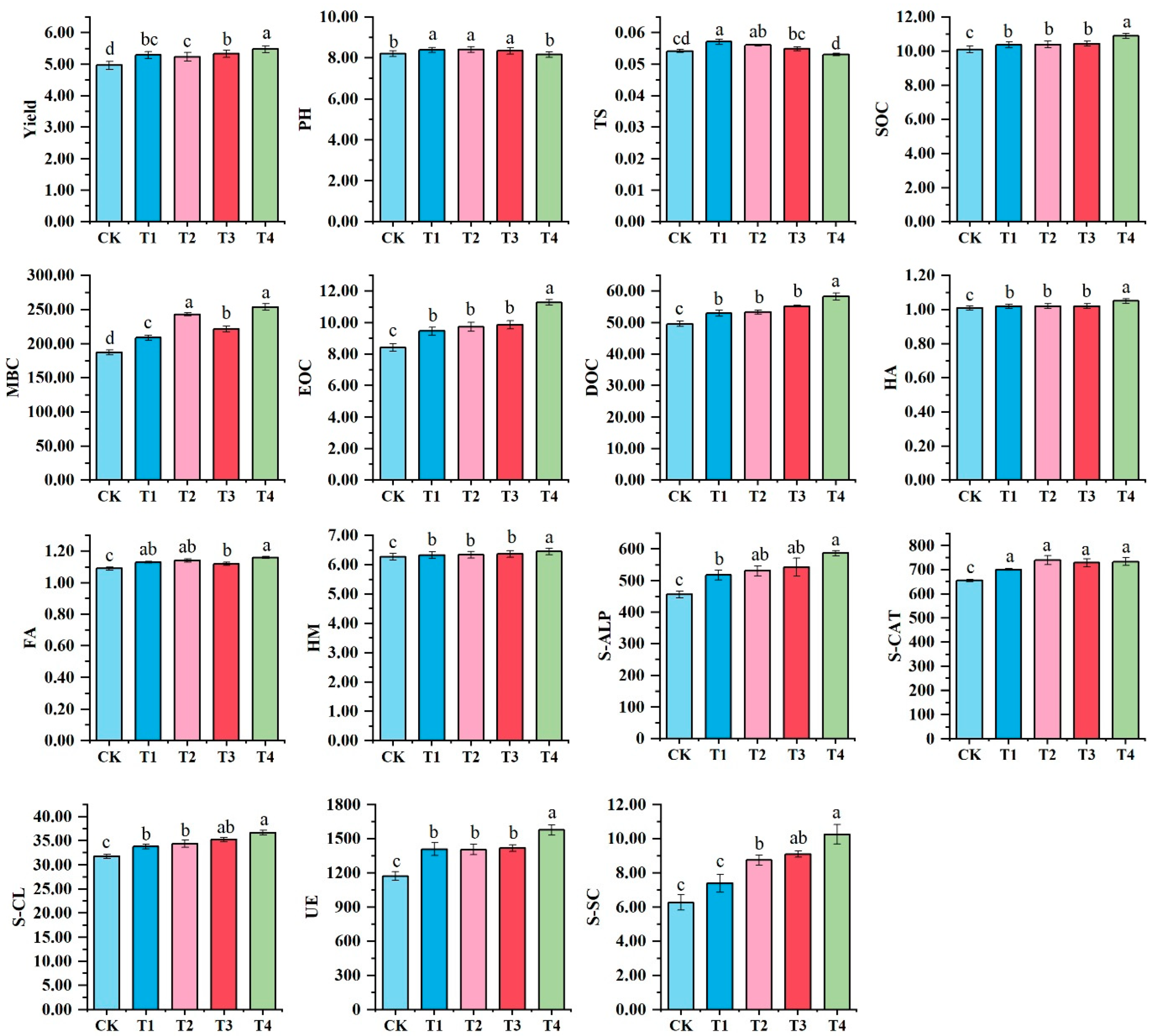
3.1. Maize Yield and Soil Physicochemical Factors
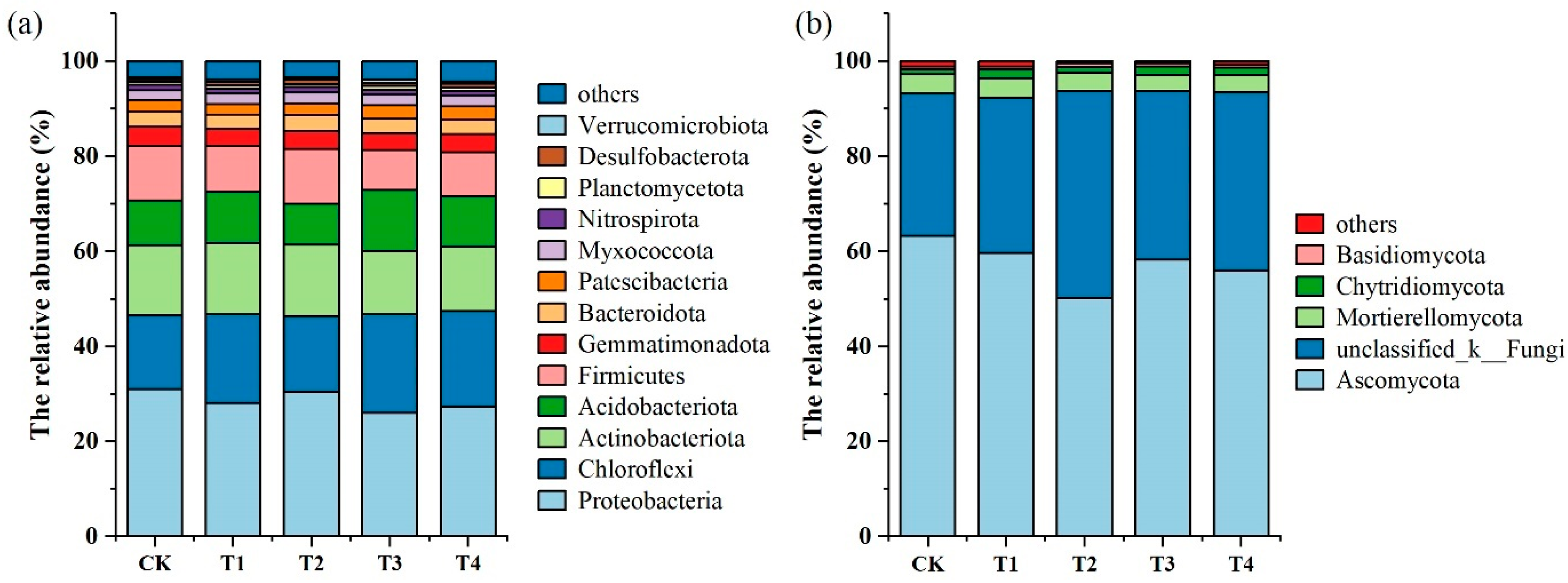
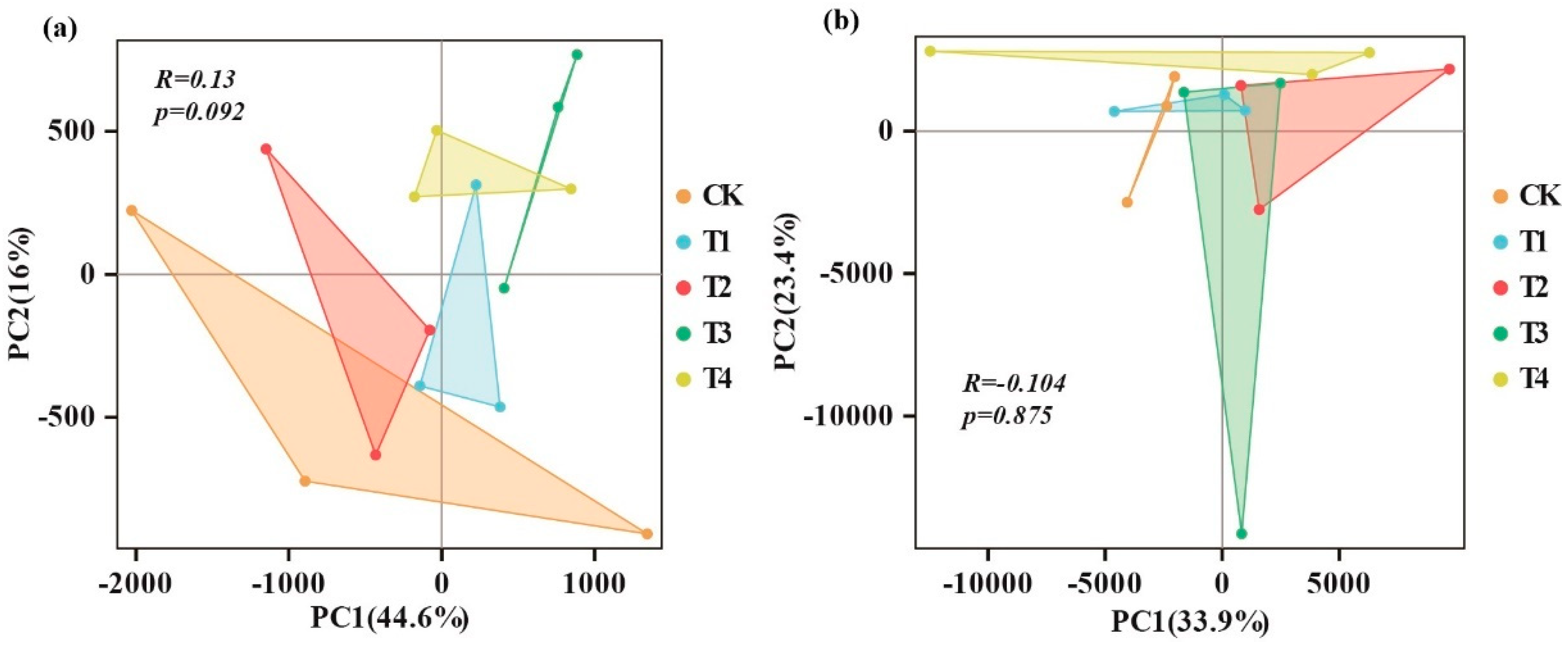
3.2. Soil Microbial Structural Composition and Community Distribution
3.3. Soil Microbial Community Diversity and Richness
3.4. Soil Microbial Community Co-Occurrence Networks
3.5. Multifunctionality of Soil Ecosystems
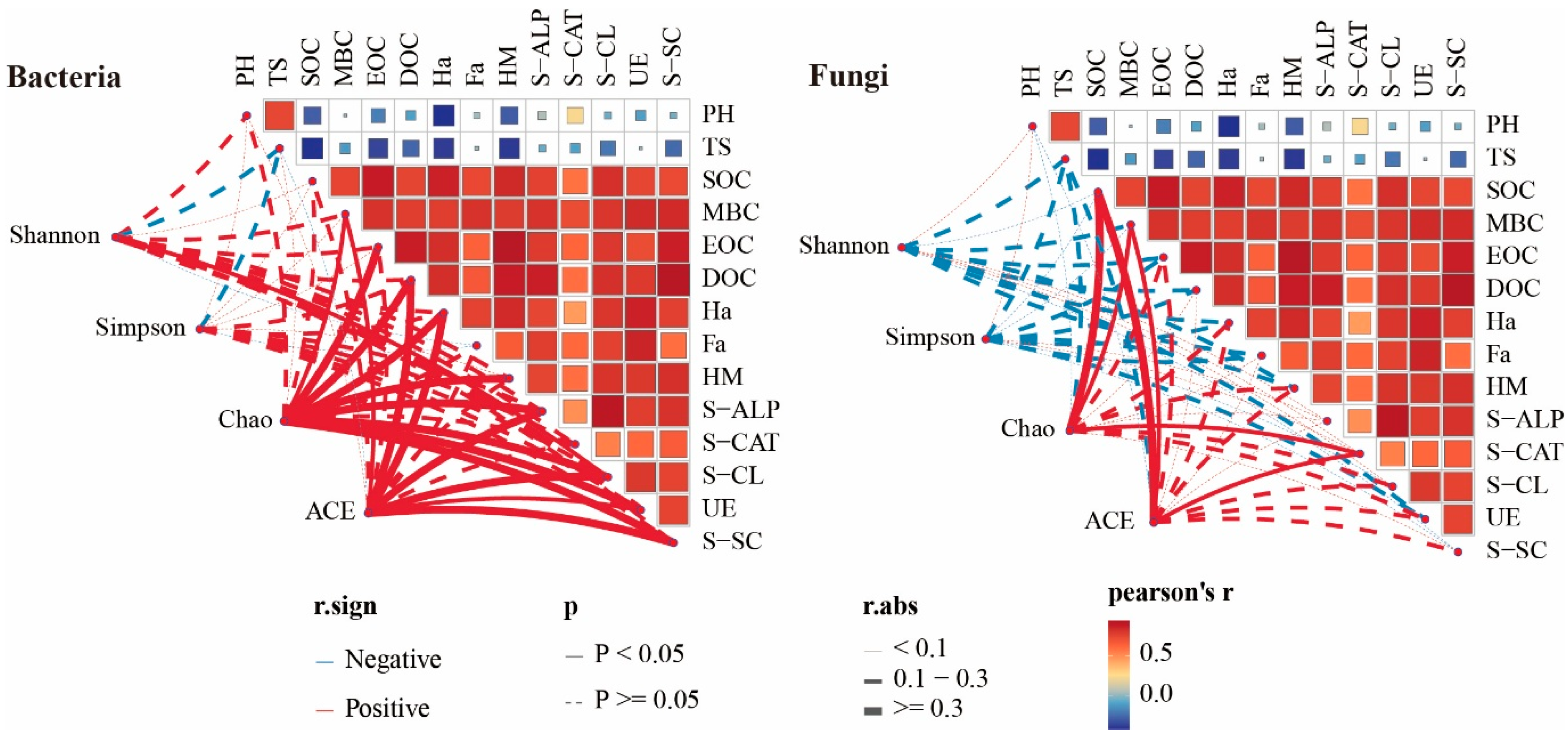
3.6. Correlation Analysis Between Soil Physicochemical Properties and Microorganisms
4. Discussion
4.1. Bio-Organic Fertilizer Changed Corn Yield by Regulating Soil Carbon Pool and Enzyme Activity
4.2. Optimum Organic Fertilization Maintained Higher Soil Microbial Community Diversity and Ecosystem Multifunctionality
4.3. Effect of Soil Properties and Microbial Diversity on Ecosystem Multifunctionality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karnatam, K.S.; Mythri, B.; Un Nisa, W.; Sharma, H.; Meena, T.K.; Rana, P.; Vikal, Y.; Gowda, M.; Dhillon, B.S.; Sandhu, S. Silage maize as a potent candidate for sustainable animal husbandry development—Perspectives and strategies for genetic enhancement. Front. Genet. 2023, 14, 1150132. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, X.; Ni, K. Laws and Regulations on Forage in China. In Research Progress on Forage Production, Processing and Utilization in China; Springer: Singapore, 2022; pp. 291–304. [Google Scholar] [CrossRef]
- Wang, E.; Cha, M.; Wang, S.; Wang, Q.; Wang, Y.; Li, S.; Wang, W. Feeding corn silage or grass hay as sole dietary forage sources: Overall mechanism of forages regulating health-promoting fatty acid status in milk of dairy cows. Foods 2023, 12, 303. [Google Scholar] [CrossRef]
- Wang, X.; Shi, M.; Wang, L. Solutions to Water Scarcity in Arid Regions: Effectiveness of Water Demand Management Policy. J. Nat. Resour. 2013, 28, 1117–1129. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Dai, C. The Adjustment of China’s Grain Planting Structure Reduced the Consumption of Cropland and Water Resources. Int. J. Environ. Res. Public Health 2021, 18, 7352. [Google Scholar] [CrossRef]
- Nikita, B.; Puneet Singh, C. Excessive and Disproportionate Use of Chemicals Cause Soil Contamination and Nutritional Stress. In Soil Contamination; Marcelo, L.L., Sonia, S., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 6. [Google Scholar] [CrossRef]
- Verma, B.C.; Pramanik, P.; Bhaduri, D. Organic Fertilizers for Sustainable Soil and Environmental Management. In Nutrient Dynamics for Sustainable Crop Production; Meena, R.S., Ed.; Springer: Singapore, 2020; pp. 289–313. [Google Scholar] [CrossRef]
- Shaji, H.; Chandran, V.; Mathew, L. Chapter 13—Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 231–245. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Li, J.; Liu, Z.; Gu, X.; Shi, L. Cow manure compost promotes maize growth and ameliorates soil quality in saline-alkali soil: Role of fertilizer addition rate and application depth. Sustainability 2022, 14, 10088. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Xu, X.; Lei, X.; Liao, S.; Li, Y.; Sun, Y. Foliar application of potassium silicate, potassium fulvate and betaine improve summer-time tomato yield by promoting plant nitrogen and potassium uptake. Folia Hortic. 2022, 34, 125–138. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus amyloliquefaciens: Harnessing its potential for industrial, medical, and agricultural applications—A comprehensive review. Microorganisms 2023, 11, 2215. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, G.; Chhabra, S.; Prasad, R. Role of soil microbes in biogeochemical cycle for enhancing soil fertility. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–157. [Google Scholar] [CrossRef]
- Sadeghi, S.; Petermann, B.J.; Steffan, J.J.; Brevik, E.C.; Gedeon, C. Predicting microbial responses to changes in soil physical and chemical properties under different land management. Appl. Soil Ecol. 2023, 188, 104878. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Cao, X.; Liu, L.; Ma, Q.; Lu, R.; Kong, H.; Kong, Y.; Zhu, L.; Zhu, C.; Tian, W.; Jin, Q.; et al. Optimum organic fertilization enhances rice productivity and ecological multifunctionality via regulating soil microbial diversity in a double rice cropping system. Field Crops Res. 2024, 318, 109569. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; Wang, G. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Ouyang, Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: A meta-analysis of field studies. Soil Biol. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Pan, J.; Shang, Y.; Zhang, W.J.; Chen, X.; Cui, Z. Improving soil quality for higher grain yields in Chinese wheat and maize production. Land Degrad. Dev. 2020, 31, 1125–1137. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Li, Y.; Han, X.; Li, B.; Zhang, X.; Li, Q.; Liang, W. Organic substitutions improve soil quality and maize yield through increasing soil microbial diversity. J. Clean. Prod. 2022, 347, 131323. [Google Scholar] [CrossRef]
- Qaswar, M.; Jing, H.; Ahmed, W.; Dongchu, L.; Shujun, L.; Lu, Z.; Cai, A.; Lisheng, L.; Yongmei, X.; Jusheng, G. Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res. 2020, 198, 104569. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.-S. The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Loeppmann, S.; Blagodatskaya, E.; Pausch, J.; Kuzyakov, Y. Substrate quality affects kinetics and catalytic efficiency of exo-enzymes in rhizosphere and detritusphere. Soil Biol. Biochem. 2016, 92, 111–118. [Google Scholar] [CrossRef]
- DeForest, J.L.; Smemo, K.A.; Burke, D.J.; Elliott, H.L.; Becker, J.C. Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry 2012, 109, 189–202. [Google Scholar] [CrossRef]
- Zhang, H.; Goll, D.S.; Wang, Y.P.; Ciais, P.; Wieder, W.R.; Abramoff, R.; Huang, Y.; Guenet, B.; Prescher, A.K.; Viscarra Rossel, R.A. Microbial dynamics and soil physicochemical properties explain large-scale variations in soil organic carbon. Glob. Change Biol. 2020, 26, 2668–2685. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Liu, C.; Cui, B.; Zeleke, K.T.; Hu, C.; Wu, H.; Cui, E.; Huang, P.; Gao, F. Risk of secondary soil salinization under mixed irrigation using brackish water and reclaimed Water. Agronomy 2021, 11, 2039. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Qin, G.; Zou, K.; He, F.; Shao, J.; Zuo, B.; Liu, J.; Liu, R.; Yang, B.; Zhao, G. Simultaneous determination of volatile phenol, cyanide, anionic surfactant and ammonia nitrogen in drinking, ground and surface water and in wastewater applying continuous flow analyzer. Sci. Rep. 2022, 13, 1829. [Google Scholar] [CrossRef]
- Ahrarai, M.; Owliaie, H.; Adhami, E.; Najafi Ghiri, M. Study of potassium status and evaluating chemical extractants for estimating available K in some soils of olive orchards of Fars Province. Water Soil 2017, 31, 835–845. [Google Scholar] [CrossRef]
- Wang, J.; Lin, C.; Han, Z.; Fu, C.; Huang, D.; Cheng, H. Dissolved nitrogen in salt-affected soils reclaimed by planting rice: How is it influenced by soil physicochemical properties? Sci. Total Environ. 2022, 824, 153863. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Q.; Wang, C.; Li, B.; Stomph, T.J.; Yang, J.; Tao, Q.; Yuan, S.; Tang, X.; Ge, J. Negative effects of urbanization on agricultural soil easily oxidizable organic carbon down the profile of the Chengdu Plain, China. Land Degrad. Dev. 2020, 31, 404–416. [Google Scholar] [CrossRef]
- Tuo, Y.; Wang, Z.; Zheng, Y.; Shi, X.; Liu, X.; Ding, M.; Yang, Q. Effect of water and fertilizer regulation on the soil microbial biomass carbon and nitrogen, enzyme activity, and saponin content of Panax notoginseng. Agric. Water Manag. 2023, 278, 108145. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Wang, K.; Li, D. Biochar drives humus formation during composting by regulating the specialized metabolic features of microbiome. Chem. Eng. J. 2023, 458, 141380. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods; Agriculture Press: Beijing, China, 1986; Volume 1986. [Google Scholar]
- Wang, X.; Li, F.Y.; Wang, Y.; Liu, X.; Cheng, J.; Zhang, J.; Baoyin, T.; Bardgett, R.D. High ecosystem multifunctionality under moderate grazing is associated with high plant but low bacterial diversity in a semi-arid steppe grassland. Plant Soil 2020, 448, 265–276. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Niu, S.; Tian, D.; Wu, Q.; Gao, X.; Schellenberg, M.P.; Han, G. Diversity of plant and soil microbes mediates the response of ecosystem multifunctionality to grazing disturbance. Sci. Total Environ. 2021, 776, 145730. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Liu, L.; Li, T.; Dou, Y.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Nitrogen fertilization weakens the linkage between soil carbon and microbial diversity: A global meta-analysis. Glob. Change Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef]
- Chen, S.; Xu, C.; Yan, J.; Zhang, X.; Zhang, X.; Wang, D. The influence of the type of crop residue on soil organic carbon fractions: An 11-year field study of rice-based cropping systems in southeast China. Agric. Ecosyst. Environ. 2016, 223, 261–269. [Google Scholar] [CrossRef]
- Hu, Q.; Thomas, B.W.; Powlson, D.; Hu, Y.; Zhang, Y.; Jun, X.; Shi, X.; Zhang, Y. Soil organic carbon fractions in response to soil, environmental and agronomic factors under cover cropping systems: A global meta-analysis. Agric. Ecosyst. Environ. 2023, 355, 108591. [Google Scholar] [CrossRef]
- Fang, Y.; Nazaries, L.; Singh, B.K.; Singh, B.P. Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob. Change Biol. 2018, 24, 2775–2790. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, Y.; Wang, Q.; Li, M.; Sun, H.; Liu, N.; Zhang, L.; Zhang, Y.; Liu, Z. Effects of potassium fulvic acid and potassium humate on microbial biodiversity in bulk soil and rhizosphere soil of Panax ginseng. Microbiol. Res. 2022, 254, 126914. [Google Scholar] [CrossRef]
- Mao, H.; Lv, Z.; Sun, H.; Li, R.; Zhai, B.; Wang, Z.; Awasthi, M.K.; Wang, Q.; Zhou, L. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour. Technol. 2018, 258, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhou, W.; Guo, S.; Zhu, R.; Qin, Y.; Sun, J. Responses of crop yields, soil enzymatic activities, and microbial communities to different long-term organic materials applied with chemical fertilizer in purple soil. Eur. J. Soil Biol. 2021, 105, 103319. [Google Scholar] [CrossRef]
- Hasan, H. Ureolytic microorganisms and soil fertility: A review. Commun. Soil Sci. Plant Anal. 2000, 31, 2565–2589. [Google Scholar] [CrossRef]
- Ma, Q.; Wen, Y.; Wang, D.; Sun, X.; Hill, P.W.; Macdonald, A.; Chadwick, D.R.; Wu, L.; Jones, D.L. Farmyard manure applications stimulate soil carbon and nitrogen cycling by boosting microbial biomass rather than changing its community composition. Soil Biol. Biochem. 2020, 144, 107760. [Google Scholar] [CrossRef]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma 2018, 319, 156–166. [Google Scholar] [CrossRef]
- Ali, A.; Liu, X.; Yang, W.; Li, W.; Chen, J.; Qiao, Y.; Gao, Z.; Yang, Z. Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat–Maize Rotation System. Agronomy 2024, 14, 1942. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, Y.; Yin, J.; Li, D.; Wang, B.; Zhang, K.; Zheng, X.; Hong, Y.; Zhang, H.; Xie, C. Productivity and quality of banana in response to chemical fertilizer reduction with bio-organic fertilizer: Insight into soil properties and microbial ecology. Agric. Ecosyst. Environ. 2021, 322, 107659. [Google Scholar] [CrossRef]
- Du, T.; Hu, Q.; He, H.; Mao, W.; Yang, Z.; Chen, H.; Sun, L.; Zhai, M. Long-term organic fertilizer and biofertilizer application strengthens the associations between soil quality index, network complexity, and walnut yield. Eur. J. Soil Biol. 2023, 116, 103492. [Google Scholar] [CrossRef]
- Rastogi, M.; Verma, S.; Kumar, S.; Bharti, S.; Kumar, G.; Azam, K.; Singh, V. Soil health and sustainability in the age of organic amendments: A review. Int. J. Environ. Clim. Chang. 2023, 13, 2088–2102. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liao, G.; Banerjee, S.; Gu, S.; Liang, J.; Guo, X.; Zhao, H.; Liang, Y.; Li, T. Long-term organic fertilization promotes the resilience of soil multifunctionality driven by bacterial communities. Soil Biol. Biochem. 2023, 177, 108922. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, J.; Wang, B.; Fan, B.; Zhou, G. Soil microbial network complexity predicts soil multifunctionality better than soil microbial diversity during grassland-farmland-shrubland conversion on the Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2025, 379, 109356. [Google Scholar] [CrossRef]
- Quan, T.; Yongqiu, X.; Chaopu, T.; Jun, S.; Wei, Z.; Chenglin, L.; Xing, Y.; Xiaoyuan, Y. Partial organic fertilizer substitution promotes soil multifunctionality by increasing microbial community diversity and complexity. Pedosphere 2023, 33, 407–420. [Google Scholar] [CrossRef]
- Idbella, M.; Baronti, S.; Giagnoni, L.; Renella, G.; Becagli, M.; Cardelli, R.; Maienza, A.; Vaccari, F.P.; Bonanomi, G. Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: Results from a 10-year field vineyard experiment. Appl. Soil Ecol. 2024, 195, 105217. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Hu, Y.; Xia, L.; Fan, K.; Zhang, Y.; Zhang, Y.; Zhou, W. Ecosystem multifunctionality and soil microbial communities in response to ecological restoration in an alpine degraded grassland. Front. Plant Sci. 2023, 14, 1173962. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Tardy, V.; Spor, A.; Mathieu, O.; Lévèque, J.; Terrat, S.; Plassart, P.; Regnier, T.; Bardgett, R.D.; van Der Putten, W.H.; Roggero, P.P. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Tunlid, A.; Floudas, D.; Koide, R.; Rineau, F. Soil organic matter decomposition mechanisms in ectomycorrhizal fungi. In Molecular Mycorrhizal Symbiosis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 257–275. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, N.; Zhang, S.; Zhu, X.; Wang, H.; Xiu, W.; Zhao, J.; Liu, H.; Zhang, H.; Yang, D. Soil bacterial community composition is altered more by soil nutrient availability than pH following long-term nutrient addition in a temperate steppe. Front. Microbiol. 2024, 15, 1455891. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, A.A.; Aburto, F.; González-Rocha, G.; Guzmán, C.M.; Schmidt, R.; Scow, K. Anthropogenic degradation alter surface soil biogeochemical pools and microbial communities in an Andean temperate forest. Sci. Total Environ. 2023, 854, 158508. [Google Scholar] [CrossRef] [PubMed]
- Fayuan, W.; Rengel, Z. Disentangling the contributions of arbuscular mycorrhizal fungi to soil multifunctionality. Pedosphere 2024, 34, 269–278. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]



| Treatments | Bacteria | Fungi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | T4 | CK | T1 | T2 | T3 | T4 | |
| Nodes | 231 | 233 | 244 | 237 | 248 | 149 | 129 | 138 | 141 | 145 |
| Links | 3676 | 4056 | 4295 | 3956 | 4487 | 1341 | 925 | 965 | 1128 | 1236 |
| Positive links | 1922 | 2123 | 2204 | 2099 | 2584 | 946 | 729 | 708 | 945 | 1017 |
| Negative links | 1754 | 1933 | 2091 | 1857 | 1903 | 395 | 196 | 257 | 183 | 219 |
| Positive links% | 52.29 | 52.34 | 51.32 | 53.06 | 57.59 | 70.54 | 78.81 | 73.37 | 83.78 | 82.28 |
| Average degree | 31.827 | 36.053 | 35.205 | 33.384 | 39.17 | 16.868 | 14.341 | 13.986 | 16 | 17.048 |
| Modularity | 0.718 | 0.722 | 0.753 | 0.809 | 0.825 | 0.831 | 0.845 | 0.873 | 0.836 | 0.875 |
| Grpah density | 0.138 | 0.161 | 0.145 | 0.141 | 0.176 | 0.107 | 0.112 | 0.102 | 0.114 | 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Nian, L.; Zhao, X.; Li, J.; Wang, Z.; Dong, L. Bio-Organic Fertilizer Application Enhances Silage Maize Yield by Regulating Soil Physicochemical and Microbial Properties. Microorganisms 2025, 13, 959. https://doi.org/10.3390/microorganisms13050959
Tang Y, Nian L, Zhao X, Li J, Wang Z, Dong L. Bio-Organic Fertilizer Application Enhances Silage Maize Yield by Regulating Soil Physicochemical and Microbial Properties. Microorganisms. 2025; 13(5):959. https://doi.org/10.3390/microorganisms13050959
Chicago/Turabian StyleTang, Ying, Lili Nian, Xu Zhao, Juan Li, Zining Wang, and Liuwen Dong. 2025. "Bio-Organic Fertilizer Application Enhances Silage Maize Yield by Regulating Soil Physicochemical and Microbial Properties" Microorganisms 13, no. 5: 959. https://doi.org/10.3390/microorganisms13050959
APA StyleTang, Y., Nian, L., Zhao, X., Li, J., Wang, Z., & Dong, L. (2025). Bio-Organic Fertilizer Application Enhances Silage Maize Yield by Regulating Soil Physicochemical and Microbial Properties. Microorganisms, 13(5), 959. https://doi.org/10.3390/microorganisms13050959





