COVID-19 Vaccine Effectiveness and Risk Factors of Booster Failure in 480,000 Patients with Diabetes Mellitus: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Outcomes
3.3. Vaccine Effectivennes
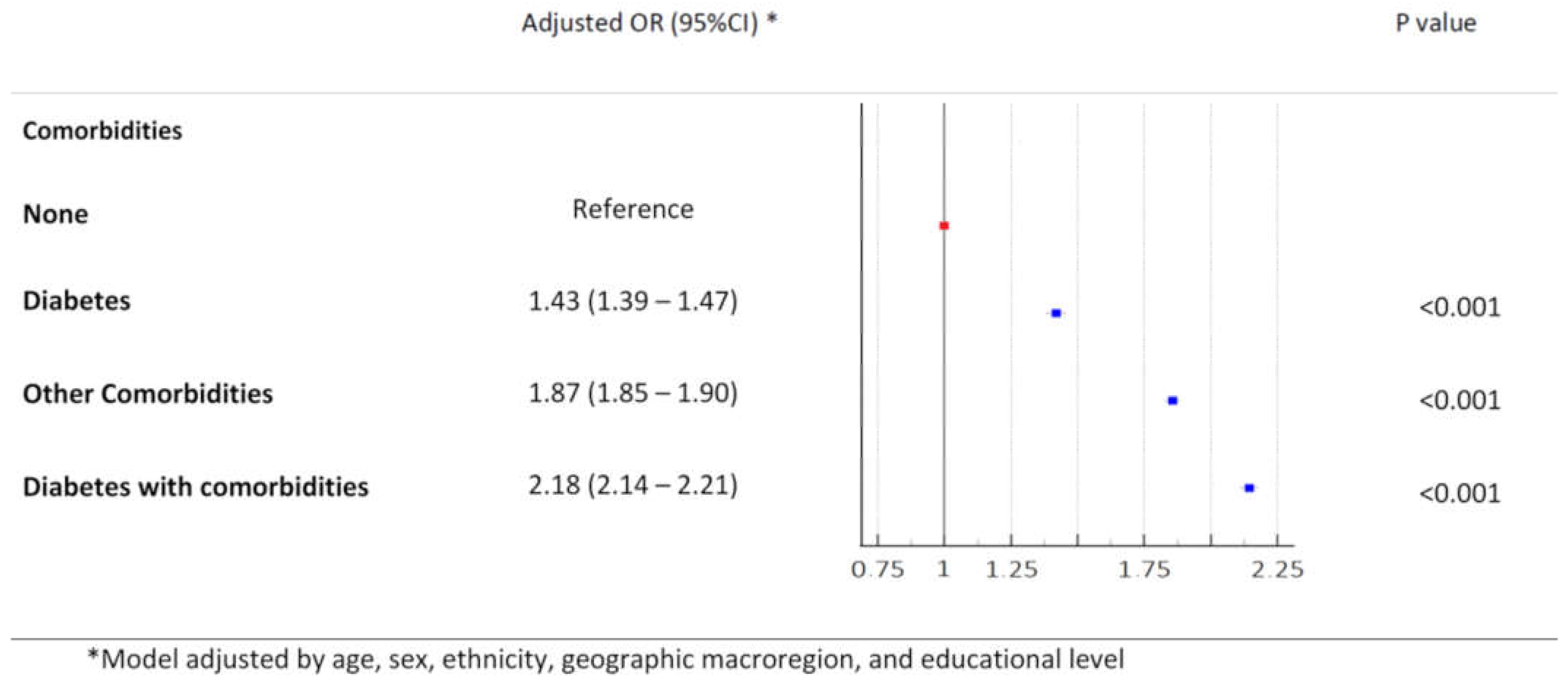
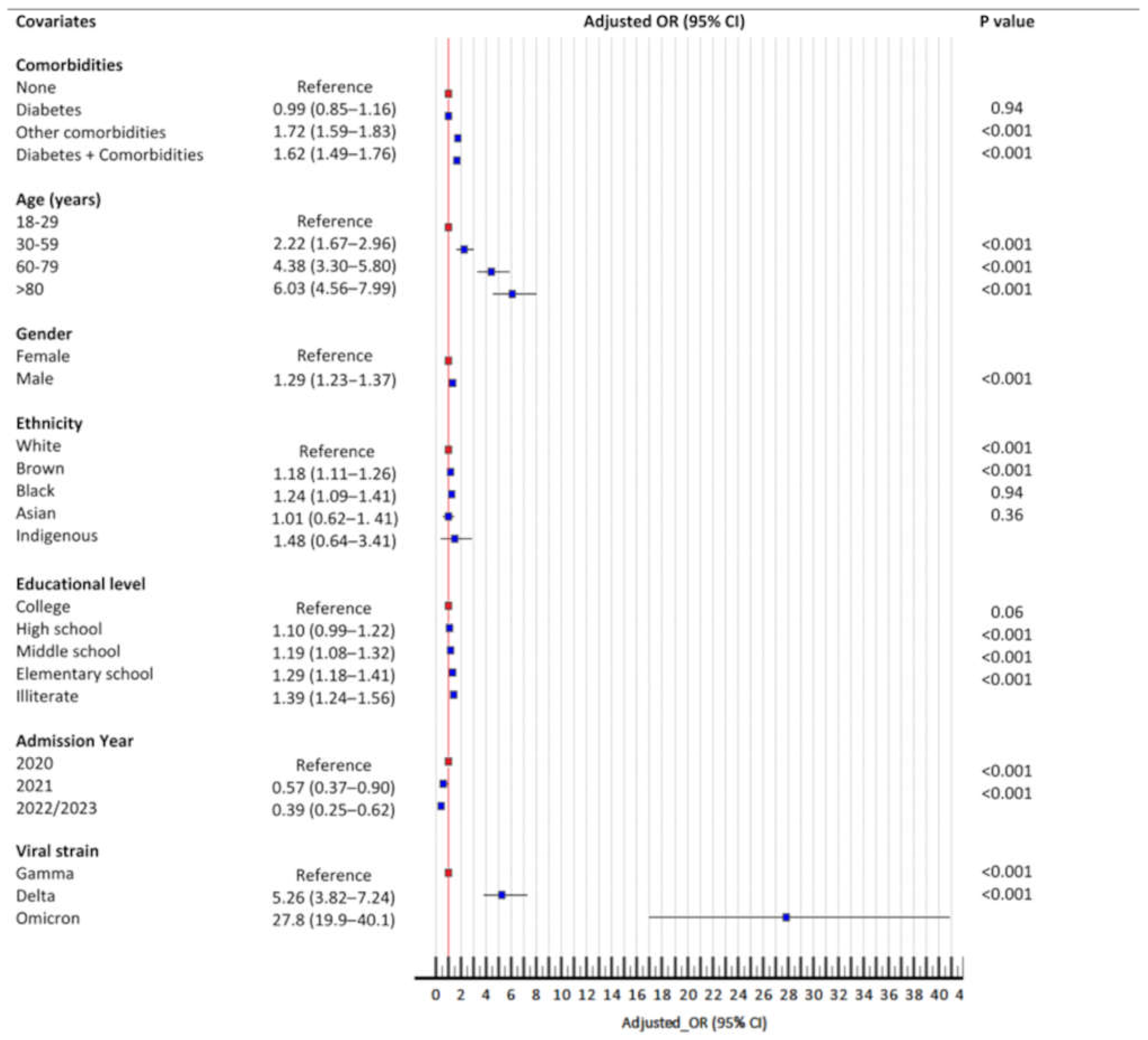
3.4. Booster Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| DM | Diabetes mellitus |
| WHO | World Health Organization |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SIVEP-Gripe | Influenza Epidemiological Surveillance Information System |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| aOR | Adjusted odds ratio |
| CI | Confidence interval |
References
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.A.; Khan, M.N.; Mustagir, M.G.; Rana, J.; Islam, M.S.; Kabir, M.I. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health 2020, 10, 020503. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; El Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: A systematic review and meta-analysis of observational studies. BMJ Open 2021, 11, e052777. [Google Scholar] [CrossRef]
- Treskova-Schwarzbach, M.; Haas, L.; Reda, S.; Pilic, A.; Borodova, A.; Karimi, K.; Koch, J.; Nygren, T.; Scholz, S.; Schonfeld, V.; et al. Pre-existing health conditions and severe COVID-19 outcomes: An umbrella review approach and meta-analysis of global evidence. BMC Med. 2021, 19, 212. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Bradley, S.A.; Banach, M.; Alvarado, N.; Smokovski, I.; Bhaskar, S.M.M. Prevalence and impact of diabetes in hospitalized COVID-19 patients: A systematic review and meta-analysis. J. Diabetes 2022, 14, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef]
- Khunti, K.; Valabhji, J.; Misra, S. Diabetes and the COVID-19 pandemic. Diabetologia 2023, 66, 255–266. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Mak, R.H.; Colosimo, E.A.; Mendonca, A.C.Q.; Vasconcelos, M.A.; Martelli-Junior, H.; Silva, L.R.; Oliveira, M.C.L.; Pinhati, C.C.; Simoes, E.S.A.C. Risk factors for COVID-19-related mortality in hospitalized children and adolescents with diabetes mellitus: An observational retrospective cohort study. Pediatr. Diabetes 2022, 23, 763–772. [Google Scholar] [CrossRef]
- Li, R.; Shen, M.; Yang, Q.; Fairley, C.K.; Chai, Z.; McIntyre, R.; Ong, J.J.; Liu, H.; Lu, P.; Hu, W.; et al. Global Diabetes Prevalence in COVID-19 Patients and Contribution to COVID-19- Related Severity and Mortality: A Systematic Review and Meta-analysis. Diabetes Care 2023, 46, 890–897. [Google Scholar] [CrossRef]
- Kass-Gergi, S.; Zhao, G.; Wong, J.; Weiner, A.I.; Adams Tzivelekidis, S.; Gentile, M.E.; Mendoza, M.; Holcomb, N.P.; Li, X.; Singh, M.; et al. Disruption of immune responses by type 1 diabetes exacerbates SARS-CoV-2 mediated lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L839–L851. [Google Scholar] [CrossRef] [PubMed]
- Bonyek-Silva, I.; Machado, A.F.A.; Cerqueira-Silva, T.; Nunes, S.; Silva Cruz, M.R.; Silva, J.; Santos, R.L.; Barral, A.; Oliveira, P.R.S.; Khouri, R.; et al. LTB(4)-Driven Inflammation and Increased Expression of ALOX5/ACE2 During Severe COVID-19 in Individuals With Diabetes. Diabetes 2021, 70, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Rockman-Greenberg, C.; Sareen, N.; Lionetti, V.; Dhingra, S. An insight into the mechanisms of COVID-19, SARS-CoV2 infection severity concerning beta-cell survival and cardiovascular conditions in diabetic patients. Mol. Cell Biochem. 2022, 477, 1681–1695. [Google Scholar] [CrossRef]
- Ashique, S.; Mishra, N.; Garg, A.; Garg, S.; Farid, A.; Rai, S.; Gupta, G.; Dua, K.; Paudel, K.R.; Taghizadeh-Hesary, F. A Critical Review on the Long-Term COVID-19 Impacts on Patients With Diabetes. Am. J. Med. 2025, 138, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Bende, F.; Fofiu, R.; Enache, A.; Pescariu, S.A.; Novacescu, D. The Impact of Metabolic Syndrome and Obesity on the Evolution of Diastolic Dysfunction in Apparently Healthy Patients Suffering from Post-COVID-19 Syndrome. Biomedicines 2022, 10, 1519. [Google Scholar] [CrossRef]
- Lee, C.H.; Gray, V.; Teo, J.M.N.; Tam, A.R.; Fong, C.H.; Lui, D.T.; Pang, P.; Chan, K.H.; Hung, I.F.; Tan, K.C.; et al. Comparing the B and T cell-mediated immune responses in patients with type 2 diabetes receiving mRNA or inactivated COVID-19 vaccines. Front. Immunol. 2022, 13, 1018393. [Google Scholar] [CrossRef]
- McGovern, A.P.; Thomas, N.J.; Vollmer, S.J.; Hattersley, A.T.; Mateen, B.A.; Dennis, J.M. The disproportionate excess mortality risk of COVID-19 in younger people with diabetes warrants vaccination prioritisation. Diabetologia 2021, 64, 1184–1186. [Google Scholar] [CrossRef]
- Boroumand, A.B.; Forouhi, M.; Karimi, F.; Moghadam, A.S.; Naeini, L.G.; Kokabian, P.; Naderi, D. Immunogenicity of COVID-19 vaccines in patients with diabetes mellitus: A systematic review. Front. Immunol. 2022, 13, 940357. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Cockram, C.S.; Ma, R.C.W.; Luk, A.O.Y. Diabetes and infection: Review of the epidemiology, mechanisms and principles of treatment. Diabetologia 2024, 67, 1168–1180. [Google Scholar] [CrossRef]
- Ekpor, E.; Akyirem, S. Global acceptance of COVID-19 vaccine among persons with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2023, 201, 110731. [Google Scholar] [CrossRef]
- Dias, C.S.; Diniz, L.M.; Oliveira, M.C.L.; Simoes, E.S.A.C.; Colosimo, E.A.; Mak, R.H.; Pinhati, C.C.; Galante, S.C.; Veloso, I.Y.; Martelli-Junior, H.; et al. Outcomes of SARS-CoV-2 and Seasonal Viruses Among Children Hospitalized in Brazil. Pediatrics 2024, 153, e2023064326. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.M.; Dias, C.S.; Oliveira, M.C.L.; Simoes e Silva, A.C.; Colosimo, E.A.; Mak, R.; Pinhati, C.C.; Galante, S.C.; Yan, I.O.; martelli-Junior, H.; et al. Outcomes of SARS-CoV-2 and seasonal viruses among 2 million adults hospitalized for severe acute respiratory infection during the COVID-19 pandemic in Brazil. J. Infect. Dis. 2024. Online ahead of print. [Google Scholar]
- Cho, K.; Park, S.; Kim, E.Y.; Koyanagi, A.; Jacob, L.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Radua, J.; Elena, D.; et al. Immunogenicity of COVID-19 vaccines in patients with diverse health conditions: A comprehensive systematic review. J. Med. Virol. 2022, 94, 4144–4155. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.M.; Remmelzwaal, S.; Blom, M.T.; van Hoek, B.; Swart, K.M.A.; Overbeek, J.A.; Burchell, G.L.; Herings, R.M.C.; Elders, P.J.M. Effectiveness of COVID-19 Vaccines in Adults with Diabetes Mellitus: A Systematic Review. Vaccines 2022, 11, 24. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Colosimo, E.A.; Simoes, E.S.A.C.; Mak, R.H.; Martelli, D.B.; Silva, L.R.; Martelli-Junior, H.; Oliveira, M.C.L. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: An analysis of a nationwide database. Lancet Child. Adolesc. Health 2021, 5, 559–568. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Oliveira, M.C.L.; Simoes, E.S.A.C.; Dias, C.S.; Diniz, L.M.; Colosimo, E.A.; Mak, R.H.; Vasconcelos, M.A.; Pinhati, C.C.; Galante, S.C.; et al. A Population-Based Epidemiologic Study of Symptomatic SARS-CoV-2 Infections and Fatalities in Brazilian Children over 3 Years. J. Pediatr. 2024, 276, 114267. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Putter, H.; Fiocco, M.; Geskus, R.B. Tutorial in biostatistics: Competing risks and multi-state models. Stat. Med. 2007, 26, 2389–2430. [Google Scholar] [CrossRef]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Cheng, B.; Chen, J.; Peng, A.; Yang, C.; Liu, C.; Xiong, M.; Deng, A.; Zhang, Y.; et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care 2020, 43, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Zaccardi, F.; Gillies, C.L.; Razieh, C.; Yates, T.; Kloecker, D.E.; Rowlands, A.V.; Davies, M.J.; Islam, N.; Seidu, S.; et al. Patterns of multimorbidity and risk of severe SARS-CoV-2 infection: An observational study in the U.K. BMC Infect. Dis. 2021, 21, 908. [Google Scholar] [CrossRef]
- Roncon, L.; Zuin, M.; Rigatelli, G.; Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020, 127, 104354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; She, Z.G.; Cheng, X.; Qin, J.J.; Zhang, X.J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e1063. [Google Scholar] [CrossRef]
- Li, C.; Islam, N.; Gutierrez, J.P.; Gutierrez-Barreto, S.E.; Castaneda Prado, A.; Moolenaar, R.L.; Lacey, B.; Richter, P. Associations of diabetes, hypertension and obesity with COVID-19 mortality: A systematic review and meta-analysis. BMJ Glob. Health 2023, 8, e012581. [Google Scholar] [CrossRef]
- Schlesinger, S.; Lang, A.; Christodoulou, N.; Linnerz, P.; Pafili, K.; Kuss, O.; Herder, C.; Neuenschwander, M.; Barbaresko, J.; Roden, M. Risk phenotypes of diabetes and association with COVID-19 severity and death: An update of a living systematic review and meta-analysis. Diabetologia 2023, 66, 1395–1412. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, F.R.; Tajada, M.; Saviron-Cornudella, R.; Sanchez-Prieto, M.; Chedraui, P.; Teran, E. Coronavirus disease 2019 and gender-related mortality in European countries: A meta-analysis. Maturitas 2020, 141, 59–62. [Google Scholar] [CrossRef]
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.R.; Franssen, G.H.L.; Hendriks, S.; Richters, A.; Venemans-Jellema, A.; Zalpuri, S.; et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef]
- Romero Starke, K.; Reissig, D.; Petereit-Haack, G.; Schmauder, S.; Nienhaus, A.; Seidler, A. The isolated effect of age on the risk of COVID-19 severe outcomes: A systematic review with meta-analysis. BMJ Glob. Health 2021, 6, e006434. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Mathioudakis, A.G.; Nikitara, K.; Stamatelopoulos, K.; Georgiopoulos, G.; Phalkey, R.; Leonardi-Bee, J.; Fernandez, E.; Carnicer-Pont, D.; Vestbo, J.; et al. Prognostic factors for mortality, intensive care unit and hospital admission due to SARS-CoV-2: A systematic review and meta-analysis of cohort studies in Europe. Eur. Respir. Rev. 2022, 31. [Google Scholar] [CrossRef]
- Baqui, P.; Bica, I.; Marra, V.; Ercole, A.; van der Schaar, M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: A cross-sectional observational study. Lancet Glob. Health 2020, 8, e1018–e1026. [Google Scholar] [CrossRef] [PubMed]
- Baqui, P.; Marra, V.; Alaa, A.M.; Bica, I.; Ercole, A.; van der Schaar, M. Comparing COVID-19 risk factors in Brazil using machine learning: The importance of socioeconomic, demographic and structural factors. Sci. Rep. 2021, 11, 15591. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.; Simoes, E.S.A.C.; Oliveira, M.C.L.; Colosimo, E.A.; Mak, R.H.; Vasconcelos, M.A.; Miranda, D.M.; Martelli, D.B.; Silva, L.R.; Pinhati, C.C.; et al. Comparison of the First and Second Waves of the Coronavirus Disease 2019 Pandemic in Children and Adolescents in a Middle-Income Country: Clinical Impact Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Gamma Lineage. J. Pediatr. 2022, 244, 178–185.e173. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Poland, G.A. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care 2000, 23, 95–108. [Google Scholar] [CrossRef]
- Ceriello, A. Diabetes, SARS-CoV-2/COVID-19 vaccines and glycemic control: Call for data. Diabetes Res. Clin. Pract. 2021, 174, 108741. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K.; Misra, A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab. Syndr. 2021, 15, 505–508. [Google Scholar] [CrossRef]
- Whitaker, H.J.; Tsang, R.S.M.; Byford, R.; Andrews, N.J.; Sherlock, J.; Sebastian Pillai, P.; Williams, J.; Button, E.; Campbell, H.; Sinnathamby, M.; et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response amongst individuals in clinical risk groups. J. Infect. 2022, 84, 675–683. [Google Scholar] [CrossRef]
- Molnar, G.A.; Voko, Z.; Suto, G.; Rokszin, G.; Nagy, D.; Surjan, G.; Surjan, O.; Nagy, P.; Kenessey, I.; Weber, A.; et al. Effectiveness of SARS-CoV-2 primary vaccines and boosters in patients with type 2 diabetes mellitus in Hungary (HUN-VE 4 Study). BMJ Open Diabetes Res. Care 2024, 12, e003777. [Google Scholar] [CrossRef]
- Perez-Alos, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Moller, D.L.; Fogh, K.; et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Houhamdi, L.; Hoang, V.T.; Stoupan, D.; Fournier, P.E.; Raoult, D.; Colson, P.; Gautret, P. High rate of reinfection with the SARS-CoV-2 Omicron variant. J. Infect. 2022, 85, 174–211. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O'Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espie, E.; Feikin, D.R.; Knoll, M.D. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Vos, E.R.A.; van Hagen, C.C.E.; Wong, D.; Smits, G.; Kuijer, M.; Wijmenga-Monsuur, A.J.; Kaczorowska, J.; van Binnendijk, R.S.; van der Klis, F.R.M.; den Hartog, G.; et al. SARS-CoV-2 Seroprevalence Trends in the Netherlands in the Variant of Concern Era: Input for Future Response. Influenza Other Respir. Viruses 2024, 18, e13312. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Dabrera, G.; Ramsay, M.; Lopez Bernal, J. Effectiveness of the Sanofi/GSK (VidPrevtyn Beta) and Pfizer-BioNTech (Comirnaty Original/Omicron BA.4-5) bivalent vaccines against hospitalisation in England. EClinicalMedicine 2024, 71, 102587. [Google Scholar] [CrossRef] [PubMed]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Paul Chourdhury, R.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef]
- Rahmati, M.; Shamsi, M.M.; Khoramipour, K.; Malakoutinia, F.; Woo, W.; Park, S.; Yon, D.K.; Lee, S.W.; Shin, J.I.; Smith, L. Baseline physical activity is associated with reduced mortality and disease outcomes in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2022, 32, e2349. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Naqvi, R.A.; Alizadehsani, R.; Hussain, S.; Moqurrab, S.A.; Lee, S.W. An adaptive ensemble deep learning framework for reliable detection of pandemic patients. Comput. Biol. Med. 2024, 168, 107836. [Google Scholar] [CrossRef]



| Covariates a | Group 1 (%) 911,270 (42.8) | Group 2 (%) 737,142 (34.6) | Group 3 (%) 100,849 (4.7) | Group 4 (%) 381,828 (17.9) |
|---|---|---|---|---|
| Age (years) | ||||
| Median (IQR) | 51.83 (40.1–65.25) | 64.6 (51.66–76.91) | 61.6 (51.58–71.83) | 68.08 (58.83–76.91) |
| Mean (SD) | 53.35 (17.28) | 63.71 (17.1) | 61.41 (14.76) | 67.44 (13.23) |
| Age group (years) | ||||
| 18–29.9 | 69,554 (7.6) | 20,811 (2.8) | 1945 (1.9) | 1862 (0.5) |
| 30–59.9 | 537,178 (58.9) | 279,165 (37.9) | 44,252 (43.9) | 103,662 (27.1) |
| 60–79.9 | 228,173 (25) | 293,043 (39.8) | 43,535 (43.2) | 207,714 (54.4) |
| >80 | 76,365 (8.4) | 144,123 (19.6) | 11,117 (11.0) | 68,590 (18.0) |
| Sex (n = 2,131,073) | ||||
| Male | 533,422 (58.5) | 393,956 (53.4) | 56,109 (55.6) | 189,280 (49.6) |
| Female | 377,839 (41.5) | 343,183 (46.6) | 44,738 (44.4) | 192,546 (50.4) |
| Region | ||||
| Southeast | 433,950 (47.6) | 374,041 (50.7) | 50,569 (50.1) | 193,660 (50.7) |
| South | 144,818 (15.9) | 138,439 (18.8) | 13,710 (13.6) | 66,197 (17.3) |
| Central-West | 104,479 (11.5) | 69,435 (9.4) | 9303 (9.2) | 32,642 (8.5) |
| Northeast | 152,970 (16.8) | 117,259 (15.9) | 19,213 (19.1) | 69,816 (18.3) |
| North | 75,053 (8.2) | 37,968 (5.2) | 8054 (8) | 19,513 (5.1) |
| Ethnicity (n = 1,739,886) | ||||
| White | 363,718 (49.8) | 334,908 (54.9) | 41,753 (49.6) | 167,992 (53.3) |
| Brown | 323,001 (44.2) | 233,366 (38.3) | 36,268 (43.1) | 122,952 (39.0) |
| Black | 32,919 (4.5) | 33,676 (5.5) | 4671 (5.6) | 19,673 (6.2) |
| Asian | 9230 (1.3) | 6910 (1.1) | 1165 (1.4) | 3869 (1.2) |
| Indigenous | 2045 (0.3) | 1009 (0.2) | 244 (0.3) | 517 (0.2) |
| Educational level (n = 754,185) | ||||
| Illiterate | 14,574 (4.7) | 20,962 (7.8) | 2584 (7.0) | 11,999 (8.7) |
| Elementary | 63,500 (20.5) | 81,871 (30.3) | 11,268 (30.3) | 47,969 (34.9) |
| Middle-School | 53,693 (17.4) | 51,813 (19.2) | 7161 (19.3) | 27,769 (20.2) |
| High-School | 120,259 (38.9) | 78,782 (29.2) | 11,346 (30.5) | 34,693 (25.2) |
| College | 57,371 (18.5) | 36,629 (13.6) | 4793 (12.9) | 15,149 (11.0) |
| Signs/symptoms at presentation | ||||
| Fever | 525,529 (57.7) | 398,280 (54.0) | 56,592 (56.1) | 198,376 (52.0) |
| Cough | 625,239 (68.6) | 499,164 (67.7) | 71,337 (70.7) | 259,120 (67.9) |
| Dyspnea | 600,330 (65.9) | 531,067 (72.0) | 70,560 (70.0) | 281,396 (73.7) |
| Odynophagia | 186,518 (20.5) | 112,622 (15.3) | 18,360 (18.2) | 56,415 (14.8) |
| Oxygen saturation <95% (n = 1,763,683) | 508,688 (70.8) | 490,602 (77.7) | 63,647 (75.1) | 263,152 (79.8) |
| Number of comorbidities | ||||
| None | 911,270 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | 0 (0.0) | 526,822 (71.5) | 100,849 (100) | 0 (0.0) |
| 2 | 0 (0.0) | 170,916 (23.2) | 0 (0.0) | 243,726 (63.8) |
| 3 | 0 (0.0) | 39,404 (5.3) | 0 (0.0) | 138,102 (36.2) |
| Major comorbidities | ||||
| Cardiology | 0 (0.0) | 408,461 (55.4) | 0 (0.0) | 274,329 (71.8) |
| Hypertension | 0 (0.0) | 169,165 (23.0) | 0 (0.0) | 106,296 (27.8) |
| Obesity | 0 (0.0) | 125,903 (17.1) | 0 (0.0) | 59,979 (15.7) |
| Neurologic | 0 (0.0) | 76,911 (10.4) | 0 (0.0) | 32,669 (8.6) |
| Pulmonary | 0 (0.0) | 62,110 (8.4) | 0 (0.0) | 23,492 (6.2) |
| Renal | 0 (0.0) | 41,944 (5.7) | 0 (0.0) | 28,498 (7.5) |
| Immunosuppression | 0 (0.0) | 37,762 (5.1) | 0 (0.0) | 11,088 (2.9) |
| Oncology | 0 (0.0) | 29,882 (4.1) | 0 (0.0) | 7130 (1.9) |
| Hematology | 0 (0.0) | 14,675 (2.0) | 0 (0.0) | 4737 (1.2) |
| Nosocomial | ||||
| No | 899,664 (98.7) | 718,522 (97.5) | 99,327 (98.5) | 372,195 (97.5) |
| Yes | 11,606 (1.3) | 18,620 (2.5) | 1522 (1.5) | 9633 (2.5) |
| SARS-CoV-2 strain | ||||
| Ancestral (predominant in 2020) | 276,520 (30.3) | 38,379 (38.1) | 242,250 (32,9) | 139,114 (36,4) |
| Gamma (more prevalent in 2021) | 509,287 (55.9) | 47,994 (47.6) | 351,679 (47,7) | 170,823 (44,7) |
| Delta (more prevalent in 2021) | 45,467 (5.0) | 4988 (4.9) | 41,267 (5,6) | 23,313 (6,1) |
| Omicron (predominant in 2022 and 2023) | 79,996 (8.8) | 9488 (9.4) | 101,946 (13,8) | 48,578 (12,7) |
| Admission Year | ||||
| 2020 | 276,520 (30.3) | 242,250 (32.9) | 38,379 (38.1) | 139,114 (36.4) |
| 2021 | 559,932 (61.4) | 397,527 (53.9) | 53,695 (53.2) | 196,728 (51.5) |
| 2022/2023 | 74,818 (8.2) | 97,365 (13.2) | 8775 (8.7) | 45,986 (12.0) |
| Vaccine doses (n = 1,887,890) | ||||
| None | 664,759 (72.9) | 490,768 (66.6) | 72,370 (80.1) | 251,177 (73.6) |
| One | 38,292 (4.2) | 38,930 (5.3) | 5126 (5.7) | 21,426 (6.3) |
| Two | 64,008 (7.0) | 81,028 (11.0) | 8731 (9.7) | 45,759 (13.4) |
| Three | 32,684 (3.6) | 45,788 (6.2) | 4107 (4.5) | 22,937 (6.7) |
| ICU (n = 1,836,099) | ||||
| No | 518,821 (69.7) | 386,078 (58.5) | 56,054 (64.2) | 185,766 (53.9) |
| Yes | 225,110 (30.3) | 274,360 (41.5) | 31,235 (35.8) | 158,675 (46.1) |
| Ventilatory support (n = 1,809,738) | ||||
| None | 193,705 (26.5) | 120,912 (18.5) | 18,355 (21.3) | 53,953 (15.9) |
| Non-invasive | 426,169 (58.3) | 381,401 (58.5) | 50,857 (59.0) | 194,575 (57.3) |
| Invasive | 111,639 (15.3) | 149,846 (23) | 17,045 (19.8) | 91,281 (26.9) |
| Mortality rate | ||||
| No | 705,799 (77.5) | 459,048 (62.3) | 67,905 (67.3) | 216,656 (56.7) |
| Yes | 205,471 (22.5) | 278,094 (37.7) | 32,944 (32.7) | 165,172 (43.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.C.L.; Martelli, D.R.; Simões e Silva, A.C.; Dias, C.S.; Diniz, L.M.; Colosimo, E.A.; Pinhati, C.C.; Galante, S.C.; Duelis, F.N.; Carvalho, L.E.; et al. COVID-19 Vaccine Effectiveness and Risk Factors of Booster Failure in 480,000 Patients with Diabetes Mellitus: A Population-Based Cohort Study. Microorganisms 2025, 13, 979. https://doi.org/10.3390/microorganisms13050979
Oliveira MCL, Martelli DR, Simões e Silva AC, Dias CS, Diniz LM, Colosimo EA, Pinhati CC, Galante SC, Duelis FN, Carvalho LE, et al. COVID-19 Vaccine Effectiveness and Risk Factors of Booster Failure in 480,000 Patients with Diabetes Mellitus: A Population-Based Cohort Study. Microorganisms. 2025; 13(5):979. https://doi.org/10.3390/microorganisms13050979
Chicago/Turabian StyleOliveira, Maria Christina L., Daniella R. Martelli, Ana Cristina Simões e Silva, Cristiane S. Dias, Lilian M. Diniz, Enrico A. Colosimo, Clara C. Pinhati, Stella C. Galante, Fernanda N. Duelis, Laura E. Carvalho, and et al. 2025. "COVID-19 Vaccine Effectiveness and Risk Factors of Booster Failure in 480,000 Patients with Diabetes Mellitus: A Population-Based Cohort Study" Microorganisms 13, no. 5: 979. https://doi.org/10.3390/microorganisms13050979
APA StyleOliveira, M. C. L., Martelli, D. R., Simões e Silva, A. C., Dias, C. S., Diniz, L. M., Colosimo, E. A., Pinhati, C. C., Galante, S. C., Duelis, F. N., Carvalho, L. E., Coelho, L. G., Bernardes, M. E. T., Martelli-Júnior, H., de Oliveira, F. E. S., Mak, R. H., & Oliveira, E. A. (2025). COVID-19 Vaccine Effectiveness and Risk Factors of Booster Failure in 480,000 Patients with Diabetes Mellitus: A Population-Based Cohort Study. Microorganisms, 13(5), 979. https://doi.org/10.3390/microorganisms13050979






