Climate Change-Related Temperature Impact on Human Health Risks of Vibrio Species in Bathing and Surface Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling
2.3. Analyses
2.4. Statistical Analyses
2.5. Quantitative Microbial Risk Assessments (QMRA)
2.6. Climate Change Scenarios
3. Results
3.1. Vibrio Concentrations and Water Temperature
3.2. Vibrio Species
3.3. Vibrio Concentrations and Risk of Illness in Different Scenarios
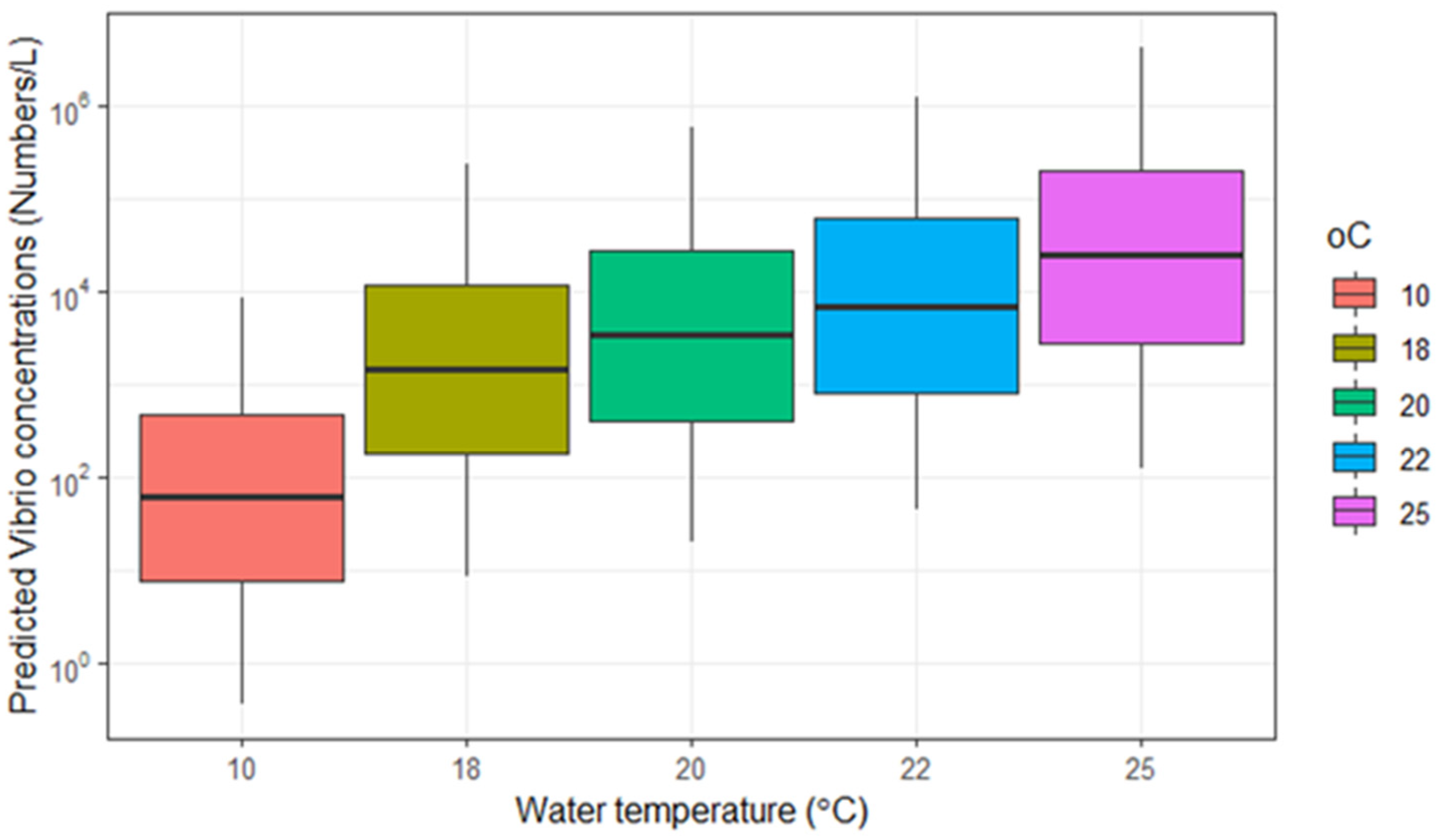
3.3.1. Water Temperature Scenarios
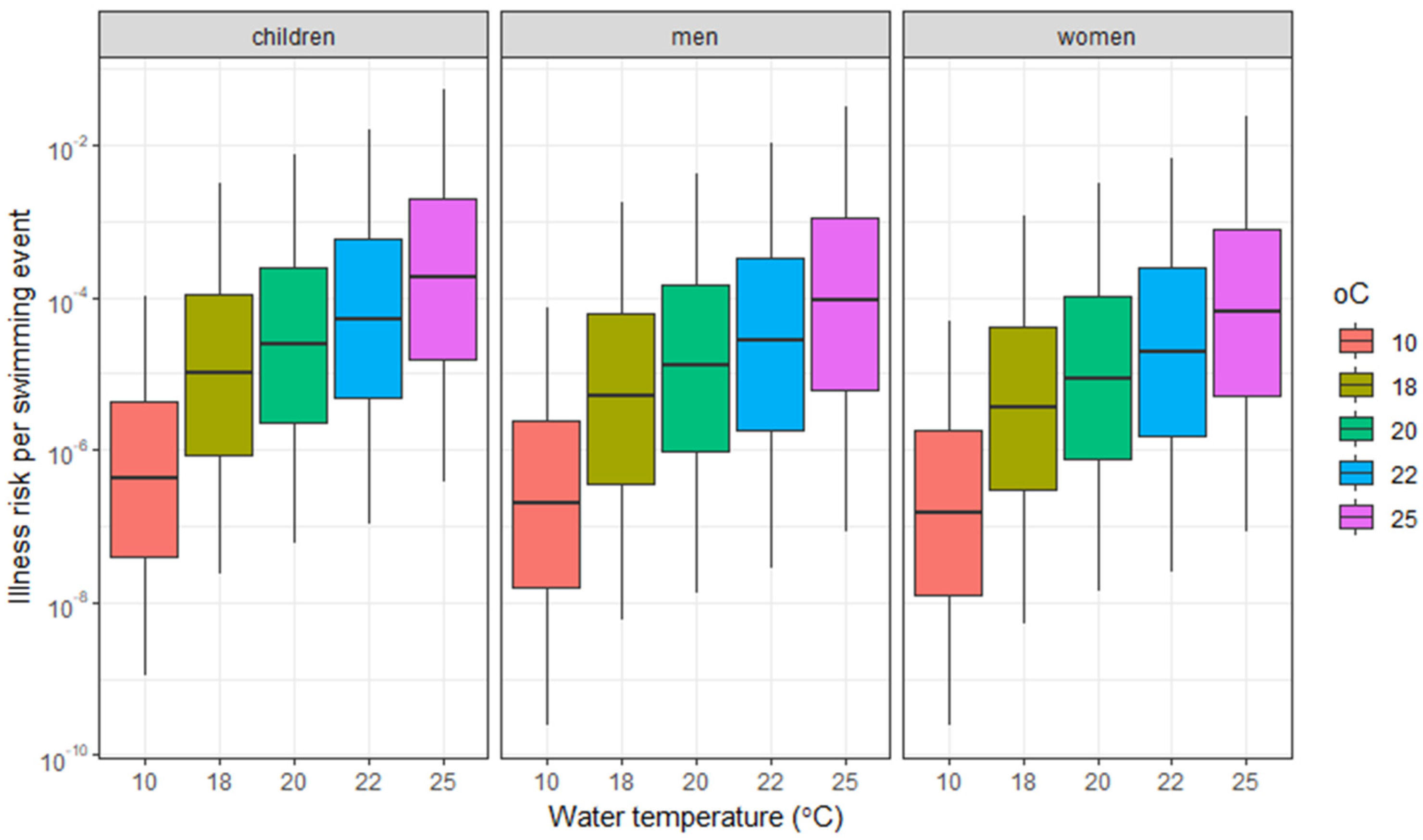
3.3.2. Climate Change Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KNMI | Royal Dutch Meteorological Institute |
| ASPW | Alkaline Saline Peptone Water |
| TCBS | Thiosulphate Citrate Bile Sucrose Agar |
| SNA | Saline Nutrient Agar |
| PCR | Polymerase Chain Reaction |
| MPN | Most Probable Number |
| QMRA | Quantitative Microbial Risk Assessment |
References
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Brumfield, K.D.; Chen, A.J.; Gangwar, M.; Usmani, M.; Hasan, N.A.; Jutla, A.S.; Huq, A.; Colwell, R.R. Environmental factors influencing occurrence of Vibrio parahaemolyticus and Vibrio vulnificus. Appl. Environ. Microbiol 2023, 89, e0030723. [Google Scholar] [CrossRef]
- Brumfield, K.D.; Usmani, M.; Chen, K.M.; Gangwar, M.; Jutla, A.S.; Huq, A.; Colwell, R.R. Environmental parameters associated with incidence and transmission of pathogenic Vibrio spp. Environ. Microbiol. 2021, 23, 7314–7340. [Google Scholar] [CrossRef]
- Gangwar, M.; Usmani, M.; Jamal, Y.; Brumfield, K.D.; Huq, A.; Unnikrishnan, A.; Colwell, R.R.; Jutla, A.S. Environmental factors associated with incidence and distribution of V. parahaemolyticus and V. vulnificus in Chesapeake Bay, Maryland, USA: A three-year case study. bioRxiv 2023. [Google Scholar] [CrossRef]
- Namadi, P.; Deng, Z. Optimum environmental conditions controlling prevalence of Vibrio parahaemolyticus in marine environment. Mar. Environ. Res. 2023, 183, 105828. [Google Scholar] [CrossRef]
- Norfolk, W.A.; Shue, C.; Henderson, W.M.; Glinski, D.A.; Lipp, E.K. Vibrio alginolyticus growth kinetics and the metabolic effects of iron. Microbiol. Spectr. 2023, 11, e0268023. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Füchslin, H.P.; Hammes, F.; Egli, T. Growth of Vibrio cholerae O1 Ogawa Eltor in freshwater. Microbiology 2007, 153, 1993–2001. [Google Scholar] [CrossRef]
- Kirschner, A.K.; Schlesinger, J.; Farnleitner, A.H.; Hornek, R.; Süss, B.; Golda, B.; Herzig, A.; Reitner, B. Rapid growth of planktonic Vibrio cholerae non-O1/non-O139 strains in a large alkaline lake in Austria: Dependence on temperature and dissolved organic carbon quality. Appl. Environ. Microbiol. 2008, 74, 2004–2015. [Google Scholar] [CrossRef]
- Percival, S.L.; Williams, D.W. Vibrio. In Microbiology of Waterborne Diseases—Microbiological Aspects and Risks, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 237–248. ISBN 978-0-12-415846-7. [Google Scholar] [CrossRef]
- Morris, J.G. Cholera and other types of vibriosis: A story of human pandemics and oysters on the half shell. Clin. Infect. Dis. 2003, 37, 272–280. [Google Scholar] [CrossRef]
- Austin, B. Vibrios as causal agents of zoonoses. Vet. Microbiol. 2010, 140, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-Cholera Vibrios: The microbial barometer of climate change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.J.; Baker-Austin, C.; Osborn, T.J.; Jones, N.R.; Martínez-Urtaza, J.; Trinanes, J.; Oliver, J.D.; González, F.J.C.; Lake, I.R. Climate warming and increasing Vibrio vulnificus infections in North America. Sci. Rep. 2023, 13, 3893. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Trinanes, J.; Taylor, N.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Change 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Le Roux, F.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzón, D.; Blokesch, M.; et al. The emergence of Vibrio pathogens in Europe: Ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front. Microbiol. 2015, 6, 830. [Google Scholar] [CrossRef]
- Brehm, T.T.; Berneking, L.; Martins, M.S.; Dupke, S.; Jacob, D.; Drechsel, O.; Bohnert, J.; Becker, K.; Kramer, A.; Christner, M.; et al. Heatwave-associated Vibrio infections in Germany, 2018 and 2019. Euro Surveill. 2021, 26, 2002041. [Google Scholar] [CrossRef]
- Amato, E.; Riess, M.; Thomas-Lopez, D.; Linkevicius, M.; Pitkänen, T.; Wołkowicz, T.; Rjabinina, J.; Jernberg, C.; Hjertqvist, M.; MacDonald, E.; et al. Epidemiological and microbiological investigation of a large increase in vibriosis, northern Europe, 2018. Euro Surveill. 2022, 27, 2101088. [Google Scholar] [CrossRef]
- Gyraitė, G.; Kataržytė, M.; Bučas, M.; Kalvaitienė, G.; Kube, S.; Herlemann, D.P.; Pansch, C.; Andersson, A.F.; Pitkanen, T.; Hokajärvi, A.M.; et al. Epidemiological and environmental investigation of the ‘big four’ Vibrio species, 1994 to 2021: A Baltic Sea retrospective study. Euro Surveill. 2024, 29, 2400075. [Google Scholar] [CrossRef]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; DePaola, A. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Fuchs, B.M.; Meiners, M.; Wichels, A.; Wiltshire, K.H.; Gerdts, G. Seasonal dynamics and modeling of a Vibrio community in coastal waters of the North Sea. Microb. Ecol. 2012, 63, 543–551. [Google Scholar] [CrossRef]
- Vezzulli, L.; Pezzati, E.; Brettar, I.; Höfle, M.; Pruzzo, C. Effects of global warming on Vibrio ecology. Microbiol. Spec. 2015, 3. [Google Scholar] [CrossRef]
- Almuhaideb, E.; Chintapenta, L.K.; Abbott, A.; Parveen, S.; Ozbay, G. Assessment of Vibrio parahaemolyticus levels in oysters (Crassostrea virginica) and seawater in Delaware Bay in relation to environmental conditions and the prevalence of molecular markers to identify pathogenic Vibrio parahaemolyticus strains. PLoS ONE 2020, 15, e0242229. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Logar-Henderson, C.; Ling, R.; Tuite, A.R.; Fisman, D.N. Effects of large-scale oceanic phenomena on non-cholera vibriosis incidence in the United States: Implications for climate change. Epidemiol. Infect. 2019, 147, e243. [Google Scholar] [CrossRef] [PubMed]
- Froelich, B.A.; Daines, D.A. In hot water: Effect of climate change on Vibrio-human interactions. Environ. Microbiol. 2020, 22, 4101–4111. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D. Vibrio vulnificus: New insights into a deadly opportunistic pathogen. Environ. Microbiol. 2018, 20, 423–430. [Google Scholar] [CrossRef]
- Schets, F.M.; Van den Berg, H.H.; Marchese, A.; Garbom, S.; De Roda Husman, A.M. Potentially human pathogenic vibrios in marine and fresh bathing waters related to environmental conditions and disease outcome. Int. J. Hyg. Environ. Health 2011, 214, 399–406. [Google Scholar] [CrossRef]
- Sterk, A.; Schets, F.M.; De Roda Husman, A.M.; De Nijs, T.; Schijven, J.F. Effect of climate change on the concentration and associated risks of Vibrio spp. in Dutch recreational waters. Risk Anal. 2015, 35, 1717–1729. [Google Scholar] [CrossRef]
- Van den Hurk, B.; Siegmund, P.; Klein Tank, A.; Attema, J.; Bakker, A.; Beersma, J.; Bessembinder, J.; Boers, R.; Brandsma, T.; van den Brink, H.; et al. KNMI’14: Climate Change Scenarios for the 21st Century—A Netherlands Perspective, 2014, KNMI Number: WR-2014-01. Available online: https://cdn.knmi.nl/knmi/pdf/bibliotheek/knmipubWR/WR2014-01.pdf (accessed on 1 July 2025).
- Hackbusch, S.; Wichels, A.; Gimenez, L.; Döpke, H.; Gerdts, G. Potentially human pathogenic Vibrio spp. in a coastal transect: Occurrence and multiple virulence factors. Sci. Total Environ. 2020, 707, 136113. [Google Scholar] [CrossRef]
- Sacheli, R.; Philippe, C.; Meex, C.; Mzougui, S.; Melin, P.; Hayette, M. Occurrence of Vibrio spp. in selected recreational water bodies in Belgium during 2021 bathing season. Int. J. Environ. Res. Public Health 2023, 20, 6932. [Google Scholar] [CrossRef]
- Bonadonna, L.; Briancesco, R.; Suffredini, E.; Coccia, A.; Della Libera, S.; Carducci, A.; Verani, M.; Federigi, I.; Iaconelli, M.; Bonanno Ferraro, G.; et al. Enteric viruses, somatic coliphages and Vibrio species in marine bathing and non-bathing waters in Italy. Mar. Pollut. Bull. 2019, 149, 110570. [Google Scholar] [CrossRef]
- Rehm, C.; Lippert, K.; Indra, A.; Kolarević, S.; Kračun-Kolarević, M.; Leopold, M.; Steinbacher, S.; Schachner, I.; Campostrini, L.; Risslegger, A.; et al. First report on the occurrence of Vibrio cholerae nonO1/nonO139 in natural and artificial lakes and ponds in Serbia: Evidence for a long-distance transfer of strains and the presence of Vibrio paracholerae. Environ. Microbiol. Rep. 2023, 15, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Van Dorland, R.; Beersma, J.; Bessembinder, J.; Bloemendaal, N.; van den Brink, H.; Brotons Blanes, M.; Drijfhout, S.; Haarsma, R.; Keizer, I.; Krikken, F.; et al. KNMI National Climate Scenarios 2023 for the Netherlands, 2023, KNMI Number: WR-23-02. Available online: https://cdn.knmi.nl/system/data_center_publications/files/000/071/902/original/KNMI23_climate_scenarios_scientific_report_WR23-02.pdf?1710489430 (accessed on 1 July 2025).
- ISO 19458; Water Quality—Sampling for Microbiological Analysis. International Organization for Standardisation (ISO): Geneva, Switzerland, 2006.
- ISO 21872-1; Microbiology of the Food Chain—Horizontal Method for the Determination of Vibrio spp. Part 1: Detection of Potentially Enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. International Organization for Standardisation (ISO): Geneva, Switzerland, 2017.
- Cai, T.; Jiang, L.; Yang, C.; Huang, K. Application of real-time PCR for quantitative detection of Vibrio parahaemolyticus from seafood in eastern China. FEMS Immunol. Med. Microbiol. 2006, 46, 180–186. [Google Scholar] [CrossRef]
- Panicker, G.; Bej, A.K. Real-time PCR detection of Vibrio vulnificus in oysters: Comparison of oligonucleotide primers and probes targeting vvhA. Appl. Environ. Microbiol. 2005, 71, 5702–5709. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Teunis, P.F.; Nagelkerke, N.J.; Haas, C.N. Dose response models for infectious gastroenteritis. Risk Anal. 1999, 19, 1251–1260. [Google Scholar] [CrossRef]
- FDA. Quantitative Risk Assessment on the Public Health Impact of Pathogenic Vibrio parahaemolyticus in Raw Oysters; Food and Drug Administration: Silver Spring, MD, USA, 2005. Available online: https://www.fda.gov/food/risk-and-safety-assessments-food/quantitative-risk-assessment-public-health-impact-pathogenic-vibrio-parahaemolyticus-raw-oysters (accessed on 1 July 2025).
- Huang, Y.; Hwang, C.; Huang, L.; Wu, V.C.; Hsiao, H. The risk of Vibrio parahaemolyticus infections associated with consumption of raw oysters as affected by processing and distribution conditions in Taiwan. Food Control 2018, 86, 101–109. [Google Scholar] [CrossRef]
- Schets, F.M.; Schijven, J.F.; De Roda Husman, A.M. Exposure assessment for swimmers in bathing waters and swimming pools. Water Res. 2011, 45, 2392–2400. [Google Scholar] [CrossRef]
- Böer, S.I.; Heinemeyer, E.A.; Luden, K.; Erler, R.; Gerdts, G.; Janssen, F.; Brennholt, N. Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German north sea. Microb. Ecol. 2013, 65, 1052–1067. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, S.; Herrig, I.; Wesp, J.; Stiedl, J.; Reifferscheid, G.; Strauch, E.; Alter, T.; Brennholt, N. Prevalence and distribution of potentially human pathogenic Vibrio spp. on German North and Baltic Sea coasts. Front. Cell. Infect. Microbiol. 2022, 12, 846819. [Google Scholar] [CrossRef]
- Takemura, A.F.; Chien, D.M.; Polz, M.F. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 2014, 5, 38. [Google Scholar] [CrossRef]
- Ministerie van Infrastructuur en Waterstaat, Inspectie Leefomgeving en Transport. Richtsnoer Analyse Microbiologische Veiligheid Drinkwater (AMVD); Inspectie Leefomgeving en Transport (ILT): Hoofddorp, The Netherlands, 2020; Available online: https://www.ilent.nl/documenten/leefomgeving-en-wonen/drinkwater/drinkwater/publicaties/richtsnoer-analyse-microbiologische-veiligheid-drinkwater-amvd (accessed on 1 July 2025). (In Dutch)
- Uh, Y.; Park, J.S.; Hwang, G.Y.; Jang, I.H.; Yoon, K.J.; Park, H.C.; Hwang, S.O. Vibrio alginolyticus acute gastroenteritis: Report of two cases. Clin. Microbiol. Infect. 2001, 7, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.D.; Reilly, C.A.; Smith, E.G.; Baker-Austin, C. Vibrio alginolyticus-associated wound infection acquired in British waters, Guernsey, July 2011. Euro Surveill. 2011, 16, 19994. [Google Scholar] [CrossRef] [PubMed]
- Jacobs Slifka, K.M.; Newton, A.E.; Mahon, B.E. Vibrio alginolyticus infections in the USA, 1988–2012. Epidemiol. Infect. 2017, 145, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Hoefler, F.; Pouget-Abadie, X.; Roncato-Saberan, M.; Lemarié, R.; Takoudju, E.M.; Raffi, F.; Corvec, S.; Le Bras, M.; Cazanave, C.; Lehours, P.; et al. Clinical and epidemiologic characteristics and therapeutic management of patients with Vibrio infections, Bay of Biscay, France, 2001–2019. Emerg. Infect. Dis. 2022, 28, 2367–2373. [Google Scholar] [CrossRef]
- Schets, F.M.; Pol-Hofstad, I.E.; van den Berg, H.H.J.L.; Lynch, G.; Serafim, F.; van Overbeek, W.M.; Schijven, J.F. Risico’s van Vibrio-Besmetting in Zwemwater, Schelpdierproductiewater en Schelpdieren; RIVM report 2022-0081; Rijksinstituut voor Volksgezondheid en Milieu: Utrecht, The Netherlands, 2023. (In Dutch) [Google Scholar] [CrossRef]
- Deeb, R.; Tufford, D.; Scott, G.I.; Moore, J.G.; Dow, K. Impact of Climate Change on Vibrio vulnificus abundance and exposure risk. Estuaries Coasts 2018, 41, 2289–2303. [Google Scholar] [CrossRef]
- Trinanes, J.; Martinez-Urtaza, J. Future scenarios of risk of Vibrio infections in a warming planet: A global mapping study. Lancet Planet. Health 2021, 5, e426–e435. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021: The Physical Science Basis. Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 1 July 2025).


| Model Parameter | Dimension | Value | Mean | Median | 5–95% | References |
|---|---|---|---|---|---|---|
| variable | ||||||
| a0 | 10log(MPN/L) | N 2 (0.021, 0.36) | Equation (1) | |||
| a1 | 10log(MPN/L)/°C | N (0.17, 0.017) | Equation (1) | |||
| ε0 | 10log(MPN/L) | 0.89 | Equation (1) | |||
| ε1 | 10log(MPN/L) | 0.90 | Equation (1) | |||
| dose response 1 | ||||||
| α | −0.6 | [40,41] | ||||
| β | 1.3 × 106 | [40,41] | ||||
| water ingestion | ||||||
| Vchild | mL | G 3 (0.64, 58) | 38 | 20 | 0.51–133 | [42] |
| Vwoman | mL | G (0.45, 60) | 18 | 8.1 | 0.076–67 | [42] |
| Vman | mL | G (0.51, 35) | 27 | 11 | 0.062–110 | [42] |
| Site | North Sea—Bathing Site | Eastern Scheldt—Bathing Site | Eastern Scheldt—Open Sea | Wadden Sea—Bathing Site | Wadden Sea—Open Sea | Veerse Meer—Bathing Site/Open Sea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Year | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2021 | 2019 | 2020 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 |
| no. samples | 20 | 13 | 19 | 13 | 6 | 10 | 7 | 19 | 13 | 8 | 11 | 10 | 8 | 12 | 11 | |
| Vibrio concentration (MPN/L) | min | 0 | 9.2 | 2.3 × 102 | 4.7 × 101 | 1.5 × 102 | 3.2 | 0 | 5.8 × 102 | 0 | 5.5 × 101 | 0 | 0 | 1.2 × 103 | 0 | 0 |
| max | 8.4 × 104 | 3.9 × 104 | 4.2 × 105 | 4.3 × 106 | 2.2 × 104 | 1.2 × 104 | 1.5 × 104 | 4.6 × 105 | 1.5 × 106 | 1.2 × 103 | 9.2 × 103 | 4.3 × 103 | 1.4 × 104 | 2.0 × 105 | 1.4 × 104 | |
| average | 9.6 × 103 | 5.3 × 103 | 6.6 × 104 | 3.9 × 105 | 4.9 × 103 | 2.5 × 103 | 3.4 × 103 | 1.3 × 105 | 2.8 × 105 | 4.2 × 102 | 1.9 × 103 | 7.8 × 102 | 4.7 × 103 | 2.5 × 105 | 2.3 × 103 | |
| median | 2.8 × 103 | 1.9 × 103 | 2.8 × 103 | 4.2 × 103 | 1.5 × 103 | 2.0 × 103 | 2.2 × 102 | 4.5 × 104 | 3.2 × 104 | 3.0 × 102 | 6.6 × 102 | 1.5 × 102 | 3.6 × 103 | 1.9 × 103 | 3.9 × 102 | |
| Water temperature (°C) | min | 11.0 | 9.9 | 12.4 | 8.6 | 12.6 | 7.2 | 7.2 | 10.0 | 6.9 | 12.1 | 6.1 | 7.3 | 14.3 | 6.2 | 7.2 |
| max | 20.6 | 21.1 | 23.8 | 27.2 | 21.4 | 24.2 | 20.7 | 19.2 | 22.8 | 23.0 | 23.5 | 20.8 | 23.4 | 24.5 | 22.2 | |
| average | 17.1 | 16.0 | 19.3 | 18.0 | 16.8 | 17.0 | 16.8 | 16.1 | 15.0 | 18.2 | 17.0 | 16.9 | 19.4 | 19.0 | 18.0 | |
| median | 17.0 | 17.0 | 19.6 | 17.7 | 16.8 | 17.6 | 18.7 | 16.4 | 16.0 | 18.0 | 17.8 | 18.0 | 19.8 | 19.1 | 19.1 | |
| acidity (pH) | min | 7.9 | 8.0 | 8.0 | 8.0 | - | 5.6 | 7.2 | 6.8 | 7.5 | - | 5.1 | 6.4 | - | 7.3 | 6.6 |
| max | 8.3 | 8.2 | 8.6 | 8.4 | - | 8.3 | 8.4 | 8.0 | 8.0 | - | 8.3 | 8.4 | - | 8.6 | 8.6 | |
| average | 8.2 | 8.0 | 8.3 | 8.0 | - | 8.0 | 7.9 | 7.8 | 8.0 | - | 8.0 | 7.6 | - | 8.0 | 7.8 | |
| median | 8.2 | 8.1 | 8.3 | 8.2 | - | 8.0 | 8.1 | 7.8 | 7.9 | - | 8.1 | 7.6 | - | 8.3 | 8.2 | |
| conductivity (mS/cm) | min | 42.4 | 40.7 | - | 42.6 | - | - | 47.7 | 28.9 | 17.2 | - | - | 47.6 | - | - | 43.4 |
| max | 51.0 | 43.6 | 50.0 | 46.5 | - | - | 56.9 | 47.2 | 41.8 | - | - | 59.2 | - | - | 56.3 | |
| average | 49.0 | 43.0 | 50.0 | 45.0 | - | - | 52.6 | 37.2 | 31.0 | - | - | 53.2 | - | - | 49.4 | |
| median | 49.6 | 43.2 | 50.0 | 45.0 | - | - | 53.5 | 28.1 | 29.4 | - | - | 53.9 | - | - | 50.5 | |
| Site | Year | Number (%) of Isolates Per Species | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vibrio Total | V. alginolyticus | V. parahaemolyticus | V. vulnificus | V. fluvialis | V. cholerae Non-O1\non-O139 | V. mimicus | Vibrio spp. | ||
| 2019 | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | - | ||
| North Sea 1 | 103 | 91 (88) | 9 (8.7) | 1 (1.0) | 1 (1.0) | - | - | 1 (1.0) | |
| Eastern Scheldt 1 | 108 | 100 (93) | 7 (6.5) | - | - | 1 (0.9) | - | - | |
| Wadden Sea 1 | 105 | 48 (46) | 52 (50) | 1 (1.0) | 1 (1.0) | 2 (1.9) | 1 (1.0) | - | |
| 2020 | |||||||||
| North Sea 1 | 52 | 48 (92) | 3 (5.8) | - | 1 (1.9) | - | - | - | |
| Eastern Scheldt 1 | 66 | 61 (92) | 3 (4.5) | - | 2 (3.0) | - | - | - | |
| Wadden Sea 1 | 58 | 31 (53) | 18 (31) | 1 (1.7) | 3 (5.2) | 1 (1.7) | - | 4 (6.9) | |
| Total 1 | 492 | 379 (77) | 92 (19) | 3 (0.6) | 8 (1.6) | 4 (0.8) | 1 (0.2) | 5 (1.0) | |
| 2019 | |||||||||
| Veerse Meer 1,2 | 43 | 22 (51) | 21 (49) | - | - | - | - | - | |
| Eastern Scheldt 2 | 40 | 36 (90) | 3 (7.5) | - | 1 (2.5) | - | - | - | |
| Wadden Sea 2 | 41 | 26 (63) | 14 (34) | 1 (2.4) | - | - | - | - | |
| 2020 | |||||||||
| Veerse Meer 1,2 | 46 | 34 (74) | 11 (24) | - | - | - | - | 1 (2.2) | |
| Eastern Scheldt 2 | 44 | 38 (86) | 5 (11) | - | 1 (2.3) | - | - | - | |
| Wadden Sea 2 | 45 | 37 (82) | 4 (8.9) | 1 (2.2) | - | - | - | 3 (6.7) | |
| 2021 | |||||||||
| Veerse Meer 1,2 | 35 | 16 (46) | 16 (46) | - | 1 (2.9) | - | - | 2 (5.7) | |
| Eastern Scheldt 2 | 25 | 24 (96) | - | - | 1 (4.0) | - | - | - | |
| Wadden Sea 2 | 34 | 22 (65) | 10 (29) | 1 (2.9) | 1 (2.9) | - | - | - | |
| Total 1,2 | 353 | 255 (72) | 84 (24) | 3 (0.8) | 5 (1.4) | - | - | 6 (1.7) | |
| total | 845 | 634 (75) | 176 (21) | 6 (0.7) | 13 (1.5) | 4 (0.5) | 1 (0.1) | 11 (1.3) | |
| 2009–2012 | |||||||||
| North Sea 1 | 265 | 172 (65) | 65 (24) | 5 (1.9) | 2 (0.8) | 8 (3.0) | - | 6 (2.3) | |
| Eastern Scheldt 1 | 310 | 275 (89) | 26 (8.4) | - | 1 (0.3) | 2 (0.6) | - | 6 (1.9) | |
| Wadden Sea 1 | 173 | 106 (61) | 41 (24) | - | 8 (4.6) | 12 (6.9) | - | 6 (3.4) | |
| Total 1 | 748 | 553 (74) | 132 (18) | 5 (0.7) | 11 (1.5) | 22 (2.9) | - | 18 (2.4) | |
| Water Temperature Scenario | 5% | Median | Mean | 95% |
|---|---|---|---|---|
| 10 °C | 0.36 | 61 | 8.9 × 103 | 8.5 × 103 |
| 18 °C | 8.8 | 1.3 × 103 | 1.6 × 105 | 2.5 × 105 |
| 20 °C | 19 | 3.3 × 103 | 3.2 × 105 | 6.0 × 105 |
| 22 °C | 39 | 7.0 × 103 | 8.2 × 105 | 1.2 × 106 |
| 25 °C | 120 | 2.4 × 104 | 2.3 × 106 | 4.6 × 106 |
| Water Temperature Scenario | 5% | Median | Average | 95% |
|---|---|---|---|---|
| Child | ||||
| 10 °C | 9.2 × 10−10 | 4.3 × 10−7 | 7.9 × 10−5 | 1.1 × 10−4 |
| 18 °C | 2.1 × 10−8 | 9.2 × 10−6 | 1.7 × 10−3 | 2.9 × 10−3 |
| 20 °C | 3.8 × 10−8 | 2.3 × 10−5 | 2.8 × 10−3 | 6.7 × 10−3 |
| 22 °C | 9.8 × 10−8 | 4.9 × 10−5 | 5.6 × 10−3 | 1.4 × 10−2 |
| 25 °C | 2.6 × 10−7 | 1.7 × 10−4 | 1.3 × 10−2 | 5.0 × 10−2 |
| Man | ||||
| 10 °C | 2.2 × 10−10 | 5.2 × 10−6 | 9.0 × 10−5 | 7.1 × 10−5 |
| 18 °C | 5.5 × 10−9 | 4.9 × 10−6 | 1.2 × 10−3 | 1.8 × 10−3 |
| 20 °C | 1.0 × 10−8 | 1.2 × 10−5 | 2.3 × 10−3 | 5.5 × 10−3 |
| 22 °C | 2.7 × 10−8 | 2.6 × 10−5 | 4.1 × 10−3 | 9.8 × 10−3 |
| 25 °C | 8.6 × 10−8 | 8.9 × 10−5 | 1.1 × 10−2 | 3.9 × 10−2 |
| Woman | ||||
| 10 °C | 2.2 × 10−10 | 1.7 × 10−7 | 5.4 × 10−5 | 5.1 × 10−5 |
| 18 °C | 5.2 × 10−9 | 3.8 × 10−6 | 9.0 × 10−4 | 1.3 × 10−3 |
| 20 °C | 1.2 × 10−8 | 9.5 × 10−6 | 1.7 × 10−3 | 3.5 × 10−3 |
| 22 °C | 2.8 × 10−8 | 2.0 × 10−5 | 3.2 × 10−3 | 7.1 × 10−3 |
| 25 °C | 8.0 × 10−8 | 7.4 × 10−5 | 7.7 × 10−3 | 2.5 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schets, F.M.; Pol-Hofstad, I.E.; van den Berg, H.H.J.L.; Schijven, J.F. Climate Change-Related Temperature Impact on Human Health Risks of Vibrio Species in Bathing and Surface Water. Microorganisms 2025, 13, 1893. https://doi.org/10.3390/microorganisms13081893
Schets FM, Pol-Hofstad IE, van den Berg HHJL, Schijven JF. Climate Change-Related Temperature Impact on Human Health Risks of Vibrio Species in Bathing and Surface Water. Microorganisms. 2025; 13(8):1893. https://doi.org/10.3390/microorganisms13081893
Chicago/Turabian StyleSchets, Franciska M., Irene E. Pol-Hofstad, Harold H. J. L. van den Berg, and Jack F. Schijven. 2025. "Climate Change-Related Temperature Impact on Human Health Risks of Vibrio Species in Bathing and Surface Water" Microorganisms 13, no. 8: 1893. https://doi.org/10.3390/microorganisms13081893
APA StyleSchets, F. M., Pol-Hofstad, I. E., van den Berg, H. H. J. L., & Schijven, J. F. (2025). Climate Change-Related Temperature Impact on Human Health Risks of Vibrio Species in Bathing and Surface Water. Microorganisms, 13(8), 1893. https://doi.org/10.3390/microorganisms13081893





