Helicobacter pylori Stress-Response: Definition of the HrcA Regulon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. RNA Extraction
2.3. RNA-Sequencing: Library Preparation, Sequencing and Analysis
2.4. Quantitative Real Time PCR (qRT-PCR) Analysis
2.5. Immunoblot Analysis
3. Results
3.1. RNA–Seq Analysis Reveals the HrcA-Dependent Transcriptome
3.2. Comparison of the HrcA regulon with the HspR and Heat-Shock Regulons
3.3. RNA-Seq Data Validation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gisbert, J.P.; Calvet, X. Review article: Common misconceptions in the management of Helicobacter pylori-associated gastric MALT-lymphoma. Aliment. Pharmacol. Ther. 2011, 34, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.R.; Hartung, M.L.; Muller, A. Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013, 11, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, D.; Scarlato, V. The interplay between two transcriptional repressors and chaperones orchestrates Helicobacter pylori heat-shock response. Int. J. Mol. Sci. 2018, 19, 1702. [Google Scholar] [CrossRef]
- Roncarati, D.; Scarlato, V. Regulation of heat-shock genes in bacteria: From signal sensing to gene expression output. FEMS Microbiol. Rev. 2017, 41, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Spohn, G.; Scarlato, V. The autoregulatory HspR repressor protein governs chaperone gene transcription in Helicobacter pylori. Mol. Microbiol. 1999, 34, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Spohn, G.; Danielli, A.; Roncarati, D.; Delany, I.; Rappuoli, R.; Scarlato, V. Dual control of Helicobacter pylori heat shock gene transcription by HspR and HrcA. J. Bacteriol. 2004, 186, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, D.; Danielli, A.; Spohn, G.; Delany, I.; Scarlato, V. Transcriptional regulation of stress response and motility functions in Helicobacter pylori is mediated by HspR and HrcA. J. Bacteriol. 2007, 189, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, D.; Danielli, A.; Scarlato, V. CbpA acts as a modulator of HspR repressor DNA binding activity in Helicobacter pylori. J Bacteriol. 2011, 193, 5629–5636. [Google Scholar] [CrossRef] [PubMed]
- Pepe, S.; Pinatel, E.; Fiore, E.; Puccio, S.; Peano, C.; Brignoli, T.; Vannini, A.; Danielli, A.; Scarlato, V.; Roncarati, D. The Helicobacter pylori heat-shock repressor HspR: Definition of its direct regulon and characterization of the cooperative DNA-binding mechanism on its own promoter. Front. Microbiol. 2018, 9, 1887. [Google Scholar] [CrossRef]
- Xiang, Z.; Censini, S.; Bayeli, P.F.; Telford, J.L.; Figura, N.; Rappuoli, R.; Covacci, A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 1995, 63, 94–98. [Google Scholar]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatusov, R.L.; Koonin, E.V.; Lipman, D.J. A genomic perspective on protein families. Science 1997, 278, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, D.; Pelliciari, S.; Doniselli, N.; Maggi, S.; Vannini, A.; Valzania, L.; Mazzei, L.; Zambelli, B.; Rivetti, C.; Danielli, A. Metal-responsive promoter DNA compaction by the ferric uptake regulator. Nat. Commun. 2016, 7, 12593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncarati, D.; Spohn, G.; Tango, N.; Danielli, A.; Delany, I.; Scarlato, V. Expression, purification and characterization of the membrane-associated HrcA repressor protein of Helicobacter pylori. Protein Expr. Purif. 2007, 51, 267–275. [Google Scholar] [CrossRef]
- Pelliciari, S.; Pinatel, E.; Vannini, A.; Peano, C.; Puccio, S.; De Bellis, G.; Danielli, A.; Scarlato, V.; Roncarati, D. Insight into the essential role of the Helicobacter pylori HP1043 orphan response regulator: Genome-wide identification and characterization of the DNA-binding sites. Sci. Rep. 2017, 7, 41063. [Google Scholar] [CrossRef]
- Spohn, G.; Delany, I.; Rappuoli, R.; Scarlato, V. Characterization of the HspR-mediated stress response in Helicobacter pylori. J. Bacteriol. 2002, 184, 2925–2930. [Google Scholar] [CrossRef]
- Roncarati, D.; Danielli, A.; Scarlato, V. The HrcA repressor is the thermosensor of the heat-shock regulatory circuit in the human pathogen Helicobacter pylori. Mol. Microbiol. 2014, 92, 910–920. [Google Scholar] [CrossRef]
- Hu, Y.; Oliver, H.F.; Raengpradub, S.; Palmer, M.E.; Orsi, R.H.; Wiedmann, M.; Boor, K.J. Transcriptomic and phenotypic analyses suggest a network between the transcriptional regulators HrcA and sigmaB in Listeria monocytogenes. Appl. Environ. Microbiol. 2007, 73, 7981–7991. [Google Scholar] [CrossRef]
- Van Bokhorst-van de Veen, H.; Bongers, R.S.; Wels, M.; Bron, P.A.; Kleerebezem, M. Transcriptome signatures of class I and III stress response deregulation in Lactobacillus plantarum reveal pleiotropic adaptation. Microb. Cell. Fact. 2013, 12, 112. [Google Scholar] [CrossRef]
- Andersen, M.T.; Brondsted, L.; Pearson, B.M.; Mulholland, F.; Parker, M.; Pin, C.; Wells, J.M.; Ingmer, H. Diverse roles for HspR in Campylobacter jejuni revealed by the proteome, transcriptome and phenotypic characterization of an hspR mutant. Microbiology 2005, 151, 905–915. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Ottemann, K.M. Helicobacter pylori biofilm involves a multigene stress-biased response, including a structural role for flagella. mBio 2018, 9, e01973-18. [Google Scholar] [CrossRef]
- Domka, J.; Lee, J.; Bansal, T.; Wood, T.K. Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol. 2007, 9, 332–346. [Google Scholar] [CrossRef]
- Skoog, E.C.; Padra, M.; Åberg, A.; Gideonsson, P.; Obi, I.; Quintana-Hayashi, M.P.; Arnqvist, A.; Linden, S.K. BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS. Sci. Rep. 2017, 7, 40656. [Google Scholar] [CrossRef]
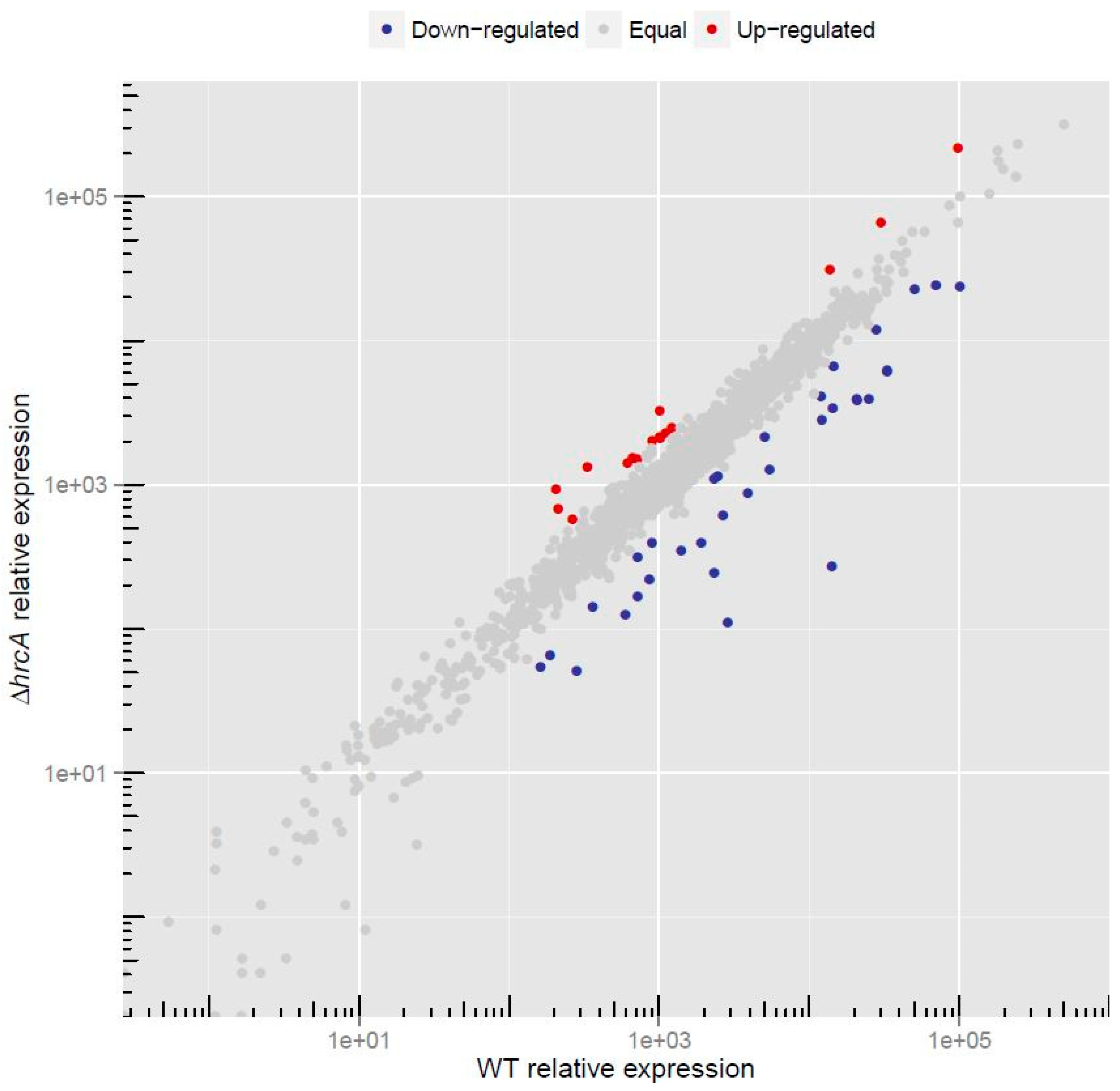
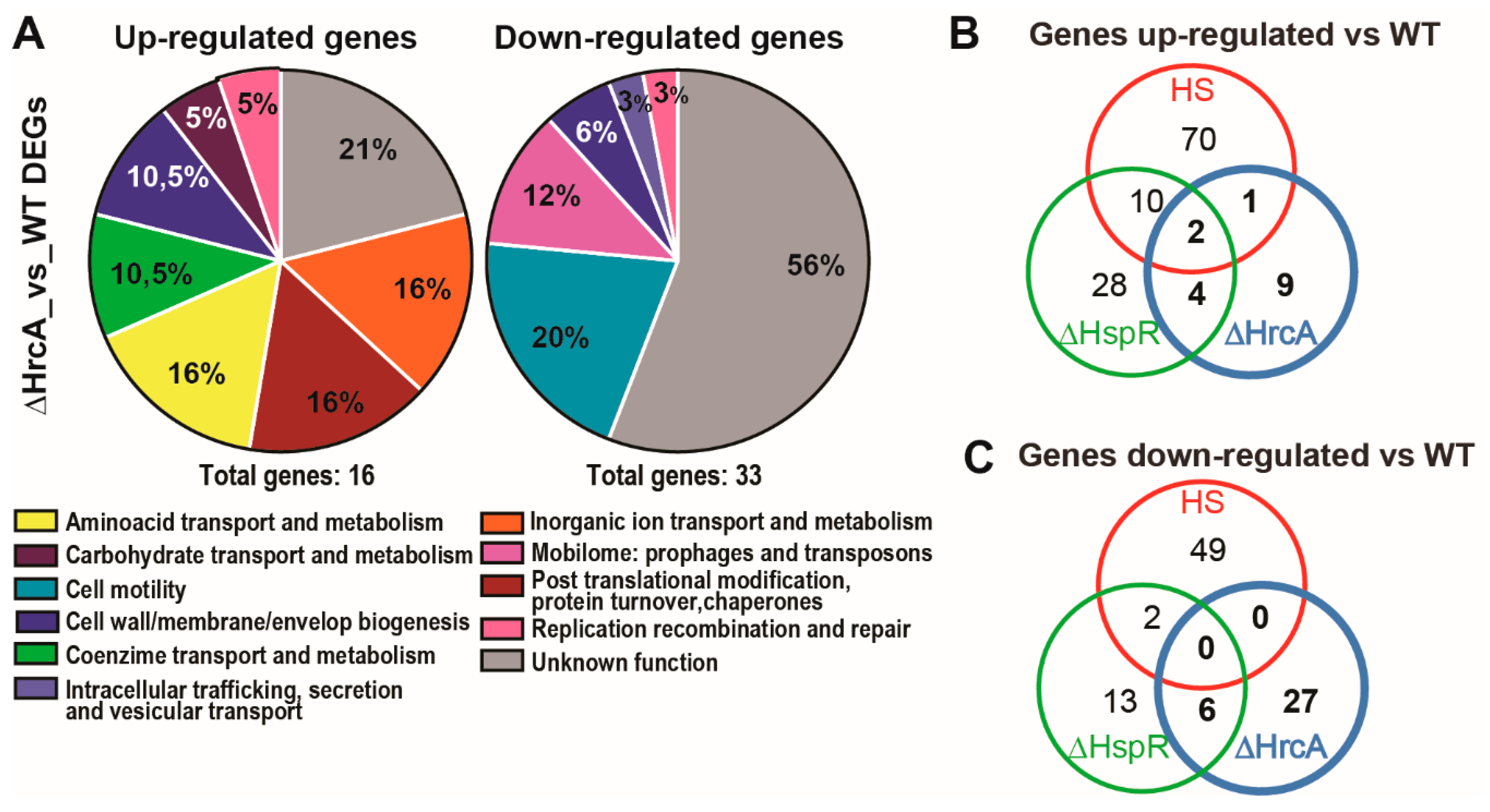
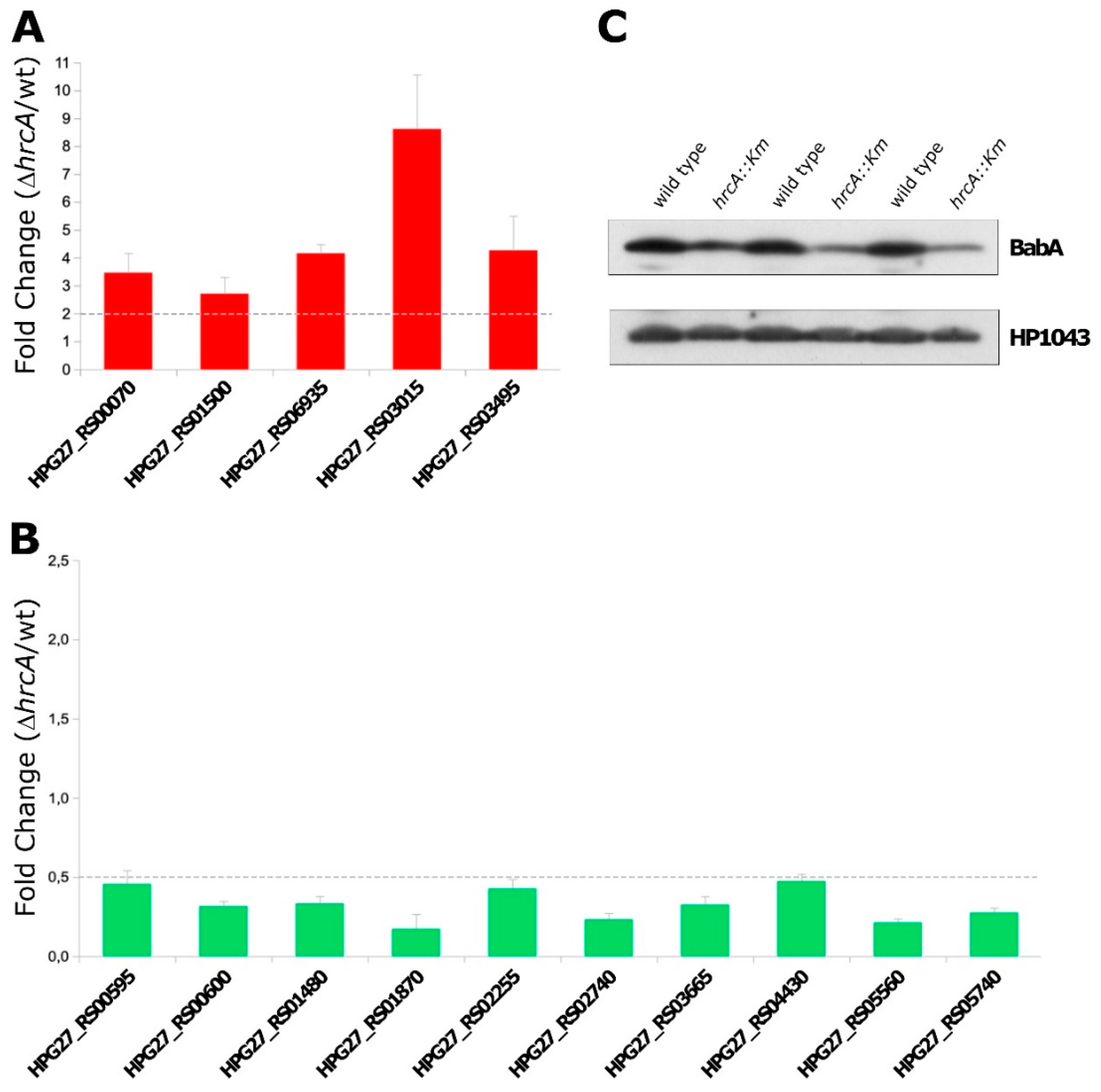
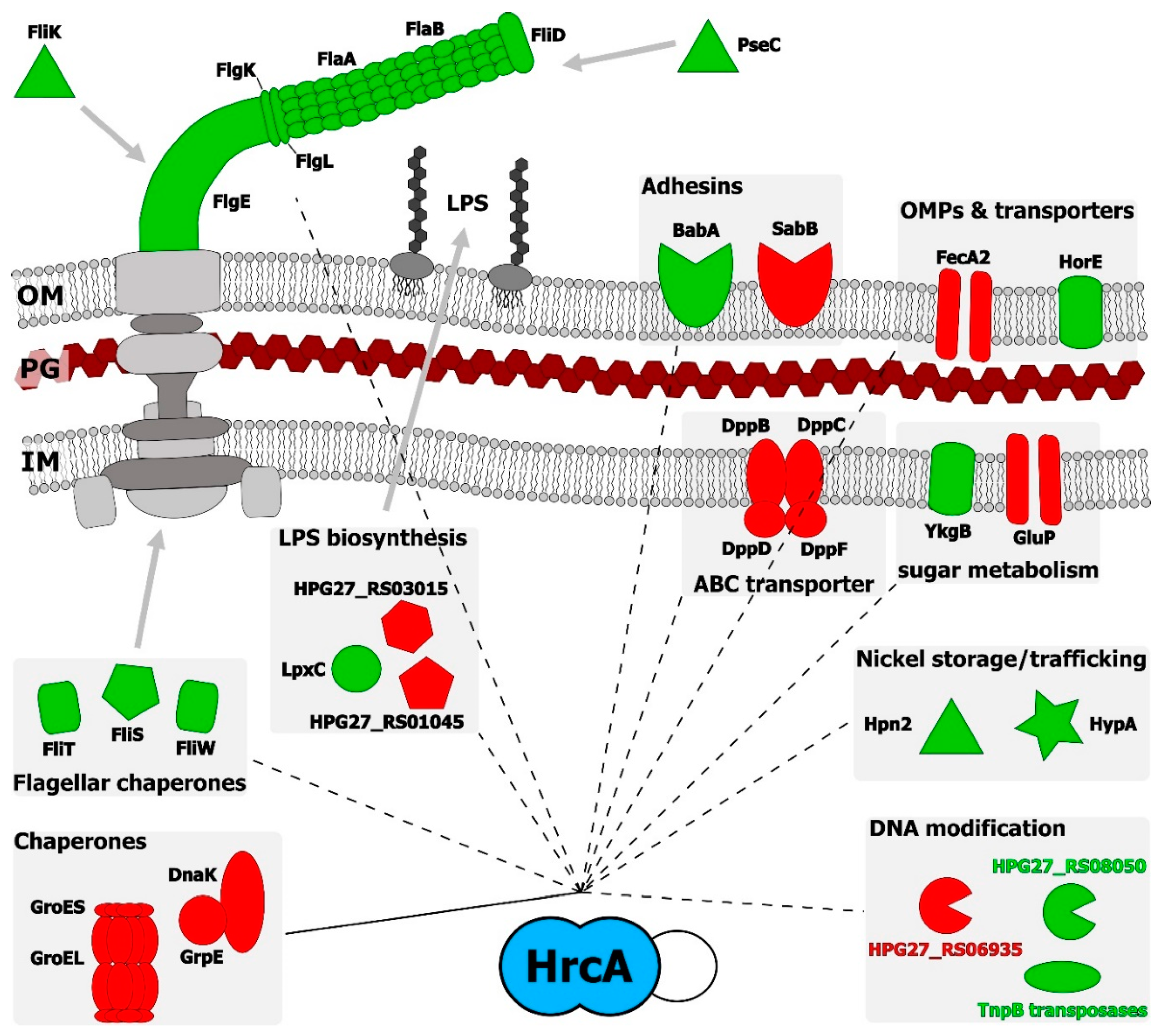
| Gene Names | Log2 FC | Common Names | Description |
|---|---|---|---|
| HPG27_RS00240 | −2.4 | tnpB | transposase |
| HPG27_RS00595 | −2.2 | HPG27_RS00595 | motility accessory factor |
| HPG27_RS00600 | −2.1 | flaB | flagellin B |
| HPG27_RS01480 | −2.1 | flgL | flagellar hook-associated protein FlgL |
| HPG27_RS01595 | −1.2 | babA, omp28 | membrane protein (adhesin) |
| HPG27_RS01870 | −1.9 | HPG27_RS01870 | hypothetical protein |
| HPG27_RS00070 | 1.1 | groEL, hspB, hsp60 | molecular chaperone GroEL |
| HPG27_RS00075 | 1.1 | groES, hspA, hsp10 | co-chaperone GroES |
| HPG27_RS00625 | 1.0 | HPG27_RS00625 | hypothetical protein |
| HPG27_RS01045 | 1.0 | HPG27_RS01045 | LPS biosynthesis protein |
| HPG27_RS01500 | 1.0 | dppB | peptide ABC transporter permease |
| HPG27_RS01505 | 1.2 | dppC | peptide ABC transporter |
| HPG27_RS01510 | 1.1 | dppD | ABC transporter ATP-binding protein |
| HPG27_RS01515 | 1.1 | dppF | ABC transporter ATP-binding protein |
| HPG27_RS03015 | 2.0 | HPG27_RS03015 | LPS biosynthesis protein |
| HPG27_RS03495 | 1.2 | hopO, omp16, sabB | Membrane protein (adhesin) |
| HPG27_RS03705 | 1.0 | HPG27_RS03705 | 5-formyltetrahydrofolate cyclo-ligase |
| HPG27_RS03940 | 1.2 | fecA2, fecA, fecA_2 | ligand-gated channel |
| HPG27_RS05840 | 1.0 | gluP | glucose/galactose MFS transporter |
| HPG27_RS06930 | 1.6 | HPG27_RS06930 | hypothetical protein |
| HPG27_RS06935 | 1.9 | HPG27_RS06935 | restriction endonuclease |
| HPG27_RS08000 | 1.7 | HPG27_RS08000 | hypothetical protein |
| HPG27_RS01870 | −1.9 | HPG27_RS01870 | hypothetical protein |
| HPG27_RS01990 | −1.1 | lpxC, envA | UDP-3-O-[3-hydroxymyristoyl] N-acetylglucosamine deacetylase |
| HPG27_RS02255 | −2.0 | horE, omp11 | membrane protein |
| HPG27_RS02575 | −2.4 | HPG27_RS02575 | transposase |
| HPG27_RS02740 | −3.1 | ykgB | membrane protein |
| HPG27_RS02925 | −1.5 | flaA | flagellin A |
| HPG27_RS03550 | −2.4 | tnpB | transposase |
| HPG27_RS03660 | −1.2 | fliD | flagellar filament capping protein FliD |
| HPG27_RS03665 | −1.1 | fliS | flagellar chaperone protein FliS |
| HPG27_RS03670 | −1.2 | fliT | flagellar chaperone protein FliT |
| HPG27_RS04250 | −1.1 | hypA | hydrogenase/urease Ni incorporation protein HypA |
| HPG27_RS04255 | −2.1 | flgE | flagellar hook protein FlgE |
| HPG27_RS04430 | −1.9 | fliK | flagellar hook-length control protein FliK |
| HPG27_RS04645 | −2.4 | tnpB | transposase |
| HPG27_RS04725 | −4.5 | HPG27_RS04725 | ATPase |
| HPG27_RS04730 | −3.2 | HPG27_RS04730 | hypothetical protein |
| HPG27_RS04735 | −2.6 | tnpB | transposase |
| HPG27_RS04920 | −1.2 | HPG27_RS04920 | hypothetical protein |
| HPG27_RS05380 | −1.1 | pseC | UDP-4-amino-4,6-dideoxy-N-acetyl-β-L-altrosamine transaminase (flagellar modification) |
| HPG27_RS05560 | −2.1 | flgK | flagellar hook-associated protein FlgK |
| HPG27_RS05565 | −2.1 | HPG27_RS05565 | hypothetical protein |
| HPG27_RS05740 | −1.1 | fliW | flagellar assembly protein FliW |
| HPG27_RS06175 | −1.8 | HPG27_RS06175 | hypothetical protein |
| HPG27_RS07055 | −1.1 | HPG27_RS07055 | hypothetical protein |
| HPG27_RS07080 | −1.5 | HPG27_RS07080 | nickel transporter |
| HPG27_RS08050 | −2.0 | HPG27_RS08050 | exonuclease VII large subunit |
| HPG27_RS08160 | −2.0 | HPG27_RS08160 | hypothetical protein |
| HPG27_RS08320 | −1.2 | HPG27_RS08320 | hypothetical protein |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roncarati, D.; Pinatel, E.; Fiore, E.; Peano, C.; Loibman, S.; Scarlato, V. Helicobacter pylori Stress-Response: Definition of the HrcA Regulon. Microorganisms 2019, 7, 436. https://doi.org/10.3390/microorganisms7100436
Roncarati D, Pinatel E, Fiore E, Peano C, Loibman S, Scarlato V. Helicobacter pylori Stress-Response: Definition of the HrcA Regulon. Microorganisms. 2019; 7(10):436. https://doi.org/10.3390/microorganisms7100436
Chicago/Turabian StyleRoncarati, Davide, Eva Pinatel, Elisabetta Fiore, Clelia Peano, Stefany Loibman, and Vincenzo Scarlato. 2019. "Helicobacter pylori Stress-Response: Definition of the HrcA Regulon" Microorganisms 7, no. 10: 436. https://doi.org/10.3390/microorganisms7100436







