Variation in Soil Fungal Composition Associated with the Invasion of Stellera chamaejasme L. in Qinghai–Tibet Plateau Grassland
Abstract
1. Introduction
- (1)
- Do soil fungal communities and edaphic properties differ between S. chamaejasme invaded and uninvaded sites?
- (2)
- Does S. chamaejasme abundance or edaphic conditions explain variations in fungal communities?
- (3)
- Are there differences in functional groups or particular fungal species that might relate to the presence of S. chamaejasme?
2. Materials and Methods
2.1. Experimental Sites
2.2. Main Sampling and Analyses
2.3. Data Analysis
3. Results
3.1. Soil Physiochemical Properties
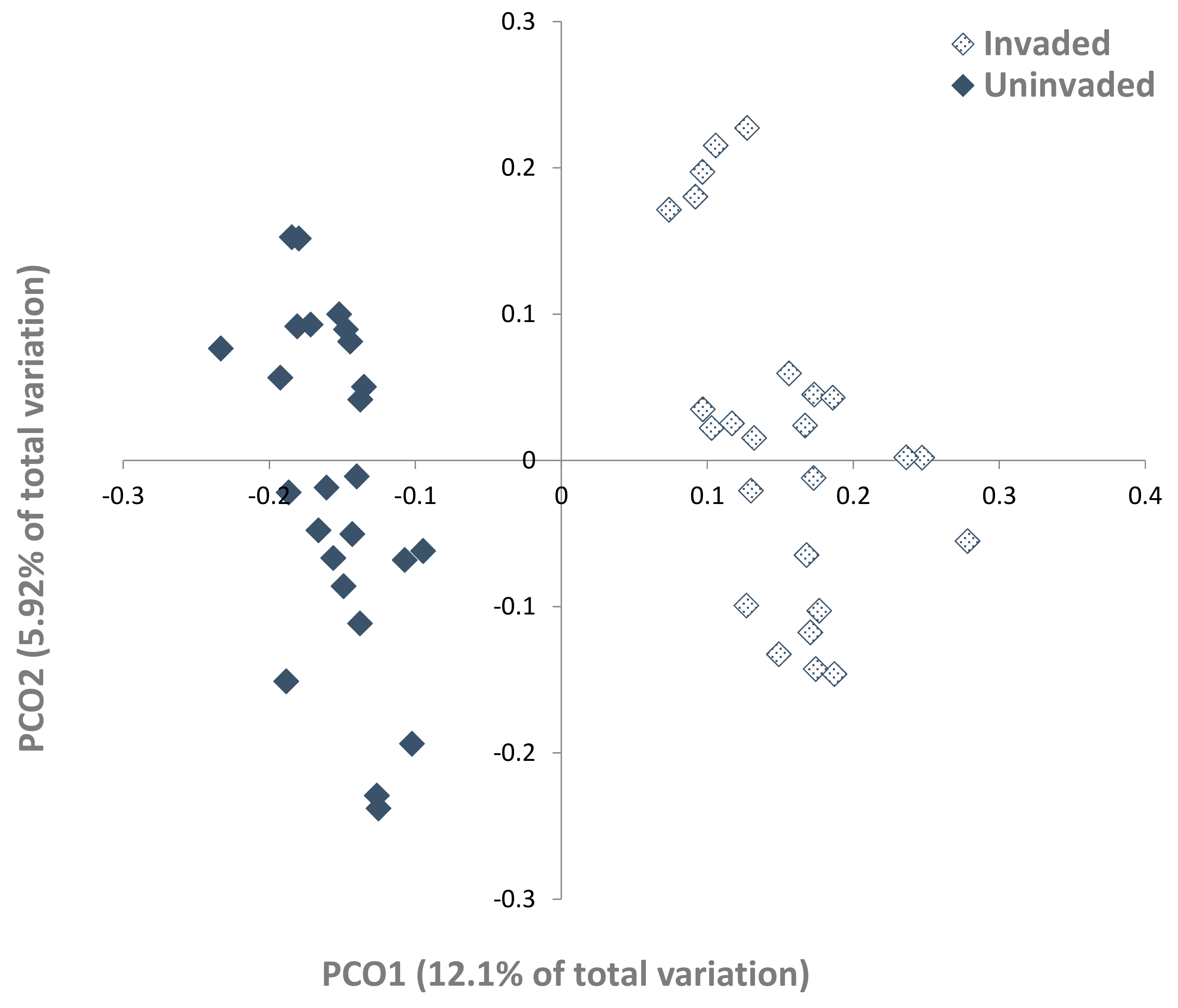
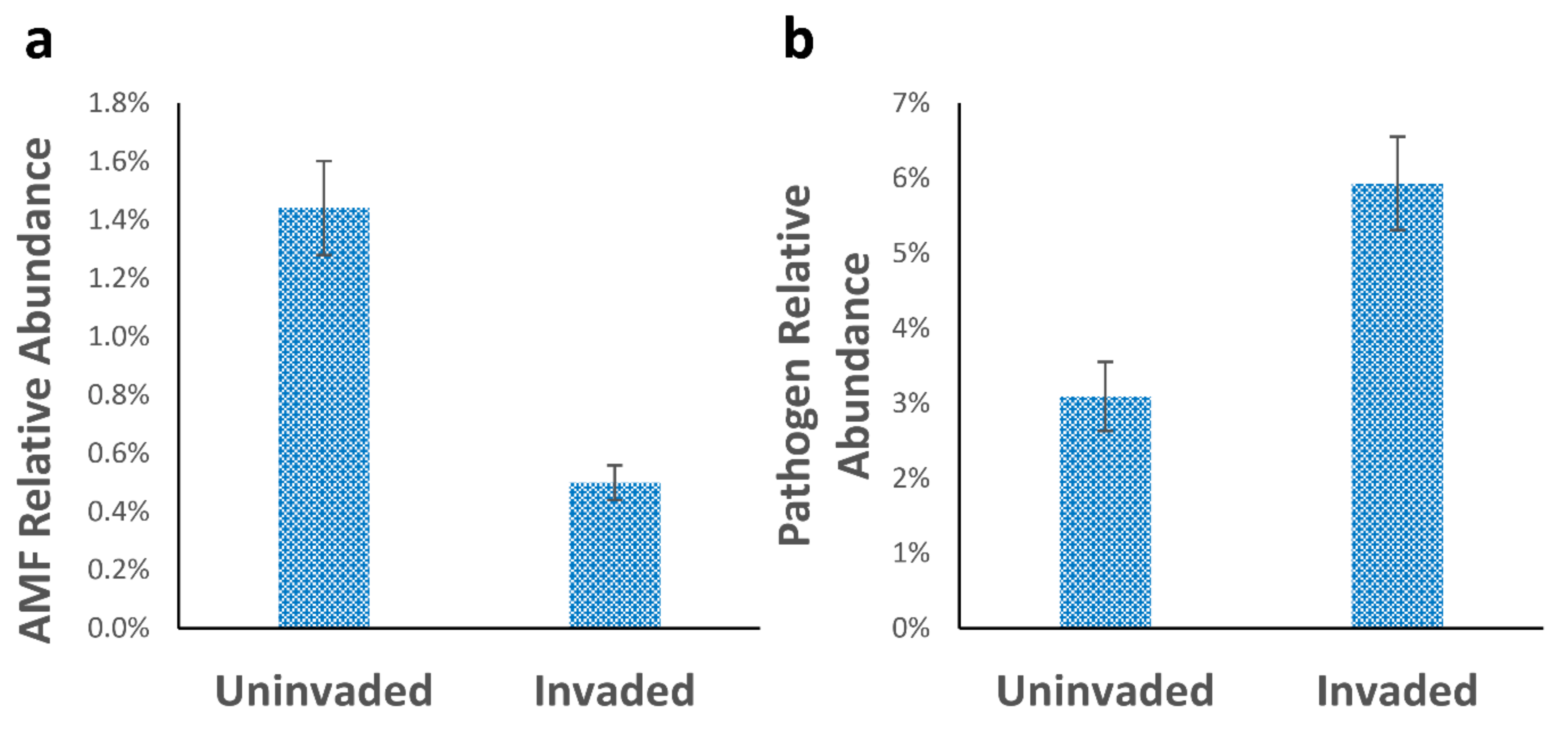
3.2. Fungal Populations
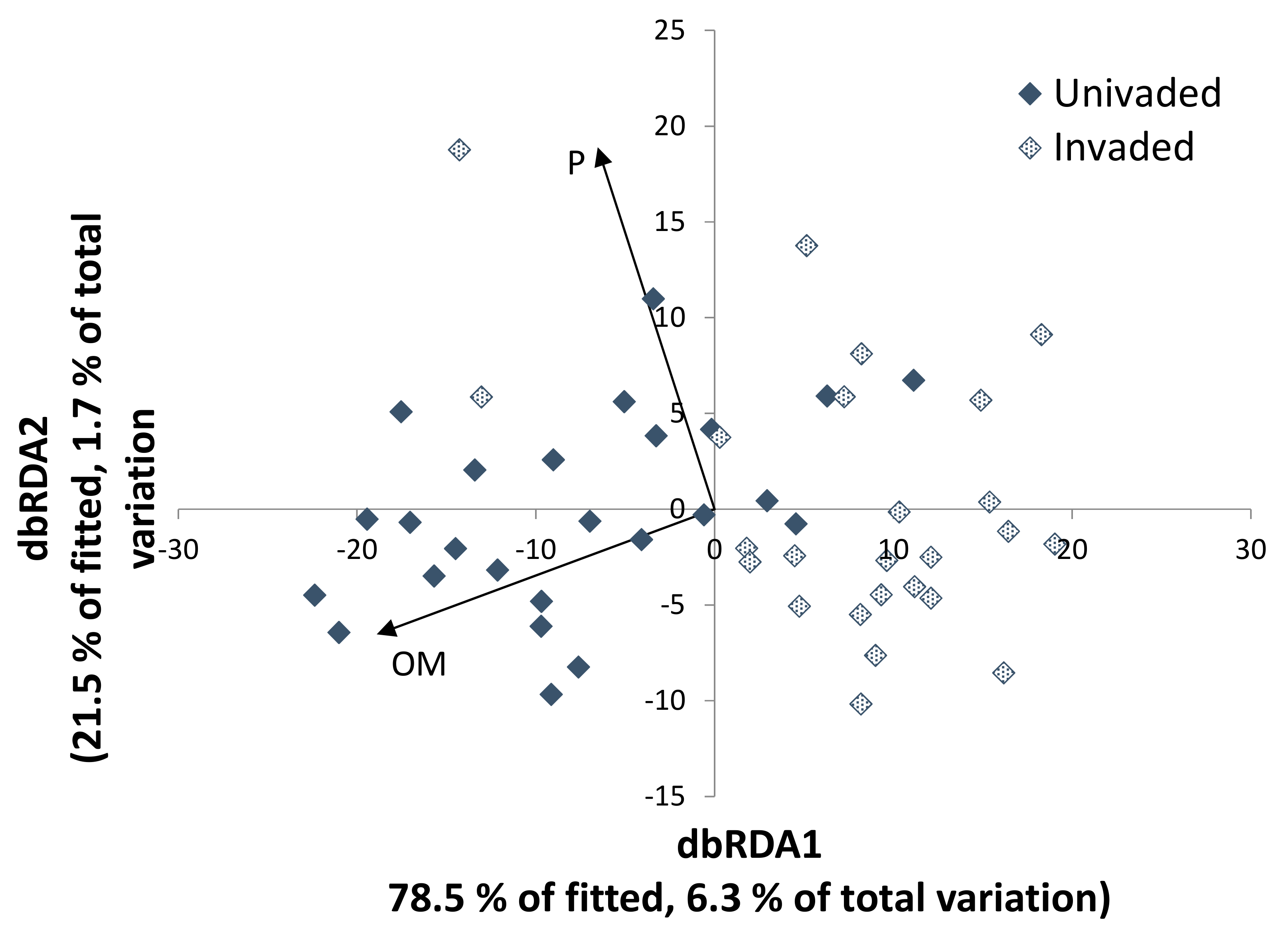
3.3. Fungal–Soil and S. chamaejasme Interrelationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.H.; Volis, S.; Sun, H. Chloroplast phylogeny and phylogeography of Stellera chamaejasme on the Qinghai-Tibet Plateau and in adjacent regions. Mol. Phylogenet. Evol. 2010, 57, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.R.; Zeng, L.M.; Yan, Z.Q.; Jin, H.; Li, X.Z.; Guan, J.F.; Qin, B. Allelochemical from the root exudates of Stellera chamaejasme L. and its degradation. Allelopath. J. 2016, 38, 119–190. [Google Scholar]
- Zhao, M.L.; Gao, X.L.; Wang, J.; He, X.L.; Han, B. A review of the most economically important poisonous plants to the livestock industry on temperate grasslands of China. J. Appl. Toxicol. 2013, 33, 9–17. [Google Scholar] [CrossRef]
- Ren, G.; Shang, Z.; Long, R.; Hou, Y.; Deng, B. The relationship of vegetation and soil differentiation during the formation of black-soil-type degraded meadows in the headwater of the Qinghai-Tibetan Plateau, China. Environ. Earth Sci. 2013, 69, 235–245. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Wittig, R. Growth parameters of characteristic species of Stipa steppes in Northern China as indicators of the grazing intensity. J. Appl. Bot. Angew. Bot. 2003, 77, 68–74. [Google Scholar]
- Klein, J.A.; Harte, J.; Zhao, X.-Q. Experimental warming, not grazing, decreases rangeland quality on the Tibetan Plateau. Ecol. Appl. 2007, 17, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Cui, J.; Zhang, S.T.; Zhang, W.G.; Liu, Y.H.; Liu, S.R.; An, S.Q. Differential water uptake among plant species in humid alpine meadows. J. Veg. Sci. 2013, 24, 138–147. [Google Scholar] [CrossRef]
- Guo, H.; Cui, H.; Jin, H.; Yan, Z.; Ding, L.; Qin, B. Potential allelochemicals in root zone soils of Stellera chamaejasme L. and variations at different geographical growing sites. Plant Growth Regul. 2015, 77, 335–342. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.Z.; Yan, Z.Q.; Guo, H.R.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure-activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef]
- Yan, L.F.; Xu, C.; Liu, Q.; Gu, A.H.; Jiang, Z.Y. Altered profile of gut microbiota after subchronic exposure to neochamaejasmin A in rats. Environ. Toxicol. Pharm. 2015, 39, 927–933. [Google Scholar] [CrossRef]
- Sun, G.; Luo, P.; Wu, N.; Qiu, P.; Gao, Y.; Chen, H.; Shi, F. Stellera chamaejasme L. increases soil N availability, turnover rates and microbial biomass in an alpine meadow ecosystem on the eastern Tibetan Plateau of China. Soil Biol. Biochem. 2009, 41, 86–91. [Google Scholar] [CrossRef]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 731. [Google Scholar] [CrossRef] [PubMed]
- Maron, J.L.; Klironomos, J.; Waller, L.; Callaway, R.M. Invasive plants escape from suppressive soil biota at regional scales. J. Ecol. 2014, 102, 19–27. [Google Scholar] [CrossRef]
- Rout, M.E.; Callaway, R.M. Interactions between exotic invasive plants and soil microbes in the rhizosphere suggest that ‘everything is not everywhere’. Ann. Bot. 2012, 110, 213–222. [Google Scholar] [CrossRef]
- Xiao, H.F.; Feng, Y.L.; Schaefer, D.A.; Yang, X.D. Soil fungi rather than bacteria were modified by invasive plants, and that benefited invasive plant growth. Plant Soil 2014, 378, 253–264. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Rodgers, V.L.; Stinson, K.A.; Pringle, A. The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. J. Ecol. 2008, 96, 777–783. [Google Scholar] [CrossRef]
- North, B.A.; Torzilli, A.P. Characterization of the root and soil mycobiome associated with invasive Microstegium vimineum in the presence and absence of a native plant community. Botany 2017, 95, 513–520. [Google Scholar] [CrossRef]
- Jordan, N.R.; Aldrich-Wolfe, L.; Huerd, S.C.; Larson, D.L.; Muehlbauer, G. Soil-occupancy effects of invasive and native grassland plant species on composition and diversity of mycorrhizal associations. Invasive Plant Sci. Manag. 2012, 5, 494–505. [Google Scholar] [CrossRef]
- Broz, A.K.; Manter, D.K.; Vivanco, J.M. Soil fungal abundance and diversity: Another victim of the invasive plant Centaurea maculosa. ISME J. 2007, 1, 763. [Google Scholar] [CrossRef]
- Gaggini, L.; Rusterholz, H.-P.; Baur, B. The invasive plant Impatiens glandulifera affects soil fungal diversity and the bacterial community in forests. Appl. Soil Ecol. 2018, 124, 335–343. [Google Scholar] [CrossRef]
- Si, C.; Liu, X.; Wang, C.; Wang, L.; Dai, Z.; Qi, S.; Du, D. Different degrees of plant invasion significantly affect the richness of the soil fungal community. PLoS ONE 2013, 8, e85490. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.M.; Lekberg, Y.; Mummey, D.L.; Sangwan, N.; Ramsey, P.W.; Gilbert, J.A. Invasive plants rapidly reshape soil properties in a grassland ecosystem. MSystems 2017, 2, e00178-16. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Lu, D.; Jin, H.; Yan, Z.; Chen, J.; Li, X.; Qin, B. Isolation and characterization of bacteria from the rhizosphere and bulk soil of Stellera chamaejasme L. Can. J. Microbiol. 2014, 61, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, X.; Liu, R.; Yan, Z.; Li, X.; Li, X.; Su, A.; Zhao, Y.; Qin, B. Bacterial community structure associated with the rhizosphere soils and roots of Stellera chamaejasme L. along a Tibetan elevation gradient. Ann. Microbiol. 2018, 68, 273–286. [Google Scholar] [CrossRef]
- Jin, H.; Yan, Z.; Liu, Q.; Yang, X.; Chen, J.; Qin, B. Diversity and dynamics of fungal endophytes in leaves, stems and roots of Stellera chamaejasme L. in northwestern China. Antonie Van Leeuwenhoek 2013, 104, 949–963. [Google Scholar] [CrossRef]
- Jin, H.; Yang, X.; Lu, D.; Li, C.; Yan, Z.; Li, X.; Zeng, L.; Qin, B. Phylogenic diversity and tissue specificity of fungal endophytes associated with the pharmaceutical plant, Stellera chamaejasme L. revealed by a cultivation-independent approach. Antonie Van Leeuwenhoek 2015, 108, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Tao, K.; Zhou, W.; Hou, T. Isolation of antifungal compound against phytophthora infestans from Stellera chamaejasme L. Asian J. Chem. 2013, 25, 4058–4060. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, Z. Research of Stelleara chamaejasme community characteristics in Qilian County and the relationship between them and the soil moisture of different depth. China Popul. Resour. Environ. 2014, 24, 168–170. [Google Scholar]
- Kang, L.; Han, X.G.; Zhang, Z.B.; Sun, O.J. Grassland ecosystems in China: Review of current knowledge and research advancement. Philos. Trans. R. Soc. B 2007, 362, 997–1008. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, H.; Huang, L.; Chen, C.; Lin, X.S.; Hu, Z.J.; Li, J.L. Grassland degradation remote sensing monitoring and driving factors quantitative assessment in China from 1982 to 2010. Ecol. Indic. 2017, 83, 303–313. [Google Scholar] [CrossRef]
- Li, X. Study on the Relationship between the Distribution of Stellera chamaejasme and the Soil Properties Based on GIS. Master’s Thesis, Northwest University, Xian, China, 2017. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Detheridge, A.P.; Brand, G.; Fychan, R.; Crotty, F.V.; Sanderson, R.; Griffith, G.W.; Marley, C.L. The legacy effect of cover crops on soil fungal populations in a cereal rotation. Agric. Ecosyst. Environ. 2016, 228, 49–61. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Hoiland, K.; Kjoller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Rosling, A.; Cox, F.; Cruz-Martinez, K.; Ihrmark, K.; Grelet, G.-A.; Lindahl, B.D.; Menkis, A.; James, T.Y. Archaeorhizomycetes: Unearthing an ancient class of ubiquitous soil fungi. Science 2011, 333, 876–879. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Q.; Wang, Q.; Wang, X.; Gange, A.C. Mycorrhizal-induced growth depression in plants. Symbiosis 2017, 72, 81–88. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.Q.; Wang, X.J.; Dou, C.Y.; Chen, M.; Lin, S.S.; Li, Y.Y. Arbuscular mycorrhiza regulate inter-specific competition between a poisonous plant, Ligularia virgaurea, and a co-existing grazing grass, Elymus nutans, in Tibetan Plateau Alpine meadow ecosystem. Symbiosis 2011, 55, 29–38. [Google Scholar] [CrossRef]
- Nijjer, S.; Rogers, W.E.; Lee, C.-T.A.; Siemann, E. The effects of soil biota and fertilization on the success of Sapium sebiferum. Appl. Soil Ecol. 2008, 38, 1–11. [Google Scholar] [CrossRef]
- Bary, F.; Gange, A.C.; Crane, M.; Hagley, K.J. Fungicide levels and arbuscular mycorrhizal fungi in golf putting greens. J. Appl. Ecol. 2005, 42, 171–180. [Google Scholar] [CrossRef]
- Gange, A.C.; Lindsay, D.E.; Ellis, L.S. Can arbuscular mycorrhizal fungi be used to control the undesirable grass Poa annua on golf courses? J. Appl. Ecol. 1999, 36, 909–919. [Google Scholar] [CrossRef]
- Bray, S.R.; Kitajima, K.; Sylvia, D.M. Mycorrhizae differentially alter growth, physiology, and competitive ability of an invasive shrub. Ecol. Appl. 2003, 13, 565–574. [Google Scholar] [CrossRef]
- Hooker, J.; Jaizme-Vega, M.; Atkinson, D. Biocontrol of Plant Pathogens Using Arbuscular Mycorrhizal Fungi. In Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems; Springer, Birkhäuser: Basel, Switzerland, 1994; pp. 191–200. [Google Scholar]
- Herre, E.A.; Mejía, L.C.; Kyllo, D.A.; Rojas, E.; Maynard, Z.; Butler, A.; Van Bael, S.A. Ecological implications of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology 2007, 88, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Vigo, C.; Norman, J.; Hooker, J. Biocontrol of the pathogen Phytophthora parasitica by arbuscular mycorrhizal fungi is a consequence of effects on infection loci. Plant Pathol. 2000, 49, 509–514. [Google Scholar] [CrossRef]
- Liang, M.; Liu, X.; Etienne, R.S.; Huang, F.; Wang, Y.; Yu, S. Arbuscular mycorrhizal fungi counteract the Janzen-Connell effect of soil pathogens. Ecology 2015, 96, 562–574. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef]
- Zhou, H.S.; Yang, G.W.; Zhang, Y.J. The impact of soil available phosphorus on the AM fungi and the organic acids exudation at different patches in northern steppe of China. J. Anim. Vet. Adv. 2012, 11, 4553–4558. [Google Scholar] [CrossRef]
- Bala, A.; Jain, J.; Kumari, A.; Singh, B. Production of an extracellular phytase from a thermophilic mould Humicola nigrescens in solid state fermentation and its application in dephytinization. Biocatal. Agric. Biotechnol. 2014, 3, 259–264. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef]
- Zhang, H.S.; Qin, F.F.; Qin, P.; Pan, S.M. Evidence that arbuscular mycorrhizal and phosphate-solubilizing fungi alleviate NaCl stress in the halophyte Kosteletzkya virginica: Nutrient uptake and ion distribution within root tissues. Mycorrhiza 2014, 24, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Ludwig, A.; Furch, A.; Mithöfer, A.; Scholz, S.S.; Reichelt, M.; Oelmüller, R. The beneficial root-colonizing fungus Mortierella hyalina promotes the aerial growth of Arabidopsis and activates calcium-dependent responses which restrict Alternaria brassicae-induced disease development in roots. Mol. Plant Microbe Interact. 2018, 32, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.X.; Jin, H.; Yang, X.Y.; Zhang, D.H.; Yan, Z.Q.; Li, X.Z.; Zhao, Y.H.; Han, R.B.; Qin, B. Characterization of rhizosphere and endophytic fungal communities from roots of Stipa purpurea in alpine steppe around Qinghai Lake. Can. J. Microbiol. 2016, 62, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jin, H.; Liu, Q.; Yan, Z.; Ding, L.; Qin, B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest Manag. Sci. 2014, 70, 827–835. [Google Scholar] [CrossRef]
- Sa, W.; An, L. Changes in plant community diversity and aboveground biomass along with altitude within an alpine meadow on the Three-River source region. Chin. Sci. Bull. 2012, 57, 3573–3577. [Google Scholar] [CrossRef]




| Uninvaded | Invaded | p-Value | |
|---|---|---|---|
| % K | 1.96 ± 0.016 | 2.02 ± 0.017 | 0.0487 |
| % P | 0.06 ± 0.001 | 0.05 ± 0.002 | 0.0042 |
| % N | 0.29 ± 0.043 | 0.22 ± 0.015 | 0.0009 |
| % OM | 5.72 ± 0.217 | 4.02 ± 0.287 | 0.0002 |
| C:N ratio | 11.4 ± 0.081 | 10.7 ± 0.089 | <0.0001 |
| % H2O | 26.8 ± 0.816 | 28.2 ± 0.514 | 0.136 |
| pH | 7.85 ± 0.061 | 7.98 ± 0.082 | 0.176 |
| Uninvaded | Invaded | |
|---|---|---|
| Population diversity indices | ||
| OTU Count | 265 ± 8.38a | 192 ± 7.08b |
| Inverse Simpson Index (OTU) | 21.36 ± 1.72 | 17.58 ± 1.42 |
| Shannon Index (OTU) | 3.85 ± 0.06a | 3.57 ± 0.08b |
| % Fungi identified to phylum | ||
| Ascomycota | 51.85 ± 1.87b | 63.36 ± 2.09a |
| Basidiomycota | 23.78 ± 1.89 | 19.35 ± 1.80 |
| Zygomycota | 16.8 ± 0.99a | 12.8 ± 1.14b |
| Glomeromycota | 1.44 ± 0.12a | 0.49 ± 0.05b |
| Chytridiomycota | 0.29 ± 0.05 | 0.22 ± 0.09 |
| Unclassified fungi | 4.06 ± 0.92 | 3.22 ± 0.54 |
| Fungi unidentified | 1.70 ± 0.43 | 0.54 ± 0.14 |
| Species | UNITE SH ID | Uninvaded Av.Ab | Invaded Av.Ab | Av.Di | Diss/SD | Contr% | Cum.% |
|---|---|---|---|---|---|---|---|
| Archaeorhizomyces sp. | SH197151.06FU | 7.86% | 6.55% | 5.14 | 0.93 | 6.52 | 6.52 |
| Mortierella sp. | SH211066.06FU | 4.28% | 4.83% | 2.32 | 1.13 | 2.94 | 9.46 |
| Mortierella polygonia | SH211068.06FU | 4.59% | 3.24% | 2.26 | 1.03 | 2.87 | 12.33 |
| Sordariomycetes OTU 12 | 1.60% | 2.33% | 1.69 | 0.38 | 2.15 | 14.48 | |
| Mortierellales OTU 8 | 3.36% | 0.01% | 1.68 | 1.28 | 2.13 | 16.61 | |
| Ascomycota sp. | SH209335.06FU | 1.63% | 2.64% | 1.42 | 0.75 | 1.80 | 18.41 |
| Humicola nigrescens | SH234919.06FU | 0.37% | 2.97% | 1.40 | 0.82 | 1.78 | 20.19 |
| Wallemia sp. | SH230273.06FU | 2.31% | 1.14% | 1.39 | 0.41 | 1.76 | 21.95 |
| Hygrocybe sp. | SH190651.06FU | 1.34% | 1.50% | 1.29 | 0.47 | 1.64 | 23.59 |
| Agaricales OTU 20 | 2.47% | 0.00% | 1.23 | 0.48 | 1.57 | 25.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Detheridge, A.; Liu, Y.; Wang, L.; Wei, H.; Griffith, G.W.; Scullion, J.; Wei, Y. Variation in Soil Fungal Composition Associated with the Invasion of Stellera chamaejasme L. in Qinghai–Tibet Plateau Grassland. Microorganisms 2019, 7, 587. https://doi.org/10.3390/microorganisms7120587
He W, Detheridge A, Liu Y, Wang L, Wei H, Griffith GW, Scullion J, Wei Y. Variation in Soil Fungal Composition Associated with the Invasion of Stellera chamaejasme L. in Qinghai–Tibet Plateau Grassland. Microorganisms. 2019; 7(12):587. https://doi.org/10.3390/microorganisms7120587
Chicago/Turabian StyleHe, Wei, Andrew Detheridge, Yongmei Liu, Lei Wang, Haochen Wei, Gareth W. Griffith, John Scullion, and Yahui Wei. 2019. "Variation in Soil Fungal Composition Associated with the Invasion of Stellera chamaejasme L. in Qinghai–Tibet Plateau Grassland" Microorganisms 7, no. 12: 587. https://doi.org/10.3390/microorganisms7120587
APA StyleHe, W., Detheridge, A., Liu, Y., Wang, L., Wei, H., Griffith, G. W., Scullion, J., & Wei, Y. (2019). Variation in Soil Fungal Composition Associated with the Invasion of Stellera chamaejasme L. in Qinghai–Tibet Plateau Grassland. Microorganisms, 7(12), 587. https://doi.org/10.3390/microorganisms7120587





