Inhibition–Disruption of Candida glabrata Biofilms: Symmetrical Selenoesters as Potential Anti-Biofilm Agents
Abstract
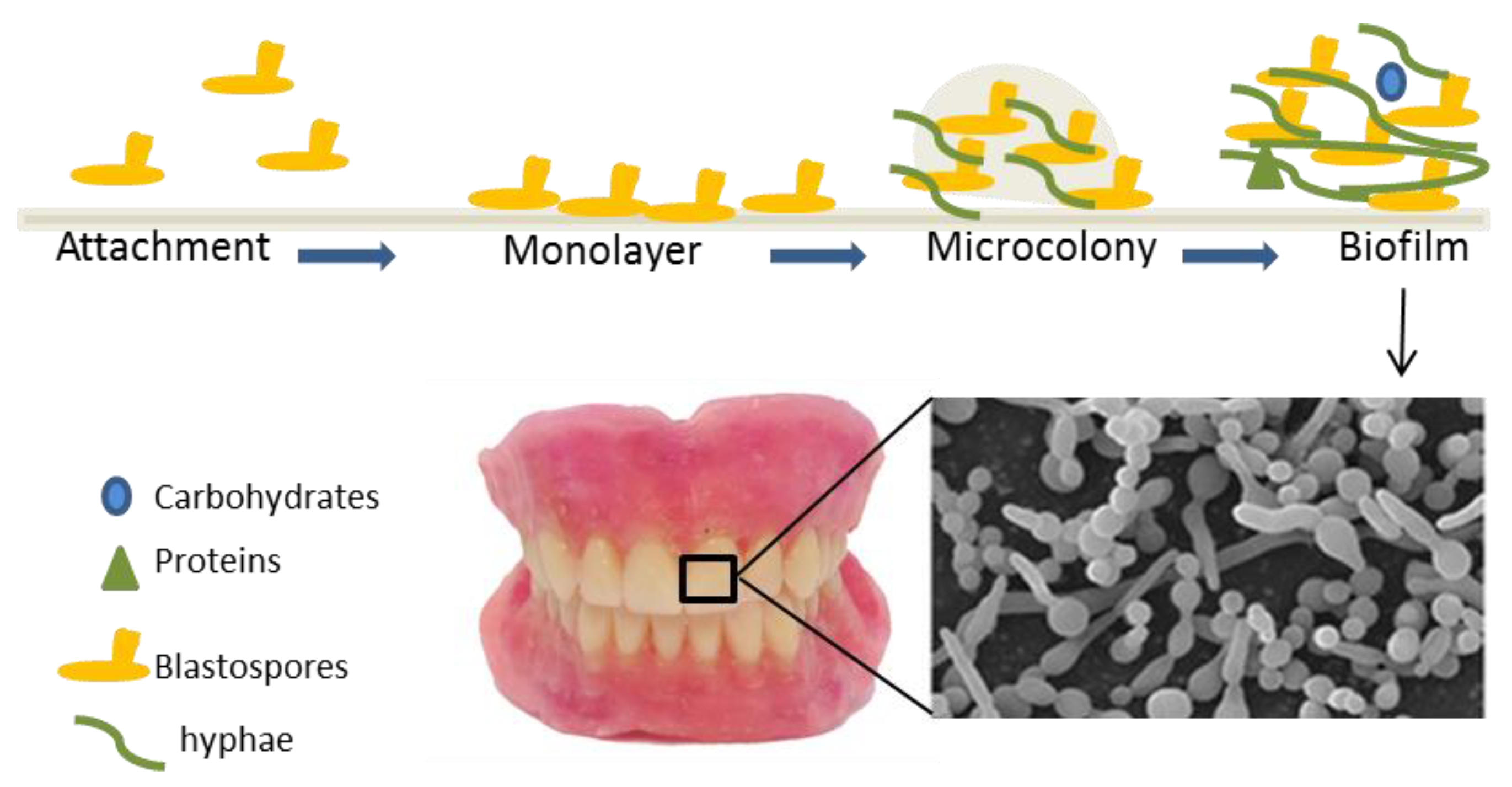
1. Introduction
2. Materials and Methods
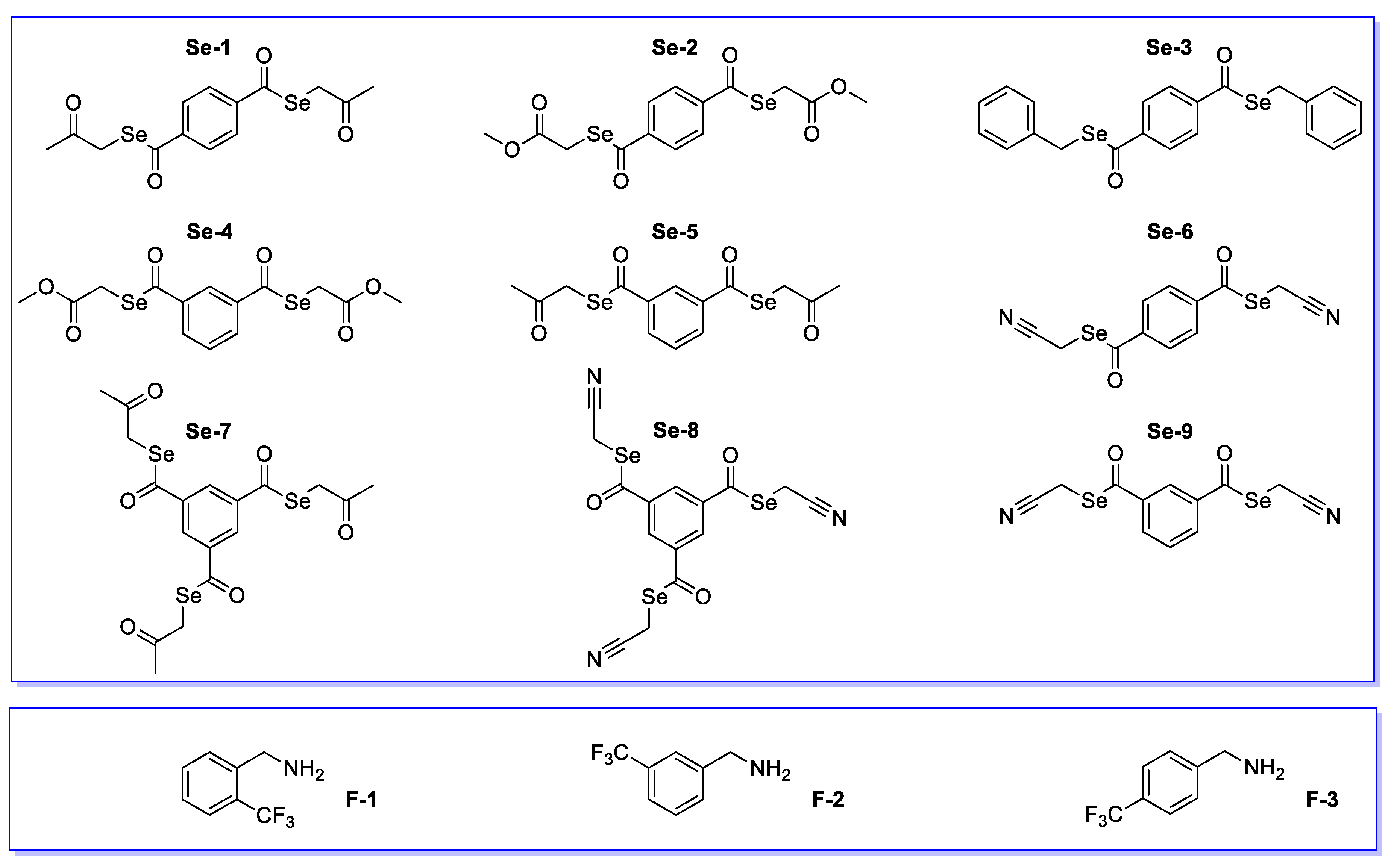
2.1. Tested Compounds
2.2. Growth Conditions
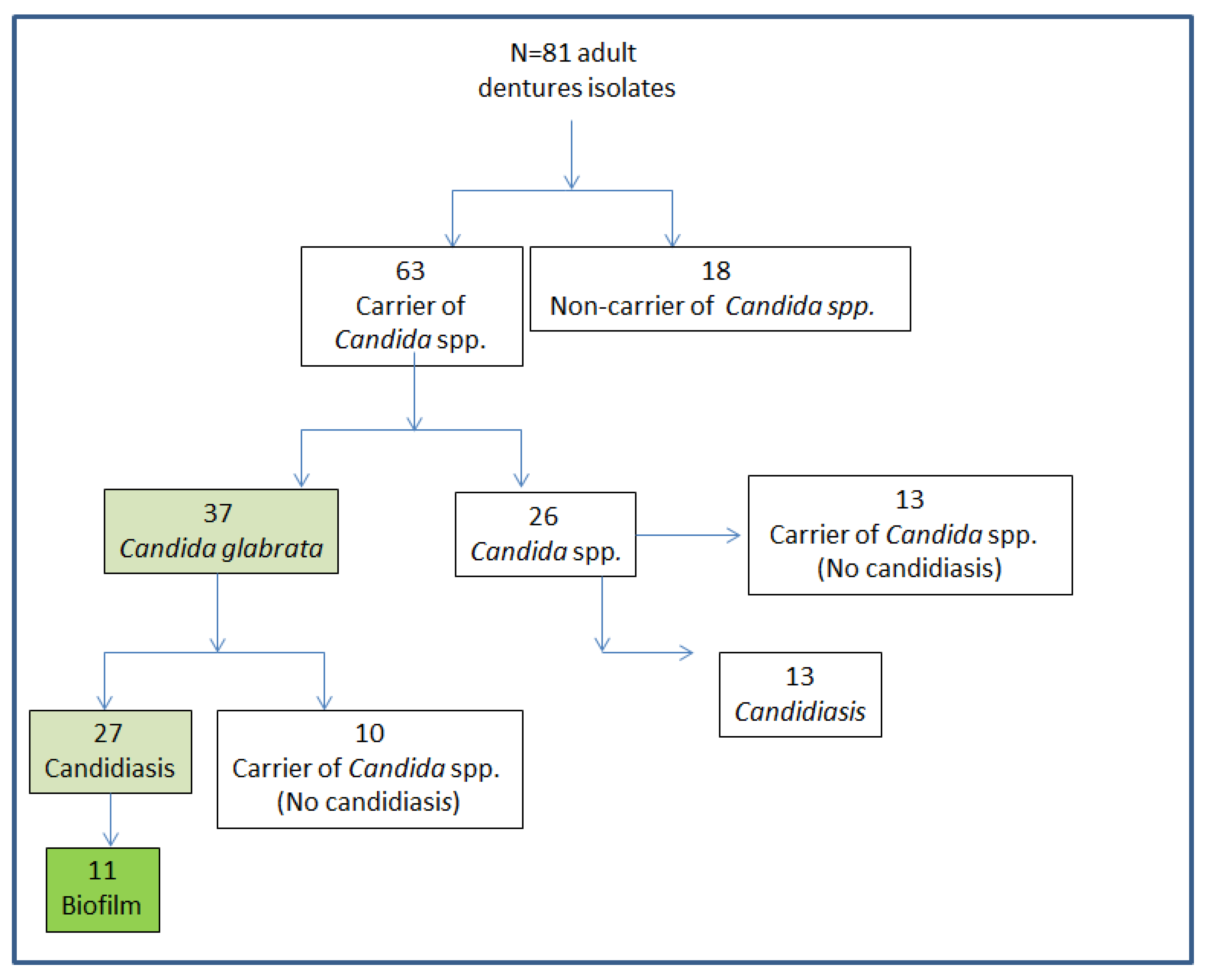
2.3. Isolation and Identification of Candida Strains
2.4. Antifungal Assay
2.5. Minimal Inhibitory Concentrations (MICs)
2.6. Biofilm Inhibition Assay
2.7. Biofilm Disruption Assay
2.8. Data Analysis
3. Results and Discussion
3.1. Antifungal Screening
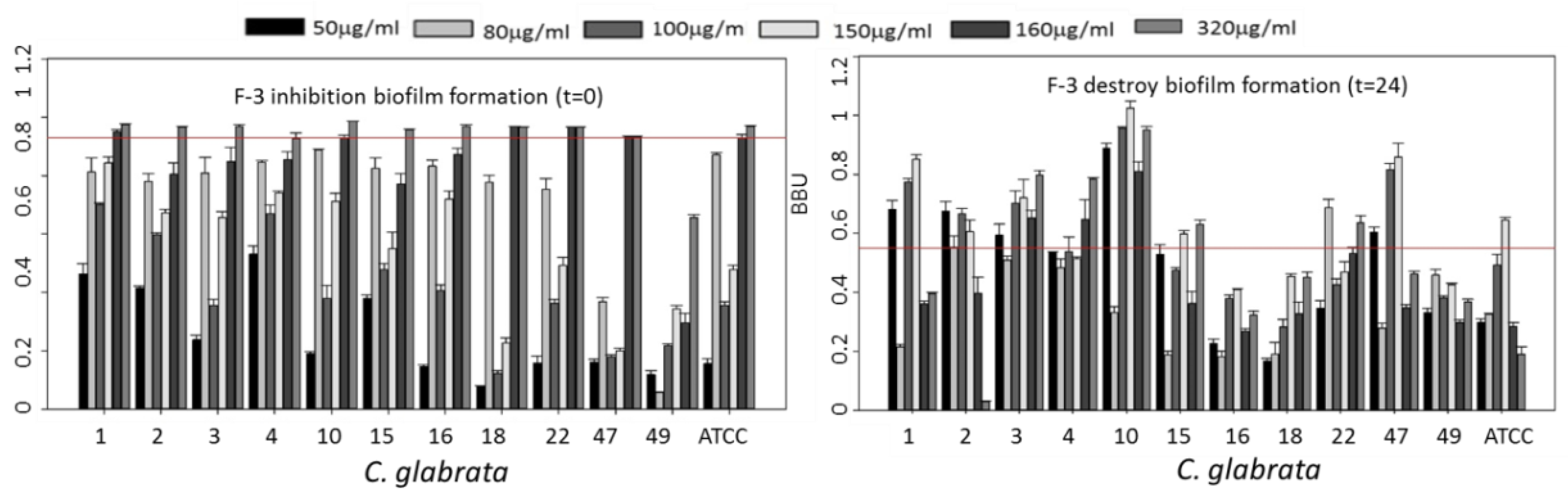
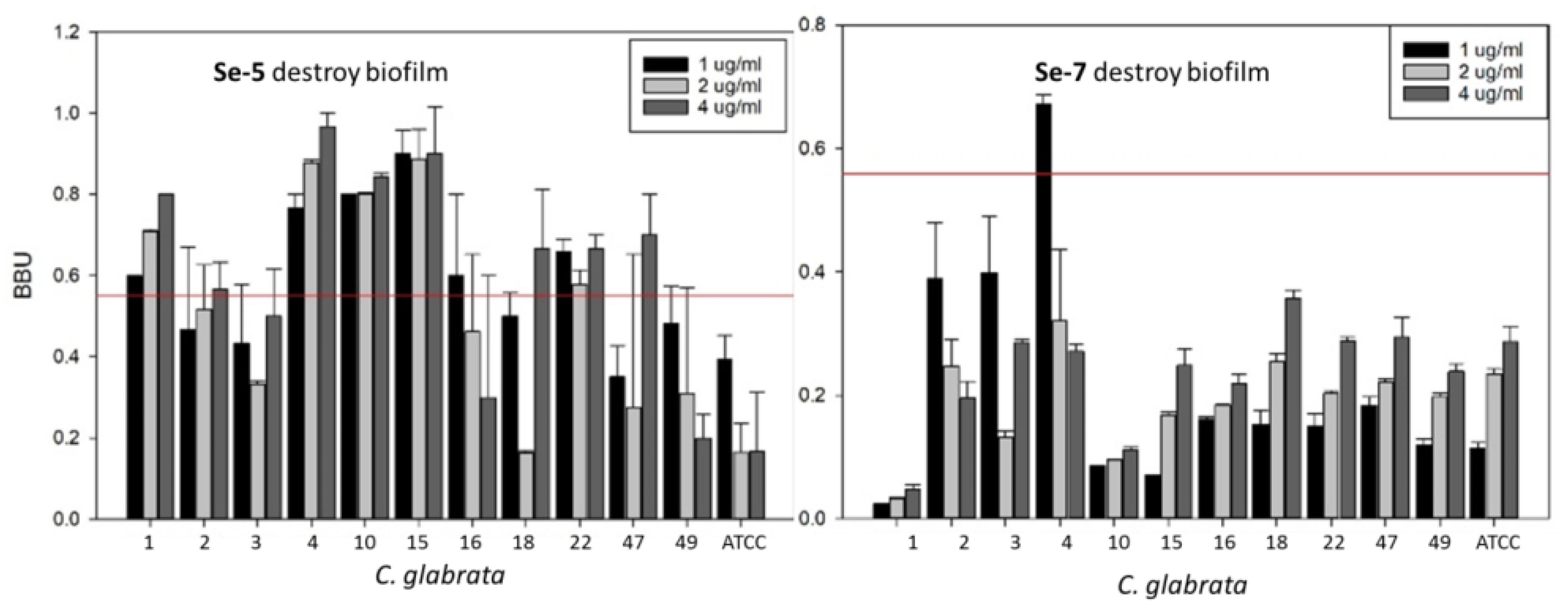
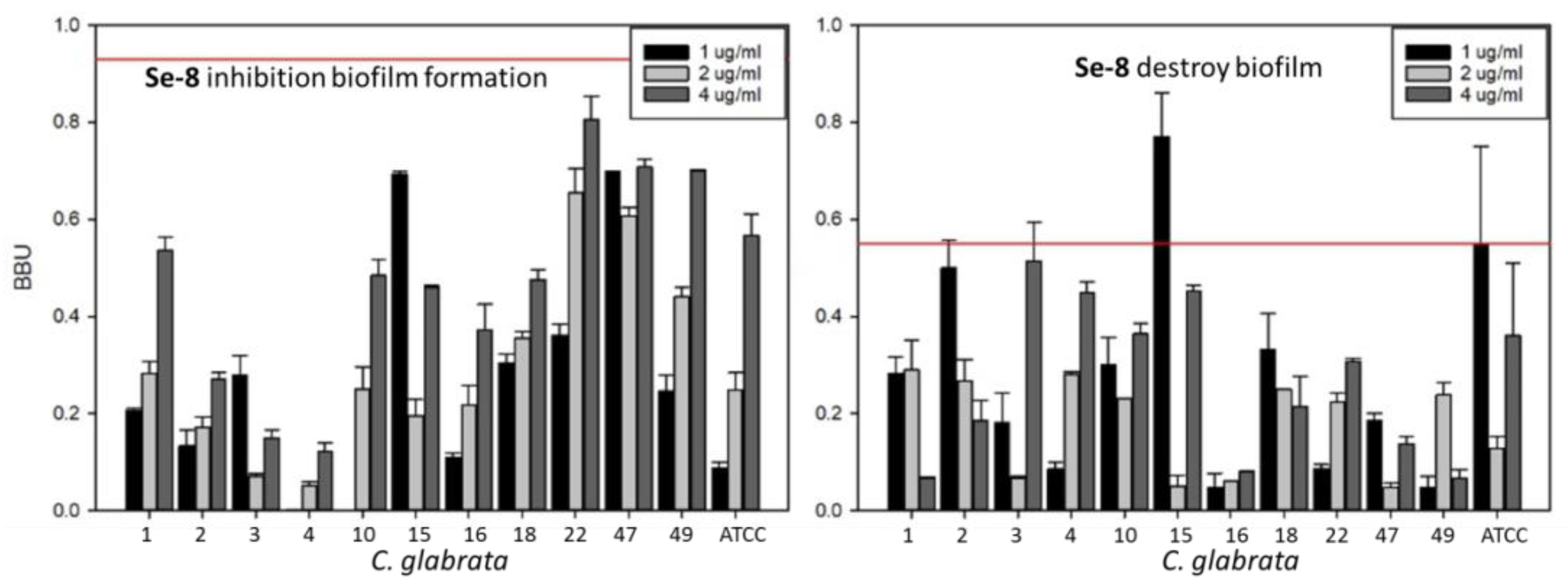
3.2. Inhibition of Biofilm Growth and Disruption of Preformed Biofilm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Wade, W.G. The human oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Paster, B.J.; Olsen, I.; Aas, J.A.; Dewhirst, F.E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2000 2006, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Corte, L.; Pierantoni, D.C.; Tascini, C.; Roscini, L.; Cardinali, G. Biofilm Specific Activity: A Measure to Quantify Microbial Biofilm. Microorganisms 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.F.; Douglas, L.J. Interactions between Candida species and bacteria in mixed infections. In Polymicrobial Diseases, 2nd ed.; Brogden, K.A., Guthmiller, J.M., Eds.; ASM Press: Washington, DC, USA, 2002; Chapter 18; pp. 357–373. [Google Scholar]
- Budtz-Jorgensen, E. Candida-associated denture stomatitis and angular cheilitis. In Oral Candidosis; Samaranayake, L.P., MacFarlane, T.W., Eds.; John Wright: Guilford, UK, 1990; pp. 156–183. ISBN 0723609837. [Google Scholar]
- Nikawa, H.; Hamada, T.; Yamamoto, T. Denture plaque past and recent concerns. J. Dent. 1998, 26, 299–304. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J. Candida biofilm resistance. Drug Resist. Updates 2004, 7, 301–309. [Google Scholar] [CrossRef]
- Lang, N.P.; Lindhe, J. (Eds.) Clinical Periodontology and Implant Dentistry, 2nd Volume Book, 6th ed.; Blackwell Publishing Company: Oxford, UK, 1998; ISBN 978-0-470-67248-8. [Google Scholar]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Figueiral, M.H.; Sousa-Rodrigues, P.; Fernandes, M.H.; Scully, C. The effect of denture adhesives on Candida albicans growth in vitro. Gerodontology 2012, 29, 348–356. [Google Scholar] [CrossRef]
- Lee, M.X.; Cajas, C.N.; Gómez, C.L.; Vergara, N.C.; Ivankovic, S.M.; Astorga, B.E. Ocurrencia de levaduras del género Cándida y estomatitis protésica antes y después del tratamiento rehabilitador basado en prótesis removible. Rev. Clin. Periodoncia Implantol. Rehabil. Oral. 2015, 8, 31–37. [Google Scholar] [CrossRef]
- Loster, B.W.; Loster, J.; Wieczorek, A.; Ryniewicz, W. Mycological analysis of the oral cavity of patients using acrylic removable dentures. Gastroenterol. Res. Pract. 2012, 2012, 951572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marra, J.; Paleari, A.G.; Rodriguez, L.S.; Leite, A.R.P.; Pero, A.C.; Compagnoni, M.A. Effect of an acrylic resin combined with an antimicrobial polymer on biofilm formation. J. Appl. Oral Sci. 2012, 20, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Gjodsbol, K.; Christensen, J.J.; Karlsmark, T.; Jorgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kirketerp-Moller, K.; Jensen, P.O.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef]
- Castrillon, L.; Palma, A.; Padilla, M. Biopelículas fúngicas. Dermatol. Rev. Mex. 2013, 57, 350–361. [Google Scholar]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, G.A.; Petruzzi, M.; Serpico, R. Cándida-associated denture stomatitis. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, e139–e143. [Google Scholar] [CrossRef]
- Abraham, C.M. Advances and emerging techniques in the identification, diagnosis and treatment of oral candidiasis. Open. Pathol. J. 2011, 5, 8–12. [Google Scholar] [CrossRef][Green Version]
- Dangi, Y.S.; Soni, M.L.; Namdeo, K.P. Oral candidiasis: A review. Int. J. Pharm. Pharm. Sci. 2010, 2, 3641. [Google Scholar]
- Vediyappan, G.; Rossignol, T.; d’Enfert, C. Interaction of Candida albicans biofilms with antifungals: Transcriptional response and binding of antifungals to beta-glucans. Antimicrob. Agents Chemother. 2010, 54, 2096–2111. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Hoiby, N. Pharmacokinetics/Pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2011, 55, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.O.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Rendekova, J.; Vlasakova, D.; Arsenyan, P.; Vasiljeva, J.; Nasim, M.J.; Witek, K.; Dominguez-Alvarez, E.; Zeslawska, E.; Manikova, D.; Tejchman, W.; et al. The Selenium-Nitrogen Bond as Basis for Reactive Selenium Species with Pronounced Antimicrobial Activity. Curr. Org. Synth. 2017, 14, 1082–1090. [Google Scholar] [CrossRef]
- Gularte, M.S.; Anghinoni, J.M.; Abenante, L.; Voss, G.T.; de Oliveira, R.L.; Vaucher, R.A.; Luchese, C.; Wilhelm, E.A.; Lenardão, E.J.; Fajardo, A.R. Synthesis of chitosan derivatives with organoselenium and organosulfur compounds: Characterization, antimicrobial properties and application as biomaterials. Carbohydr. Polym. 2019, 219, 240–250. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 2017, 13, 1095–1103. [Google Scholar] [CrossRef]
- Salin, H.; Fardeau, V.; Piccini, E.; Lelandais, G.; Tanty, V.; Lemoine, S.; Jacq, C.; Devaux, F. Structure and properties of transcriptional networks driving selenite stress response in yeasts. BMC Genom. 2008, 9, 333. [Google Scholar] [CrossRef]
- Csonka, A.; Kincses, A.; Nové, M.; Vadas, Z.; Sanmartín, C.; Domínguez-Álvarez, E.; Spengler, G. Selenoesters and Selenoanhydrides as Novel Agents Against Resistant Breast Cancer. Anticancer Res. 2019, 39, 3777–3783. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium Compounds as Novel Adjuvants of Chemotherapy Drugs-A Promising Approach to Fight Cancer Drug Resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef]
- Mosolygó, T.; Kincses, A.; Csonka, A.; Tönki, Á.S.; Witek, K.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Kieć-Kononowicz, K.; Domínguez-Álvarez, E.; et al. Selenocompounds as Novel Antibacterial Agents and Bacterial Efflux Pump Inhibitors. Molecules 2019, 24, 1487. [Google Scholar] [CrossRef]
- Ali, W.; Álvarez-Pérez, M.; Marć, M.A.; Salardón-Jiménez, N.; Handzlik, J.; Domínguez-Álvarez, E. The anticancer and chemopreventive activity of selenocyanate-containing compounds. Curr. Pharmacol. Rep. 2018, 4, 468–481. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, G.; Zhu, X.; Jiang, K.; Wu, H.; Deng, G.; Qiu, C. Sodium selenite induces apoptosis via ROS-mediated NF-κB signaling and activation of the Bax-caspase-9-caspase-3 axis in 4T1 cells. J. Cell. Physiol. 2019, 234, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Jamier, V.; Ba, L.A.; Jacob, C. Selenium- and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chemistry 2010, 16, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Staicu, L.; Oremland, R.; Tobe, R.; Mihara, H. Bacteria Versus Selenium. In Selenium in Plants. Plant Ecophysiology; Pilon-Smits, E., Winkel, L., Lin, Z.Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 11, Chapter 6. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Spengler, G.; Jacob, C.; Sanmartín, C. Seleno Ester-Containing Compounds for Use in the Treatment of Microbial Infections or Colorectal Cancer. European Patent Application EP17382693, 28 September 2018. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 7th ed.; CLSI Document M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- O’Toole, G.; Kolter, R. Initiation of biofilm formation in Pseudomona florescens WCS365 proceeds via múltiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 3. [Google Scholar] [CrossRef]
- Ramage, G.; Vande, W.; Wickes, B.; Lopez-Ribot, J. Standardized Method for in vitro Antifungal Susceptibility Testing of Cándida albicans Biofilms. Antibiocrob. Agents Chemother. 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, M. Factores de Riesgo Asociados a la Resistencia de Cándida spp. a Fluconazol y Voriconazol en el Tratamiento de Candidiasis Subprotesica, en Adultos Portadores de Protesis Dental que Residen en el Hogar “25 de Mayo” Sucre 2012. Tesis Para Optar a Magister Farmacologia Básica y Clínica. Master’s Thesis, Farmacologia básica y Clínica, Universidad Andina Simón Bolivar, Sucre, Bolivia, 2013. [Google Scholar]
- Budtz-Jorgensen, E. Ecology of Candida-associated denture stomatitis. Microb. Ecol. Health Dis. 2000, 12, 170–185. [Google Scholar] [CrossRef][Green Version]
- Canuto, M.M.; Rodero, F.G. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Davies, D. Understanding Biofilm Resistance to Antibacterial. Agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Rogers, S.A.; Huigens, R.W., III; Melander, C. A 2-Aminobenzimidazole That Inhibits and Disperses Gram-Positive Biofilms through a Zinc-Dependent Mechanism. J. Am. Chem. Soc. 2009, 131, 9868–9869. [Google Scholar] [CrossRef] [PubMed]




| Species | Interpretive Categories and Zone Diameter (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antifungal Agents | Fluoride Compounds | Selenoesters | |||||||
| FLZ | VOR | CAS | F-1 | F-2 | F-3 | Se-5 | Se-7 | Se-8 | |
| (25 μg) | (1 μg) | (5 μg/mL) | (500 μg) | (2 μg) | |||||
| C. glabrata ATCC2001 | 20 | 21 | 21 | 21 | 24 | 21 | 20 | 22 | 26 |
| C. krusei ATCC6258 | 6 | 23 | 20 | 10 | 19 | 10 | 36 | 37 | 29 |
| C. parapsilosis ATCC22019 | 24 | 31 | 14 | 10 | 10 | 10 | 36 | 40 | 28 |
| Compounds | MIC (µg/mL) | |||
|---|---|---|---|---|
| C. glabrata ATCC 2001 | C. krusei ATCC 6258 | C. parapsilosis ATCC 22019 | C. glabrata isolates (N = 37) | |
| VOR | <0.5 | 0.5 | 0.12 | 0.12–1 |
| FLZ | 32 | 16 | 2 | 0.1–64 |
| CAS | 0.12 | 0.19 | 1 | 0.12–8 |
| Se-1 | 8 | 32 | 32 | 32–64 |
| Se-2 | 64 | 64 | 64 | 32–64 |
| Se-3 | 64 | 64 | 64 | 32–64 |
| Se-4 | 64 | 64 | 64 | 64–128 |
| Se-5 | 4 | 2 | 1 | 0.5–4 |
| Se-6 | 64 | 64 | 64 | 64–128 |
| Se-7 | 2 | 1 | 2 | 0.5–4 |
| Se-8 | 5 | 1 | 0.5 | 10–32 |
| Se-9 | 64 | 64 | 64 | 16–64 |
| MICs (µg/mL), C. glabrata Dental Isolates (n = 11) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain Number | |||||||||||
| Compounds | 1 | 2 | 3 | 5 | 10 | 15 | 16 | 18 | 22 | 47 | 49 |
| VRZ | 0.25 | 0.25 | 0.25 | O.5 | 0.25 | 0.25 | 0.25 | ≤0.12 | ≤0.12 | 0.25 | ≤0.12 |
| CAS | 0.25 | 0.25 | 0.25 | >8 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Se-5 | 1 | 4 | 2 | 4 | 2 | 2 | 1 | 1 | 2 | 2 | 4 |
| Se-7 | 1 | 2 | 6 | 2 | 2 | 1 | 2 | 16 | 2 | 1 | >2 |
| Se-8 | 16 | 16 | 4 | 32 | 16 | 16 | 20 | 8 | 32 | 16 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Cruz-Claure, M.L.; Cèspedes-Llave, A.A.; Ulloa, M.T.; Benito-Lama, M.; Domínguez-Álvarez, E.; Bastida, A. Inhibition–Disruption of Candida glabrata Biofilms: Symmetrical Selenoesters as Potential Anti-Biofilm Agents. Microorganisms 2019, 7, 664. https://doi.org/10.3390/microorganisms7120664
De la Cruz-Claure ML, Cèspedes-Llave AA, Ulloa MT, Benito-Lama M, Domínguez-Álvarez E, Bastida A. Inhibition–Disruption of Candida glabrata Biofilms: Symmetrical Selenoesters as Potential Anti-Biofilm Agents. Microorganisms. 2019; 7(12):664. https://doi.org/10.3390/microorganisms7120664
Chicago/Turabian StyleDe la Cruz-Claure, María L., Ariel A. Cèspedes-Llave, María T. Ulloa, Miguel Benito-Lama, Enrique Domínguez-Álvarez, and Agatha Bastida. 2019. "Inhibition–Disruption of Candida glabrata Biofilms: Symmetrical Selenoesters as Potential Anti-Biofilm Agents" Microorganisms 7, no. 12: 664. https://doi.org/10.3390/microorganisms7120664
APA StyleDe la Cruz-Claure, M. L., Cèspedes-Llave, A. A., Ulloa, M. T., Benito-Lama, M., Domínguez-Álvarez, E., & Bastida, A. (2019). Inhibition–Disruption of Candida glabrata Biofilms: Symmetrical Selenoesters as Potential Anti-Biofilm Agents. Microorganisms, 7(12), 664. https://doi.org/10.3390/microorganisms7120664







