Nontuberculous Mycobacteria Persistence in a Cell Model Mimicking Alveolar Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mycobacterial Strains
2.2. THP-1 Cells
2.3. Macrophage Infection
2.4. Phagosome Maturation
2.5. NTM Growth at Different PHs
2.6. Nitric Oxide
2.7. Apoptosis Mediated by Caspase 8 and Caspases 3/7
2.8. Statistical Analysis
2.9. Whole-Genome Sequencing, Assembly and Annotation
2.10. Overview of the Accessory Genome of Same-Species Reference and Clinical Isolates
2.11. In silico Screening of Virulence and Antibiotic Resistance Genes
3. Results
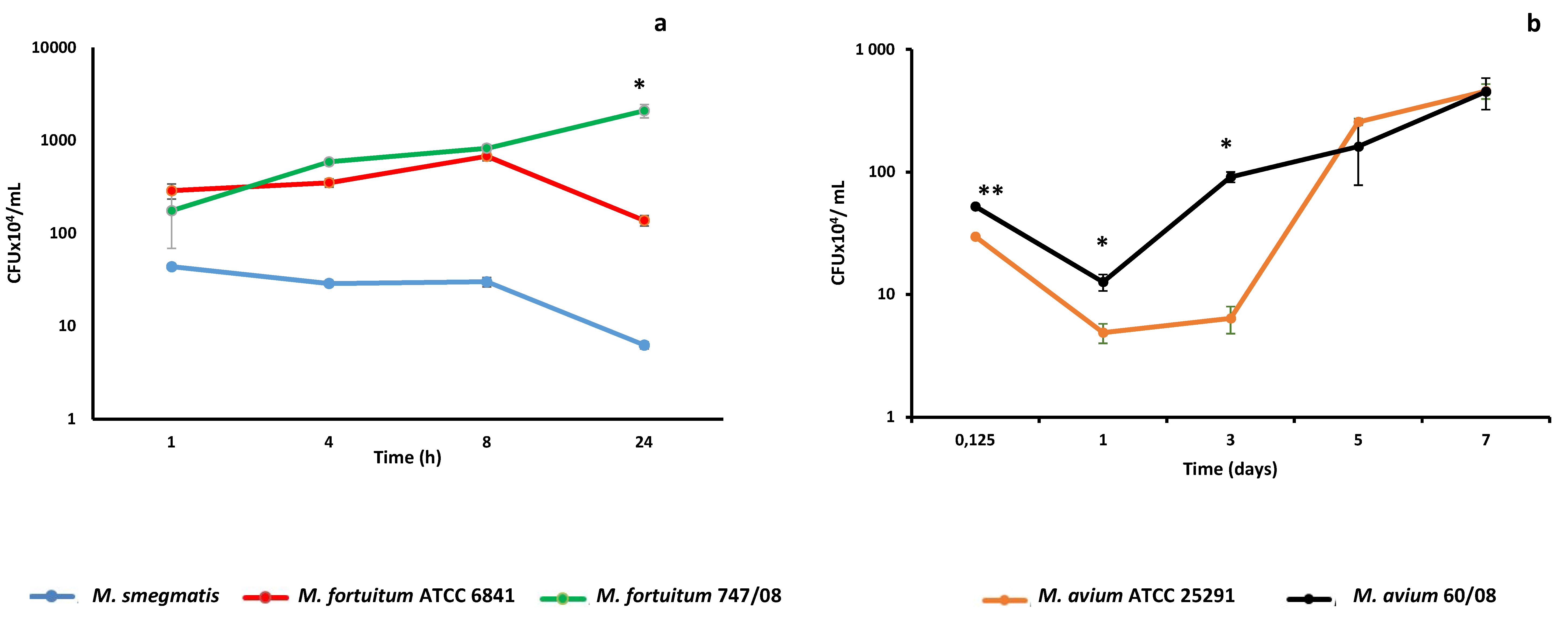
3.1. Kinetics of NTM Survival in Macrophages
3.2. NTM Phagosome Maturation and pH
3.3. Nitric Oxide
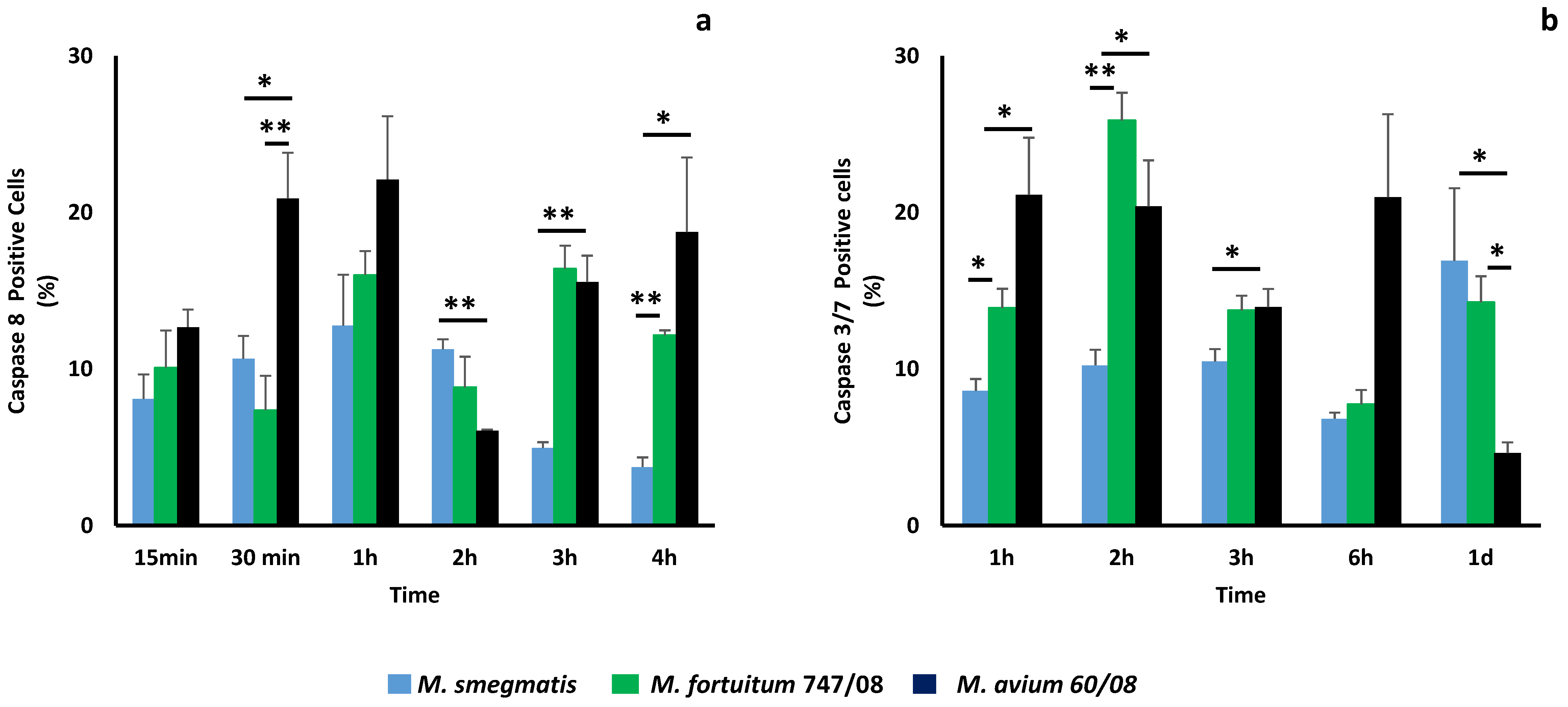
3.4. Apotosis
3.5. Main Genetic Differences between Same-Species Reference and Clinical Isolates
3.6. Repertoire of Virulence and Antibiotic Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassidy, P.M.; Hedberg, K.; Saulson, A.; McNelly, E.; Winthrop, K.L. Nontuberculous Mycobacterial Disease Prevalence and Risk Factors: A Changing Epidemiology. Clin. Infect. Dis. 2009, 49, e124–e129. [Google Scholar] [CrossRef]
- Fusco da Costa, A.R.; Lopes, M.L.; de Sousa, M.S.; Suffys, P.N.; Helena, L.; Sales, M.; Valéria, K.; Lima, B. Pulmonary nontuberculous mycobacterial infections in the State of Para, an endemic region for tuberculosis in North of Brazil. In Pulmonary Infection; IntechOpen: London, UK, 2012; ISBN 978-953-51-0286-1. [Google Scholar]
- Daley, C.L. Nontuberculous (environmental) mycobacterial disease. In Breathing in America: Disease Progress, and Hope; Schraufnagel, D.E., Ed.; The American Thoracic Society: New York, NY, USA, 2010; pp. 121–129. [Google Scholar]
- Antunes, A.; Viveiros, F.; Carvalho, A.; Duarte, R. Micobacterioses não-tuberculosas—Das manifestações clínicas ao tratamento. Arq. Med. 2012, 26, 25–30. [Google Scholar]
- Primm, T.P.; Lucero, C.A.; Falkinham, J.O., III. Health impacts of environmental mycobacteria. Clin. Microbiol. Rev. 2004, 17, 98–106. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [PubMed]
- Hussein, Z.; Landt, O.; Wirths, B.; Wellinghausen, N. Detection of non-tuberculous mycobacteria in hospital water by culture and molecular methods. Int. J. Med. Microbiol. 2009, 299, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.D.; Armstrong, J.A.; Brown, C.A.; Draper, P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect. Immun. 1972, 5, 803–807. [Google Scholar] [PubMed]
- Russell, D.G. Mycobacterium tuberculosis: Here today, and here tomorrow. Nat. Rev. Mol. Cell Biol. 2001, 2, 569–577. [Google Scholar] [CrossRef]
- Jordao, L.; Vieira, O.V. Tuberculosis: New aspects of an old disease. Int. J. Cell Biol. 2011, 2011, 403623. [Google Scholar] [CrossRef] [PubMed]
- Joao, I.; Cristovao, P.; Antunes, L.; Nunes, B.; Jordao, L. Identification of nontuberculous mycobacteria by partial gene sequencing and public databases. Int. J. Mycobact. 2014, 3, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes—Second Edition: Approved Standard M24-A2 2011. Available online: https://clsi.org/media/1463/m24a2_sample.pdf (accessed on 2 October 2018).
- Jordao, L.; Bleck, C.K.E.; Mayorga, L.; Griffiths, G.; Anes, E. On the killing of mycobacteria by macrophages. Cell. Microbiol. 2008, 10, 529–548. [Google Scholar] [CrossRef]
- Benjak, A.; Sala, C.; Hartkoorn, R.C. Whole-Genome Sequencing for Comparative Genomics and De Novo Genome Assembly. In Methods in Molecular Biology (Clifton, N.J.); Springer: Berlin, Germany, 2015; Volume 1285, pp. 1–16. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. chewBBACA: A complete suite for gene-by-gene schema creation and strain identification. Microb. Genomics 2018, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leimbach, A. Bac-Genomics-Scripts: Bovine, E. coli Mastitis Comparative Genomics Edition. 2016. Available online: https://zenodo.org/record/215824#.XMF5wmhL-Uk (accessed on 21 December 2016).
- Fedrizzi, T.; Meehan, C.J.; Grottola, A.; Giacobazzi, E.; Fregni Serpini, G.; Tagliazucchi, S.; Fabio, A.; Bettua, C.; Bertorelli, R.; De Sanctis, V.; et al. Genomic characterization of Nontuberculous Mycobacteria. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Danelishvili, L.; Babrack, L.; Pham, T. Evidence for genes associated with the ability of Mycobacterium avium subsp. hominissuis to escape apoptotic macrophages. Front. Cell. Infect. Microbiol. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Danelishvili, L.; Bermudez, L.E. Mycobacterium avium MAV_2941 mimics phosphoinositol-3-kinase to interfere with macrophage phagosome maturation. Microbes Infect. 2015, 17, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.L.; Velmurugan, K.; Cowan, M.J.; Briken, V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog. 2010, 6, e1000864. [Google Scholar] [CrossRef]
- Velmurugan, K.; Chen, B.; Miller, J.L.; Azogue, S.; Gurses, S.; Hsu, T.; Glickman, M.; Jacobs, W.R.; Porcelli, S.A.; Briken, V. Mycobacterium tuberculosis nuoG Is a Virulence Gene That Inhibits Apoptosis of Infected Host Cells. PLoS Pathog. 2007, 3, e110. [Google Scholar] [CrossRef]
- Hinchey, J.; Lee, S.; Jeon, B.Y.; Basaraba, R.J.; Venkataswamy, M.M.; Chen, B.; Chan, J.; Braunstein, M.; Orme, I.M.; Derrick, S.C.; et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Investig. 2007, 117, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-I.; Choi, H.-G.; Son, Y.-J.; Whang, J.; Kim, K.; Jeon, H.S.; Park, H.-S.; Back, Y.W.; Choi, S.; Kim, S.-W.; et al. Mycobacterium avium MAV2052 protein induces apoptosis in murine macrophage cells through Toll-like receptor 4. Apoptosis 2016, 21, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-I.; Whang, J.; Choi, H.-G.; Son, Y.-J.; Jeon, H.S.; Back, Y.W.; Park, H.-S.; Paik, S.; Park, J.-K.; Choi, C.H.; et al. Mycobacterium avium MAV2054 protein induces macrophage apoptosis by targeting mitochondria and reduces intracellular bacterial growth. Sci. Rep. 2016, 6, 37804. [Google Scholar] [CrossRef] [PubMed]
- Paroha, R.; Chourasia, R.; Mondal, R.; Chaurasiya, S.K. PknG supports mycobacterial adaptation in acidic environment. Mol. Cell. Biochem. 2018, 443, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Forrellad, M.A.; Bianco, M.V.; Blanco, F.C.; Nuñez, J.; Klepp, L.I.; Vazquez, C.L.; de la Paz Santangelo, M.; Rocha, R.V.; Soria, M.; Golby, P.; et al. Study of the in vivo role of Mce2R, the transcriptional regulator of mce2 operon in Mycobacterium tuberculosis. BMC Microbiol. 2013, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2004, 33, D325–D328. [Google Scholar] [CrossRef] [PubMed]
- Brunello, F.; Ligozzi, M.; Cristelli, E.; Bonora, S.; Tortoli, E.; Fontana, R. Identification of 54 mycobacterial species by PCR-restriction fragment length polymorphism analysis of the hsp65 gene. J. Clin. Microbiol. 2001, 39, 2799–2806. [Google Scholar] [CrossRef]
- Shin, S.J.; Lee, B.S.; Koh, W.J.; Manning, E.J.B.; Anklam, K.; Sreevatsan, S.; Lambrecht, R.S.; Collins, M.T. Efficient differentiation of Mycobacterium avium complex species and subspecies by use of five-target multiplex PCR. J. Clin. Microbiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, Y.; Dean, S.; Morris, J.G.; Salfinger, M.; Johnson, J.A. Multiple-genome comparison reveals new loci for Mycobacterium species identification. J. Clin. Microbiol. 2011, 49, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Theus, S.A.; Cave, M.D.; Eisenach, K.D. Activated THP-1 cells: An attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect. Immun. 2004, 72, 1169–1173. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Hoefsloot, W.; Van Ingen, J.; Andrejak, C.; Ängeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Durão, V.; Silva, A.; Macedo, R.; Durão, P.; Santos-Silva, A.; Duarte, R. Portuguese in vitro antibiotic susceptibilities favor current nontuberculous mycobacteria treatment guidelines. Pulmonology 2018. [Google Scholar] [CrossRef]
- Anes, E.; Peyron, P.; Staali, L.; Jordao, L.; Gutierrez, M.G.; Kress, H.; Hagedorn, M.; Maridonneau-Parini, I.; Skinner, M.A.; Wildeman, A.G.; et al. Dynamic life and death interactions between Mycobacterium smegmatis and J774 macrophages. Cell. Microbiol. 2006, 8, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Control of Phagocytosis by Microbial Pathogens. Front. Immunol. 2017, 8, 1368. [Google Scholar] [PubMed]
- de Chastellier, C.; Lang, T.; Thilo, L. Phagocytic processing of the macrophage endoparasite, Mycobacterium avium, in comparison to phagosomes which contain Bacillus subtilis or latex beads. Eur. J. Cell Biol. 1995, 68, 167–182. [Google Scholar] [PubMed]
- Vieira, O.V.; Botelho, R.J.; Grinstein, S. Phagosome maturation: Aging gracefully. Biochem. J. 2002, 366, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Yates, R.M.; Hermetter, A.; Russell, D.G. The Kinetics of Phagosome Maturation as a Function of Phagosome/Lysosome Fusion and Acquisition of Hydrolytic Activity. Traffic 2005, 6, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Ando, M.; Akaike, T.; Suga, M.; Sato, K.; Maeda, H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect. Immun. 1993, 61, 1980–1989. [Google Scholar] [PubMed]
- Denis, M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: Killing effector mechanism depends on the generation of reactive nitrogen intermediates. J. Leukoc. Biol. 1991, 49, 380–387. [Google Scholar]
- Datta, D.; Khatri, P.; Banerjee, C.; Singh, A.; Meena, R.; Saha, D.R.; Raman, R.; Rajamani, P.; Mitra, A.; Mazumder, S. Calcium and Superoxide-Mediated Pathways Converge to Induce Nitric Oxide-Dependent Apoptosis in Mycobacterium fortuitum-Infected Fish Macrophages. PLoS ONE 2016, 11, e0146554. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.R.M.; De Freitas, J.R.; Silva, Q.C.; Figueira, C.P.; Roxo, E.; Leão, S.C.; De Freitas, L.A.R.; Veras, P.S.T. Virulent Mycobacterium fortuitum restricts NO production by a gamma interferon-activated J774 cell line and phagosome-lysosome fusion. Infect. Immun. 2002, 70, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Akaki, T.; Sato, K.; Shimizu, T.; Sano, C.; Kajitani, H.; Dekio, S.; Tomioka, H. Effector molecules in expression of the antimicrobial activity of macrophages against Mycobacterium avium complex: Roles of reactive nitrogen intermediates, reactive oxygen intermediates, and free fatty acids. J. Leukoc. Biol. 1997, 62, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.S.; Flórido, M.; Pais, T.F.; Appelberg, R. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J. Immunol. 1999, 162, 6734–6739. [Google Scholar] [PubMed]
- Clemens, D.L.; Horwitz, M.A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 1995, 181, 257–270. [Google Scholar] [CrossRef]
- Nathan, C. Inducible Nitric Oxide Synthase in the Tuberculous Human Lung. Am. J. Respir. Crit. Care Med. 2002, 166, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Chan, J.; Schluger, N.W. What is the Role of Nitric Oxide in Murine and Human Host Defense against Tuberculosis? Am. J. Respir. Cell Mol. Biol. 2001, 25, 606–612. [Google Scholar] [CrossRef]
- MacMicking, J.D.; Taylor, G.A.; McKinney, J.D. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 2003, 302, 654–659. [Google Scholar] [CrossRef]
- Lee, J.-S.; Yang, C.-S.; Shin, D.-M.; Yuk, J.-M.; Son, J.-W.; Jo, E.-K. Nitric Oxide Synthesis is Modulated by 1,25-Dihydroxyvitamin D3 and Interferon-gamma in Human Macrophages after Mycobacterial Infection. Immune Netw. 2009, 9, 192–202. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Gey van Pittius, N.C.; DiGiuseppe Champion, P.A.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.J.E.; Appelmelk, B.J.; Bitter, W. Type VII secretion mycobacteria show the way. Nat. Rev. Microbiol. 2007, 5, 883–891. [Google Scholar] [CrossRef]
- Serafini, A.; Boldrin, F.; Palù, G.; Manganelli, R. Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: Essentiality and rescue by iron and zinc. J. Bacteriol. 2009, 191, 6340–6344. [Google Scholar] [CrossRef]
- Dumas, E.; Christina Boritsch, E.; Vandenbogaert, M.; Rodríguez de la Vega, R.C.; Thiberge, J.-M.; Caro, V.; Gaillard, J.-L.; Heym, B.; Girard-Misguich, F.; Brosch, R.; et al. Mycobacterial Pan-Genome Analysis Suggests Important Role of Plasmids in the Radiation of Type VII Secretion Systems. Genome Biol. Evol. 2016, 8, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Srivastava, M.; Dasgupta, A.; Singh, M.P.; Srivastava, R.; Srivastava, B.S. Increased virulence of Mycobacterium tuberculosis H37Rv overexpressing LipY in a murine model. Tuberculosis (Edinb.) 2014, 94, 252–261. [Google Scholar] [CrossRef]
- Blasco, B.; Chen, J.M.; Hartkoorn, R.; Sala, C.; Uplekar, S.; Rougemont, J.; Pojer, F.; Cole, S.T. Virulence Regulator EspR of Mycobacterium tuberculosis Is a Nucleoid-Associated Protein. PLoS Pathog. 2012, 8, e1002621. [Google Scholar] [CrossRef]
- Guo, S.; Xue, R.; Li, Y.; Wang, S.M.; Ren, L.; Xu, J.J. The CFP10/ESAT6 complex of Mycobacterium tuberculosis may function as a regulator of macrophage cell death at different stages of tuberculosis infection. Med. Hypotheses 2012, 78, 389–392. [Google Scholar] [CrossRef]
- Clark, R.R.; Judd, J.; Lasek-Nesselquist, E.; Montgomery, S.A.; Hoffmann, J.G.; Derbyshire, K.M.; Gray, T.A. Direct cell–cell contact activates SigM to express the ESX-4 secretion system in Mycobacterium smegmatis. Proc. Natl. Acad. Sci. USA 2018, 115, E6595–E6603. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, J.-P. Mammalian cell entry gene family of Mycobacterium tuberculosis. Mol. Cell. Biochem. 2011, 352, 1–10. [Google Scholar] [CrossRef]
- Nash, K.A.; Zhang, Y.; Brown-Elliott, B.A.; Wallace, R.J. Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum. J. Antimicrob. Chemother. 2005, 55, 170–177. [Google Scholar] [CrossRef]
- Rudra, P.; Hurst-Hess, K.; Lappierre, P.; Ghosh, P. High Levels of Intrinsic Tetracycline Resistance in Mycobacterium abscessus Are Conferred by a Tetracycline-Modifying Monooxygenase. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, S.; Long, C.D.; Small, P.M.; Van, T.; Schoolnik, G.K.; Bohannan, B.J.M. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 2006, 312, 1944–1946. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, A.H.; Wong, A.; Kassen, R. The fitness costs of antibiotic resistance mutations. Evol. Appl. 2015, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Perdigao, J.; Alverca, E.; de Matos, A.P.A.; Carvalho, P.A.; Portugal, I.; Jordao, L. Exploring the Contribution of Mycobacteria Characteristics in Their Interaction with Human Macrophages. Microsc. Microanal. 2013, 19, 1159–1169. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Hart, P.D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 1971, 134, 713–740. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Herget, T.; Klebl, B.; Ullrich, A. Interplay between mycobacteria and host signalling pathways. Nat. Rev. Microbiol. 2004, 2, 189–202. [Google Scholar] [CrossRef]
- Vandal, O.H.; Pierini, L.M.; Schnappinger, D.; Nathan, C.F.; Ehrt, S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 2008, 14, 849–854. [Google Scholar] [CrossRef]
- Livanainen, E. Isolation of mycobacteria from acidic forest soil samples: Comparison of culture methods. J. Appl. Bacteriol. 1995, 78, 663–668. [Google Scholar] [CrossRef]
- Iivanainen, E.K.; Martikainen, P.J.; Räisänen, M.L.; Katila, M.-L. Mycobacteria in boreal coniferous forest soils. FEMS Microbiol. Ecol. 1997, 23, 325–332. [Google Scholar] [CrossRef]
- Kirschner, R.A.; Parker, B.C.; Falkinham, J.O. Epidemiology of Infection by Nontuberculous Mycobacteria: Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in Acid, Brown-Water Swamps of the Southeastern United States and Their Association with Environment. Am. Rev. Respir. Dis. 1992, 145, 271–275. [Google Scholar] [CrossRef]
- Vandal, O.H.; Nathan, C.F.; Ehrt, S. Acid Resistance in Mycobacterium tuberculosis. J. Bacteriol. 2009, 191, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Kissing, S.; Saftig, P.; Haas, A. Vacuolar ATPase in phago(lyso)some biology. Int. J. Med. Microbiol. 2018, 308, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J.E.; Torrado, E.; Tighe, M.; Fountain, J.J.; Solache, A.; Strutt, T.; Swain, S.; Appelberg, R.; Cooper, A.M. Nitric oxide inhibits the accumulation of CD4 + CD44 hi Tbet + CD69 lo T cells in mycobacterial infection. Eur. J. Immunol. 2012, 42, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Fratazzi, C.; Arbeit, R.D.; Carini, C.; Balcewicz-Sablinska, M.K.; Keane, J.; Kornfeld, H.; Remold, H.G. Macrophage apoptosis in mycobacterial infections. J. Leukoc. Biol. 1999, 66, 763–764. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Gan, H.; Remold, H.G. A mechanism of virulence: Virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J. Immunol. 2006, 176, 3707–3716. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pathak, S.; Basak, C.; Law, S.; Kundu, M.; Basu, J. Execution of Macrophage Apoptosis by Mycobacterium avium through Apoptosis Signal-regulating Kinase 1/p38 Mitogen-activated Protein Kinase Signaling and Caspase 8 Activation. J. Biol. Chem. 2003, 278, 26517–26525. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, L.E.; Parker, A.; Petrofsky, M. Apoptosis of Mycobacterium avium-infected macrophages is mediated by both tumour necrosis factor (TNF) and Fas, and involves the activation of caspases. Clin. Exp. Immunol. 1999, 116, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Catanzaro, A.; Rao, S.P. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect. Immun. 1997, 65, 5262–5271. [Google Scholar]
- Datta, D.; Khatri, P.; Singh, A.; Saha, D.R.; Verma, G.; Raman, R.; Mazumder, S. Mycobacterium fortuitum-induced ER-Mitochondrial calcium dynamics promotes calpain/caspase-12/caspase-9 mediated apoptosis in fish macrophages. Cell death Discov. 2018, 4, 30. [Google Scholar] [CrossRef]
- Bohsali, A.; Abdalla, H.; Velmurugan, K.; Briken, V. The non-pathogenic mycobacteria M. smegmatis and M. fortuitum induce rapid host cell apoptosis via a caspase-3 and TNF dependent pathway. BMC Microbiol. 2010, 10, 237. [Google Scholar]
- Oh, S.-M.; Lim, Y.-J.; Choi, J.-A.; Lee, J.; Cho, S.-N.; Go, D.; Kim, S.-H.; Song, C.-H. TNF-α–mediated ER stress causes elimination of Mycobacterium fortuitum reservoirs by macrophage apoptosis. FASEB J. 2018, 32, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Obregón-Henao, A.; Duque-Correa, M.A.; Rojas, M.; García, L.F.; Brennan, P.J.; Ortiz, B.L.; Belisle, J.T. Stable extracellular RNA fragments of Mycobacterium tuberculosis induce early apoptosis in human monocytes via a caspase-8 dependent mechanism. PLoS ONE 2012, 7, e29970. [Google Scholar] [CrossRef] [PubMed]
- Early, J.; Fischer, K.; Bermudez, L.E. Mycobacterium avium uses apoptotic macrophages as tools for spreading. Microb. Pathog. 2011, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Mwandumba, H.C.; Rhoades, E.E. Mycobacterium and the coat of many lipids. J Cell Biol 2002, 158, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Boulon, R.; Ducoux, M.; Gavalda, S.; Laval, F.; Jamet, S.; Eynard, N.; Lemassu, A.; Cam, K.; Bousquet, M.-P.; et al. HadD, a novel fatty acid synthase type II protein, is essential for alpha- and epoxy-mycolic acid biosynthesis and mycobacterial fitness. Sci. Rep. 2018, 8, 6034. [Google Scholar] [CrossRef] [PubMed]


| Nontuberculous Mycobacteria ID | |||||
|---|---|---|---|---|---|
| pH | M. smegmatis | M. fortuitum | M. avium | ||
| ATCC 6841 | 747/08 | ATCC 25291 | 60/08 | ||
| 4.6 | - | + | + | + | + |
| 5.4 | + | + | + | + | + |
| 6.7 | + | + | + | + | + 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.; Borges, V.; Joao, I.; Gomes, J.P.; Jordao, L. Nontuberculous Mycobacteria Persistence in a Cell Model Mimicking Alveolar Macrophages. Microorganisms 2019, 7, 113. https://doi.org/10.3390/microorganisms7050113
Sousa S, Borges V, Joao I, Gomes JP, Jordao L. Nontuberculous Mycobacteria Persistence in a Cell Model Mimicking Alveolar Macrophages. Microorganisms. 2019; 7(5):113. https://doi.org/10.3390/microorganisms7050113
Chicago/Turabian StyleSousa, Sara, Vítor Borges, Ines Joao, João Paulo Gomes, and Luisa Jordao. 2019. "Nontuberculous Mycobacteria Persistence in a Cell Model Mimicking Alveolar Macrophages" Microorganisms 7, no. 5: 113. https://doi.org/10.3390/microorganisms7050113
APA StyleSousa, S., Borges, V., Joao, I., Gomes, J. P., & Jordao, L. (2019). Nontuberculous Mycobacteria Persistence in a Cell Model Mimicking Alveolar Macrophages. Microorganisms, 7(5), 113. https://doi.org/10.3390/microorganisms7050113






