Arbuscular Mycorrhiza Enhances Biomass Production and Salt Tolerance of Sweet Sorghum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil, AMF Inoculum, and Plants
2.2. Experimental Design and Procedure
2.3. Plant and Soil Analysis
2.4. Data Analysis
3. Results and Discussion
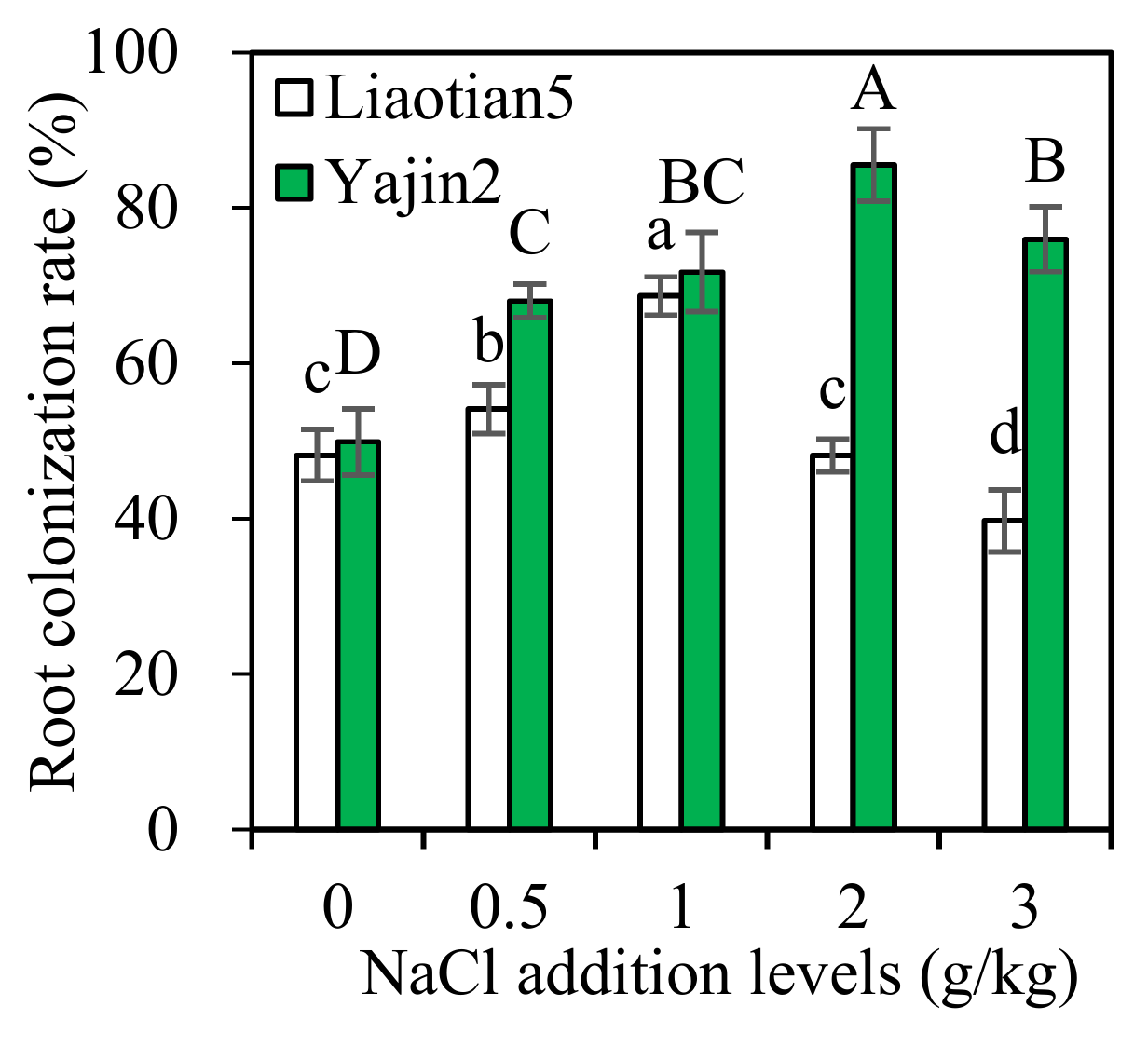
3.1. Root Colonization Rate
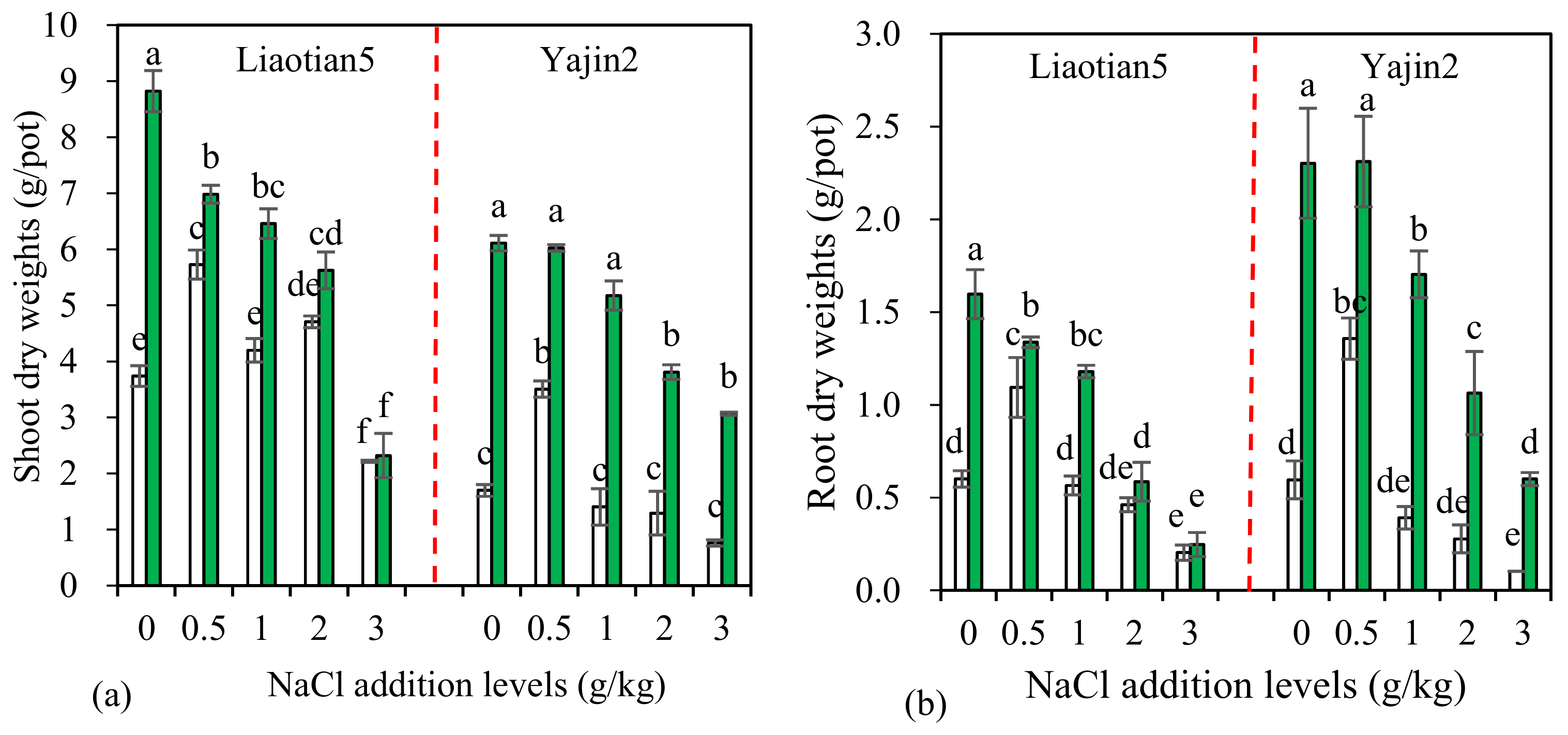
3.2. Plant Biomass and Mycorrhizal Response (MR)
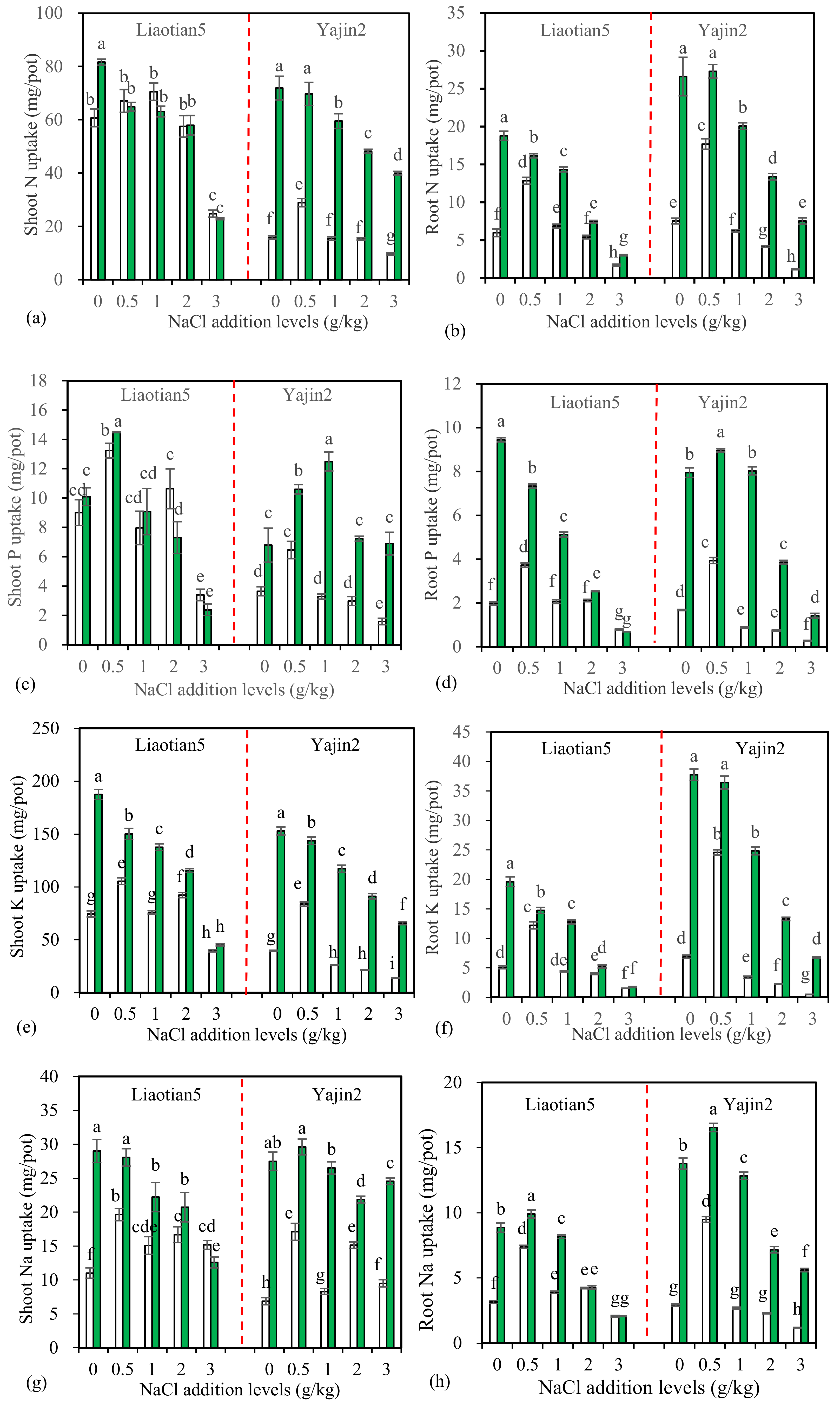
3.3. Plant Mineral Uptake and K+/Na+ Ratio
3.4. Plant Antioxidant Enzymes
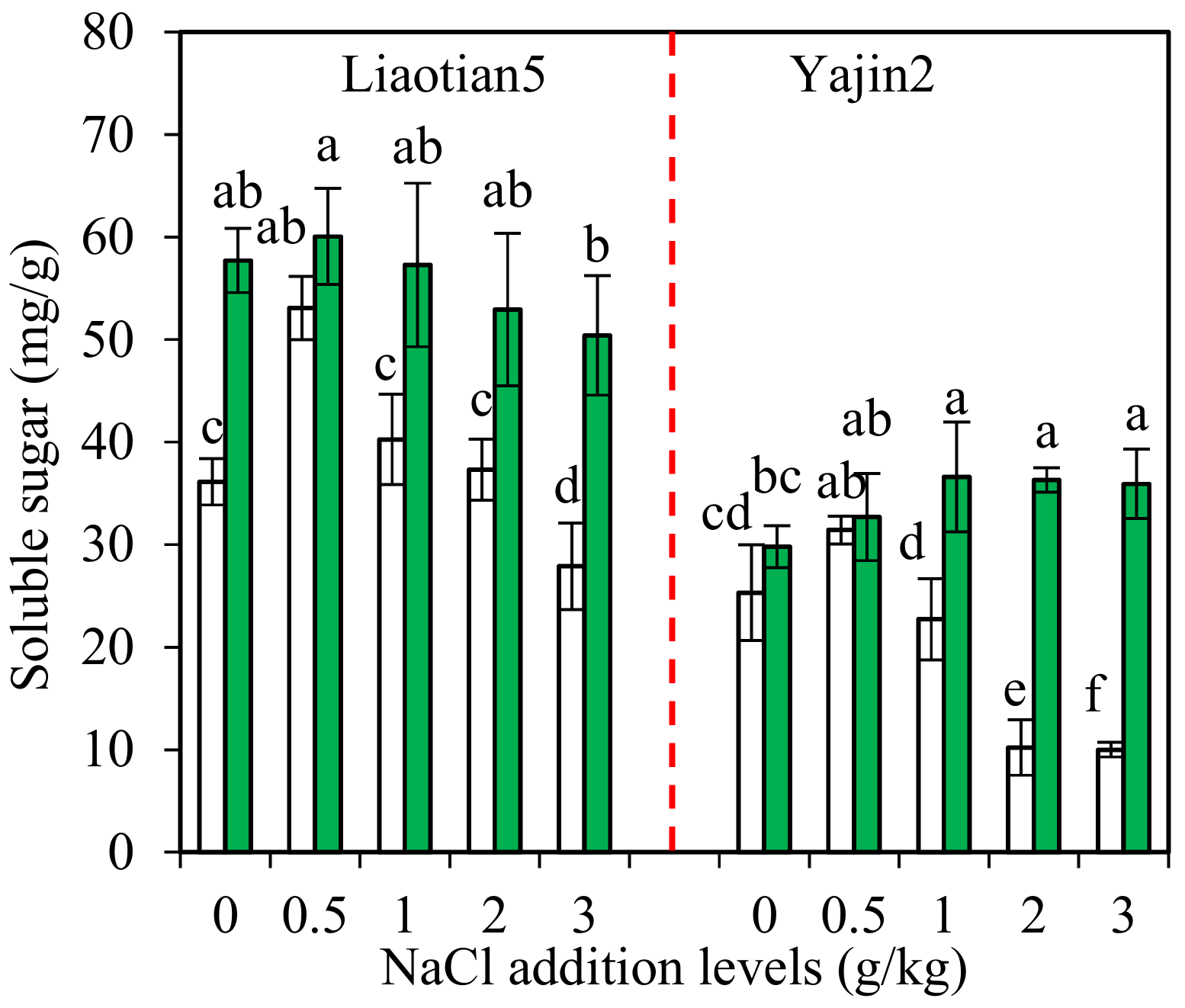
3.5. Soluble Sugar in Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | arbuscular mycorrhizal |
| AMF | arbuscular mycorrhizal fungi |
| CAT | catalase |
| DWs | dry weights |
| FDs | fresh weights |
| MR | mycorrhizal response |
| POD | peroxidase |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Arzani, A.; Ashraf, M. Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. Crit. Rev. Plant Sci. 2016, 35, 146–189. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.A.; Dar, E.A.; Kaur, A.; Phutela, U.G. Sweet sorghum—A promising alternative feedstock for biofuel production. Renew. Sust. Energy Rev. 2018, 82, 4070–4090. [Google Scholar]
- Zhuang, D.; Jiang, D.; Liu, L.; Huang, Y. Assessment of bioenergy potential on marginal land in China. Renew. Sust. Energy Rev. 2011, 15, 1050–1056. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant. Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Manuel Ruiz-Lozano, J. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Yamato, M.; Ikeda, S.; Iwase, K. Community of arbuscular mycorrhizal fungi in a coastal vegetation on Okinawa island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza 2008, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Paula, M.A.; Reis, V.M.; Döbereiner, J. Interactions of Glomus clarum with Acetobacter diazotrophicus in infection of sweet potato (Ipomoea batatas), sugarcane (Saccharum spp.), and sweet sorghum (Sorghum vulgare). Biol. Fertil. Soils 1991, 11, 111–115. [Google Scholar] [CrossRef]
- Deepadevi, M.; Basu, M.J.; Santhaguru, K. Response of Sorghum bicolor (L.) Monech to dual inoculation with Glomus fasciculatum and Herbaspirillum seropedicae. General. Appl. Plant Physiol. 2010, 36, 176–182. [Google Scholar]
- Wang, F.Y.; Lin, X.G.; Yin, R. Inoculation with arbuscular mycorrhizal fungus Acaulospora mellea decreases Cu phytoextraction by maize from Cu-contaminated soil. Pedobiologia 2007, 51, 99–109. [Google Scholar] [CrossRef]
- Wang, F.Y.; Shi, Z.Y.; Tong, R.J.; Xu, X.F. Dynamics of phoxim residues in green onion and soil as influenced by arbuscular mycorrhizal fungi. J. Hazard. Mater. 2011, 185, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Wensheng, S.; Zhian, L.; Bin, L.; Jintian, L.; Jingsong, S. Removal of metals by sorghum plants from contaminated land. J. Environ. Sci. 2009, 21, 1432–1437. [Google Scholar] [CrossRef]
- Tian, C.Y.; Feng, G.; Li, X.L.; Zhang, F.S. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl. Soil Ecol. 2004, 26, 143–148. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [PubMed]
- Lu, R. Analytical Methods for Soils and Agricultural Chemistry; China Agricultural Science and Technology Press: Bejing, China, 2000. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Pütter, J.; Becker, R. Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; p. 286. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Sanchez, F.J.; Manzanares, M.; Efde, A.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Wang, F.; Jin, X.; Adams, C.A.; Shi, Z.; Sun, Y. Decreased ZnO nanoparticles phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ. Sci. Pollut. Res. 2018, 25, 23736–23747. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Adams, C.A.; Shi, Z.; Sun, Y. Combined effects of ZnO NPs and Cd on sweet sorghum as influenced by an arbuscular mycorrhizal fungus. Chemosphere 2018, 209, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Tawaraya, K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci. Plant Nutr. 2003, 49, 655–668. [Google Scholar] [CrossRef]
- Bothe, H. Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 2012, 58, 7–16. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Hammad, R.; Rusan, M. Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 2001, 11, 43–47. [Google Scholar] [CrossRef]
- Aliasgharzadeh, N.; Rastin, S.N.; Towfighi, H.; Alizadeh, A. Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 2001, 11, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Saita, A.; Sortino, O. Comparative effects of salt and water stress on seed germination and early embryo growth in two cultivars of sweet sorghum. J. Agron. Crop Sci. 2013, 199, 30–37. [Google Scholar] [CrossRef]
- Shakeri, E.; Emam, Y. Selectable traits in sorghum genotypes for tolerance to salinity stress. J. Agric. Sci. Technol. 2017, 27, 1319–1332. [Google Scholar]
- Milford, G.F.J.; Cormack, W.F.; Durrant, M.J. Effects of sodium chloride on water status and growth of sugar beet. J. Exp. Bot. 1977, 28, 1380–1388. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A functional plant nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar]
- Rao, D.L.N.; Pathak, H. Ameliorative influence of organic matter on biological activity of salt-affected soils. Arid Soil Res. Rehabil. 1996, 10, 311–319. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Karagiannidis, N.; Gatsis, T. Sweet sorghum productivity for biofuels under increased soil salinity and reduced irrigation. Field Crops Res. 2011, 120, 38–46. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, F.; Gillessen, M.; Hutter, I.; Schneider, C.; Feldmann, F.; Alford, D.V.; Furk, C. Should we breed for effective mycorrhiza symbioses? In Crop Plant Resistance To Biotic and Abiotic Factors: Current Potential and Future Demands, Proceedings of the International Symposium on Plant Protection and Plant Health in Europe, Julius Kühn-Institut, Berlin-Dahlem, Germany, 14–16 May 2009; DPG-Publisher: Braunschweig, Germany, 2019. [Google Scholar]
- Hetrick, B.A.D.; Wilson, G.W.T.; Todd, T.C. Differential responses of C3 and C4 grasses to mycorrhizal symbiosis. Can. J. Bot. 1990, 68, 461–467. [Google Scholar] [CrossRef]
- Wang, F.Y.; Lin, X.G.; Yin, R.; Wu, L.H. Effects of arbuscular mycorrhizal inoculation on the growth of Elsholtzia splendens and Zea mays and the activities of phosphatase and urease in a multi-metal-contaminated soil under unsterilized conditions. Appl. Soil Ecol. 2006, 31, 110–119. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Mishra, S. Influence of arbuscular mycorrhizal (AM) fungi and salinity on seedling growth, solute accumulation, and mycorrhizal dependency of Jatropha curcas L. J. Plant Growth Regul. 2010, 29, 297–306. [Google Scholar] [CrossRef]
- Alkaraki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Ghorbanli, M.; Ebrahimzadeh, H.; Sharifi, M. Effects of NaCl and mycorrhizal fungi on antioxidative enzymes in soybean. Biol. Plant. 2004, 48, 575–581. [Google Scholar] [CrossRef]
- Alamgir, A.N.M.; Ali, M.Y. Effect of salinity on leaf pigments, sugar and protein concentrations and chloroplast ATPase activity of rice (Oryza sativa L.). Bangladesh J. Bot. 1999, 28, 145–149. [Google Scholar]
- Feng, G.; Zhang, F.; Li, X.; Tian, C.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar]
- Almodares, A.; Hadi, M.R.; Ahmadpour, H. Sorghum stem yield and soluble carbohydrates under different salinity levels. Afr. J. Biotechnol. 2010, 7, 4051–4055. [Google Scholar]
- Hammer, E.C.; Rillig, M.C. The influence of different stresses on glomalin levels in an arbuscular mycorrhizal fungus—Salinity increases glomalin content. PLoS ONE 2011, 6, e28426. [Google Scholar] [CrossRef]


| Cultivars | Variables | AM | NaCl | AM × NaCl |
|---|---|---|---|---|
| Liaotian5 | Shoot dry weights | 87.57 ** | 52.21 ** | 17.59 ** |
| Root dry weights | 56.63 ** | 42.50 ** | 10.03 ** | |
| Shoot N uptake | 5.51 ** | 75.21 ** | 6.03 ** | |
| Root N uptake | 46.22 ** | 30.37 ** | 7.33 ** | |
| Shoot P uptake | 100.50 ** | 287.50 ** | 148.94 ** | |
| Root P uptake | 1239.95 ** | 528.92 ** | 271.13 ** | |
| Shoot K uptake | 127.99 ** | 11.93 ** | 6.02 ** | |
| Root K uptake | 139.20 ** | 142.84 ** | 63.41 ** | |
| Shoot Na uptake | 57.96 ** | 30.02 ** | 23.45 ** | |
| Root Na uptake | 99.32 ** | 602.32 ** | 104.72 ** | |
| Soluble sugar | 114.53 ** | 13.05 ** | 3.15 * | |
| Yajin2 | Shoot dry weights | 205.268 ** | 21.297 ** | 3.619 ** |
| Root dry weights | 141.251 ** | 35.910 ** | 5.646 ** | |
| Shoot N uptake | 645.61 ** | 25.96 ** | 12.47 ** | |
| Root N uptake | 119.94 ** | 35.08 ** | 4.21 * | |
| Shoot P uptake | 640.37 ** | 56.23 ** | 26.02 ** | |
| Root P uptake | 1984.91 ** | 367.96 ** | 112.77 ** | |
| Shoot K uptake | 632.71 ** | 205.78 ** | 88.19 ** | |
| Root K uptake | 1503.20 ** | 940.96 ** | 247.30 ** | |
| Shoot Na uptake | 5.58 ** | 684.83 ** | 1284.87 ** | |
| Root Na uptake | 43.35 ** | 874.67 ** | 129.67 ** | |
| Soluble sugar | 160.91 ** | 8.93 ** | 20.12 ** |
| NaCl Addition Levels (g/kg) | Liaotian5 | Yajin2 | ||
|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | |
| 0 | 136 | 165.8 | 260 | 286.3 |
| 0.5 | 21.9 | 22.2 | 71.9 | 70.3 |
| 1 | 53.8 | 108.6 | 268.9 | 335.6 |
| 2 | 19.5 | 26.9 | 194.6 | 283.1 |
| 3 | 4.6 | 21.6 | 303.1 | 485.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Sun, Y.; Shi, Z. Arbuscular Mycorrhiza Enhances Biomass Production and Salt Tolerance of Sweet Sorghum. Microorganisms 2019, 7, 289. https://doi.org/10.3390/microorganisms7090289
Wang F, Sun Y, Shi Z. Arbuscular Mycorrhiza Enhances Biomass Production and Salt Tolerance of Sweet Sorghum. Microorganisms. 2019; 7(9):289. https://doi.org/10.3390/microorganisms7090289
Chicago/Turabian StyleWang, Fayuan, Yuhuan Sun, and Zhaoyong Shi. 2019. "Arbuscular Mycorrhiza Enhances Biomass Production and Salt Tolerance of Sweet Sorghum" Microorganisms 7, no. 9: 289. https://doi.org/10.3390/microorganisms7090289
APA StyleWang, F., Sun, Y., & Shi, Z. (2019). Arbuscular Mycorrhiza Enhances Biomass Production and Salt Tolerance of Sweet Sorghum. Microorganisms, 7(9), 289. https://doi.org/10.3390/microorganisms7090289






