Pathobiology of Aspergillus Fumigatus Endophthalmitis in Immunocompetent and Immunocompromised Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Ethics Statement
2.2. Preparation of Fungal Spores
2.3. Induction of Aspergillus Endophthalmitis
2.4. Fungal Burden Estimation
2.5. Cytokine/Chemokine ELISA
2.6. RNA Extraction and qRT-PCR
2.7. Immunofluorescence and TUNEL Staining
2.8. Histological Assay
2.9. PMN Infiltration
2.10. Neutrophil Depletion
2.11. Statistical Analysis
3. Results
3.1. Intravitreal Inoculation of AF Spores Causes Endophthalmitis in C57BL/6 Murine Eyes
3.2. AF Infected Eyes Exhibited a Temporal Decrease in Fungal Burden and Neutrophil Infiltration
3.3. AF Infected Retina Exhibited Increased Inflammatory Mediators and Induced Expression of Toll-Like Receptors (TLRs)
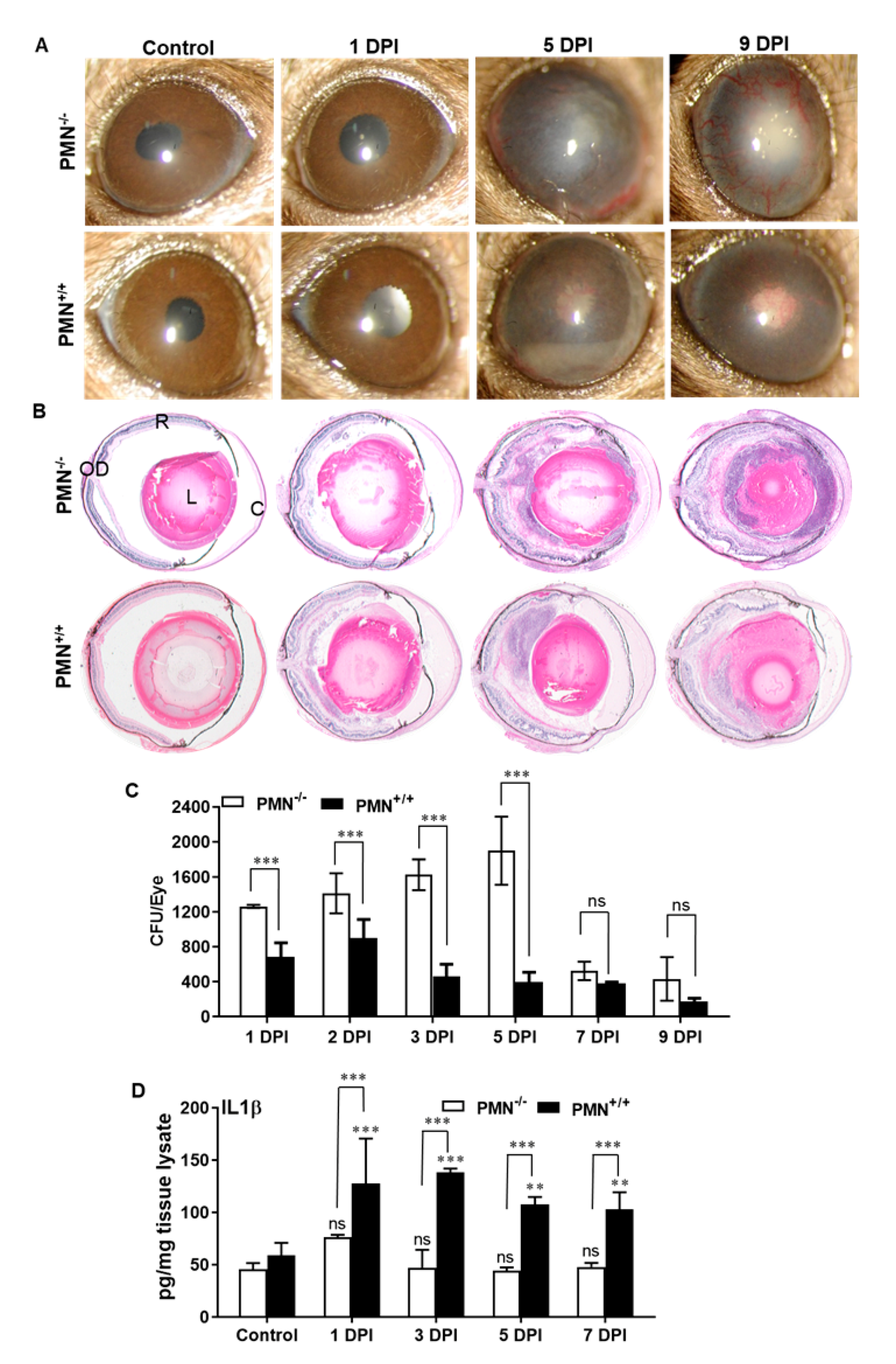
3.4. Neutropenic Mice Are More Susceptible to AF Endophthalmitis
4. Discussion
Author Contributions
Funding
Acknowledgments
Disclaimer
Conflicts of Interest
References
- Sadaka, A.; Durand, M.L.; Gilmore, M.S. Bacterial endophthalmitis in the age of outpatient intravitreal therapies and cataract surgeries: Host-microbe interactions in intraocular infection. Prog. Retin. Eye Res. 2012, 31, 316–331. [Google Scholar] [CrossRef]
- Miller, F.C.; Coburn, P.S.; Huzzatul, M.M.; LaGrow, A.L.; Livingston, E.; Callegan, M.C. Targets of immunomodulation in bacterial endophthalmitis. Prog. Retin. Eye Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, R.K.; Miller, L.J.; Singh, P.K.; Kanwar, M. Muller glia in retinal innate immunity: A perspective on their roles in endophthalmitis. Crit. Rev. Immunol. 2013, 33, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, D.; Chakrabarti, M.; Jayasudha, R.; Hasnat Ali, M.; Tyagi, M.; Sharma, S.; Joseph, J. Elevated cytokine levels in vitreous as biomarkers of disease severity in infectious endophthalmitis. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Brockhaus, L.; Goldblum, D.; Eggenschwiler, L.; Zimmerli, S.; Marzolini, C. Revisiting systemic treatment of bacterial endophthalmitis: A review of intravitreal penetration of systemic antibiotics. Clin. Microbiol. Infec. 2019. [Google Scholar] [CrossRef] [PubMed]
- Dave, T.V.; Dave, V.P.; Sharma, S.; Karolia, R.; Joseph, J.; Pathengay, A.; Pappuru, R.R.; Das, T. Infectious endophthalmitis leading to evisceration: Spectrum of bacterial and fungal pathogens and antibacterial susceptibility profile. J. Ophthalmic Inflamm. Infect. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.L. Bacterial and Fungal Endophthalmitis. Clin. Microbiol. Rev. 2017, 30, 597–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, J.; Flynn, H.W., Jr.; Kuriyan, A.E.; Miller, D.; Albini, T. Endogenous fungal endophthalmitis: Risk factors, clinical features, and treatment outcomes in mold and yeast infections. J. Ophthalmic Inflamm. Infect. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Marangon, F.B.; Miller, D.; Giaconi, J.A.; Alfonso, E.C. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am. J. Ophthalmol. 2004, 137, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ertugrul, B.; Gultekin, B.; Uyar, G.; Kir, E. Treatment of two postoperative endophthalmitis cases due to Aspergillus flavus and Scopulariopsis spp. with local and systemic antifungal therapy. BMC Infect. Dis. 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.M.; Singh, P.K.; Revankar, S.G.; Chandrasekar, P.H.; Kumar, A. Isavuconazole for Treatment of Experimental Fungal Endophthalmitis Caused by Aspergillus fumigatus. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latgé, J.-P. Aspergillus fumigatus and Aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Gruener, A.M.; Allen, F.; Stanford, M.R.; Graham, E.M. Aspergillus fumigatus Endophthalmitis with Necrotizing Scleritis following Pars Plana Vitrectomy. Case Rep. Ophthalmol. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Ho, S.; Krishnan, P.; Teoh, S.C. Aspergillus terreus endogenous endophthalmitis in a nonimmunocompromised patient with a history of bronchiectasis. Ocul. Immunol. Inflamm. 2013, 21, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, P.K.; Roy, R.; Pal, S.S.; Mukherjee, A.; Lobo, A. Aspergillus terreus endogenous endophthalmitis: Report of a case and review of literature. Indian J. Ophthalmol. 2014, 62, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Dogra, M.; Akella, M.; Dogra, M.R.; Gupta, A. Presumably contaminated intravenous infusion-induced Aspergillus terreus endogenous endophthalmitis presenting with posterior hypopyon. Indian J. Ophthalmol. 2018, 66, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.B.; Vaz, F.; Rodrigues, A.; Donato, S. Intravitreal voriconazole as primary treatment for endogenous Aspergillus endophthalmitis. BMJ Case Rep. 2009, 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkanci, A.; Ozdek, S. Ocular fungal infections. Curr. Eye Res. 2011, 36, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Sontam, B.; Guda, S.J.M.; Gandhi, J.; Sharma, S.; Tyagi, M.; Dave, V.P.; Das, T. Trends in microbiological spectrum of endophthalmitis at a single tertiary care ophthalmic hospital in India: A review of 25 years. Eye (Lond) 2019. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Flynn, H.W., Jr.; Miller, D.; Scott, I.U.; Alfonso, E.C. Exogenous Fungal Endophthalmitis: Microbiology and Clinical Outcomes. Ophthalmology 2008, 115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cheng, Y.; Song, X.; Wang, C.; Su, G.; Liu, Z. A Comparative Treatment Study of Intravitreal Voriconazole and Liposomal Amphotericin B in an Aspergillus fumigatus Endophthalmitis ModelComparison of Effects of VCZ and Liposomal Amp-B. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7369–7376. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, X.; Wu, X.; Xie, L.; Min, X. Intravitreally implantable voriconazole delivery system for experimental fungal endophthalmitis. Retina 2011, 31, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.M.; Glickman, R.D.; Ballentine, C.S.; Trigo, Y.; Pena, M.A.; Kurian, P.; Najvar, L.K.; Kumar, N.; Patel, A.H.; Sponsel, W.E.; et al. Retinal function assessed by ERG before and after induction of ocular aspergillosis and treatment by the anti-fungal, micafungin, in rabbits. Doc. Ophthalmol. 2005, 110, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Whiston, E.A.; Sugi, N.; Kamradt, M.C.; Sack, C.; Heimer, S.R.; Engelbert, M.; Wawrousek, E.F.; Gilmore, M.S.; Ksander, B.R.; Gregory, M.S. αB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect. Immun. 2008, 76, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.T.; Ramirez, R.; Novosad, B.D.; Callegan, M.C. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr. Eye Res. 2006, 31, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Talreja, D.; Singh, P.K.; Kumar, A. In Vivo Role of TLR2 and MyD88 Signaling in Eliciting Innate Immune Responses in Staphylococcal Endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2015, 56, 1719–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Kasetti, R.B.; Zode, G.S.; Goyal, A.; Juzych, M.S.; Kumar, A. Zika Virus Infects Trabecular Meshwork and Causes Trabeculitis and Glaucomatous Pathology in Mouse Eyes. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, A. Retinal Photoreceptor Expresses Toll-Like Receptors (TLRs) and Elicits Innate Responses Following TLR Ligand and Bacterial Challenge. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Singh, P.K.; Donovan, D.M.; Kumar, A. Intravitreal Injection of the Chimeric Phage Endolysin Ply187 Protects Mice from Staphylococcus aureus Endophthalmitis. Antimicrob. Agents Chemother. 2014, 58, 4621–4629. [Google Scholar] [CrossRef] [Green Version]
- Talreja, D.; Kaye, K.S.; Yu, F.S.; Walia, S.K.; Kumar, A. Pathogenicity of ocular isolates of Acinetobacter baumannii in a mouse model of bacterial endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2014, 55, 2392–2402. [Google Scholar] [CrossRef]
- Thomas, P.A. Current Perspectives on Ophthalmic Mycoses. Clin. Microbiol. Rev. 2003, 16, 730–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, S.A.; Penn, C.C.; Negvesky, G.J.; Butrus, S.I. Fungal and Parasitic Infections of the Eye. Clin. Microbiol. Rev. 2000, 13, 662–685. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.P.; Majji, A.B.; Suma, N.; Pappuru, R.R. A rare case of Aspergillus terreus endogenous endophthalmitis in a patient of acute lymphoid leukemia with good clinical outcome. Eye (Lond) 2011, 25, 1094–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalina, P.H.; Campbell, R.J. Aspergillus terreus endophthalmitis in a patient with chronic lymphocytic leukemia. Arch. Ophthalmol. 1991, 109, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.G. Endogenous Aspergillus-induced endophthalmitis. Successful treatment without systemic antifungal medication. Retina 1992, 12, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Kramer, M.R.; Blau, H.; Bishara, J.; Axer-Siegel, R.; Weinberger, D. Intravitreal voriconazole for the treatment of endogenous Aspergillus endophthalmitis. Ophthalmology 2006, 113, 1184–1186. [Google Scholar] [CrossRef] [PubMed]
- Riddell Iv, J.; McNeil, S.A.; Johnson, T.M.; Bradley, S.F.; Kazanjian, P.H.; Kauffman, C.A. Endogenous Aspergillus endophthalmitis: Report of 3 cases and review of the literature. Medicine (Baltimore) 2002, 81, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Vohra, R.; Kaur, R.; Singh, S.; Ashapritpal; Vartika. Excellent outcome of Aspergillous endophthalmitis in a case of allergic bronchopulmonary aspergillosis. Indian J. Ophthalmol. 2014, 62, 352–354. [Google Scholar] [CrossRef]
- Callegan, M.C.; Kane, S.T.; Cochran, D.C.; Novosad, B.; Gilmore, M.S.; Gominet, M.; Lereclus, D. Bacillus endophthalmitis: Roles of bacterial toxins and motility during infection. Invest. Ophthalmol. Vis. Sci. 2005, 46, 3233–3238. [Google Scholar] [CrossRef]
- Callegan, M.C.; Gilmore, M.S.; Gregory, M.; Ramadan, R.T.; Wiskur, B.J.; Moyer, A.L.; Hunt, J.J.; Novosad, B.D. Bacterial endophthalmitis: Therapeutic challenges and host-pathogen interactions. Prog. Retin. Eye Res. 2007, 26, 189–203. [Google Scholar] [CrossRef]
- Moyer, A.L.; Ramadan, R.T.; Novosad, B.D.; Astley, R.; Callegan, M.C. Bacillus cereus-induced permeability of the blood-ocular barrier during experimental endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wilhelmus, K.R. Corneal neovascularization during experimental fungal keratitis. Mol. Vis. 2009, 15, 1988–1996. [Google Scholar] [PubMed]
- Callegan, M.C.; Jett, B.D.; Hancock, L.E.; Gilmore, M.S. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect. Immun. 1999, 67, 3357–3366. [Google Scholar] [PubMed]
- Callegan, M.C.; Booth, M.C.; Jett, B.D.; Gilmore, M.S. Pathogenesis of gram-positive bacterial endophthalmitis. Infect. Immun. 1999, 67, 3348–3356. [Google Scholar] [PubMed]
- Shamsuddin, N.; Kumar, A. TLR2 mediates the innate response of retinal Muller glia to Staphylococcus aureus. J. Immunol. 2011, 186, 7089–7097. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Guest, J.M.; Kanwar, M.; Boss, J.; Gao, N.; Juzych, M.S.; Abrams, G.W.; Yu, F.S.; Kumar, A. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2010, 58, 253–263. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, C.N.; Glybina, I.V.; Mahmoud, T.H.; Yu, F.S. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J. Infect. Dis. 2010, 201, 255–263. [Google Scholar] [CrossRef]
- Fuchs, K.; Cardona Gloria, Y.; Wolz, O.O.; Herster, F.; Sharma, L.; Dillen, C.A.; Taumer, C.; Dickhofer, S.; Bittner, Z.; Dang, T.M.; et al. The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size. Embo Rep. 2018, 19. [Google Scholar] [CrossRef]
- Bourgeois, C.; Kuchler, K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Front. Cell Infect. Microbiol. 2012, 2. [Google Scholar] [CrossRef]
- Netea, M.G.; Warris, A.; Van der Meer, J.W.; Fenton, M.J.; Verver-Janssen, T.J.; Jacobs, L.E.; Andresen, T.; Verweij, P.E.; Kullberg, B.J. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 2003, 188, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Gow, N.A.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerda, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Kirschning, C.J.; Nikolaus, T.; Wagner, H.; Heesemann, J.; Ebel, F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell. Microbiol. 2003, 5, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.; Kullberg, B.J.; Vonk, A.G.; Warris, A.; Cambi, A.; Latge, J.P.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G. Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infect. Immun. 2009, 77, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Balloy, V.; Si-Tahar, M.; Takeuchi, O.; Philippe, B.; Nahori, M.A.; Tanguy, M.; Huerre, M.; Akira, S.; Latge, J.P.; Chignard, M. Involvement of toll-like receptor 2 in experimental invasive pulmonary aspergillosis. Infect. Immun. 2005, 73, 5420–5425. [Google Scholar] [CrossRef] [PubMed]
- Marquart, M.E. Animal models of bacterial keratitis. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.T.; Moyer, A.L.; Callegan, M.C. A role for tumor necrosis factor-alpha in experimental Bacillus cereus endophthalmitis pathogenesis. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4482–4489. [Google Scholar] [CrossRef]
- Astley, R.A.; Coburn, P.S.; Parkunan, S.M.; Callegan, M.C. Modeling intraocular bacterial infections. Prog. Retin. Eye Res. 2016, 54, 30–48. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kumar, A. Role of Staphylococcus aureus Virulence Factors in Inducing Inflammation and Vascular Permeability in a Mouse Model of Bacterial Endophthalmitis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Clark, H.L.; Abbondante, S.; Minns, M.S.; Greenberg, E.N.; Sun, Y.; Pearlman, E. Protein Deiminase 4 and CR3 Regulate Aspergillus fumigatus and beta-Glucan-Induced Neutrophil Extracellular Trap Formation, but Hyphal Killing Is Dependent Only on CR3. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Leal, S.M., Jr.; Cowden, S.; Hsia, Y.C.; Ghannoum, M.A.; Momany, M.; Pearlman, E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. Plos Pathog. 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.D.; Chan, C.C.; Derevjanik, N.L.; Mahlow, J.; Chiu, C.; Peng, B.; Tobe, T.; Campochiaro, P.A.; Vinores, S.A. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: Comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J. Neurosci. Res. 1997, 49, 268–280. [Google Scholar] [CrossRef]
- De Vos, A.F.; van Haren, M.A.; Verhagen, C.; Hoekzema, R.; Kijlstra, A. Tumour necrosis factor-induced uveitis in the Lewis rat is associated with intraocular interleukin 6 production. Exp. Eye Res. 1995, 60, 199–207. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Singh, P.K.; Revankar, S.G.; Chandrasekar, P.H.; Kumar, A. Pathobiology of Aspergillus Fumigatus Endophthalmitis in Immunocompetent and Immunocompromised Mice. Microorganisms 2019, 7, 297. https://doi.org/10.3390/microorganisms7090297
Gupta N, Singh PK, Revankar SG, Chandrasekar PH, Kumar A. Pathobiology of Aspergillus Fumigatus Endophthalmitis in Immunocompetent and Immunocompromised Mice. Microorganisms. 2019; 7(9):297. https://doi.org/10.3390/microorganisms7090297
Chicago/Turabian StyleGupta, Neha, Pawan Kumar Singh, Sanjay G. Revankar, Pranatharthi H. Chandrasekar, and Ashok Kumar. 2019. "Pathobiology of Aspergillus Fumigatus Endophthalmitis in Immunocompetent and Immunocompromised Mice" Microorganisms 7, no. 9: 297. https://doi.org/10.3390/microorganisms7090297
APA StyleGupta, N., Singh, P. K., Revankar, S. G., Chandrasekar, P. H., & Kumar, A. (2019). Pathobiology of Aspergillus Fumigatus Endophthalmitis in Immunocompetent and Immunocompromised Mice. Microorganisms, 7(9), 297. https://doi.org/10.3390/microorganisms7090297






