The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans
Abstract
:1. Introduction
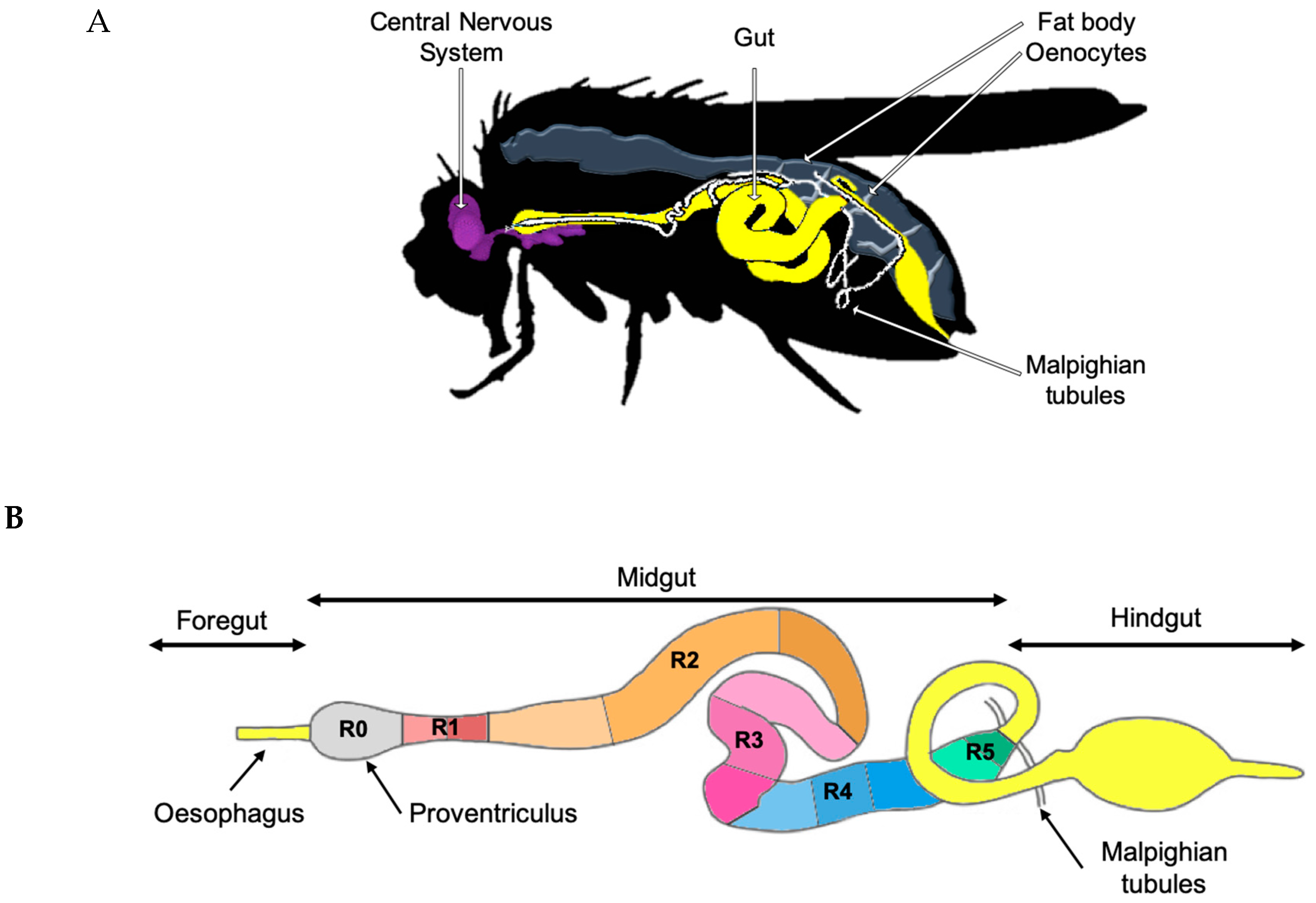
2. Gut Physiology and Homeostasis in Drosophila melanogaster
2.1. Intestinal Stem Cells and Organ Plasticity
2.2. Microbiota Composition and Manipulation in the Laboratory
2.3. Pathogenic Bacterial Interactions with the Host Gut
3. The Detection of Bacterial Bioproducts by Enterocytes
3.1. Peptidoglycan Detection in Drosophila melanogaster’s Gut
3.2. Uracil Detection in Drosophila melanogaster
3.3. Host–Commensal Interactions: Short-Chain Fatty Acids (SCFAs)
4. Immunometabolism in the Drosophila melanogaster Intestinal Epithelium
4.1. Immunometabolic Signaling during Enteric Infection
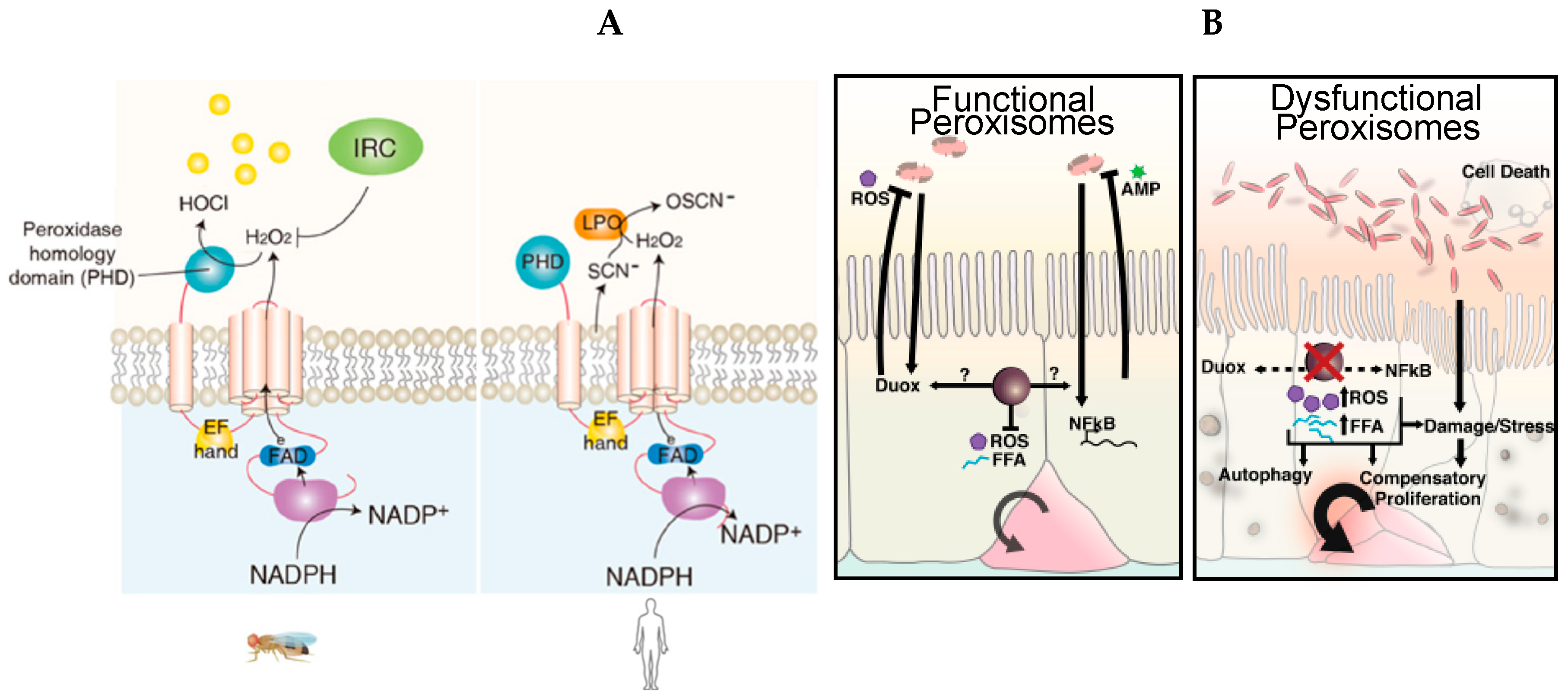
4.2. The Essential Role of Peroxisomes as Immunometabolic Organelles in Intestinal Epithelial Integrity and Homeostasis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| clAP1 | Cellular Inhibitor of Apoptosis Protein 1; |
| Diap2 | Drosophila inhibitor of apoptosis 2; |
| Dredd | death-related ced-3/Nedd2-like protein; |
| FADD | Fas-associated protein with Death Domain; |
| IKK | IκB kinase; |
| Imd | Immune Deficiency; |
| NF-κB | Nuclear factor κB; |
| PGN | Peptidoglycan; |
| PGRP | PeptidoGlycan Recognition Protein; |
| Rel | Relish; |
| RIPK2 | Receptor-Interacting Serine-Threonine Kinase 2; |
| Tab | Tak1-associated binding protein; |
| Tak1 | TGF-β activated kinase 1; |
| κB | inhibitor of κB; |
| XIAP | X-linked IAP. |
References
- Raz, E. Mucosal immunity: Aliment and ailments. Mucosal Immunol. 2009, 3, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satsu, H. Molecular and cellular studies on the absorption, function, and safety of food components in intestinal epithelial cells. Biosci. Biotechnol. Biochem. 2017, 81, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Porte, D.; Schwartz, M.W. Signals that regulate food intake and energy homeostasis. Science 1998, 280, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Kabouridis, P.S.; Pachnis, V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J. Clin. Investig. 2015, 125, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Abot, A.; Cani, P.D.; Knauf, C. Impact of Intestinal Peptides on the Enteric Nervous System: Novel Approaches to Control Glucose Metabolism and Food Intake. Front. Endocrinol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Frosali, S.; Pagliari, D.; Gambassi, G.; Landolfi, R.; Pandolfi, F.; Cianci, R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. J. Immunol. Res. 2015, 2015, 489821. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.A.; Lemaitre, B. Gut homeostasis in a microbial world: Insights from Drosophila melanogaster. Nat. Rev. Microbiol. 2013, 11, 615–626. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.-P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Giorgio, V.; Alberelli, M.A.; De Candia, E.; Gasbarrini, A.; Grieco, A. Impact of Gut Microbiota on Obesity, Diabetes, and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2015, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A Systematic Analysis of Human Disease-Associated Gene Sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef]
- Trinder, M.; Daisley, B.A.; Dube, J.S.; Reid, G. Drosophila melanogaster as a High-Throughput Model for Host-Microbiota Interactions. Front. Microbiol. 2017, 8, 751. [Google Scholar] [CrossRef]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Osman, D. All for one and one for all: Regionalization of the Drosophila intestine. Insect Biochem. Mol. Biol. 2015, 67, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Micchelli, C.A.; Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006, 439, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ohlstein, B.; Spradling, A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 2007, 315, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kopp, Z.A.; Jain, U.; Van Limbergen, J.; Stadnyk, A.W. Do antimicrobial peptides and complement collaborate in the intestinal mucosa? Front. Immunol. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R. Physiology of the Gastrointestinal Tract, 4th ed.; Elsevier: San Diego, CA, USA, 2006. [Google Scholar]
- Reiher, W.; Shirras, C.; Kahnt, J.; Baumeister, S.; Isaac, R.E.; Wegener, C. Peptidomics and peptide hormone processing in the Drosophila midgut. J. Proteome Res. 2011, 10, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Bonfini, A.; Liu, X.; Buchon, N. From pathogens to microbiota: How Drosophila intestinal stem cells react to gut microbes. Dev. Comp. Immunol. 2016, 64, 22–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasov, W.H.; Douglas, A.E. Comparative digestive physiology. Compr. Physiol. 2013, 3, 741–783. [Google Scholar]
- Overend, G.; Luo, Y.; Henderson, L.; Douglas, A.E.; Davies, S.A.; Dow, J.A.T. Molecular mechanism and functional significance of acid generation in the Drosophila midgut. Sci. Rep. 2016, 6, 27242. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, S.; Tripathi, S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J. Exp. Biol. 2009, 212, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. Know your neighbor: Microbiota and host epithelial cells interact locally to control intestinal function and physiology. Bioessays News Rev. Mol. Cell. Dev. Biol. 2016, 38, 455–464. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pais, I.S.; Valente, R.S.; Sporniak, M.; Teixeira, L. Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria. PLoS Biol. 2018, 16, e2005710. [Google Scholar] [CrossRef] [PubMed]
- Staubach, F.; Baines, J.F.; Künzel, S.; Bik, E.M.; Petrov, D.A. Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS ONE 2013, 8, e70749. [Google Scholar] [CrossRef]
- Wong, C.N.A.; Ng, P.; Douglas, A.E. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 2011, 13, 1889–1900. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Qi, Y.; Jasper, H. Preventing Age-Related Decline of Gut Compartmentalization Limits Microbiota Dysbiosis and Extends Lifespan. Cell Host Microbe 2016, 19, 240–253. [Google Scholar] [CrossRef]
- Storelli, G.; Strigini, M.; Grenier, T.; Bozonnet, L.; Schwarzer, M.; Daniel, C.; Matos, R.; Leulier, F. Drosophila Perpetuates Nutritional Mutualism by Promoting the Fitness of Its Intestinal Symbiont Lactobacillus plantarum. Cell Metab. 2018, 27, 362–377. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.V.; Ambort, D.; Pelaseyed, T.; Schütte, A.; Gustafsson, J.K.; Ermund, A.; Subramani, D.B.; Holmén-Larsson, J.M.; Thomsson, K.A.; Bergström, J.H.; et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 2011, 68, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.A.; Härd, T.; Uv, A.; van Dijk-Härd, I.F. A potential role for Drosophila mucins in development and physiology. PLoS ONE 2008, 3, e3041. [Google Scholar] [CrossRef] [PubMed]
- Kuraishi, T.; Binggeli, O.; Opota, O.; Buchon, N.; Lemaitre, B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2011, 108, 15966–15971. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Lemaitre, B.; Miguel-Aliaga, I. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 2013, 47, 377–404. [Google Scholar] [CrossRef]
- Jiang, H.; Edgar, B.A. Intestinal stem cell function in Drosophila and mice. Curr. Opin. Genet. Dev. 2012, 22, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.H.; Dutta, D.; Edgar, B.A. Niche appropriation by Drosophila intestinal stem cell tumours. Nat. Cell Biol. 2015, 17, 1182–1192. [Google Scholar] [CrossRef]
- Tian, A.; Benchabane, H.; Ahmed, Y. Wingless/Wnt Signaling in Intestinal Development, Homeostasis, Regeneration and Tumorigenesis: A Drosophila Perspective. J. Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- White, B.D.; Chien, A.J.; Dawson, D.W. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219–232. [Google Scholar] [CrossRef]
- Tian, A.; Benchabane, H.; Wang, Z.; Ahmed, Y. Regulation of Stem Cell Proliferation and Cell Fate Specification by Wingless/Wnt Signaling Gradients Enriched at Adult Intestinal Compartment Boundaries. PLoS Genet. 2016, 12, e1005822. [Google Scholar] [CrossRef] [PubMed]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Ohlstein, B. Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 2015, 350, aab0988. [Google Scholar] [CrossRef] [PubMed]
- Petkau, K.; Ferguson, M.; Guntermann, S.; Foley, E. Constitutive Immune Activity Promotes Tumorigenesis in Drosophila Intestinal Progenitor Cells. Cell Rep. 2017, 20, 1784–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fre, S.; Pallavi, S.K.; Huyghe, M.; Laé, M.; Janssen, K.-P.; Robine, S.; Artavanis-Tsakonas, S.; Louvard, D. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc. Natl. Acad. Sci. USA 2009, 106, 6309–6314. [Google Scholar] [CrossRef] [Green Version]
- Rodilla, V.; Villanueva, A.; Obrador-Hevia, A.; Robert-Moreno, A.; Fernández-Majada, V.; Grilli, A.; López-Bigas, N.; Bellora, N.; Albà, M.M.; Torres, F.; et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 6315–6320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakula, M. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J. Invertebr. Pathol. 1969, 14, 365–374. [Google Scholar] [CrossRef]
- Chandler, J.A.; Lang, J.M.; Bhatnagar, S.; Eisen, J.A.; Kopp, A. Bacterial communities of diverse Drosophila species: Ecological context of a host-microbe model system. PLoS Genet. 2011, 7, e1002272. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Pontaroli, A.C.; Shimkets, L.J.; Bennetzen, J.L.; Habel, K.E.; Promislow, D.E.L. Geographical Distribution and Diversity of Bacteria Associated with Natural Populations of Drosophila melanogaster. Appl. Environ. Microbiol. 2007, 73, 3470–3479. [Google Scholar] [CrossRef]
- Blum, J.E.; Fischer, C.N.; Miles, J.; Handelsman, J. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. MBio 2013, 4, e00860-13. [Google Scholar] [CrossRef]
- Erkosar, B.; Storelli, G.; Defaye, A.; Leulier, F. Host-intestinal microbiota mutualism: learning on the fly. Cell Host Microbe 2013, 13, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chaston, J.M.; Newell, P.D.; Douglas, A.E. Metagenome-wide association of microbial determinants of host phenotype in Drosophila melanogaster. MBio 2014, 5, e01631-14. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Téfit, M.A.; Leulier, F. Lactobacillus plantarum favors the early emergence of fit and fertile adult Drosophila upon chronic undernutrition. J. Exp. Biol. 2017, 220, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.N.; Trautman, E.P.; Crawford, J.M.; Stabb, E.V.; Handelsman, J.; Broderick, N.A. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. Elife 2017, 6, e18855. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W.; et al. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.C.-N.; Chaston, J.M.; Douglas, A.E. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J. 2013, 7, 1922–1932. [Google Scholar] [CrossRef] [Green Version]
- Dobson, A.J.; Chaston, J.M.; Douglas, A.E. The Drosophila transcriptional network is structured by microbiota. BMC Genom. 2016, 17, 975. [Google Scholar] [CrossRef]
- Early, A.M.; Shanmugarajah, N.; Buchon, N.; Clark, A.G. Drosophila Genotype Influences Commensal Bacterial Levels. PLoS ONE 2017, 12, e0170332. [Google Scholar] [CrossRef]
- Heys, C.; Lizé, A.; Blow, F.; White, L.; Darby, A.; Lewis, Z.J. The effect of gut microbiota elimination in Drosophila melanogaster: A how-to guide for host-microbiota studies. Ecol. Evol. 2018, 8, 4150–4161. [Google Scholar] [CrossRef]
- Broderick, N.A.; Lemaitre, B. Gut-associated microbes of Drosophila melanogaster. Gut Microbes 2012, 3, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandler, J.A.; James, P.M.; Jospin, G.; Lang, J.M. The bacterial communities of Drosophila suzukii collected from undamaged cherries. PeerJ 2014, 2, e474. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.R.; Gilmore, M.S. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect. Immun. 2007, 75, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Webster, P.; Finkel, S.E.; Tower, J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007, 6, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Leulier, F. The importance of being persistent: The first true resident gut symbiont in Drosophila. PLoS Biol. 2018, 16, e2006945. [Google Scholar] [CrossRef]
- Koyle, M.L.; Veloz, M.; Judd, A.M.; Wong, A.C.-N.; Newell, P.D.; Douglas, A.E.; Chaston, J.M. Rearing the Fruit Fly Drosophila melanogaster Under Axenic and Gnotobiotic Conditions. J. Vis. Exp. JoVE 2016, 113, e54219. [Google Scholar] [CrossRef]
- Broderick, N.A.; Buchon, N.; Lemaitre, B. Microbiota-induced changes in drosophila melanogaster host gene expression and gut morphology. MBio 2014, 5, e01117-14. [Google Scholar] [CrossRef]
- Erkosar, B.; Erkosar Combe, B.; Defaye, A.; Bozonnet, N.; Puthier, D.; Royet, J.; Leulier, F. Drosophila microbiota modulates host metabolic gene expression via IMD/NF-κB signaling. PLoS ONE 2014, 9, e94729. [Google Scholar]
- Martino, M.E.; Ma, D.; Leulier, F. Microbial influence on Drosophila biology. Curr. Opin. Microbiol. 2017, 38, 165–170. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Sannino, D.R.; Dobson, A.J.; Edwards, K.; Angert, E.R.; Buchon, N. The Drosophila melanogaster Gut Microbiota Provisions Thiamine to Its Host. MBio 2018, 9, e00155-18. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster: From resistance to resilience. Curr. Opin. Immunol. 2013, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Panayidou, S.; Ioannidou, E.; Apidianakis, Y. Human pathogenic bacteria, fungi, and viruses in Drosophila: Disease modeling, lessons, and shortcomings. Virulence 2014, 5, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Fast, D.; Kostiuk, B.; Foley, E.; Pukatzki, S. Commensal pathogen competition impacts host viability. Proc. Natl. Acad. Sci. USA 2018, 115, 7099–7104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apidianakis, Y.; Pitsouli, C.; Perrimon, N.; Rahme, L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Acad. Sci. USA 2009, 106, 20883–20888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulcahy, H.; Sibley, C.D.; Surette, M.G.; Lewenza, S. Drosophila melanogaster as an animal model for the study of Pseudomonas aeruginosa biofilm infections in vivo. PLoS Pathog. 2011, 7, e1002299. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.D.; Duan, K.; Fischer, C.; Parkins, M.D.; Storey, D.G.; Rabin, H.R.; Surette, M.G. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008, 4, e1000184. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Z.; Lestradet, M.; Socha, C.; Schirmeier, S.; Schmitz, A.; Spenlé, C.; Lefebvre, O.; Keime, C.; Yamba, W.M.; Bou Aoun, R.; et al. Enterocyte Purge and Rapid Recovery Is a Resilience Reaction of the Gut Epithelium to Pore-Forming Toxin Attack. Cell Host Microbe 2016, 20, 716–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benguettat, O.; Jneid, R.; Soltys, J.; Loudhaief, R.; Brun-Barale, A.; Osman, D.; Gallet, A. The DH31/CGRP enteroendocrine peptide triggers intestinal contractions favoring the elimination of opportunistic bacteria. PLoS Pathog. 2018, 14, e1007279. [Google Scholar] [CrossRef] [PubMed]
- Leulier, F.; Parquet, C.; Pili-Floury, S.; Ryu, J.-H.; Caroff, M.; Lee, W.-J.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat. Immunol. 2003, 4, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.; Chevalier, G.; Eberl, G.; Gomperts Boneca, I. The biology of bacterial peptidoglycans and their impact on host immunity and physiology. Cell. Microbiol. 2014, 16, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Randich, A.M.; Brun, Y.V. Molecular mechanisms for the evolution of bacterial morphologies and growth modes. Front. Microbiol. 2015, 6, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.W.; Fisher, J.F.; Mobashery, S. Bacterial cell-wall recycling. Ann. N. Y. Acad. Sci. 2013, 1277, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Buckley, K.M.; Rast, J.P. Diversity of animal immune receptors and the origins of recognition complexity in the deuterostomes. Dev. Comp. Immunol. 2015, 49, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Poidevin, M.; Kwon, H.-M.; Guillou, A.; Sottas, V.; Lee, B.-L.; Lemaitre, B. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 12442–12447. [Google Scholar] [CrossRef] [PubMed]
- Kleino, A.; Silverman, N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2014, 42, 25–35. [Google Scholar] [CrossRef]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila imd signaling pathway. J. Immunol. Baltim. Md 1950 2014, 192, 3455–3462. [Google Scholar] [CrossRef]
- Capo, F.; Charroux, B.; Royet, J. Bacteria sensing mechanisms in Drosophila gut: Local and systemic consequences. Dev. Comp. Immunol. 2016, 64, 11–21. [Google Scholar] [CrossRef]
- Choe, K.-M.; Werner, T.; Stöven, S.; Hultmark, D.; Anderson, K.V. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 2002, 296, 359–362. [Google Scholar] [CrossRef]
- Gottar, M.; Gobert, V.; Michel, T.; Belvin, M.; Duyk, G.; Hoffmann, J.A.; Ferrandon, D.; Royet, J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 2002, 416, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Rämet, M.; Manfruelli, P.; Pearson, A.; Mathey-Prevot, B.; Ezekowitz, R.A.B. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 2002, 416, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Kim, M.-S.; Kim, H.-E.; Yano, T.; Oshima, Y.; Aggarwal, K.; Goldman, W.E.; Silverman, N.; Kurata, S.; Oh, B.-H. Structural basis for preferential recognition of diaminopimelic acid-type peptidoglycan by a subset of peptidoglycan recognition proteins. J. Biol. Chem. 2006, 281, 8286–8295. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Gold, D.; Hartenstein, V. Stem cells and lineages of the intestine: A developmental and evolutionary perspective. Dev. Genes Evol. 2013, 223, 85–102. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.E.; Soliman, S.S.; Li, X.; Bilder, D. Altered modes of stem cell division drive adaptive intestinal growth. Cell 2011, 147, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-J.; Choi, J.-M. Sex-specific regulation of immune responses by PPARs. Exp. Mol. Med. 2017, 49, e364. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A. Gender differences in metabolism; nutrition and supplements. J. Sci. Med. Sport 2000, 3, 287–298. [Google Scholar] [CrossRef]
- Brant, S.R.; Nguyen, G.C. Is there a gender difference in the prevalence of Crohn’s disease or ulcerative colitis? Inflamm. Bowel Dis. 2008, 14, S2–S3. [Google Scholar] [CrossRef]
- Rolston, V.S.; Boroujerdi, L.; Long, M.D.; McGovern, D.P.B.; Chen, W.; Martin, C.F.; Sandler, R.S.; Carmichael, J.D.; Dubinsky, M.; Melmed, G.Y. The Influence of Hormonal Fluctuation on Inflammatory Bowel Disease Symptom Severity-A Cross-Sectional Cohort Study. Inflamm. Bowel Dis. 2018, 24, 387–393. [Google Scholar] [CrossRef]
- Neyen, C.; Bretscher, A.J.; Binggeli, O.; Lemaitre, B. Methods to study Drosophila immunity. Methods 2014, 68, 116–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponton, F.; Wilson, K.; Cotter, S.C.; Raubenheimer, D.; Simpson, S.J. Nutritional immunology: A multi-dimensional approach. PLoS Pathog. 2011, 7, e1002223. [Google Scholar] [CrossRef] [PubMed]
- Vijendravarma, R.K.; Narasimha, S.; Chakrabarti, S.; Babin, A.; Kolly, S.; Lemaitre, B.; Kawecki, T.J. Gut physiology mediates a trade-off between adaptation to malnutrition and susceptibility to food-borne pathogens. Ecol. Lett. 2015, 18, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.D.W.; Partridge, L.; Raubenheimer, D.; Simpson, S.J. Dietary restriction and aging: A unifying perspective. Cell Metab. 2011, 14, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Galenza, A.; Hutchinson, J.; Campbell, S.D.; Hazes, B.; Foley, E. Glucose modulates Drosophila longevity and immunity independent of the microbiota. Biol. Open 2016, 5, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Dudzic, J.P.; Li, X.; Collas, E.J.; Boquete, J.-P.; Lemaitre, B. Remote Control of Intestinal Stem Cell Activity by Haemocytes in Drosophila. PLoS Genet. 2016, 12, e1006089. [Google Scholar] [CrossRef] [PubMed]
- Troha, K.; Buchon, N. Methods for the study of innate immunity in Drosophila melanogaster. Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e344. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Cahn, L.R.Y.; Huey, R.B. Life history consequences of temperature transients in Drosophila melanogaster. J. Exp. Biol. 2007, 210, 2897–2904. [Google Scholar] [CrossRef]
- Klepsatel, P.; Wildridge, D.; Gáliková, M. Temperature induces changes in Drosophila energy stores. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bosco-Drayon, V.; Poidevin, M.; Boneca, I.G.; Narbonne-Reveau, K.; Royet, J.; Charroux, B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe 2012, 12, 153–165. [Google Scholar] [CrossRef]
- Neyen, C.; Poidevin, M.; Roussel, A.; Lemaitre, B. Tissue-and ligand-specific sensing of gram-negative infection in drosophila by PGRP-LC isoforms and PGRP-LE. J. Immunol. Baltim. Md 1950 2012, 189, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Yano, T.; Aggarwal, K.; Lim, J.-H.; Ueda, K.; Oshima, Y.; Peach, C.; Erturk-Hasdemir, D.; Goldman, W.E.; Oh, B.-H.; et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 2006, 7, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Capo, F.; Chaduli, D.; Viallat-Lieutaud, A.; Charroux, B.; Royet, J. Oligopeptide Transporters of the SLC15 Family Are Dispensable for Peptidoglycan Sensing and Transport in Drosophila. J. Innate Immun. 2017, 9, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.; Monahan, A.; Caffrey, D.R.; Elling, R.; Goldman, W.E.; Silverman, N. SLC46 Family Transporters Facilitate Cytosolic Innate Immune Recognition of Monomeric Peptidoglycans. J. Immunol. Baltim. Md 1950 2017, 199, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Mita, S.; Ohmori, H.; Oshima, Y.; Fujimoto, Y.; Ueda, R.; Takada, H.; Goldman, W.E.; Fukase, K.; Silverman, N.; et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat. Immunol. 2008, 9, 908–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2018, 670, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Charroux, B.; Capo, F.; Kurz, C.L.; Peslier, S.; Chaduli, D.; Viallat-Lieutaud, A.; Royet, J. Cytosolic and Secreted Peptidoglycan-Degrading Enzymes in Drosophila Respectively Control Local and Systemic Immune Responses to Microbiota. Cell Host Microbe 2018, 23, 215–228.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costechareyre, D.; Capo, F.; Fabre, A.; Chaduli, D.; Kellenberger, C.; Roussel, A.; Charroux, B.; Royet, J. Tissue-Specific Regulation of Drosophila NF-x03BA; B Pathway Activation by Peptidoglycan Recognition Protein SC. J. Innate Immun. 2016, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Neyen, C.; Runchel, C.; Schüpfer, F.; Meier, P.; Lemaitre, B. The regulatory isoform rPGRP-LC induces immune resolution via endosomal degradation of receptors. Nat. Immunol. 2016, 17, 1150–1158. [Google Scholar] [CrossRef] [Green Version]
- Dworkin, J. The medium is the message: Interspecies and interkingdom signaling by peptidoglycan and related bacterial glycans. Annu. Rev. Microbiol. 2014, 68, 137–154. [Google Scholar] [CrossRef]
- Boneca, I.G. The role of peptidoglycan in pathogenesis. Curr. Opin. Microbiol. 2005, 8, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Cundell, D.R.; Kanthakumar, K.; Taylor, G.W.; Goldman, W.E.; Flak, T.; Cole, P.J.; Wilson, R. Effect of tracheal cytotoxin from Bordetella pertussis on human neutrophil function in vitro. Infect. Immun. 1994, 62, 639–643. [Google Scholar] [PubMed]
- Bouskra, D.; Brézillon, C.; Bérard, M.; Werts, C.; Varona, R.; Boneca, I.G.; Eberl, G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008, 456, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, W.; Dao, D.; Lau, T.C.; Henriksbo, B.D.; Cavallari, J.F.; Foley, K.P.; Schertzer, J.D. Bacterial peptidoglycan stimulates adipocyte lipolysis via NOD1. PLoS ONE 2014, 9, e97675. [Google Scholar] [CrossRef] [PubMed]
- Schertzer, J.D.; Tamrakar, A.K.; Magalhães, J.G.; Pereira, S.; Bilan, P.J.; Fullerton, M.D.; Liu, Z.; Steinberg, G.R.; Giacca, A.; Philpott, D.J.; et al. NOD1 activators link innate immunity to insulin resistance. Diabetes 2011, 60, 2206–2215. [Google Scholar] [CrossRef]
- Lee, K.-A.; Kim, S.-H.; Kim, E.-K.; Ha, E.-M.; You, H.; Kim, B.; Kim, M.-J.; Kwon, Y.; Ryu, J.-H.; Lee, W.-J. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell 2013, 153, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, W.-J. Role of DUOX in gut inflammation: Lessons from Drosophila model of gut-microbiota interactions. Front. Cell. Infect. Microbiol. 2014, 3, 116. [Google Scholar] [CrossRef]
- You, H.; Lee, W.J.; Lee, W.-J. Homeostasis between gut-associated microorganisms and the immune system in Drosophila. Curr. Opin. Immunol. 2014, 30, 48–53. [Google Scholar] [CrossRef]
- Garavaglia, M.; Rossi, E.; Landini, P. The pyrimidine nucleotide biosynthetic pathway modulates production of biofilm determinants in Escherichia coli. PLoS ONE 2012, 7, e31252. [Google Scholar] [CrossRef]
- Ha, E.-M.; Oh, C.-T.; Bae, Y.S.; Lee, W.-J. A direct role for dual oxidase in Drosophila gut immunity. Science 2005, 310, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Choi, M.K.; Lee, W.-J. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010, 31, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009, 23, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.-M.; Lee, K.-A.; Park, S.H.; Kim, S.-H.; Nam, H.-J.; Lee, H.-Y.; Kang, D.; Lee, W.-J. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev. Cell 2009, 16, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.-M.; Lee, K.-A.; Seo, Y.Y.; Kim, S.-H.; Lim, J.-H.; Oh, B.-H.; Kim, J.; Lee, W.-J. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat. Immunol. 2009, 10, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Hoidal, J.R. Reactive Oxygen Species and Cell Signaling. Am. J. Respir. Cell Mol. Biol. 2001, 25, 661–663. [Google Scholar] [CrossRef]
- Lee, K.-A.; Kim, B.; Bhin, J.; Kim, D.H.; You, H.; Kim, E.-K.; Kim, S.-H.; Ryu, J.-H.; Hwang, D.; Lee, W.-J. Bacterial uracil modulates Drosophila DUOX-dependent gut immunity via Hedgehog-induced signaling endosomes. Cell Host Microbe 2015, 17, 191–204. [Google Scholar] [CrossRef]
- Di Cara, F.; Bülow, M.H.; Simmonds, A.J.; Rachubinski, R.A. Dysfunctional peroxisomes compromise gut structure and host defense by increased cell death and Tor-dependent autophagy. Mol. Biol. Cell 2018, 29, 2766–2783. [Google Scholar] [CrossRef]
- Grasberger, H.; Gao, J.; Nagao-Kitamoto, H.; Kitamoto, S.; Zhang, M.; Kamada, N.; Eaton, K.A.; El-Zaatari, M.; Shreiner, A.B.; Merchant, J.L.; et al. Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine. Gastroenterology 2015, 149, 1849–1859. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-A.; Cho, K.-C.; Kim, B.; Jang, I.-H.; Nam, K.; Kwon, Y.E.; Kim, M.; Hyeon, D.Y.; Hwang, D.; Seol, J.-H.; et al. Inflammation-Modulated Metabolic Reprogramming Is Required for DUOX-Dependent Gut Immunity in Drosophila. Cell Host Microbe 2018, 23, 338–352. [Google Scholar] [CrossRef]
- Chandler, J.D.; Day, B.J. Thiocyanate: A potentially useful therapeutic agent with host defense and antioxidant properties. Biochem. Pharmacol. 2012, 84, 1381–1387. [Google Scholar] [CrossRef]
- Johnson, K.R.; Marden, C.C.; Ward-Bailey, P.; Gagnon, L.H.; Bronson, R.T.; Donahue, L.R. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol. Endocrinol. 2007, 21, 1593–1602. [Google Scholar] [CrossRef]
- Grisham, M.B.; Granger, D.N. Neutrophil-mediated mucosal injury. Dig. Dis. Sci. 1988, 33, 6S–15S. [Google Scholar] [CrossRef]
- Razzell, W.; Evans, I.R.; Martin, P.; Wood, W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 2013, 23, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kamareddine, L.; Robins, W.P.; Berkey, C.D.; Mekalanos, J.J.; Watnick, P.I. The Drosophila Immune Deficiency Pathway Modulates Enteroendocrine Function and Host Metabolism. Cell Metab. 2018, 28, 449–462.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauber, A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003, 4, 897–901. [Google Scholar] [CrossRef]
- Feingold, K.R.; Soued, M.; Staprans, I.; Gavin, L.A.; Donahue, M.E.; Huang, B.J.; Moser, A.H.; Gulli, R.; Grunfeld, C. Effect of tumor necrosis factor (TNF) on lipid metabolism in the diabetic rat. Evidence that inhibition of adipose tissue lipoprotein lipase activity is not required for TNF-induced hyperlipidemia. J. Clin. Investig. 1989, 83, 1116–1121. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Yuan, M.; Konstantopoulos, N.; Lee, J.; Hansen, L.; Li, Z.W.; Karin, M.; Shoelson, S.E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001, 293, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Lee, W.-J. Immune-metabolic interactions during systemic and enteric infection in Drosophila. Curr. Opin. Insect Sci. 2018, 29, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.N.; Vanhove, A.S.; Watnick, P.I. The interplay between intestinal bacteria and host metabolism in health and disease: Lessons from Drosophila melanogaster. Dis. Model. Mech. 2016, 9, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Liu, Y.; Luo, J. Insulin/IGF signaling and its regulation in Drosophila. Gen. Comp. Endocrinol. 2015, 221, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Musselman, L.P.; Fink, J.L.; Grant, A.R.; Gatto, J.A.; Tuthill, B.F.; Baranski, T.J. A Complex Relationship between Immunity and Metabolism in Drosophila Diet-Induced Insulin Resistance. Mol. Cell. Biol. 2018, 38, e00259-17. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.-H.; Jones, R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef]
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001, 292, 104–106. [Google Scholar] [CrossRef]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. Edinb. Scotl. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- DiAngelo, J.R.; Bland, M.L.; Bambina, S.; Cherry, S.; Birnbaum, M.J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20853–20858. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.C.; Kim, S.-H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.-A.; Yoon, J.-H.; Ryu, J.-H.; Lee, W.-J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Purdy, A.E.; Robins, W.P.; Wang, Z.; Mandal, M.; Chang, S.; Mekalanos, J.J.; Watnick, P.I. The acetate switch of an intestinal pathogen disrupts host insulin signaling and lipid metabolism. Cell Host Microbe 2014, 16, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.A.; Waterham, H.R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 2006, 75, 295–332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.D.; Baes, M.; Van Veldhoven, P.P. Degradation of very long chain dicarboxylic polyunsaturated fatty acids in mouse hepatocytes, a peroxisomal process. Biochim. Biophys. Acta 2008, 1781, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, F.; Sheshachalam, A.; Braverman, N.E.; Rachubinski, R.A.; Simmonds, A.J. Peroxisome-Mediated Metabolism Is Required for Immune Response to Microbial Infection. Immunity 2017, 47, 93–106.e7. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, I.J.; Semenkovich, C.F. Peroxisomes: A nexus for lipid metabolism and cellular signaling. Cell Metab. 2014, 19, 380–392. [Google Scholar] [CrossRef]
- Bergman, P.; Seyedoleslami Esfahani, S.; Engström, Y. Drosophila as a Model for Human Diseases-Focus on Innate Immunity in Barrier Epithelia. Curr. Top. Dev. Biol. 2017, 121, 29–81. [Google Scholar]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capo, F.; Wilson, A.; Di Cara, F. The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans. Microorganisms 2019, 7, 336. https://doi.org/10.3390/microorganisms7090336
Capo F, Wilson A, Di Cara F. The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans. Microorganisms. 2019; 7(9):336. https://doi.org/10.3390/microorganisms7090336
Chicago/Turabian StyleCapo, Florence, Alexa Wilson, and Francesca Di Cara. 2019. "The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans" Microorganisms 7, no. 9: 336. https://doi.org/10.3390/microorganisms7090336
APA StyleCapo, F., Wilson, A., & Di Cara, F. (2019). The Intestine of Drosophila melanogaster: An Emerging Versatile Model System to Study Intestinal Epithelial Homeostasis and Host-Microbial Interactions in Humans. Microorganisms, 7(9), 336. https://doi.org/10.3390/microorganisms7090336




