Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface
Abstract
:1. Introduction
2. Adenoviral Entry and Trafficking
2.1. Clathrin-mediated Endocytosis
2.2. Caveolin-Mediated Endocytosis
2.3. Macropinocytosis
2.4. Uncoating and Nuclear Trafficking
3. Adenovirus Trafficking and Innate Immunity
4. Ocular Immune Response to Adenovirus Infection
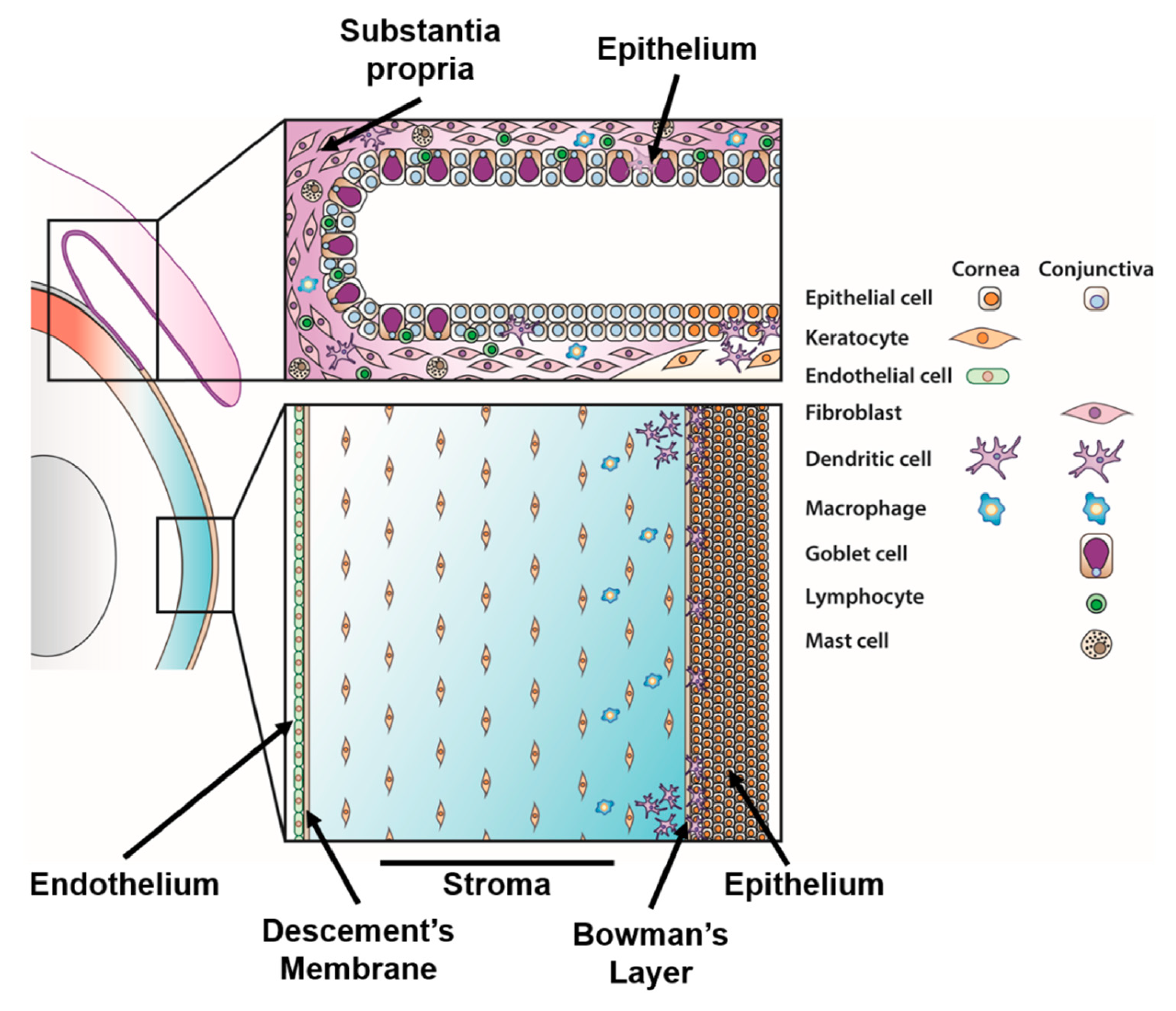
4.1. Corneal Immunity
4.1.1. Corneal Epithelial Cell Responses
4.1.2. Corneal Keratocyte Responses
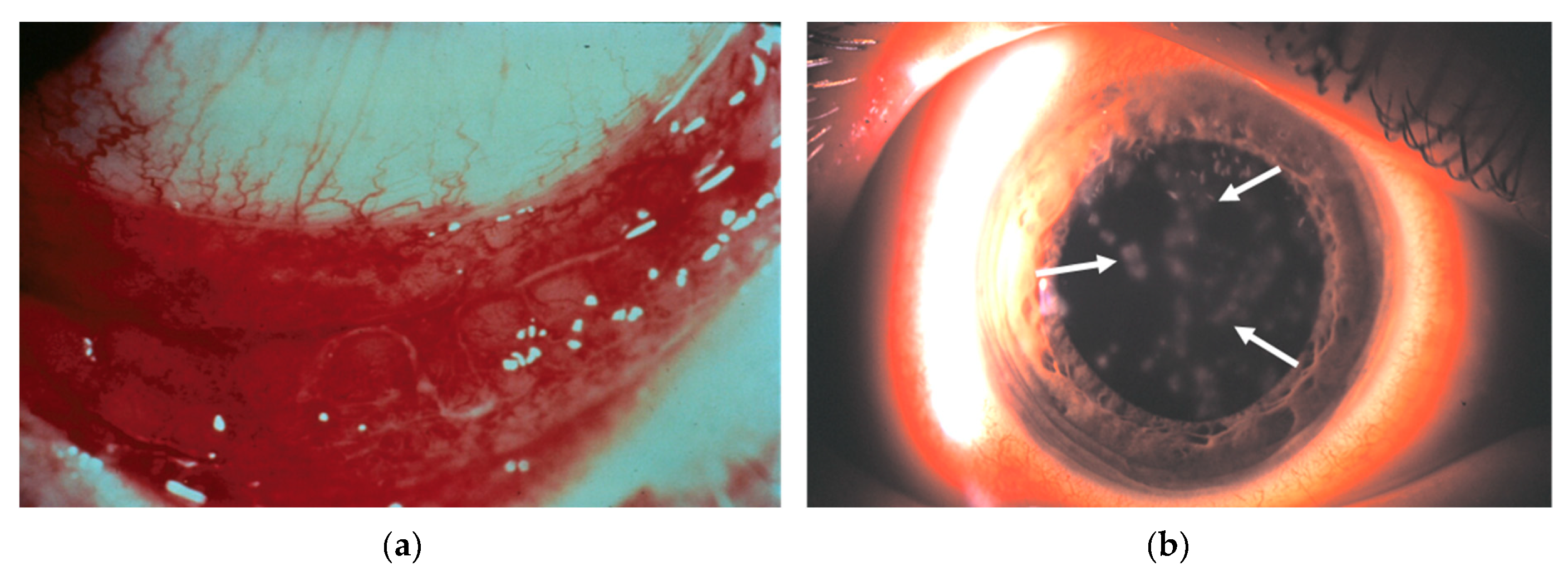
4.1.3. Formation of Subepithelial Infiltrates
4.1.4. Corneal Resident Immune Cells
4.2. Conjunctival Immunity
4.2.1. Infection of the Conjunctiva
4.2.2. Natural Killer Cells
4.2.3. Conjunctival Mucins and Conjunctival Goblet Cells
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- McDonnell, P.J. How do general practitioners manage eye disease in the community? Br. J. Ophthalmol. 1988, 72, 733–736. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, J.H.; Wilson, A.D.; Vernon, S.A.; Sheldrick, C.M. Management of ophthalmic disease in general practice. Br. J. Gen. Pract. 1993, 43, 459–462. [Google Scholar]
- Smith, A.F.; Waycaster, C. Estimate of the direct and indirect annual cost of bacterial conjunctivitis in the United States. BMC Ophthalmol. 2009, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Shields, T.; Sloane, P.D. A comparison of eye problems in primary care and ophthalmology practices. Fam. Med. 1991, 23, 544–546. [Google Scholar] [PubMed]
- Azari, A.A.; Barney, N.P. Conjunctivitis: A systematic review of diagnosis and treatment. JAMA 2013, 310, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.L.; Abbott, R.L. Conjunctivitis. Am. Fam. Phys. 1995, 57, 735–746. [Google Scholar]
- Hierholzer, J.C. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 1992, 5, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.A. Adenoviral disease in pediatric solid organ transplant recipients. Pediatr. Transplant. 2006, 10, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, L.; De Clercq, E.; Naesens, L. Clinical features and treatment of adenovirus infections. Rev. Med. Virol. 2008, 18, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Wold, W.S.M.; Horwitz, M.S. Adenoviruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 2395–2436. [Google Scholar]
- Masterton, S.; Ahearne, M. Mechanobiology of the corneal epithelium. Exp. Eye Res. 2018, 177, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Brissette-Storkus, C.S.; Reynolds, S.M.; Lepisto, A.J.; Hendricks, R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2264–2271. [Google Scholar]
- Knickelbein, J.E.; Watkins, S.C.; McMenamin, P.G.; Hendricks, R.L. Stratification of antigen-presenting cells within the normal cornea. Ophthalmol. Eye Dis. 2009, 1, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Gomes, J.A.P.; Donoso, L.A.; Laibson, P.R. The ocular surface as part of the mucosal immune system: Conjunctival mucosa-specific lymphocytes in ocular surface pathology. Eye 1995, 9, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knop, E.; Knop, N. Anatomy and immunology of the ocular surface. In Immune Response and the Eye; Karger: Basel, Switzerland, 2007; pp. 36–49. [Google Scholar]
- Dartt, D.A.; Masli, S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 464–470. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, T.P.; Jeng, B.H.; McDonald, M.; Raizman, M.B. Acute conjunctivitis: Truth and misconceptions. Curr. Med. Res. Opin. 2009, 25, 1953–1961. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Aoki, K.; Kinchington, P.R. Adenovirus keratoconjunctivitis. In Ocular Infection and Immunity; Pepose, J.S., Holland, G.N., Wilhelmus, K.R., Eds.; Mosby: St. Louis, MO, USA, 1996; pp. 877–894. [Google Scholar]
- Jhanji, V.; Chan, T.C.Y.; Li, E.Y.M.; Agarwal, K.; Vajpayee, R.B. Adenoviral keratoconjunctivitis. Surv. Ophthalmol. 2015, 60, 435–443. [Google Scholar] [CrossRef]
- Chigbu, D.I.; Labib, B.A. Pathogenesis and management of adenoviral keratoconjunctivitis. Infect. Drug Resist. 2018, 11, 981–993. [Google Scholar] [CrossRef]
- Ford, E.; Nelson, K.E.; Warren, D. Epidemiology of epidemic keratoconjunctivitis. Epidemiol. Rev. 1987, 9, 244–261. [Google Scholar] [CrossRef]
- Zhou, X.; Robinson, C.M.; Rajaiya, J.; Dehghan, S.; Seto, D.; Jones, M.S.; Dyer, D.W.; Chodosh, J. Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2804–2811. [Google Scholar] [CrossRef]
- Butt, A.L.; Chodosh, J. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea 2006, 25, 199–202. [Google Scholar] [CrossRef]
- Dosso, A.A.; Rungger-Brändle, E. Clinical course of epidemic keratoconjunctivitis. Cornea 2008, 27, 263–268. [Google Scholar] [CrossRef]
- Subaşı, S.; Yüksel, N.; Toprak, M.; Yılmaz Tuğan, B. In vivo confocal microscopy analysis of the corneal layers in adenoviral epidemic keratoconjunctivitis. Turk. J. Ophthalmol. 2018, 48, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Migita, H.; Ueno, T.; Tsukahara-Kawamura, T.; Saeki, Y.; Fujimoto, T.; Uchio, E. Clinical and virological analysis of epidemic keratoconjunctivitis caused by adenovirus type 54 in a regional ophthalmic clinic in Kyushu, Japan. Clin. Ophthalmol. 2018, 12, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Trelstad, R.L.; Coulombre, A.J. Morphogenesis of the collagenous stroma in the chick cornea. J. Cell Biol. 1971, 50, 840–858. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for “corneal crystallins”. J. Cell Sci. 1999, 112, 613–622. [Google Scholar] [PubMed]
- Tsai, J.C.; Garlinghouse, G.; McDonnell, P.J.; Trousdale, M.D. An experimental animal model of adenovirus-induced ocular disease. The cotton rat. Arch. Ophthalmol. 1992, 110, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Trousdale, M.D.; Nóbrega, R.; Wood, R.L.; Stevenson, D.; dos Santos, P.M.; Klein, D.; McDonnell, P.J. Studies of adenovirus-induced eye disease in the rabbit model. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2740–2748. [Google Scholar]
- Chintakuntlawar, A.; Astley, R.; Chodosh, J. Adenovirus type 37 keratitis in the C57BL/6J mouse. Investig. Ophthalmol. Vis. Sci. 2007, 48, 781–788. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zhou, X.; Rajaiya, J.; Chodosh, J. Ultrastructure of adenovirus keratitis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 472–477. [Google Scholar] [CrossRef]
- Duke-Elder, S.; MacFaul, P. System of Ophthalmology, Vol. VIII, Diseases of the Outer Eye, Part 1. Conjunctiva, 1st ed.; CV Mosby: St. Louis, MO, USA, 1965; pp. 352–357. [Google Scholar]
- Aydin Kurna, S.; Altun, A.; Oflaz, A.; Karatay Arsan, A. Evaluation of the impact of persistent subepithelial corneal infiltrations on the visual performance and corneal optical quality after epidemic keratoconjunctivitis. Acta Ophthalmol. 2015, 93, 377–382. [Google Scholar] [CrossRef]
- Tekin, K.; Kiziltoprak, H.; Koc, M.; Goker, Y.S.; Kocer, A.M.; Yilmazbas, P. The effect of corneal infiltrates on densitometry and higher-order aberrations. Clin. Exp. Optom. 2019, 102, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, A.Y.; Akileswaran, L.; Stroman, D.; Najafi-Tagol, K.; Kleiboeker, S.; Chodosh, J.; Magaret, A.; Wald, A.; Van Gelder, R.N.; et al. Determinants of outcomes of adenoviral keratoconjunctivitis. Ophthalmology 2018, 125, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, S.; Kapoor-Munshi, A.; Nemerow, G. Adenovirus internalization and infection require dynamin. J. Virol. 1998, 72, 3455–3458. [Google Scholar] [PubMed]
- Lee, J.S.; Ismail, A.M.; Lee, J.Y.; Zhou, X.; Materne, E.C.; Chodosh, J.; Rajaiya, J. Impact of dynamin 2 on adenovirus nuclear entry. Virology 2019, 529, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Meier, O.; Boucke, K.; Hammer, S.V.; Keller, S.; Stidwill, R.P.; Hemmi, S.; Greber, U.F. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 2002, 158, 1119–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Chiang, L.; Contreras, J.; Wu, K.; Garner, J.A.; Medina-Kauwe, L.; Hamm-Alvarez, S.F. Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini. J. Virol. 2006, 80, 11833–11851. [Google Scholar] [CrossRef]
- Kälin, S.; Amstutz, B.; Gastaldelli, M.; Wolfrum, N.; Boucke, K.; Havenga, M.; DiGennaro, F.; Liska, N.; Hemmi, S.; Greber, U.F. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J. Virol. 2010, 84, 5336–5350. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.A.; Zhou, X.; Mukherjee, S.; Chintakuntlawar, A.V.; Lee, J.Y.; Ramke, M.; Chodosh, J.; Rajaiya, J. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS ONE 2013, 8, e77462. [Google Scholar] [CrossRef]
- Iacobelli-Martinez, M.; Nemerow, G.R. Preferential activation of toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 2007, 81, 1305–1312. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Yang, Y. Innate immune response to adenoviral vectors is mediated by both toll-like receptor-dependent and -independent pathways. J. Virol. 2007, 81, 3170–3180. [Google Scholar] [CrossRef]
- Nociari, M.; Ocheretina, O.; Schoggins, J.W.; Falck-Pedersen, E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 2007, 81, 4145–4157. [Google Scholar] [CrossRef] [PubMed]
- Muruve, D.A.; Pétrilli, V.; Zaiss, A.K.; White, L.R.; Clark, S.A.; Ross, P.J.; Parks, R.J.; Tschopp, J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 2008, 452, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Flatt, J.W.; Greber, U.F. Misdelivery at the nuclear pore complex-stopping a virus dead in its tracks. Cells 2015, 4, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Flatt, J.W.; Butcher, S.J. Adenovirus flow in host cell networks. Open Biol. 2019, 9, 190012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [PubMed]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef]
- Tomko, R.P.; Xu, R.; Philipson, L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 1997, 94, 3352–3356. [Google Scholar] [CrossRef] [Green Version]
- Segerman, A.; Atkinson, J.P.; Marttila, M.; Dennerquist, V.; Wadell, G.; Arnberg, N. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 2003, 77, 9183–9191. [Google Scholar] [CrossRef]
- Nilsson, E.C.; Storm, R.J.; Bauer, J.; Johansson, S.M.C.; Lookene, A.; Ångström, J.; Hedenström, M.; Eriksson, T.L.; Frängsmyr, L.; Rinaldi, S.; et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011, 17, 105–109. [Google Scholar] [CrossRef]
- Reddy, V.S.; Nemerow, G.R. Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection. Proc. Natl. Acad. Sci. USA 2014, 111, 11715–11720. [Google Scholar] [CrossRef] [Green Version]
- Wu, E.; Pache, L.; Von Seggern, D.J.; Mullen, T.-M.; Mikyas, Y.; Stewart, P.L.; Nemerow, G.R. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 2003, 77, 7225–7235. [Google Scholar] [CrossRef] [PubMed]
- Cupelli, K.; Stehle, T. Viral attachment strategies: The many faces of adenoviruses. Curr. Opin. Virol. 2011, 1, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chandra, N.; Frängsmyr, L.; Imhof, S.; Caraballo, R.; Elofsson, M.; Arnberg, N. Sialic acid-containing glycans as cellular receptors for ocular human adenoviruses: Implications for tropism and treatment. Viruses 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Trauger, S.A.; Pache, L.; Mullen, T.-M.; von Seggern, D.J.; Siuzdak, G.; Nemerow, G.R. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 2004, 78, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- Storm, R.J.; Persson, B.D.; Skalman, L.N.; Frängsmyr, L.; Lindström, M.; Rankin, G.; Lundmark, R.; Domellöf, F.P.; Arnberg, N. Human adenovirus type 37 uses αVβ1 and α3β1 integrins for infection of human corneal cells. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Rayner, S.A.; Gallop, J.L.; George, A.J.T.; Larkin, D.F.P. Distribution of integrins αvβ5, αvβ3 and αv in normal human cornea: Possible implications in clinical and therapeutic adenoviral infection. Eye 1998, 12, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Reddy, V.; Dasgupta, N.; Nemerow, G.R. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J. Virol. 1999, 73, 2798–2802. [Google Scholar]
- Ismail, A.M.; Lee, J.S.; Dyer, D.W.; Seto, D.; Rajaiya, J.; Chodosh, J. Selection pressure in the human adenovirus fiber knob drives cell specificity in epidemic keratoconjunctivitis. J. Virol. 2016, 90, 9598–9607. [Google Scholar] [CrossRef]
- Leopold, P.; Crystal, R. Intracellular trafficking of adenovirus: Many means to many ends. Adv. Drug Deliv. Rev. 2007, 59, 810–821. [Google Scholar] [CrossRef]
- Meier, O.; Greber, U.F. Adenovirus endocytosis. J. Gene Med. 2004, 6, S152–S163. [Google Scholar] [CrossRef]
- Bartlett, J.S.; Wilcher, R.; Samulski, R.J. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 2000, 74, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Medina-Kauwe, L.K. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Deliv. Rev. 2003, 55, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Bantel-Schaal, U.; Hub, B.; Kartenbeck, J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J. Virol. 2002, 76, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Dales, S. An electron microscope study of the early association between two mammalian viruses and their hosts. J. Cell Biol. 1962, 13, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Rosenkranz, H.S.; Mednis, B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J. Virol. 1969, 4, 777–796. [Google Scholar] [PubMed]
- Chardonnet, Y.; Dales, S. Early events in the interaction of adenoviruses with Hela cells: I. Penetration of type 5 and intracellular release of the DNA genome. Virology 1970, 40, 462–477. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Padmanabhan, R.; Pastan, I.; Willingham, M.C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell 1983, 32, 607–617. [Google Scholar] [CrossRef]
- Brodsky, F.M.; Chen, C.Y.; Knuehl, C.; Towler, M.C.; Wakeham, D.E. Biological basket weaving: Formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001, 17, 517–568. [Google Scholar] [CrossRef]
- Young, A. Structural insights into the clathrin coat. Semin. Cell Dev. Biol. 2007, 18, 448–458. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Redelmeier, T.E.; Damke, H.; Tisdale, E.J.; Meyerowitz, E.M.; Schmid, S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993, 122, 553–563. [Google Scholar] [CrossRef]
- Shpetner, H.S.; Vallee, R.B. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell 1989, 59, 421–432. [Google Scholar] [CrossRef]
- Tanabe, K.; Takei, K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J. Cell Biol. 2009, 185, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Nakata, T.; Noda, Y.; Sato-Yoshitake, R.; Hirokawa, N. Interaction of dynamin with microtubules: Its structure and GTPase activity investigated by using highly purified dynamin. Mol. Biol. Cell 1992, 3, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, M.; Imelli, N.; Boucke, K.; Amstutz, B.; Meier, O.; Greber, U.F. Infectious adenovirus type 2 transport through early but not late endosomes. Traffic 2008, 9, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, B.; Gastaldelli, M.; Kälin, S.; Imelli, N.; Boucke, K.; Wandeler, E.; Mercer, J.; Hemmi, S.; Greber, U.F. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008, 27, 956–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, B.B.; Zhou, X.; Spurr-Michaud, S.; Rajaiya, J.; Chodosh, J.; Gipson, I.K. Epidemic keratoconjunctivitis-causing adenoviruses induce MUC16 ectodomain release to infect ocular surface epithelial cells. mSphere 2016, 1. [Google Scholar] [CrossRef]
- Singh, G.; Zhou, X.; Lee, J.Y.; Yousuf, M.A.; Ramke, M.; Ismail, A.M.; Lee, J.S.; Robinson, C.M.; Seto, D.; Dyer, D.W.; et al. Recombination of the epsilon determinant and corneal tropism: Human adenovirus species D types 15, 29, 56, and 69. Virology 2015, 485, 452–459. [Google Scholar] [CrossRef]
- Robinson, C.M.; Zhou, X.; Rajaiya, J.; Yousuf, M.A.; Singh, G.; DeSerres, J.J.; Walsh, M.P.; Wong, S.; Seto, D.; Dyer, D.W.; et al. Predicting the next eye pathogen: Analysis of a novel adenovirus. MBio 2013, 4. [Google Scholar] [CrossRef]
- Bailey, C.J.; Crystal, R.G.; Leopold, P.L. Association of adenovirus with the microtubule organizing center. J. Virol. 2003, 77, 13275–13287. [Google Scholar] [CrossRef]
- Kiss, A.L.; Botos, E. Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell. Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef]
- Pelkmans, L.; Helenius, A. Endocytosis via caveolae. Traffic 2002, 3, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Smart, E.J. Caveolae structure and function. J. Cell. Mol. Med. 2008, 12, 796–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnitzer, J.E. Caveolae: From basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv. Drug Deliv. Rev. 2001, 49, 265–280. [Google Scholar] [CrossRef]
- Scherer, P.E.; Okamoto, T.; Chun, M.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc. Natl. Acad. Sci. USA 1996, 93, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Scherer, P.E.; Okamoto, T.; Song, K.; Chu, C.; Kohtz, D.S.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996, 271, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Bürli, T.; Zerial, M.; Helenius, A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004, 118, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.; McIntosh, D.P.; Schnitzer, J.E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 1998, 141, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.R.; Krueger, E.W.A.; Oswald, B.J.; McNiven, M.A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998, 141, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chen, J.; Cao, H.; Orth, J.D.; McCaffery, J.M.; Stan, R.V.; McNiven, M.A. Caveolin-1 interacts directly with dynamin-2. J. Mol. Biol. 2005, 348, 491–501. [Google Scholar] [CrossRef]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef]
- Pelkmans, L.; Püntener, D.; Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Brown, J.C.; Choudhury, A.; Peterson, T.E.; Holicky, E.; Marks, D.L.; Simari, R.; Parton, R.G.; Pagano, R.E. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol. Biol. Cell 2004, 15, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Kiyota, A.; Suzaki, E.; Kataoka, K.; Ohe, Y.; Miyamoto, K.; Senda, T.; Fujimoto, T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004, 78, 8701–8708. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Radulescu, C.; Lazar, C.; Petrescu, S.; Durantel, D.; Dwek, R.A.; Zitzmann, N.; Nichita, N.B. Hepatitis B virus requires intact caveolin-1 function for productive infection in HepaRG cells. J. Virol. 2010, 84, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Bousarghin, L.; Touze, A.; Sizaret, P.Y.; Coursaget, P. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J. Virol. 2003, 77, 3846–3850. [Google Scholar] [CrossRef] [PubMed]
- Kartenbeck, J.; Stukenbrok, H.; Helenius, A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J. Cell Biol. 1989, 109, 2721–2729. [Google Scholar] [CrossRef]
- Empig, C.J.; Goldsmith, M.A. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 2002, 76, 5266–5270. [Google Scholar] [CrossRef]
- Mañes, S.; del Real, G.; Lacalle, R.A.; Lucas, P.; Gómez-Moutón, C.; Sánchez-Palomino, S.; Delgado, R.; Alcamí, J.; Mira, E.; Martínez-A, C. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000, 1, 190–196. [Google Scholar] [CrossRef]
- Guyader, M.; Kiyokawa, E.; Abrami, L.; Turelli, P.; Trono, D. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol. 2002, 76, 10356–10364. [Google Scholar] [CrossRef]
- Marjomäki, V.; Pietiäinen, V.; Matilainen, H.; Upla, P.; Ivaska, J.; Nissinen, L.; Reunanen, H.; Huttunen, P.; Hyypiä, T.; Heino, J. Internalization of echovirus 1 in caveolae. J. Virol. 2002, 76, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.D.; Eustace, H.E.; McKee, T.A.; Brown, T.D.K. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 2002, 76, 9307–9322. [Google Scholar] [CrossRef] [PubMed]
- Eash, S.; Querbes, W.; Atwood, W.J. Infection of vero cells by BK virus is dependent on caveolae. J. Virol. 2004, 78, 11583–11590. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Andersen, D.S.; Rojek, A.; Pedersen, L. Caveola-dependent endocytic entry of amphotropic murine leukemia virus. J. Virol. 2005, 79, 10776–10787. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Larocco, M.; Baxt, B. Heparan sulfate-binding foot-and-mouth disease virus enters cells via caveola-mediated endocytosis. J. Virol. 2008, 82, 9075–9085. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Liu, D.; Wu, Y.Y.; Yang, X.B.; Yang, L.S.; Mi, S.; Huang, Y.X.; Luo, Y.W.; Jia, K.T.; Liu, Z.Y.; et al. Entry of tiger frog virus (an Iridovirus) into HepG2 cells via a pH-dependent, atypical, caveola-mediated endocytosis pathway. J. Virol. 2011, 85, 6416–6426. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.; Schudel, B.R.; Maar, D.; Kozina, C.; Ikegami, T.; Tseng, C.T.; Negrete, O.A. Rift Valley fever virus strain MP-12 enters mammalian host cells via caveola-mediated endocytosis. J. Virol. 2012, 86, 12954–12970. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef]
- Canton, J. Macropinocytosis: New insights into its underappreciated role in innate immune cell surveillance. Front. Immunol. 2018, 9, 2286. [Google Scholar] [CrossRef]
- Nicola, A.V.; Hou, J.; Major, E.O.; Straus, S.E. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 2005, 79, 7609–7616. [Google Scholar] [CrossRef]
- Nicola, A.V.; McEvoy, A.M.; Straus, S.E. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 2003, 77, 5324–5332. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Knebel, S.; Schmidt, F.I.; Crouse, J.; Burkard, C.; Helenius, A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc. Natl. Acad. Sci. USA 2010, 107, 9346–9351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergelson, J.M.; Coyne, C.B. Picornavirus entry. In Viral Entry into Host Cells; Pohlmann, S., Simmons, G., Eds.; Springer: New York, NY, USA, 2013; Volume 790, pp. 24–41. [Google Scholar]
- Greber, U.F.; Suomalainen, M.; Stidwill, R.P.; Boucke, K.; Ebersold, M.W.; Helenius, A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997, 16, 5998–6007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.G.; Wiethoff, C.M.; Stewart, P.L.; Nemerow, G.R. Adenovirus. In Cell Entry by Non-Enveloped Viruses; Johnson, J., Ed.; Springer: Heidelberg, Germany, 2010; Volume 343, pp. 195–224. [Google Scholar]
- Henaff, D.; Salinas, S.; Kremer, E.J. An adenovirus traffic update: From receptor engagement to the nuclear pore. Future Microbiol. 2011, 6, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kilcher, S.; Mercer, J. DNA virus uncoating. Virology 2015, 479, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, N.; Greber, U.F. Adenovirus signalling in entry. Cell. Microbiol. 2013, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Moyer, C.L.; Besser, E.S.; Nemerow, G.R. A single maturation cleavage site in adenovirus impacts cell entry and capsid assembly. J. Virol. 2016, 90, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.J.; Suomalainen, M.; Schoenenberger, P.; Boucke, K.; Hemmi, S.; Greber, U.F. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe 2011, 10, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Strunze, S.; Engelke, M.F.; Wang, I.H.; Puntener, D.; Boucke, K.; Schleich, S.; Way, M.; Schoenenberger, P.; Burckhardt, C.J.; Greber, U.F. Kinesin-1-mediated capsid disassembly and disruption of the nuclear pore complex promote virus infection. Cell Host Microbe 2011, 10, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, M.; Nakano, M.Y.; Keller, S.; Boucke, K.; Stidwill, R.P.; Greber, U.F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 1999, 144, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Cassany, A.; Ragues, J.; Guan, T.; Bégu, D.; Wodrich, H.; Kann, M.; Nemerow, G.R.; Gerace, L. Nuclear import of adenovirus DNA involves direct interaction of hexon with an N-terminal domain of the nucleoporin Nup214. J. Virol. 2015, 89, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Trotman, L.C.; Mosberger, N.; Fornerod, M.; Stidwill, R.P.; Greber, U.F. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat. Cell Biol. 2001, 3, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Yi, H.; Desai, R.; Hand, A.R.; Haas, A.L.; Ferreira, P.A. RANBP2 is an allosteric activator of the conventional kinesin-1 motor protein, KIF5B, in a minimal cell-free system. EMBO Rep. 2009, 10, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Yu, F.S.; Kumar, A. Targeting toll-like receptor signaling as a novel approach to prevent ocular infectious diseases. Indian J. Med. Res. 2013, 138, 609–619. [Google Scholar] [PubMed]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef]
- Fejer, G.; Freudenberg, M.; Greber, U.F.; Gyory, I. Adenovirus-triggered innate signalling pathways. Eur. J. Microbiol. Immunol. 2011, 1, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Teigler, J.E.; Kagan, J.C.; Barouch, D.H. Late endosomal trafficking of alternative serotype adenovirus vaccine vectors augments antiviral innate immunity. J. Virol. 2014, 88, 10354–10363. [Google Scholar] [CrossRef]
- Teigler, J.E.; Iampietro, M.J.; Barouch, D.H. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 2012, 86, 9590–9598. [Google Scholar] [CrossRef]
- Fejer, G.; Drechsel, L.; Liese, J.; Schleicher, U.; Ruzsics, Z.; Imelli, N.; Greber, U.F.; Keck, S.; Hildenbrand, B.; Krug, A.; et al. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 2008, 4, e1000208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. Ocular immune privilege and transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rüsenberg, B.; Loderstädt, U.; Richard, G.; Kaulfers, P.M.; Gesser, C. Epidemic keratoconjunctivitis: The current situation and recommendations for prevention and treatment. Dtsch. Arztebl. Int. 2011, 108, 475–480. [Google Scholar] [PubMed]
- Boniuk, M.; Phillips, C.A.; Friedman, J.B. Chronic adenovirus type 2 keratitis in man. N. Engl. J. Med. 1965, 273, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.R.; Hanna, L.; Togni, B. Adenovirus type 8 infections in the United States. IV. Observations on the pathogenesis of lesions in severe eye disease. Arch. Ophthalmol. 1972, 87, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Maudgal, P.C. Cytopathology of adenovirus keratitis by replica technique. Br. J. Ophthalmol. 1990, 74, 670–675. [Google Scholar] [CrossRef]
- Chodosh, J.; Miller, D.; Stroop, W.G.; Pflugfelder, S.C. Adenovirus epithelial keratitis. Cornea 1995, 14, 167–174. [Google Scholar] [CrossRef]
- Oakes, J.E.; Monteiro, C.A.; Cubitt, C.L.; Lausch, R.N. Induction of interleukin-8 gene expression is associated with herpes simplex virus infection of human corneal keratocytes but not human corneal epithelial cells. J. Virol. 1993, 67, 4777–4784. [Google Scholar] [Green Version]
- Cubitt, C.L.; Tang, Q.; Monteiro, C.A.; Lausch, R.N.; Oakes, J.E. IL-8 gene expression in cultures of human corneal epithelial cells and keratocytes. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3199–3206. [Google Scholar]
- Cubitt, C.L.; Lausch, R.N.; Oakes, J.E. Differences in interleukin-6 gene expression between cultured human corneal epithelial cells and keratocytes. Investig. Ophthalmol. Vis. Sci. 1995, 36, 330–336. [Google Scholar]
- Wilson, S.E.; He, Y.G.; Lloyd, S.A. EGF, EGF receptor, basic FGF, TGF beta-1, and IL-1 alpha mRNA in human corneal epithelial cells and stromal fibroblasts. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1756–1765. [Google Scholar]
- Shirane, J.; Nakayama, T.; Nagakubo, D.; Izawa, D.; Hieshima, K.; Shimomura, Y.; Yoshie, O. Corneal epithelial cells and stromal keratocytes efficently produce CC chemokine-ligand 20 (CCL20) and attract cells expressing its receptor CCR6 in mouse herpetic stromal keratitis. Curr. Eye Res. 2004, 28, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Huang, Y.; Issekutz, A.C.; Griffith, M.; Lin, K.H.; Anderson, R. Interleukin-1α released from epithelial cells after adenovirus type 37 infection activates intercellular adhesion molecule 1 expression on human vascular endothelial cells. J. Virol. 2002, 76, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.R. The clinical features of viral keratitis and a concept of their pathogenesis. Proc. R. Soc. Med. 1958, 51, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.J.; Pels, L.; Vrensen, G.F. Novel aspects of the ultrastructural organization of human corneal keratocytes. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2557–2567. [Google Scholar]
- Fukuda, K.; Ishida, W.; Tanaka, H.; Harada, Y.; Fukushima, A. Inhibition by rebamipide of cytokine-induced or lipopolysaccharide-induced chemokine synthesis in human corneal fibroblasts. Br. J. Ophthalmol. 2014, 98, 1751–1755. [Google Scholar] [CrossRef]
- Kimura, K.; Orita, T.; Nomi, N.; Fujitsu, Y.; Nishida, T.; Sonoda, K.H. Identification of common secreted factors in human corneal fibroblasts exposed to LPS, poly(I:C), or zymosan. Exp. Eye Res. 2012, 96, 157–162. [Google Scholar] [CrossRef]
- McInnis, K.A.; Britain, A.; Lausch, R.N.; Oakes, J.E. Synthesis of α-chemokines IP-10, I-TAC, and MIG are differentially regulated in human corneal keratocytes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1668–1674. [Google Scholar] [CrossRef]
- Molesworth-Kenyon, S.J.; Milam, A.; Rockette, A.; Troupe, A.; Oakes, J.E.; Lausch, R.N. Expression, inducers andcellular sources of the chemokine MIG (CXCL 9), during primary Herpes simplex virus type-1 infection of the cornea. Curr. Eye Res. 2015, 40, 800–808. [Google Scholar] [CrossRef]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Cubitt, C.L.; Lausch, R.N.; Oakes, J.E. Differential induction of GRO alpha gene expression in human corneal epithelial cells and keratocytes exposed to proinflammatory cytokines. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1149–1158. [Google Scholar]
- Barbosa, F.L.; Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp. Eye Res. 2010, 91, 456–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.J.; Kim, M.K.; Ko, J.H.; Lee, H.J.; Jeong, H.J.; Wee, W.R.; Seong, S.Y.; Akira, S. Effect of toll-like receptor 2 and 4 of corneal fibroblasts on cytokine expression with co-cultured antigen presenting cells. Cytokine 2011, 56, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, N.; Matsuda, A.; Nakamura, S.; Matsuda, H.; Murakami, A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8549–8557. [Google Scholar] [CrossRef] [PubMed]
- Elner, V.M.; Strieter, R.M.; Pavilack, M.A.; Elner, S.G.; Remick, D.G.; Danforth, J.M.; Kunkel, S.L. Human corneal interleukin-8. IL-1 and TNF-induced gene expression and secretion. Am. J. Pathol. 1991, 139, 977–988. [Google Scholar] [PubMed]
- Bian, Z.M.; Elner, V.M.; Lukacs, N.W.; Strieter, R.M.; Kunkel, S.L.; Elner, S.G. Glycated human serum albumin induces IL-8 and MCP-1 gene expression in human corneal keratocytes. Curr. Eye Res. 1998, 17, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chodosh, J.; Astley, R.A.; Butler, M.G.; Kennedy, R.C. Adenovirus keratitis: A role for interleukin-8. Investig. Ophthalmol. Vis. Sci. 2000, 41, 783–789. [Google Scholar]
- Natarajan, K.; Rajala, M.S.; Chodosh, J. Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J. Immunol. 2003, 170, 6234–6243. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Pergolizzi, S.; Lauriano, E.R.; Santoro, G.; Spataro, F.; Cimino, F.; Speciale, A.; Nostro, A.; Bisignano, G. TLR2 activation in corneal stromal cells by Staphylococcus aureus-induced keratitis. APMIS 2015, 123, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ishida, W.; Fukushima, A.; Nishida, T. Corneal fibroblasts as sentinel cells and local immune modulators in infectious keratitis. Int. J. Mol. Sci. 2017, 18, 1831. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Tellaetxe-Isusi, M.; Elner, V.; Strieter, R.M.; Lausch, R.N.; Oakes, J.E. Proinflammatory cytokines induce RANTES and MCP-1 synthesis in human corneal keratocytes but not in corneal epithelial cells. Beta-chemokine synthesis in corneal cells. Investig. Ophthalmol. Vis. Sci. 1996, 37, 987–996. [Google Scholar]
- Ebihara, N.; Yamagami, S.; Yokoo, S.; Amano, S.; Murakami, A. Involvement of C-C chemokine ligand 2-CCR2 interaction in monocyte-lineage cell recruitment of normal human corneal stroma. J. Immunol. 2007, 178, 3288–3292. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Choi, B.K.; Kang, W.J.; Kim, Y.H.; Park, H.Y.; Kim, K.H.; Kwon, B.S. MCP-1 derived from stromal keratocyte induces corneal infiltration of CD4+ T cells in herpetic stromal keratitis. Mol. Cells 2008, 26, 67–73. [Google Scholar] [PubMed]
- Chintakuntlawar, A.V.; Chodosh, J. Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J. Interferon Cytokine Res. 2009, 29, 657–666. [Google Scholar] [CrossRef]
- Chintakuntlawar, A.V.; Zhou, X.; Rajaiya, J.; Chodosh, J. Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog. 2010, 6, e1000841. [Google Scholar] [CrossRef]
- Zhou, X.; Ramke, M.; Chintakuntlawar, A.V.; Lee, J.Y.; Rajaiya, J.; Chodosh, J. Role of MyD88 in adenovirus keratitis. Immunol. Cell Biol. 2017, 95, 108–116. [Google Scholar] [CrossRef]
- Appledorn, D.M.; Patial, S.; McBride, A.; Godbehere, S.; Van Rooijen, N.; Parameswaran, N.; Amalfitano, A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 2008, 181, 2134–2144. [Google Scholar] [CrossRef]
- Ahtiainen, L.; Mirantes, C.; Jahkola, T.; Escutenaire, S.; Diaconu, I.; Österlund, P.; Kanerva, A.; Cerullo, V.; Hemminki, A. Defects in innate immunity render breast cancer initiating cells permissive to oncolytic adenovirus. PLoS ONE 2010, 5, e13859. [Google Scholar] [CrossRef]
- Basner-Tschakarjan, E.; Gaffal, E.; O’Keeffe, M.; Tormo, D.; Limmer, A.; Wagner, H.; Hochrein, H.; Tüting, T. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-α production. J. Gene Med. 2006, 8, 1300–1306. [Google Scholar] [CrossRef]
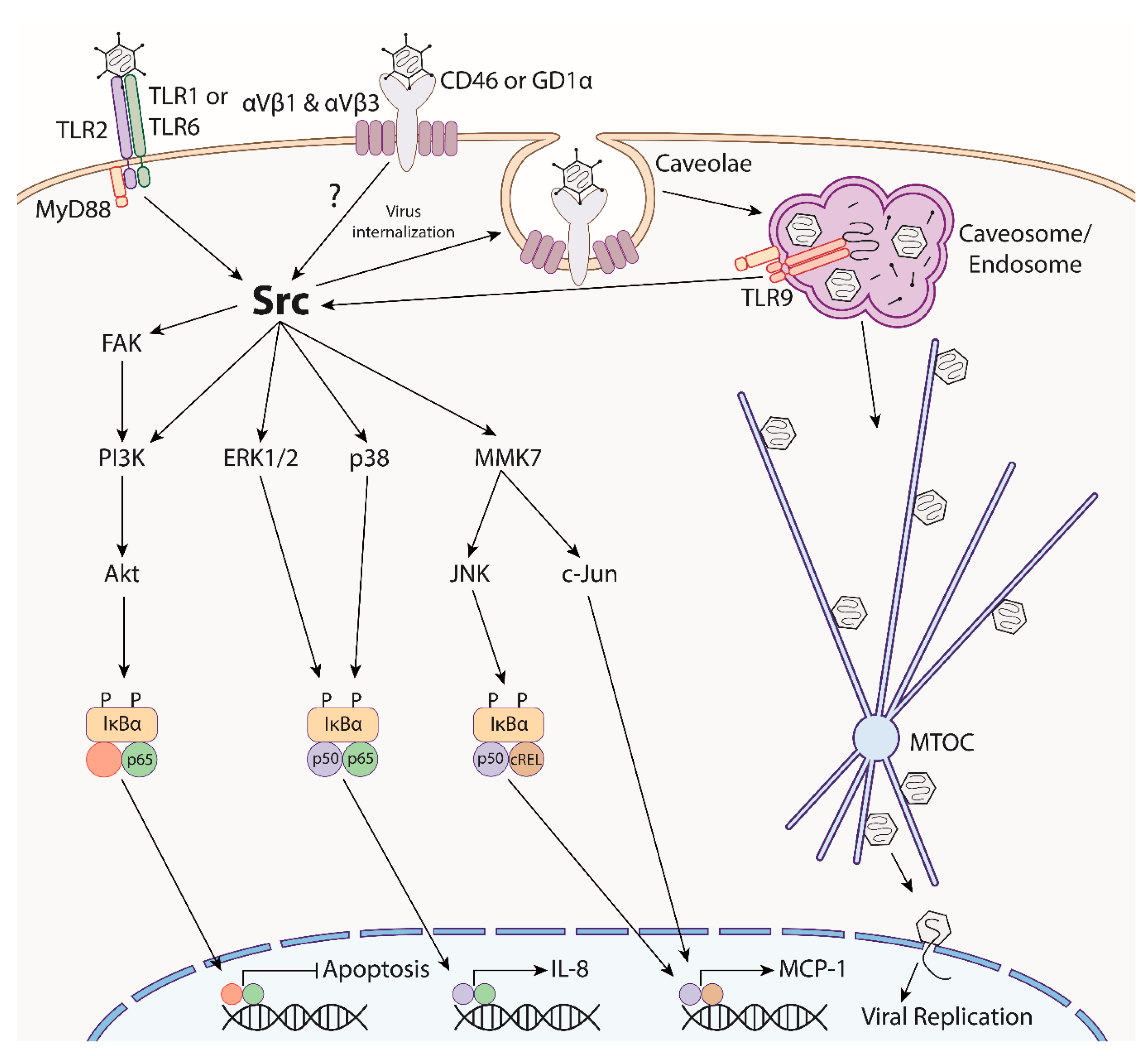
- Roskoski, R. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rajala, M.S.; Rajala, R.V.S.; Astley, R.A.; Butt, A.L.; Chodosh, J. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J. Virol. 2005, 79, 12332–12341. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Ghalayini, A.J.; Sterling, R.S.; Holbrook, R.M.; Kennedy, R.C.; Chodosh, J. Activation of focal adhesion kinase in adenovirus-infected human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2685–2690. [Google Scholar]
- Rajaiya, J.; Sadeghi, N.; Chodosh, J. Specific NFkappaB subunit activation and kinetics of cytokine induction in adenoviral keratitis. Mol. Vis. 2009, 15, 2879–2889. [Google Scholar] [PubMed]
- Rajaiya, J.; Xiao, J.; Rajala, R.V.; Chodosh, J. Human adenovirus type 19 infection of corneal cells induces p38 MAPK-dependent interleukin-8 expression. Virol. J. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chodosh, J. JNK regulates MCP-1 expression in adenovirus type 19–infected human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3777–3782. [Google Scholar] [CrossRef]
- Lund, O.E.; Stefani, F.H. Corneal histology after epidemic keratoconjunctivitis. Arch. Ophthalmol. 1978, 96, 2085–2088. [Google Scholar] [CrossRef]
- Tullo, A.B.; Ridgway, A.E.; Lucas, D.R.; Richmond, S. Histopathology of adenovirus type 8 keratitis. Cornea 1987, 6, 234. [Google Scholar] [CrossRef]
- Oka, M.; Norose, K.; Matsushima, K.; Nishigori, C.; Herlyn, M. Overexpression of IL-8 in the cornea induces ulcer formation in the SCID mouse. Br. J. Ophthalmol. 2006, 90, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Rajaiya, J.; Zhou, X.; Barequet, I.; Gilmore, M.S.; Chodosh, J. Novel model of innate immunity in corneal infection. Vitr. Cell Dev. Biol. Anim. 2015, 51, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Conrady, C.D.; Zheng, M.; Mandal, N.A.; van Rooijen, N.; Carr, D.J.J. IFN-α-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013, 6, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Li, Z.; Smith, C.W. Neutrophil migration in the wounded cornea: The role of the keratocyte. Ocul. Surf. 2005, 3, S173–S176. [Google Scholar] [CrossRef]
- Hodge, W.; Wohl, T.; Whitcher, J.P.; Margolis, T.P. Corneal subepithelial infiltrate recurrence sine adenovirus. Cornea 1995, 14, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, E.G.; Roba, L.A.; Wiley, L.; Araullo-Cruz, T.; Gordon, Y.J. The effects of corticosteroids of adenoviral replication. Arch. Ophthalmol. 1996, 114, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Araullo-Cruz, T.; Romanowski, E.G. The effects of topical nonsteroidal anti-inflammatory drugs on adenoviral replication. Arch. Ophthalmol. 1998, 116, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, E.G.; Yates, K.A.; Gordon, Y.J. Short-term treatment with a potent topical corticosteroid of an acute ocular adenoviral infection in the New Zealand white rabbit. Cornea 2001, 20, 657–660. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Pless, P.; Yates, K.A.; Gordon, Y.J. Topical cyclosporine A inhibits subepithelial immune infiltrates but also promotes viral shedding in experimental adenovirus models. Cornea 2005, 24, 86–91. [Google Scholar] [CrossRef]
- Chodosh, J. Human adenovirus type 37 and the BALB/c mouse: Progress toward a restricted adenovirus keratitis model (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 346–365. [Google Scholar]
- Natarajan, K.; Shepard, L.A.; Chodosh, J. The use of DNA array technology in studies of ocular viral pathogenesis. DNA Cell Biol. 2002, 21, 483–490. [Google Scholar] [CrossRef]
- Hartman, Z.C.; Kiang, A.; Everett, R.S.; Serra, D.; Yang, X.Y.; Clay, T.M.; Amalfitano, A. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 2007, 81, 1796–1812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dahlö, M.; Isaksson, A.; Syvänen, A.C.; Pettersson, U. The transcriptome of the adenovirus infected cell. Virology 2012, 424, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, B.; Toth, K.; Spencer, J.F.; Aurora, R.; Wold, W.S.M. Transcriptome sequencing and development of an expression microarray platform for liver infection in adenovirus type 5-infected Syrian golden hamsters. Virology 2015, 485, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Xu, Z.; Huang, L.; Qin, E.Q.; Zhang, J.L.; Zhao, P.; Tu, B.; Shi, L.; Li, W.G.; Chen, W.W. Transcriptome sequencing identifies novel immune response genes highly related to the severity of human adenovirus type 55 infection. Front. Microbiol. 2019, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, J.E.; Buela, K.A.; Hendricks, R.L. Antigen-presenting cells are stratified within normal human corneas and are rapidly mobilized during ex vivo viral infection. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1118–1123. [Google Scholar] [CrossRef]
- Gillette, T.E.; Chandler, J.W.; Greiner, J.V. Langerhans cells of the ocular surface. Ophthalmology 1982, 89, 700–711. [Google Scholar] [CrossRef]
- Buela, K.A.G.; Hendricks, R.L. Cornea-infiltrating and lymph node dendritic cells contribute to CD4 + T Cell expansion after herpes simplex virus-1 ocular infection. J. Immunol. 2015, 194, 379–387. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, X.; Stuart, P.M.; Leib, D.A. Dendritic cell autophagy contributes to herpes simplex virus-driven stromal keratitis and immunopathology. MBio 2015, 6. [Google Scholar] [CrossRef]
- Lee, H.S.; Amouzegar, A.; Dana, R. Kinetics of corneal antigen presenting cells in experimental dry eye disease. BMJ Open Ophthalmol. 2017, 1, e000078. [Google Scholar] [CrossRef]
- Kwon, M.S.; Carnt, N.A.; Truong, N.R.; Pattamatta, U.; White, A.J.; Samarawickrama, C.; Cunningham, A.L. Dendritic cells in the cornea during Herpes simplex viral infection and inflammation. Surv. Ophthalmol. 2018, 63, 565–578. [Google Scholar] [CrossRef]
- Sun, L.; Chen, C.; Wu, J.; Dai, C.; Wu, X. TSLP-activated dendritic cells induce T helper type 2 inflammation in Aspergillus fumigatus keratitis. Exp. Eye Res. 2018, 171, 120–130. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Carlson, E.C.; Sun, Y.; Lin, M.; Burnett, S.H.; Perez, V.L.; McMenamin, P.G.; Pearlman, E. Bone marrow chimeras and c-fms conditional ablation (Mafia) mice reveal an essential role for resident myeloid cells in lipopolysaccharide/TLR4-induced corneal inflammation. J. Immunol. 2009, 182, 2738–2744. [Google Scholar] [CrossRef] [PubMed]
- Ramke, M.; Zhou, X.; Materne, E.C.; Rajaiya, J.; Chodosh, J. Resident corneal c-fms+ macrophages and dendritic cells mediate early cellular infiltration in adenovirus keratitis. Exp. Eye Res. 2016, 147, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A.; Willcox, M.D.P. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, K.; Shimizu, H.; Yoshida, A.; Nagaoka, E.; Nishio, O.; Okuda, K. Monitoring of adenovirus from conjunctival scrapings in Japan during 2005–2006. J. Med. Virol. 2008, 80, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhu, Z.; Wang, B.M.C.; Mei, H.; Li, H.; Ga, D.Z.G.; Jie, G.; Chi, M.M.B.; Zhang, S.; Ma, C.; et al. Outbreaks of epidemic keratoconjunctivitis caused by human adenovirus type 8 in the Tibet Autonomous Region of China in 2016. PLoS ONE 2017, 12, e0185048. [Google Scholar] [CrossRef]
- Migita, H.; Ueno, T.; Tsukahara-Kawamura, T.; Saeki, Y.; Hanaoka, N.; Fujimoto, T.; Uchio, E. Evaluation of adenovirus amplified detection of immunochromatographic test using tears including conjunctival exudate in patients with adenoviral keratoconjunctivitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 815–820. [Google Scholar] [CrossRef]
- Schwartz, H.S.; Vastine, D.W.; Yamashiroya, H.; West, C.E. Immunofluorescent detection of adenovirus antigen in epidemic keratoconjunctivitis. Investig. Ophthalmol. 1976, 15, 199–207. [Google Scholar]
- Kaye, S.B.; Lloyd, M.; Williams, H.; Yuen, C.; Scott, J.A.; O’Donnell, N.; Batterbury, M.; Hiscott, P.; Hart, C.A. Evidence for persistence of adenovirus in the tear film a decade following conjunctivitis. J. Med. Virol. 2005, 77, 227–231. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Yarber, F.A.; Mazurek, C.; Trousdale, M.D.; Medina-Kauwe, L.K.; Kasahara, N.; Hamm-Alvarez, S.F. Adenoviral capsid modulates secretory compartment organization and function in acinar epithelial cells from rabbit lacrimal gland. Gene Ther. 2004, 11, 970–981. [Google Scholar] [CrossRef]
- Lavappa, K.S. Survey of ATCC stocks of human cell lines for HeLa contamination. Vitro 1978, 14, 469–475. [Google Scholar] [CrossRef]
- Harvey, S.A.K.; Romanowski, E.G.; Yates, K.A.; Gordon, Y.J. Adenovirus-directed ocular innate immunity: The role of conjunctival defensin-like chemokines (IP-10, I-TAC) and phagocytic human defensin-α. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3657–3665. [Google Scholar] [CrossRef]
- Chintakuntlawar, A.V.; Chodosh, J. Cellular and tissue architecture of conjunctival membranes in epidemic keratoconjunctivitis. Ocul. Immunol. Inflamm. 2010, 18, 341–345. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M. Defensins and other antimicrobial peptides at the ocular surface. Ocul. Surf. 2004, 2, 229–247. [Google Scholar] [CrossRef]
- Yawata, N.; Selva, K.J.; Liu, Y.C.; Tan, K.P.; Lee, A.W.; Siak, J.; Lan, W.; Vania, M.; Arundhati, A.; Tong, L.; et al. Dynamic change in natural killer cell type in the human ocular mucosa in situ as means of immune evasion by adenovirus infection. Mucosal Immunol. 2016, 9, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Evolutionary struggles between NK cells and viruses. Nat. Rev. Immunol. 2008, 8, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Ablamowicz, A.F.; Nichols, J.J. Ocular surface membrane-associated mucins. Ocul. Surf. 2016, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Elimova, E.; Woodward, A.M.; Argüeso, P. Glycosylation pathways of human corneal and conjunctival epithelial cell mucins. Carbohydr. Res. 2018, 470, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Chandra, N.; Liu, Y.; Liu, J.X.; Frängsmyr, L.; Wu, N.; Silva, L.; Lindström, M.; Chai, W.; Pedrosa Domellöf, F.; Feizi, T.; et al. Sulfated glycosaminoglycans as viral decoy receptors for human adenovirus type 37. Viruses 2019, 11, 247. [Google Scholar] [CrossRef]
- Chandra, N.; Frängsmyr, L.; Arnberg, N. Decoy receptor interactions as novel drug targets against EKC-causing human adenovirus. Viruses 2019, 11, 242. [Google Scholar] [CrossRef]
- Hori, Y. Secreted mucins on the ocular surface. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES151–DES156. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGilligan, V.E.; Gregory-Ksander, M.S.; Li, D.; Moore, J.E.; Hodges, R.R.; Gilmore, M.S.; Moore, T.C.B.; Dartt, D.A. Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells. PLoS ONE 2013, 8, e74010. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hodges, R.R.; Bispo, P.; Gilmore, M.S.; Gregory-Ksander, M.; Dartt, D.A. Neither non-toxigenic Staphylococcus aureus nor commensal S. epidermidi activates NLRP3 inflammasomes in human conjunctival goblet cells. BMJ Open Ophthalmol. 2017, 2, e000101. [Google Scholar] [CrossRef] [PubMed]
- Holly, M.K.; Smith, J.G. Adenovirus infection of human enteroids reveals interferon sensitivity and preferential infection of goblet cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennington, M.R.; Saha, A.; Painter, D.F.; Gavazzi, C.; Ismail, A.M.; Zhou, X.; Chodosh, J.; Rajaiya, J. Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface. Microorganisms 2019, 7, 351. https://doi.org/10.3390/microorganisms7090351
Pennington MR, Saha A, Painter DF, Gavazzi C, Ismail AM, Zhou X, Chodosh J, Rajaiya J. Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface. Microorganisms. 2019; 7(9):351. https://doi.org/10.3390/microorganisms7090351
Chicago/Turabian StylePennington, Matthew R., Amrita Saha, David F. Painter, Christina Gavazzi, Ashrafali M. Ismail, Xiaohong Zhou, James Chodosh, and Jaya Rajaiya. 2019. "Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface" Microorganisms 7, no. 9: 351. https://doi.org/10.3390/microorganisms7090351




