Nuclear Gene Transformation in the Dinoflagellate Oxyrrhis marina
Abstract
1. Introduction
2. Methods
2.1. Culturing O. marina
2.2. Testing O. marina Resistance to Selection Markers
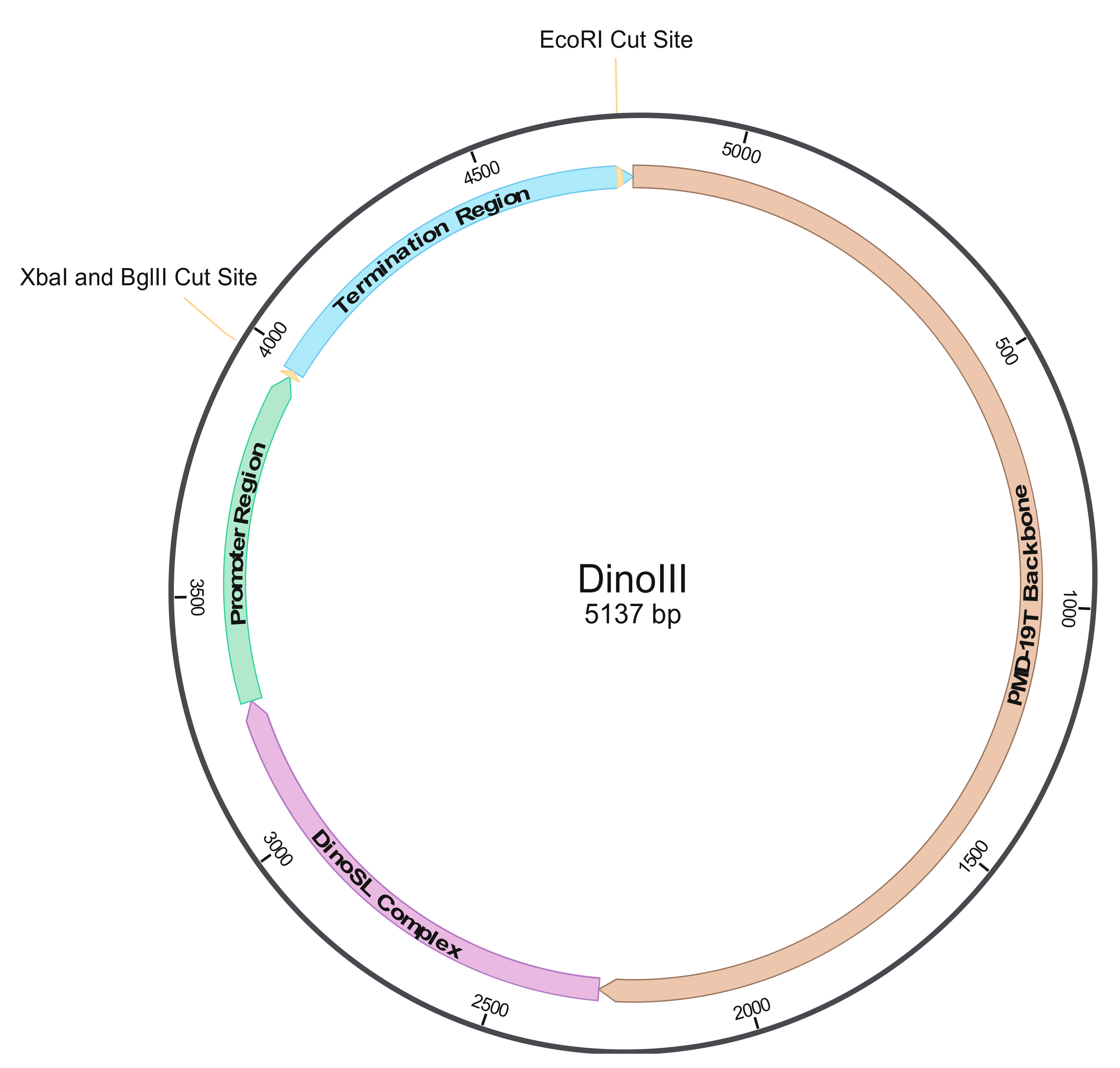
2.3. Constructing Dinoflagellate Expression Vectors
2.4. Optimizing Promoter Region
2.5. Introducing DNA into O. marina Using Lonza’s Nucleofector
2.6. Detecting the Transformed Gene and Its Expression
2.7. Transforming D. tertiolecta with DinoIII Using Lonza’s Nucleofector
3. Results
3.1. Construction of Dinoflagellate Backbone Expression Vector
3.2. Transformation using Lonza’s 4D-Nucleofector™ X Unit System
3.3. GFP Expression
3.4. Rifampin Resistance as a Selection Marker
3.5. Testing the Potential Interference of Dunaliella tertiolecta in O. marina Transformation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rill, R.L.; Livolant, F.; Aldrich, H.C.; Davidson, M.W. Electron microscopy of liquid crystalline DNA: Direct evidence for cholesteric-like organization of DNA in dinoflagellate chromosomes. Chromosoma 1989, 98, 280–286. [Google Scholar] [CrossRef]
- Hou, Y.; Lin, S. Distinct gene number-genome size relationships for eukaryotes and non-eukaryotes: Gene content estimation for dinoflagellate genomes. PLoS ONE 2009, 4, e6978. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.H.; Yan, K.T.H.; Bennett, M.J.; Wong, J.T.Y. Birefringence and DNA condensation of liquid crystalline chromosomes. Eukaryot. Cell 2010, 9, 1577–1587. [Google Scholar] [CrossRef]
- Gornik, S.G.; Ford, K.L.; Mulhern, T.D.; Bacic, A.; McFadden, G.I.; Waller, R.F. Loss of nucleosomal DNA condensation coincides with appearance of a novel nuclear protein in dinoflagellates. Curr. Biol. 2012, 22, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Irwin, N.A.T.; Martin, B.J.E.; Young, B.P.; Browne, M.J.G.; Flaus, A.; Loewen, C.J.R.; Keeling, P.J.; Howe, L.J. Viral proteins as a potential driver of histone depletion in dinoflagellates. Nat. Commun. 2018, 9, 1535. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.H.; Markovic, P.; Hastings, J.W.; Jovine, R.V.M.; Morse, D. Structure and organization of the peridinin-chlorophyll a-binding protein gene in Gonyaulax polyedra. Mol. Gen. Genet. 1997, 255, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Lin, S. Genomic understanding of dinoflagellates. Res. Microbiol. 2011, 162, 551–569. [Google Scholar] [CrossRef]
- McLean, T.I. ‘Eco-omics’: A review of the application of genomics, transcriptomics, and proteomics for the study of the ecology of harmful algae. Microb. Ecol. 2013, 65, 901–915. [Google Scholar] [CrossRef]
- Murray, S.A.; Suggett, D.J.; Doblin, M.A.; Kohli, G.S.; Seymour, J.R.; Fabris, M.; Ralph, P.J. Unravelling the functional genetics of dinoflagellates: A review of approaches and opportunities. Perspect. Phycol. 2016, 3, 37–52. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, S.; Song, B.; Zhong, X.; Lin, X.; Li, W.; Li, L.; Zhang, Y.; Zhang, H.; Ji, Z.; et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 2015, 350, 691–694. [Google Scholar] [CrossRef]
- Jackson, C.J.; Gornik, S.G.; Waller, R.F. The mitochondrial genome and transcriptome of the basal dinoflagellate Hematodinium sp.: Character evolution within the highly derived mitochondrial genomes of dinoflagellates. Genome Biol. Evol. 2012, 4, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, R.G.; Howe, C.J. Integration of plastids with their hosts: Lessons learned from dinoflagellates. Proc. Natl. Acad. Sci. USA 2015, 112, 10247–10254. [Google Scholar] [CrossRef] [PubMed]
- Janouškovec, J.; Gavelis, G.S.; Burki, F.; Dinh, D.; Bachvaroff, T.R.; Gornik, S.G.; Bright, K.J.; Imanian, B.; Strom, S.L.; Delwiche, C.F.; et al. Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics. Proc. Natl. Acad. Sci. USA 2017, 114, E171–E180. [Google Scholar] [CrossRef] [PubMed]
- Erdner, D.L.; Anderson, D.M. Global transcriptional profiling of the toxic dinoflagellate Alexandrium fundyense using massively parallel signature sequencing. BMC Genom. 2006, 7, 88. [Google Scholar] [CrossRef]
- Nosenko, T.; Lidie, K.L.; Van Dolah, F.M.; Lindquist, E.; Cheng, J.F.; Bhattacharya, D. Chimeric plastid proteome in the Florida ‘red tide’ dinoflagellate Karenia brevis. Mol. Biol. Evol. 2006, 23, 2026–2038. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, Y.; Miranda, L.; Campbell, D.A.; Sturm, N.R.; Gaasterland, T.; Lin, S. Spliced leader RNA trans-splicing in dinoflagellates. Proc. Natl. Acad. Sci. USA 2007, 104, 4618–4623. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Zhuang, Y.; Tran, B.; Gill, J. Spliced leader-based metatranscriptomic analyses lead to recognition of hidden genomic features in dinoflagellates. Proc. Natl. Acad. Sci. USA 2010, 107, 20033–20038. [Google Scholar] [CrossRef]
- Moustafa, A.; Evans, A.N.; Kulis, D.M.; Hackett, J.D.; Erdner, D.L.; Anderson, D.M.; Bhattacharya, D. Transcriptome profiling of a toxic dinoflagellate reveals a gene-rich protist and a potential impact on gene expression due to bacterial presence. PLoS ONE 2010, 5, e9688. [Google Scholar] [CrossRef]
- Toulza, E.; Shin, M.S.; Blanc, G.; Audic, S.; Laabir, M.; Collos, Y.; Claverie, J.M.; Grzebyk, D. Gene expression in proliferating cells of the dinoflagellate Alexandrium catenella (Dinophyceae). Appl. Environ. Microbiol. 2010, 76, 4521–4529. [Google Scholar] [CrossRef]
- Leggat, W.; Yellowlees, D.; Medina, M. Recent progress in Symbiodinium transcriptomics. J. Exp. Mar. Biol. Ecol. 2011, 408, 120–125. [Google Scholar] [CrossRef]
- Bayer, T.; Aranda, M.; Sunagawa, S.; Yum, L.K.; DeSalvo, M.K.; Lindquist, E.; Coffroth, M.A.; Voolstra, C.R.; Medina, M. Symbiodinium transcriptomes: Genome insights into the dinoflagellate symbionts of reef-building corals. PLoS ONE 2012, 7, e35269. [Google Scholar] [CrossRef]
- Johnson, J.G.; Morey, J.S.; Neely, M.G.; Ryan, J.C.; van Dolah, F.M. Transcriptome remodeling associated with chronological aging in the dinoflagellate, Karenia brevis. Mar. Genom. 2012, 5, 15–25. [Google Scholar] [CrossRef]
- Zhang, H.; Zhuang, Y.; Gill, J.; Lin, S. Proof that dinoflagellate spliced leader (DinoSL) is a useful hook for fishing dinoflagellate transcripts from mixed microbial samples: Symbiodinium kawagutii as a case study. Protist 2013, 164, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.D.; Bentlage, B.; Gibbons, T.R.; Bachvaroff, T.R.; Delwiche, C.F. Metatranscriptome profiling of a harmful algal bloom. Harmful Algae 2014, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gavelis, G.S.; White, R.A.; Suttle, C.A.; Keeling, P.J.; Leander, B.S. Single-cell transcriptomics using spliced leader PCR: Evidence for multiple losses of photosynthesis in polykrikoid dinoflagellates. BMC Genom. 2015, 16, 528. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, H.; Hannick, L.; Lin, S. Metatranscriptome profiling reveals versatile N-nutrient utilization, CO2 limitation, oxidative stress, and active toxin production in an Alexandrium fundyense bloom. Harmful Algae 2015, 42, 60–70. [Google Scholar] [CrossRef]
- Gong, W.; Browne, J.; Hall, N.; Schruth, D.; Paerl, H.; Marchetti, A. Molecular insights into a dinoflagellate bloom. ISME J. 2017, 11, 439–452. [Google Scholar] [CrossRef]
- Jaeckisch, N.; Yang, I.; Wohlrab, S.; Glöckner, G.; Kroymann, J.; Vogel, H.; Cembella, A.; John, U. Comparative genomic and transcriptomic characterization of the toxigenic marine dinoflagellate Alexandrium ostenfeldii. PLoS ONE 2011, 6, e28012. [Google Scholar] [CrossRef]
- Shoguchi, E.; Shinzato, C.; Kawashima, T.; Gyoja, F.; Mungpakdee, S.; Koyanagi, R.; Takeuchi, T.; Hisata, K.; Tanaka, M.; Fujiwara, M.; et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 2013, 23, 1399–1408. [Google Scholar] [CrossRef]
- Aranda, M.; Li, Y.; Liew, Y.J.; Baumgarten, S.; Simakov, O.; Wilson, M.C.; Piel, J.; Ashoor, H.; Bougouffa, S.; Bajic, V.B.; et al. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 2016, 6, 39734. [Google Scholar] [CrossRef]
- Liu, H.; Stephens, T.G.; González-Pech, R.A.; Beltran, V.H.; Lapeyre, B.; Bongaerts, P.; Cooke, I.; Aranda, M.; Bourne, D.G.; Forêt, S.; et al. Symbiodinium genomes reveal adaptive evolution of functions related to coral-dinoflagellate symbiosis. Commun. Biol. 2018, 1, 95. [Google Scholar] [CrossRef] [PubMed]
- Shoguchi, E.; Beedessee, G.; Tada, I.; Hisata, K.; Kawashima, T.; Takeuchi, T.; Arakaki, N.; Fujie, M.; Koyanagi, R.; Roy, M.C.; et al. Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes. BMC Genom. 2018, 19, 458. [Google Scholar] [CrossRef] [PubMed]
- Ten Lohuis, M.R.; Miller, D.J. Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): Expression of GUS in microalgae using heterologous promoter constructs. Plant J. 1998, 13, 427–435. [Google Scholar]
- Ortiz-Matamoros, M.F.; Villanueva, M.A.; Islas-Flores, T. Transient transformation of cultured photosynthetic dinoflagellates (Symbiodinium spp.) with plant-targeted vectors | Transformación de dinoflagelados fotosintéticos del género Symbiodinium en cultivo con vectores diseñados para plantas. Cienc. Mar. 2015, 41, 21–32. [Google Scholar] [CrossRef][Green Version]
- Ortiz-Matamoros, M.F.; Islas-Flores, T.; Voigt, B.; Menzel, D.; Baluška, F.; Villanueva, M.A. Heterologous DNA uptake in cultured Symbiodinium spp. aided by Agrobacterium tumefaciens. PLoS ONE 2015, 10, e0132693. [Google Scholar] [CrossRef]
- Diao, J.; Song, X.; Zhang, X.; Chen, L.; Zhang, W. Genetic engineering of Crypthecodinium cohnii to increase growth and lipid accumulation. Front. Microbiol. 2018, 9, 492. [Google Scholar] [CrossRef]
- Nimmo, I.; McEwan, M.L.; Fast, N.M.; Taylor, F.J.R.; Keeling, P.J. Genetic transformation of the dinoflagellate chloroplast. eLife 2019, 8, e45292. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; McEwan, M.L.; Fast, N.M.; Taylor, F.J.R.; Keeling, P.J. Multiple protein phylogenies show that Oxyrrhis marina and Perkinsus marinus are early branches of the dinoflagellate lineage. Int. J. Syst. Evol. Microbiol. 2003, 53, 355–365. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S. mRNA editing and spliced-leader RNA trans-splicing groups Oxyrrhis, Noctiluca, Heterocapsa, and Amphidinium as basal lineages of dinoflagellates. J. Phycol. 2008, 44, 703–711. [Google Scholar] [CrossRef]
- Montagnes, D.J.S.; Lowe, C.D.; Martin, L.; Watts, P.C.; Downes-Tettmar, N.; Yang, Z.; Roberts, E.C.; Davidson, K. An introduction to the special issue: Oxyrrhis marina, a model organism? J. Plankton Res. 2011, 33, 549–554. [Google Scholar] [CrossRef]
- Montagnes, D.J.S.; Zhang, H.; Liu, S.; Lin, S. Oxyrrhis marina growth, sex and reproduction. J. Plankton Res. 2011, 33, 615–627. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, H.; Liu, S.; Lin, S. Biology of the marine heterotrophic dinoflagellate Oxyrrhis marina: Current status and future directions. Microorganisms 2013, 1, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Bachvaroff, T.R.; Keeling, P.J. Dinoflagellate phylogeny revisited: Using ribosomal proteins to resolve deep branching dinoflagellate clades. Mol. Phyl. Evol. 2014, 70, 314–322. [Google Scholar] [CrossRef]
- Riaz, S.; Kang, H.; Shim, J.H.; Park, J.K.; Kim, J.S.; Song, J.Y.; Choi, H.J. Distinctive Nuclear Features of Dinoflagellates with a Particular Focus on Histone and Histone-Replacement Proteins. Microorganisms 2018, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Slamovits, C.H.; Keeling, P.J. Contributions of Oxyrrhis marina to molecular biology, genomics and organelle evolution of dinoflagellates. J. Plankton Res. 2011, 33, 591–602. [Google Scholar] [CrossRef]
- Slamovits, C.H.; Saldarriaga, J.F.; Larocque, A.; Keeling, P.J. The highly reduced and fragmented mitochondrial genome of the early-branching dinoflagellate Oxyrrhis marina shares characteristics with both apicomplexan and dinoflagellate mitochondrial genomes. J. Mol. Biol. 2007, 372, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Kang, H.; Shim, J.H.; Park, J.K.; Kim, J.S.; Song, J.Y.; Choi, H.J. Interactions among the toxic dinoflagellate Amphidinium carterae, the heterotrophic dinoflagellate Oxyrrhis marina, and the calanoid copepods Acartia spp. Mar. Ecol. Prog. Ser. 2001, 218, 77–86. [Google Scholar] [CrossRef]
- Yang, Z.; Jeong, H.J.; Montagnes, D.J.S. The role of Oxyrrhis marina as a model prey: Current work and future directions. J. Plankton Res. 2011, 33, 665–675. [Google Scholar] [CrossRef]
- Roberts, E.C.; Wootton, E.C.; Davidson, K.; Jeong, H.J.; Lowe, C.D.; Montagnes, D.J.S. Feeding in the dinoflagellate Oxyrrhis marina: Linking behaviour with mechanisms. J. Plankton Res. 2011, 33, 603–614. [Google Scholar] [CrossRef]
- Kleppel, G.S.; Burkart, C.A.; Houchin, L. Nutrition and the regulation of egg production in the calanoid copepod Acartia tonsa. Limnol. Oceanogr. 1998, 43, 1000–1007. [Google Scholar] [CrossRef]
- Lund, E.D.; Chu, F.L.E.; Harvey, E.; Adlof, R. Mechanism(s) of long chain n-3 essential fatty acid production in two species of heterotrophic protists: Oxyrrhis marina and Gyrodinium dominans. Mar. Biol. 2008, 155, 23–26. [Google Scholar] [CrossRef]
- Lowe, C.D.; Mello, L.V.; Samatar, N.; Martin, L.E.; Montagnes, D.J.S.; Watts, P.C. The transcriptome of the novel dinoflagellate Oxyrrhis marina (Alveolata: Dinophyceae): Response to salinity examined by 454 sequencing. BMC Genom. 2011, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Slamovits, C.H.; Okamoto, N.; Burri, L.; James, E.R.; Keeling, P.J. A bacterial proteorhodopsin proton pump in marine eukaryotes. Nat. Commun. 2011, 2, 183. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, H.; Lin, S. Light-promoted rhodopsin expression and starvation survival in the marine dinoflagellate Oxyrrhis marina. PLoS ONE 2014, 9, e114941. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Lai, H.; Malik, S.B.; Saldarriaga, J.F.; Keeling, P.J.; Slamovits, C.H. Analysis of EST data of the marine protist Oxyrrhis marina, an emerging model for alveolate biology and evolution. BMC Genom. 2014, 15, 122. [Google Scholar] [CrossRef]
- Beja, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L.; et al. Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea. Science 2000, 289, 1902–1906. [Google Scholar] [CrossRef]
- Martinet, W.; Schrijvers, D.M.; Kockx, M.M. Nucleofection as an efficient nonviral transfection method for human monocytic cells. Biotechnol. Lett. 2003, 25, 1025–1029. [Google Scholar] [CrossRef]
- Zhang, H.; Campbell, D.A.; Sturm, N.R.; Lin, S. Dinoflagellate spliced leader RNA genes display a variety of sequences and genomic arrangements. Mol. Biol. Evol. 2009, 26, 1757–1771. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Dubois, A. Low abundance distribution of Pfiesteria piscicida in Pacific and Western Atlantic as detected by mtDNA-18S rDNA real-time polymerase chain reaction. J. Plankton Res. 2006, 28, 667–681. [Google Scholar] [CrossRef]
- Zhang, H.; Finiguerra, M.; Dama, H.G.; Huang, Y.; Xu, D.; Liu, G.; Lin, S. An improved method for achieving high-quality RNA for copepod transcriptomic studies. J. Exp. Mar. Biol. Ecol. 2013, 446, 57–66. [Google Scholar] [CrossRef]
- Baysarowich, J.; Koteva, K.; Hughes, D.W.; Ejim, L.; Griffiths, E.; Zhang, K.; Junop, M.; Wright, G. Rifamycin antibiotic resistance by ADP-ribosylation: Structure and diversity of Arr. Proc. Natl. Acad. Sci. USA 2008, 105, 4886–4891. [Google Scholar] [CrossRef]
- Strath, M.; Scott-Finnigan, T.; Gardner, M.; Williamson, D.; Wilson, I. Antimalarial activity of rifampicin in vitro and in rodent models. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 211–216. [Google Scholar] [CrossRef]
- Goodman, C.D.; Su, V.; McFadden, G.I. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 2007, 152, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Slamovits, C.H.; Keeling, P.J. Plastid-Derived Genes in the Nonphotosynthetic Alveolate Oxyrrhis marina. Mol. Biol. Evol. 2008, 25, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Covey, S.N.; Dale, P. Genetically modified plants and the 35S promoter: Assessing the risks and enhancing the debate. Microb. Ecol. Health Dis. 2000, 12, 1–5. [Google Scholar] [CrossRef][Green Version]
- Kahn, R.W.; Andersen, B.H.; Brunk, C.F. Transformation of Tetrahymena thermophila by microinjection of a foreign gene. Proc. Natl. Acad. Sci. USA 1993, 90, 9295–9299. [Google Scholar] [CrossRef]
- Crabb, B.S.; Cowman, A.F. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1996, 93, 7289–7294. [Google Scholar] [CrossRef]
- Hackett, J.D.; Anderson, D.M.; Erdner, D.L.; Bhattacharya, D. Dinoflagellates: A remarkable evolutionary experiment. Am. J. Bot. 2004, 91, 1523–1534. [Google Scholar] [CrossRef]
- Liu, L.; Hastings, J.W. Novel and rapidly diverging intergenic sequences between tandem repeats of the luciferase genes in seven dinoflagellate species. J. Phycol. 2006, 42, 96–103. [Google Scholar] [CrossRef]
- Guillebault, D.; Sasorith, S.; Derelle, E.; Wurtz, J.M.; Lozano, J.C.; Bingham, S.; Tora, L.; Moreau, H. A new class of transcription initiation factors, intermediate between TATA box-binding proteins (TBPs) and TBP-like factors (TLFs), is present in the marine unicellular organism, the dinoflagellate Crypthecodinium cohnii. J. Biol. Chem. 2002, 277, 40881–40886. [Google Scholar] [CrossRef]
- Potter, H.; Heller, R. Transfection by electroporation. Curr. Protoc. Mol. Biol. 2018, 121, 9.3.1–9.3.13. [Google Scholar] [CrossRef]
- Booth, D.S.; Szmidt-Middleton, H.; King, N. Transfection of choanoflagellates illuminates their cell biology and the ancestry of animal septins. Mol. Biol. Cell 2018, 29, 2969–3062. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Valach, M.; Peña-Diaz, P.; Moreira, S.; Keeling, P.J.; Burger, G.; Lukeš, J.; Faktorová, D. Transformation of Diplonema papillatum, the type species of the highly diverse and abundant marine microeukaryotes Diplonemida (Euglenozoa). Environ. Microbiol. 2018, 20, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.R.; Köhler, R.H. GFP imaging: Methodology and application to investigate cellular compartmentation in plants. J. Exp. Bot. 2001, 52, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Triemer, R.E. A unique mitotic variation in the marine dinoflagellate Oxyrrhis marina (pyrrophyta). J. Phycol. 1982, 18, 399–411. [Google Scholar] [CrossRef]
- Gao, X.; Li, J. Nuclear division in the marine dinoflagellate Oxyrrhis marina. J. Cell Sci. 1986, 85, 161–175. [Google Scholar]
- Sano, J.; Kato, K.H. Localization and copy number of the protein-codinggenes actin, α-tubulin, and HSP90 in the nucleus of a primitive dinoflagellate, Oxyrrhis marina. Zool. Sci. 2009, 26, 745–753. [Google Scholar]




| Amphotericin B | Ampicillin | Basta | Blasticidin | Chloramphenicol | Formaldehyde | Geneticin (G418) | Hygromycin B | Kanamycin | Paromomycin | Rifampin | Streptomycin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxyrrhis marina | n/a (1000) | n/a (1000) | n/a (500) | n/a (500) | 50 | 40 | n/a (1000) | n/a (2000) | n/a (2000) | n/a (1500) | 225 | n/a (1000) |
| Primer Name | Sequence Information | Tm | Polymerase Used |
|---|---|---|---|
| SymkaLHC5FN1 | GAGAACTAGTAAGTCCCGTGGCTGTCATATCTAG | 68 °C | Takara PrimeSTAR HS DNA Polymerase |
| SymLHC3_5R | GACTCCTGGCCGAGATCTTCTAGAGGCTCCGAAATTTGGTCTAAGCAC | 68 °C | Takara PrimeSTAR HS DNA Polymerase |
| SymLHC5_3F | CCAAATTTCGGAGCCTCTAGAAGATCTCGGCCAGGAGTCACAGAAAACAAG | 68 °C | Takara PrimeSTAR HS DNA Polymerase |
| SymkaLHC3R1 | TCTCTCGAATTCCGTGTGCTTGTGAAACTTTTATC | 68 °C | Takara PrimeSTAR HS DNA Polymerase |
| DinoSL | NCCGTAGCCATTTTGGCTCAAG | 58 °C | Takara PrimeSTAR HS DNA Polymerase |
| KbrSRP-U6R1 | CAGAGATCAAGACATGCTTCAGGAC | 58 °C | Takara PrimeSTAR HS DNA Polymerase |
| gfpNF2 | AACTAGTATGGCTAGCAAAGGAGAAGAACTTTTC | 5 cycles at 55 °C and 25 at 62 °C | Takara PrimeSTAR HS DNA Polymerase |
| gfpNR | TATGATCATCATTTGTAGAGCTCATCCATGCCA | 5 cycles at 55 °C and 25 at 62 °C | Takara PrimeSTAR HS DNA Polymerase |
| arr2F | GAGAACTAGTATGGTGAAGGA | 57 °C | Takara PrimeSTAR HS DNA Polymerase |
| arr2R | TCTCTGATCACTAATCCTCG | 57 °C | Takara PrimeSTAR HS DNA Polymerase |
| OxyRhodF2 | CACTACTTCMGNATCTTCAACTC | 60 °C | Takara PrimeSTAR HS DNA Polymerase |
| OxyrhodR | CAGAGGMACRGTCARCARCCARTC | 60 °C | Takara PrimeSTAR HS DNA Polymerase |
| Rhod_interspacerF | GAGAACTAGTAATTTTGGGAGTTGGGCT | 57 °C | Takara PrimeSTAR HS DNA Polymerase |
| Illu-DSL | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGTCCGTAGCCATTTTGGCTCAAG | 68 °C | Takara PrimeSTAR HS DNA Polymerase |
| SymkaLHC3R1 | TCTCTCGAATTCCGTGTGCTTGTGAAACTTTTATC | 68 °C | Takara PrimeSTAR HS DNA Polymerase |
| arr2Q1F | TACCACGGAACCAAGGCGAACT | 60 °C | SsoAdvanced Universal SYBR Green Supermix |
| arr2Q1R | CCAAGCCAGACAGCGACATAGC | 60 °C | SsoAdvanced Universal SYBR Green Supermix |
| arr2Q1Fa | GAGATACCACGGAACCAAGGCGAACT | 60 °C | SsoAdvanced Universal SYBR Green Supermix |
| arr2Q1Ra | GAGACCAAGCCAGACAGCGACATAGC | 60 °C | SsoAdvanced Universal SYBR Green Supermix |
| MdT | TCAACGATACGCTACGTAACGTAATACGACTCACTATAGGGTTTTTTTTTTTTTTTTVN | 42 °C | Reverse Transcriptase |
| Average Cell Counts 24 h after Electroporation (cells/well) | Success with gfp * | Number of Trials with arrO | Number of Trials with arrO with Long-Term Survival in Rifampin ** | Number of Trials with arrO-N | Number of Trials with arrO-N with Long-Term Survival in Rifampin ** | |
|---|---|---|---|---|---|---|
| DS-137 | 1150 | Yes | 8 | 2 | 2 | 0 |
| DS-134 | 2060 | No | 5 | 1 | 2 | 1 |
| ED-150 | 3830 | Less Bright | 2 | 0 | 2 | 0 |
| DS-138 | 4190 | No | 6 | 1 | 2 | 2 |
| DS-130 | 7610 | Yes | 4 | 0 | 2 | 0 |
| DS-150 | 7820 | No | 6 | 1 | 2 | 0 |
| DS-120 | 16,980 | Less Bright | 8 | 1 | 2 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprecher, B.N.; Zhang, H.; Lin, S. Nuclear Gene Transformation in the Dinoflagellate Oxyrrhis marina. Microorganisms 2020, 8, 126. https://doi.org/10.3390/microorganisms8010126
Sprecher BN, Zhang H, Lin S. Nuclear Gene Transformation in the Dinoflagellate Oxyrrhis marina. Microorganisms. 2020; 8(1):126. https://doi.org/10.3390/microorganisms8010126
Chicago/Turabian StyleSprecher, Brittany N., Huan Zhang, and Senjie Lin. 2020. "Nuclear Gene Transformation in the Dinoflagellate Oxyrrhis marina" Microorganisms 8, no. 1: 126. https://doi.org/10.3390/microorganisms8010126
APA StyleSprecher, B. N., Zhang, H., & Lin, S. (2020). Nuclear Gene Transformation in the Dinoflagellate Oxyrrhis marina. Microorganisms, 8(1), 126. https://doi.org/10.3390/microorganisms8010126






