Biochemical and Structural Characterization of OXA-405, an OXA-48 Variant with Extended-Spectrum β-Lactamase Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. PCR, Cloning, Expression, and DNA Sequencing
2.3. Protein Purification
2.4. Steady State Kinetic Determinations
2.5. Protein Crystallization and X-Ray Crystallography
2.6. Structure Analysis, Docking, Molecular Dynamics, and Water Network Analysis
2.7. PDB Deposition
3. Results
3.1. Biochemical Properties of OXA-405
3.2. X-Ray Crystallography
3.3. Structural Analysis of Covalent Protein-Ligand Complexes
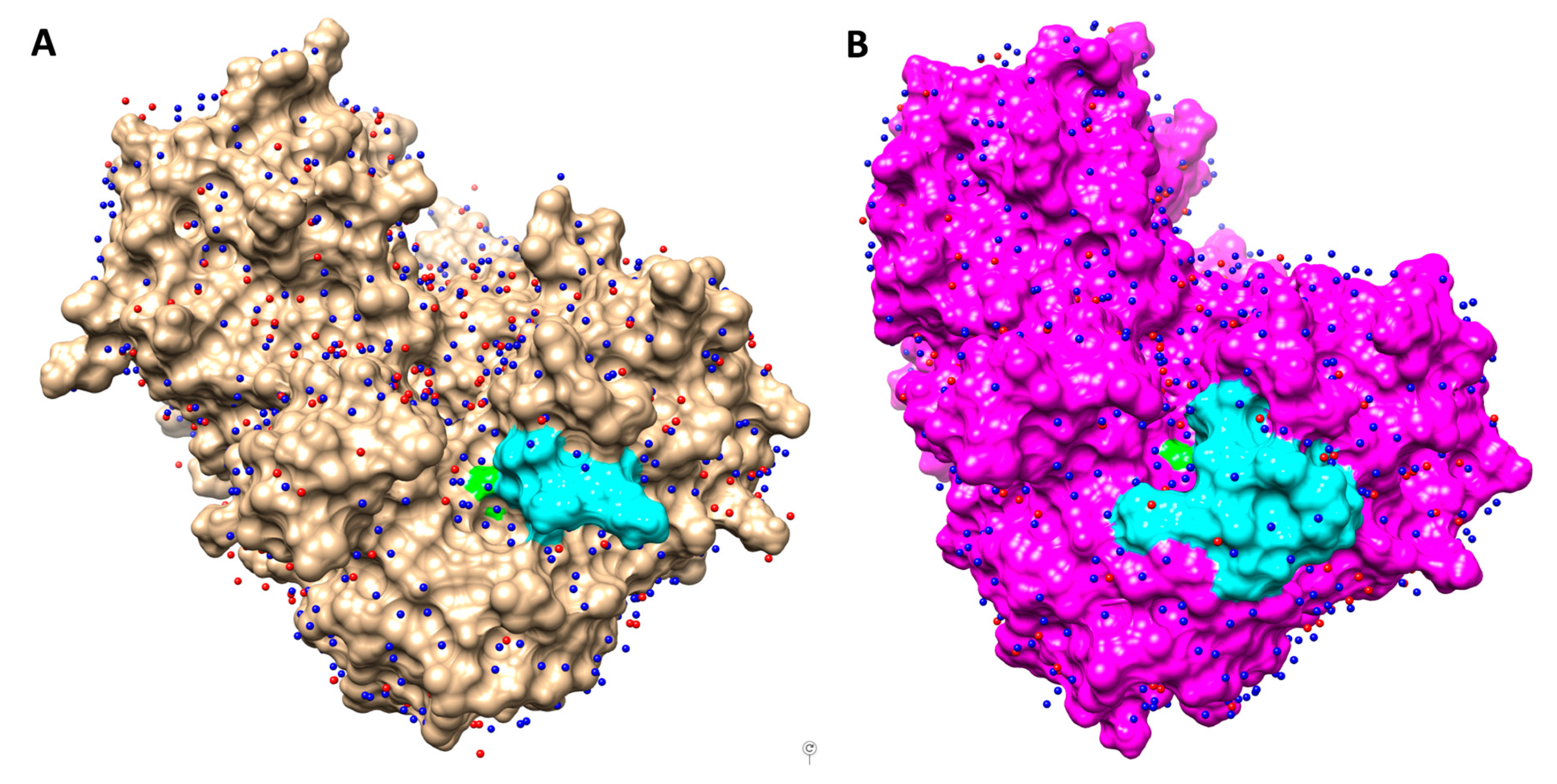
3.4. Molecular Dynamics and Water Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Queeenan, A.M.; Bush, K. Carbapenemases: The Versatile β-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Naas, T.; Nordmann, P. Diversity, epidemiology and genetics of class D beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Poirel, L.; Héritier, C.; Tolün, V.; Nordmann, P. Emergence of Oxacillinase-Mediated Resistance to Imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dabos, L.; Bogaerts, P.; Bonnin, R.A.; Zavala, A.; Sacré, P.; Iorga, B.I.; Huang, D.T.; Glupczynski, Y.; Naas, T. Genetic and Biochemical Characterization of OXA-519, a Novel OXA-48-Like β-Lactamase. Antimicrob. Agents Chemother. 2018, 62, e00469-18. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Rondinaud, E.; Nordmann, P. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Eurosurveillance 2013, 18, 20549. [Google Scholar] [CrossRef]
- Poirel, L.; Potron, A.; Nordmann, P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012, 67, 1597–1606. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)—structure and function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Oueslati, S.; Nordmann, P.; Poirel, L. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J. Antimicrob. Chemother. 2015, 70, 1059–1063. [Google Scholar] [CrossRef]
- Poirel, L.; Castanheira, M.; Carrër, A.; Rodriguez, C.P.; Jones, R.N.; Smayevsky, J.; Nordmann, P. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 2011, 55, 2546–2551. [Google Scholar] [CrossRef]
- Abdelaziz, M.O.; Bonura, C.; Aleo, A.; El-Domany, R.A.; Fasciana, T.; Mammina, C. OXA-163-producing Klebsiella pneumoniae in Cairo, Egypt, in 2009 and 2010. J. Clin. Microbiol. 2012, 50, 2489–2491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomez, S.; Pasteran, F.; Faccone, D.; Bettiol, M.; Veliz, O.; De Belder, D.; Rapoport, M.; Gatti, B.; Petroni, A.; Corso, A. Intrapatient emergence of OXA-247: A novel carbapenemase found in a patient previously infected with OXA-163-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2013, 19, E233–E235. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Oueslati, S.; Jeannot, K.; Tandé, D.; Naas, T.; Nordmann, P. Genetic and Biochemical Characterization of OXA-405, an OXA-48-Type Extended-Spectrum β-Lactamase without Significant Carbapenemase Activity. Antimicrob. Agents Chemother. 2015, 59, 3823–3828. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Vagin, A.; Teplyakov, A. MOLREP: An Automated Program for Molecular Replacement. J. Appl. Crystallogr. 1997, 30, 1022–1025. [Google Scholar] [CrossRef]
- Docquier, J.D.; Calderone, V.; De Luca, F.; Benvenuti, M.; Giuliani, F.; Bellucci, L.; Tafi, A.; Nordmann, P.; Botta, M.; Rossolini, G.M.; et al. Crystal structure of the OXA-48 beta-lactamase reveals mechanistic diversity among class D carbapenemases. Chem. Biol. 2009, 16, 540–547. [Google Scholar] [CrossRef]
- Blanc, E.; Roversi, P.; Vonrhein, C.; Flensburg, C.; Lea, S.M.; Bricogne, G. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2210–2221. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein–ligand docking using GOLD. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Beckstein, O.; Michaud-Agrawal, N.; Woolf, T.B. Quantitative Analysis of Water Dynamics in and near Proteins. Biophys. J. 2009, 96, 601a. [Google Scholar] [CrossRef][Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Stojanoski, V.; Chow, D.C.; Fryszczyn, B.; Hu, L.; Nordmann, P.; Poirel, L.; Sankaran, B.; Prasad, B.V.V.; Palzkill, T. Structural Basis for Different Substrate Profiles of Two Closely Related Class D β-Lactamases and Their Inhibition by Halogens. Biochemistry 2015, 54, 3370–3380. [Google Scholar] [CrossRef]
- Wall, M.E.; Calabró, G.; Bayly, C.I.; Mobley, D.L.; Warren, G.L. Biomolecular Solvation Structure Revealed by Molecular Dynamics Simulations. J. Am. Chem. Soc. 2019, 141, 4711–4720. [Google Scholar] [CrossRef]
- Lund, B.A.; Thomassen, A.M.; Carlsen, T.J.O.; Leiros, H.K.S. Structure, activity and thermostability investigations of OXA-163, OXA-181 and OXA-245 using biochemical analysis, crystal structures and differential scanning calorimetry analysis. Acta Crystallogr. F Struct. Biol. Commun. 2017, 73, 579–587. [Google Scholar] [CrossRef]



| Km (μM) a | kcat (s−1) | kcat/Km (mM−1 s−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Substrate | OXA-405 | OXA-163 | OXA-48 | OXA-405 | OXA-163 | OXA-48 | OXA-405 | OXA-163 | OXA-48 |
| Benzylpenicillin | 18 | 13 | ND b | 12 | 23 | ND | 667 | 1800 | ND |
| Ampicillin | 212 | 315 | 395 | 29 | 23 | 955 | 137 | 70 | 2400 |
| Oxacillin | 69 | 90 | 95 | 19 | 34 | 130 | 275 | 370 | 1400 |
| Temocillin | NH c | NH | 45 | NH | NH | 0.3 | ND | ND | 6.6 |
| Cephalothin | 18 | 10 | 195 | 8 | 3 | 44 | 444 | 300 | 225 |
| Cefotaxime | 369 | 45 | >900 | 9.7 | 10 | >9 | 26 | 230 | 10 |
| Ceftazidime | >1000 | >1000 | NH | 0.7 | 8 | NH | 0.7 | 3 | NH |
| Imipenem | 532 | 520 | 13 | 0.1 | 0.03 | 4.8 | 0.2 | 0.06 | 370 |
| Meropenem | >2000 | >2000 | 11 | 0.1 | >0.1 | 0.07 | 0.09 | 0.03 | 6.2 |
| Ertapenem | 88 | 130 | 100 | 0.04 | 0.05 | 0.13 | 0.4 | 0.3 | 1.3 |
| Inhibitor | IC50 (μM) | ||
|---|---|---|---|
| OXA-405 | OXA-163 | OXA-48 | |
| Clavulanic acid | 6 | 13.4 | 28.5 |
| Tazobactam | 1.8 | 0.75 | 20 |
| NaCl | 40 × 103 | 97 × 103 | 35 × 103 |
| OXA-405 | |
|---|---|
| Protein Data Bank code | 5FDH |
| wavelength (Å) | 1.54187 |
| Space group | P43212 |
| Asymmetric unit | 1 dimer |
| Unit cell (Å) | |
| A | 90.40 |
| B | 90.40 |
| C | 172.63 |
| α (deg) | 90.0 |
| β (deg) | 90.0 |
| γ (deg) | 90.0 |
| Resolution (Å) | 13.12–2.26 |
| Observed reflections | 35,642 (4116) a |
| Unique reflections | 10,205 (1346) |
| Completeness (%) | 98.0 (90.3) |
| I/σ (I) | 18.9 (4.6) |
| Rsym (%) | 9.7 (46.8) |
| Rcryst (%) | 17.5 |
| Rfree (%) | 21.3 |
| no. of nonhydrogen atoms | 4482 |
| Protein | 3952 |
| heterogen | 530 |
| Waters | 434 |
| no. of protein residues | 484 |
| no. of ligands | 8 SO4, 3 GOL |
| Root mean square deviation | |
| Bond lengths (Å) | 0.010 |
| Bond angles (deg) | 1.10 |
| Ramachandran | |
| favored (%) | 96 |
| outliers (%) | 0 |
| Mean B value (Å2) | |
| Protein | 38.2 (chain A), 39.1 (chain B) |
| Solvent | 46.1 (SO4) 55.2 (GOL), 45.9 (HOH) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oueslati, S.; Retailleau, P.; Marchini, L.; Dortet, L.; Bonnin, R.A.; Iorga, B.I.; Naas, T. Biochemical and Structural Characterization of OXA-405, an OXA-48 Variant with Extended-Spectrum β-Lactamase Activity. Microorganisms 2020, 8, 24. https://doi.org/10.3390/microorganisms8010024
Oueslati S, Retailleau P, Marchini L, Dortet L, Bonnin RA, Iorga BI, Naas T. Biochemical and Structural Characterization of OXA-405, an OXA-48 Variant with Extended-Spectrum β-Lactamase Activity. Microorganisms. 2020; 8(1):24. https://doi.org/10.3390/microorganisms8010024
Chicago/Turabian StyleOueslati, Saoussen, Pascal Retailleau, Ludovic Marchini, Laurent Dortet, Rémy A. Bonnin, Bogdan I. Iorga, and Thierry Naas. 2020. "Biochemical and Structural Characterization of OXA-405, an OXA-48 Variant with Extended-Spectrum β-Lactamase Activity" Microorganisms 8, no. 1: 24. https://doi.org/10.3390/microorganisms8010024
APA StyleOueslati, S., Retailleau, P., Marchini, L., Dortet, L., Bonnin, R. A., Iorga, B. I., & Naas, T. (2020). Biochemical and Structural Characterization of OXA-405, an OXA-48 Variant with Extended-Spectrum β-Lactamase Activity. Microorganisms, 8(1), 24. https://doi.org/10.3390/microorganisms8010024








