Abstract
Geobacillus sp. JF8 is a thermophilic biphenyl and naphthalene degrader. To identify the naphthalene degradation genes, cis-naphthalene dihydrodiol dehydrogenase was purified from naphthalene-grown cells, and its N-terminal amino acid sequence was determined. Using a DNA probe encoding the N-terminal region of the dehydrogenase, a 10-kb DNA fragment was isolated. Upstream of nahB, a gene for dehydrogenase, there were two open reading frames which were designated as nahAc and nahAd, respectively. The products of nahAc and nahAd were predicted to be alpha and beta subunit of ring-hydroxylating dioxygenases, respectively. Phylogenetic analysis of amino acid sequences of NahB indicated that it did not belong to the cis-dihydrodiol dehydrogenase group that includes those of classical naphthalene degradation pathways. Downstream of nahB, four open reading frames were found, and their products were predicted as meta-cleavage product hydrolase, monooxygenase, dehydrogenase, and gentisate 1,2-dioxygenase, respectively. A reverse transcriptase-PCR analysis showed that transcription of nahAcAd was induced by naphthalene. These findings indicate that we successfully identified genes involved in the upper pathway of naphthalene degradation from a thermophilic bacterium.
1. Introduction
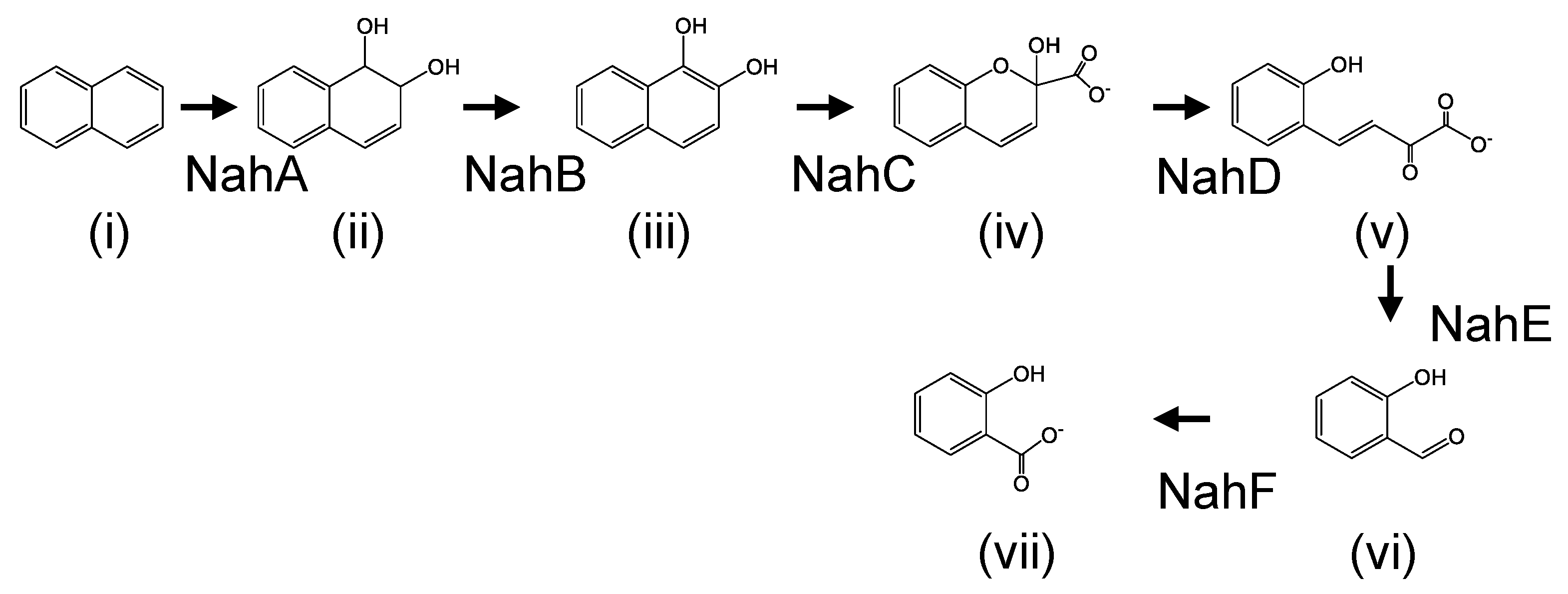
Naphthalene degradation pathways and their enzymes have been extensively studied in mesophilic bacteria including Pseudomonas species. Their degradation genes are organized in three operons: one of them encodes the enzymes involved in conversion of naphthalene to salicylate (naphthalene-degradation upper pathway); the second operon encodes the enzymes for conversion of salicylate to tricarboxylic acid cycle intermediates (pyruvate and acetyl-CoA) through the meta-cleavage pathway (naphthalene-degradation lower pathway); the third operon encodes a positive transcriptional regulator (NahR) [1,2,3,4,5,6,7]. The initial step of naphthalene degradation is the addition of dioxygen to naphthalene by naphthalene 1,2-dioxygenase (NahA enzyme), and it is converted to cis-1,2-dihydroxy-1,2-dihydronaphthalene (DDNP) (Figure 1). Secondly, DDNP is dehydrated and converted to 1,2-dihydroxynaphthalene (DHNP) by 1,2-dihydroxy-1,2-dihydronaphthalene dehydrogenase (NahB enzyme) (Figure 1). Thirdly, another dioxygen is added to DHNP by 1,2-dihydroxynaphthalene dioxygenase (NahC enzyme) and the DHNP is cleaved at the meta position on the benzene ring (Figure 1). The meta-cleavage product was converted to salicylate with some steps (Figure 1). The salicylate could be degraded via catechol or gentisate [1,8,9].

Figure 1.
Naphthalene degradation pathway in mesophilic degraders. (i) naphthalene, (ii) cis-1,2-Dihydroxy-1,2-dihydronaphthalene, (iii)1,2-dihydroxynaphthalene, (iv) 2-hydroxychromene-2-carboxylate, (v) trans-o-hydroxybenzylidenepyruvate, (vi) salicylaldehyde, (vii) salicylate. NahA, naphthalene 1,2-dioxygenase; NahB, 1,2-dihydroxy-1,2-dihydronaphthalene dehydrogenase; NahC, 1,2-dihydroxynaphthalene dioxygenase; NahD, 2-hydroxychromene-2-carboxylate isomerase; NahE, trans-o-hydroxybenzylidenepyruvate hydratase-aldolase; NahF, salicylaldehyde dehydrogenase.
In contrast, thermophilic naphthalene-degrading bacteria were rarely reported [10,11,12,13]. Geobacillus thermoleovorans Hamburg 2 degrades naphthalene through a different pathway from that known in mesophilic naphthalene degraders [11]. Previously, we isolated a thermophilic naphthalene-degrader, Geobacillus sp. JF8 [10] and successfully identified its 1,2-dihydroxynaphthalne dioxygenase (NahC) from the cell of JF8 grown with naphthalene [14]. The amino acid sequences of NahC exhibited only 20–22% identity to 1,2-dihydroxynaphthalene dioxygenase of those of mesophiles (NahC of Pseudomonas putida G7, NahC of Pseudomonas stutzeri AN10, or NagC of Ralstonia sp. U2). Currently, no genes were found around the above nahC_JF8 showing similarity to the genes for the naphthalene-degradation upper pathway.
In the present study, we successfully purified a dihydrodiol dehydrogenase of JF8 and identified genes for the naphthalene-degradation upper pathway.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Geobacillus sp. JF8 was grown at 60 °C on Castenholtz D solid medium (1.5% agar). Naphthalene was provided as vapor. The composition of Castenholtz D medium has been described previously [8]. pahAc_OUS82 and pahAd_OUS82 (encoding terminal dioxygenase of naphthalene dioxygenase from Pseudomonas putida OUS82), a kind gift from Prof. H. Kiyohara of Okayama University of Science, were on a 2.6-kb XhoI fragment in pHSG396 [15]. Escherichia coli JM109, used for construction and maintenance of plasmids, was cultured at 37 °C on LB medium [16]. Ampicillin (100 μg/mL), chloramphenicol (30 μg/mL), isopropyl -β-d-galactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used for selection of plasmids.
2.2. Southern Blot Analyses
A DNA probe spanning the determined N-terminal region of the dihydrodiol dehydrogenase was prepared by PCR with the degenerate primers, DDD-F; 5′-GARAAYAARGTiGCiTTYAT-3′ and DDD-R; 5′-ATiGCYTGYTTiACYTGYTC-3′ (R: A or G; Y: C or T, i: inosine). The probe was labeled with digoxygenin by DIG DNA Labeling and Detection Kit (Roche Diagnostics GmbH, Mannhiem, Germany). Total DNA of Geobacillus sp. JF8 was digested with BamHI, EcoRI, HindIII, SmaI, and XbaI, respectively, and the resultant DNAs were subjected to electrophoresis in 0.7% agarose gel before transfer to Hybond-N+ nylon membrane (GE Healthcare Life Sciences, Buckinghamshire HP7 9NA, England). For colony hybridization, the recombinant E. coli colonies were transferred to Hybond N+ nylon membranes. Southern hybridization was performed under high stringency as previously described [14].
2.3. Cloning and DNA Sequencing
Two DNA libraries were constructed with 9-kb SmaI or 5-kb EcoRI fragments of the chromosomal DNA of Geobacillus sp. JF8 in Charomid 9-36 (Nippon Gene Co. Ltd., Tokyo, Japan). The positive clones of E. coli with the above-mentioned DIG-probe, containing pCBEI5 (with 5-kb EcoRI fragment) and pCBSm9 (with 9-kb SmaI fragment) were obtained by colony hybridization. A 6-kb SacI-SmaI fragment was subcloned into pUC18 (Takara Bio Inc., Shiga, Japan) from pBSm9 and designated as pBSS6. Similarly, the 5-kb EcoRI fragment of pCBEI5 was subcloned into pSTV28 (Takara) designated as pBEI5. Nucleotide sequences of their inserts were determined as follows. First, nested unidirectional deletions in the target DNA, the 5 kb EcoRI and 6 kb SacI/SmaI inserts were created by the Kilo-sequence Deletion kit (Takara). Then, the nucleotide sequences of the different deleted inserts were determined using the CEQ DTCS-Quick Start Kit (Beckman Coulter Inc. Brea, CA, USA) by the dideoxy-chain termination method, with a CEQ2000 DNA sequencer (Beckman Coulter Inc.). DNA regions upstream and downstream of the cloned 7.6-kb fragment were isolated by genome walking. To obtain the downstream DNA fragment, a 0.5-kb BamHI-EcoRI fragment was used as a probe of Southern hybridization with BamHI-digested total DNA. A 4 kb BamHI fragment was isolated.
2.4. Enzymatic Assays
Enzymatic activity of NahB_JF8 was estimated by following the reduction of NAD+ using a DU-650 Spectrophotometer (Beckman Coulter Inc.) at 30 °C [17]. The extinction coefficients used for NADH were λmax = 340 nm, ε = 6.22/μM/cm. Reaction mixtures contained 50 mM phosphate buffer (pH7.5), 30 μM cis-naphthalene dihydrodiol, 2.4 mM NAD+, 1 mM ascorbate, and enzyme solution. One enzyme unit was defined as the amount of enzyme required to reduce 1.0 μmol NAD+ per min.
2.5. Purification of the Cis-Dihydrodiol Dehydrogenase
Geobacillus sp. JF8 was grown on Castenholz D agar plates in the presence of naphthalene vapors. The cells were harvested, washed and resuspended in 20 mM phosphate buffer (pH 7.5) containing 30 mM β-mercaptoethanol and 0.5 mM EDTA (Buffer A). The cell suspension was passed through a French Press (Thermo Fisher Scientific Inc. Tokyo, Japan) and centrifuged at 17,000× g for 60 min. The supernatant was applied to a DEAE-Toyopearl 650M (Tosoh) column. The enzyme was eluted with 4 L gradient of 0.0 to 0.5 M KCl. The eluted protein was dialyzed and applied to a Phenyl sepharose FF 16/10 column (GE Healthcare Life Sciences) equilibrated with Buffer A containing 0.1 M ammonium sulfate (Buffer B). The enzyme was eluted with a 400 mL gradient of 1.0 to 0.0 M ammonium sulfate. The eluted protein was dialyzed against 20 mM phosphate buffer (pH7.0) containing 30 mM β-mercaptoethanol, 0.5 mM EDTA, and 0.2 M ammonium sulfate (Buffer B2). Then the sample was applied to a Phenyl sepharose FF 16/10 column equilibrated with Buffer B2. The enzyme fraction was eluted by a 200 mL gradient of 1.0 to 0.0 M ammonium sulfate, and the enzyme was dialyzed against 20 mM phosphate buffer (pH7.0) containing 30 mM β-mercaptoethanol and 0.5 mM EDTA (Buffer A2) and water. The samples eluted by Buffer A2 and water were combined dialyzed against 20 mM Tris-HCl buffer (pH8.5) containing 30 mM β-mercaptoethanol and 2 mM EDTA (Buffer C). Finally, the resultant samples were applied to a Mono Q HR 5/5 column (GE Healthcare Life Sciences) equilibrated with Buffer C. The enzyme was eluted with a 40 mL gradient of 0.0 to 0.5 M KCl. The fractions containing enzyme activity were pooled and dialyzed against Buffer A. The N-terminal sequence of the native enzyme was determined by automated Edman degradation on a Model 492 Protein Sequencer (Thermo Fisher Scientific Inc.).
2.6. Electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the method of Laemmli [18] with Prestained Protein Marker Broad Range (New England Biolabs Japan Inc., Tokyo, Japan). Native nondenaturing PAGE was performed with HMW Native Marker Kit (GE Healthcare Life Sciences) using the same solutions without SDS. Gels were stained with Coomassie Brilliant Blue R250 [16]. The relative molecular masses were calculated from the mobilities of the marker proteins.
2.7. RNA Extraction and RT-PCR
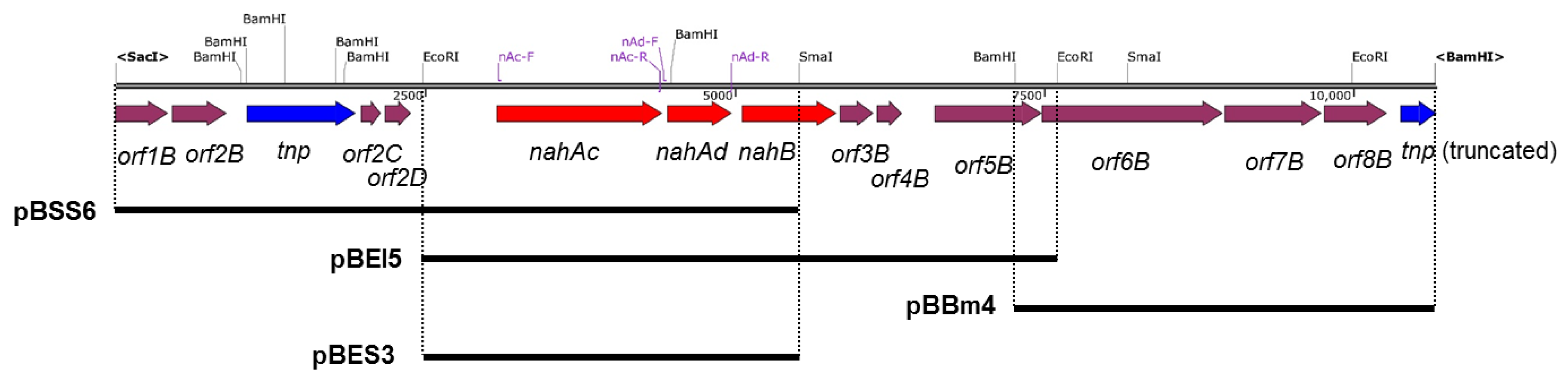
Geobacillus sp. JF8 was grown on LB plate at 60 °C for 8 h, then the resultant colonies were exposed to naphthalene vapor for 4 h to induce their degradative pathways. Total RNA was extracted from the JF8 cells using a RNeasy Mini Kit (Qiagen, Venlo, The Netherlands). The RNA samples were treated with DNase I (Thermo Fisher Scientific Inc). Reverse transcriptase (RT)-PCR was carried out with 1-ng RNA of each sample and OneStep RT-PCR kit (Qiagen) using primers; nAc-F: 5′-AGAACAAATCGAAGGCGTTT-3′ and nAc-R: 5′-TTTCCATTGACGCCAAATG-3′, and nAd-F: 5′-TGAGCTTTCGATACCGAGACA-3′ and nAd-R: 5′-ACCGCTAAGTTATCCATCCCT -3′. The primer sets of nAc-F and nAc-R were used to amplify nahAc gene and those of nAd-F and nAd-R were for nahAd gene (Figure 2). The conditions for the RT-PCR amplification were as follows; 50 °C for 30 min; 95 °C for 15 min; followed by 35 cycles of 94 °C for 30 s, 48 °C for 1 min, and 72 °C for 5 min. For negative controls, the initial incubation at 50 °C was omitted.
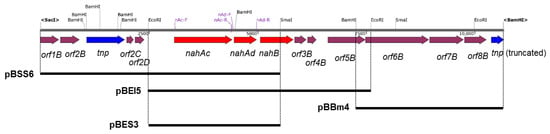
Figure 2.
Genetic organization of the nah gene cluster of strain JF8. Red, blue, and magenta pentagons indicate naphthalene-degradative genes, transposase gene, and genes for hypothetical proteins. Restriction enzyme site and positions for primers are shown. The solid black bars under the map indicate DNA regions cloned into each plasmid.
2.8. Construction of an Expression Vector of nahAcAd and Its Biotransformation Assay
E. coli cells containing pBES3, (3-kb EcoRI–SmaI fragment including nahAcAd (Figure 2) in pBluescript II KS(+) were cultivated in the presence of 1 mM IPTG in 400 mL LB medium containing 100 μg/mL ampicillin at 37 °C. Harvested cells of the recombinant E. coli were suspended in Buffer A and sonicated by Homogenizer subsonic HMO-100 (AGC Techno Glass Co., Ltd., Shizuoka, Japan) for 3 min. Cell debris was removed by centrifugation for 30 min at 18,000× g. The supernatant was referred to as the cell-free extract. E. coli cells containing pBES3 was cultured in LB medium with appropriate antibiotics. After the overnight cultivation at 37 °C, 1 mM IPTG was added and the cells were cultivated for another 2 h. The cells were harvested, washed and suspended in LB medium at an OD600 = 2. Five milliliters of the bacterial suspension was transferred to a test tube and naphthalene was added at a concentration of 1 mM. The tubes were incubated at 37 °C for 2 h. The cell suspension was acidified by the addition of 50 μL concentrated HCl, an equal volume of ethyl acetate was added and mixed for 10 min. After centrifugation at 5000× g for 10 min to extract the transformants, the ethyl acetate was concentrated under a stream of nitrogen and trimethylsilylation done using BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide) + TMCS (trimethylchlorosilane) (Sylon BFT kit, Sigma-Aldrich Japan Inc., Tokyo, Japan). The derivatized samples were analyzed by gas chromatography (Hewlett-Packard model 6890, Agilent Technologies Japan, Tokyo, Japan), equipped with an HP-5ms capillary column (50 m, 0.2 mm, 0·33 µm, Agilent Technologies Japan, Tokyo, Japan) and a mass selective detector (Hewlett-Packard, model 5972A, Agilent Technologies Japan, Tokyo, Japan). The condition of GC-MS has been described previously [10].
2.9. Nucleotide Sequence Accession Number
The genome sequence of Geoacillus sp. JF8 is deposited at CP006254- CP006255.
3. Results
3.1. Determination of N-terminal Amino Acid Sequence of Cis-Naphthalene Dihydrodiol Dehydrogenase
In order to obtain information of N-terminal amino acid sequence of the dehydrogenase, NahB_JF8 was purified from naphthalene-grown cell of Geobacillus sp. JF8. The cell extract was subjected to the five step purification procedures (Table 1). We eluted twice for the Phenyl Sepharose column with ammonium sulfate and water because both eluted fractions showed enzymatic activities. The resultant fractions were combined and subjected to the MonoQ column. The purity of NahB_JF8 was assessed by SDS-PAGE analysis, and a single protein was observed at 33 kDa during SDS-PAGE, which was larger than the calculated molecular mass (27 kDa). The N-terminal amino acid sequence of the purified enzyme was determined by Edman degradation to be TKRLENKVAFITGAAGGQGRAAAIVFAREGAKVAVVDVDAKGIEET-ARLVNEAGGE AIAIP XDVSN(E/N)EQVKQAI(I/Q)QTVN.

Table 1.
Purification of cis-naphthalene dihydrodiol dehydrogenase (NahB).
3.2. Cloning and Characterization of nah Genes
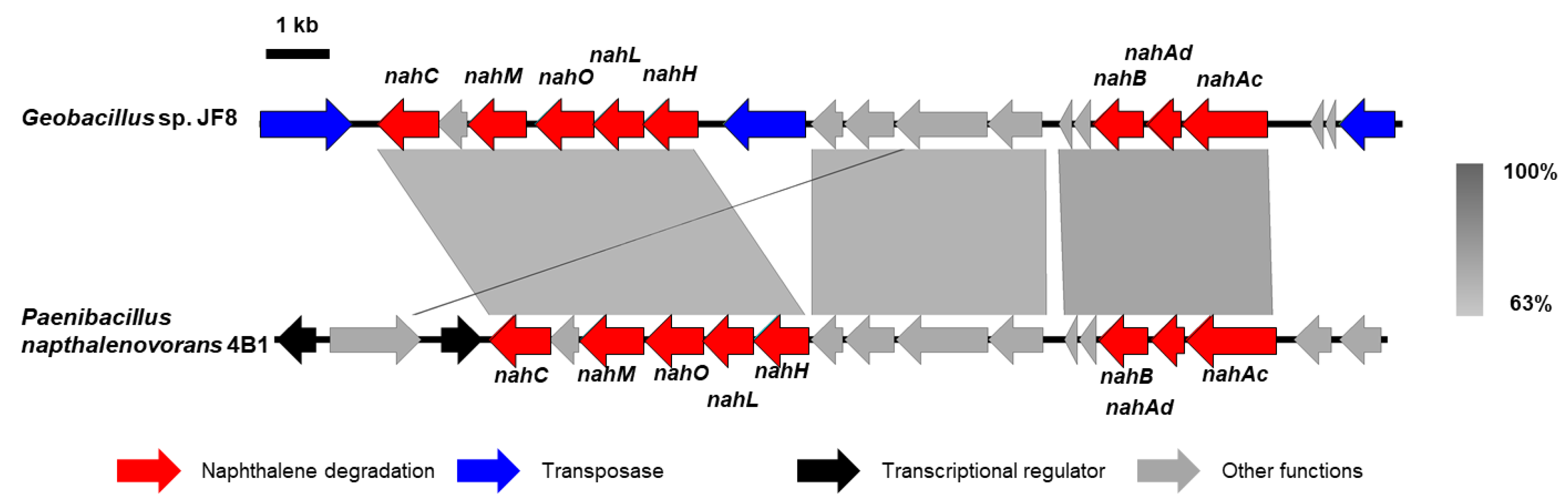
Using a DNA probe spanning the determined N-terminal region of the purified dehydrogenase, two overlapping DNA fragments, a 6-kb SacI-SmaI fragment and 5-kb EcoRI fragment, were cloned, which were designated as pBSS6 and pBEI5, respectively. By genome walking, a 4-kb BamHI fragment downstream of pBEI5 was cloned and it was designated as pBBm4. A 10-kb DNA fragment containing the flanking region of above nahB_JF8 was obtained and their nucleotide sequence was determined. Thirteen open reading frames were identified as shown in Figure 2.
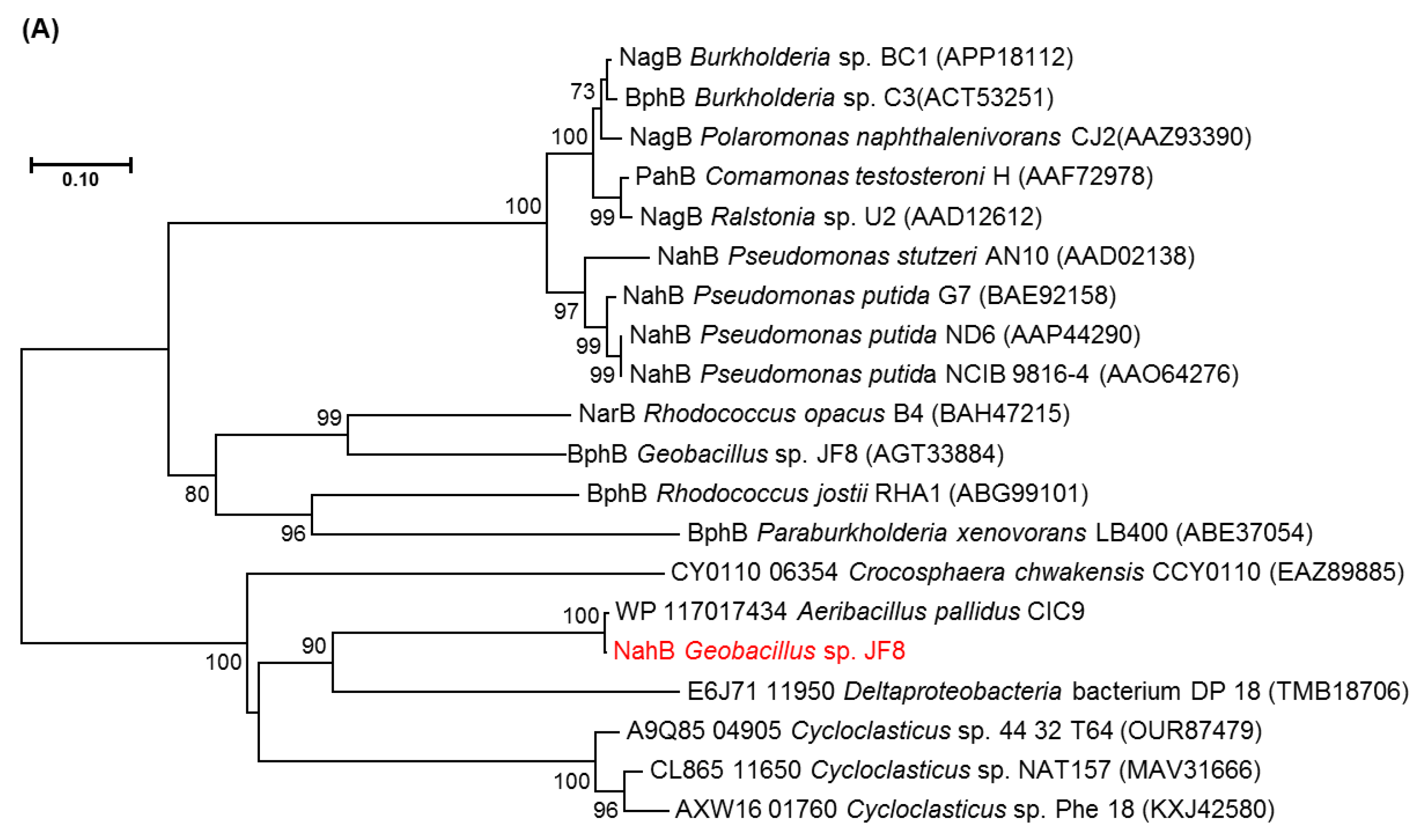
The deduced N-terminal amino acid sequence of NahB was MTKRLENKVAFITGAAGGQGR AAAIVFAREGAKVAVVDVDAKGIEETARLVNEAGGEAIAIPCDVSNEEQVKQAIQQTVN, which is identical to the N-terminal amino acid sequence of the purified dehydrogenase from naphthalene grown cell of Geobacillus sp. JF8. The amino acid sequence of NahB_JF8 exhibited 99% identity to those of a putative dehydrogenase from Aeribacillus pallidus CIC9 (WP_117017434), whereas its activity was not elucidated yet. Those of NahB_JF8 showed low identities with those of mesophilic naphthalene degraders; 46% identity to a 2,5-dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase from Crocosphaera chwakensis CCY0110(EAZ89885), and 31% identity to cis-naphthalene dihydrodiol dehydrogenase (NahB) from Pseudomonas putida G7 (BAE92158). In a phylogenetic tree of dehydrogenases, NahB_JF8 was not located in the same clade containing cis-naphthalene dihydrodiol dehydrogenase, cis-biphenyl dihydrodiol dehydrogenase, or cis-toluene dihydrodiol dehydrogenase (Figure 3A).
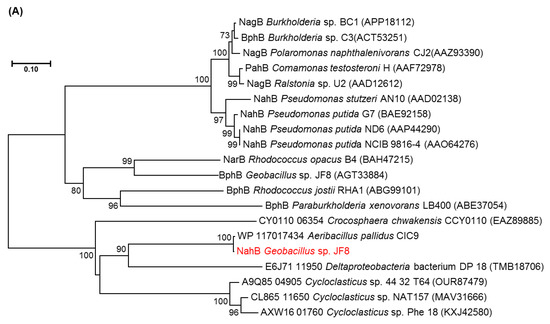

Figure 3.
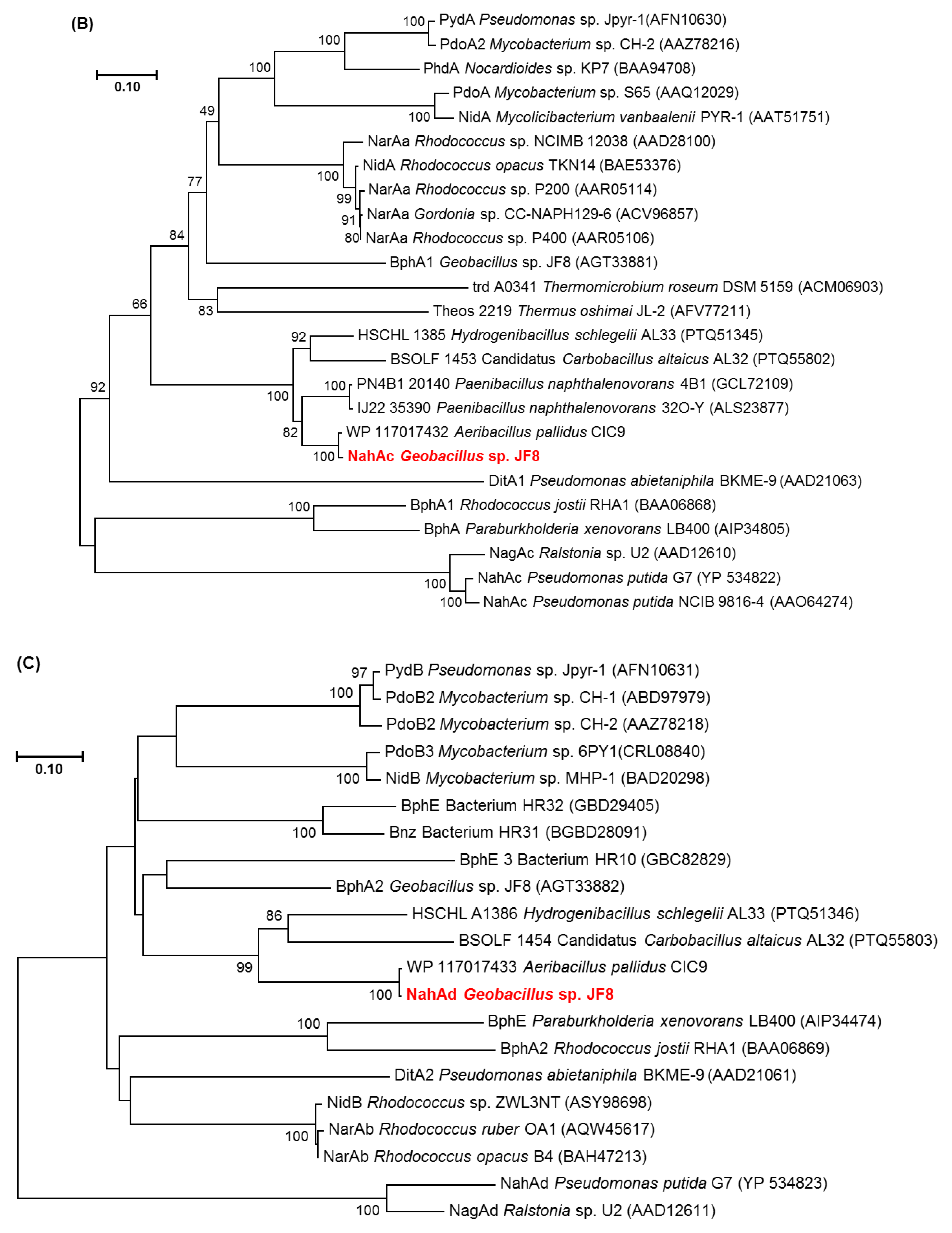
Neighbor-joining tree of amino acid sequences of (A) NahAc, (B) NahAd, and (C) NahB of JF8 (shown in red) with representative homologous proteins in mesophilic- and thermophilic-degraders. Bootstrap values (1000 replications) are shown as percentages at nodes. The tree was reconstructed using MEGA software. Bar, 0.1 substitutions per amino acid position.
There were two ORFs upstream of nahB, and they were designated as nahAc and nahAd, respectively (Figure 2). Their deduced amino acid sequences exhibited similarity to naphthalene 1,2-dioxygenase α- and β-subunit, respectively. The amino acid sequences of NahAc of strain JF8 showed 98% identity with those of a putative large subunit of aromatic dioxygenase in Aeribacillus pallidus CIC9 (WP_117017432). Those showed a 53% identity to a large subunit of naphthalene dioxygenase in Rhodococcus sp. P400 (AAR05106) [19]. The conserved amino acid sequences for a [2Fe-2S] Rieske-type cluster were found in 95–118 residues ([2Fe–2S] cluster binding residues, Cys-X-His-X15-17-Cys-X2-His). Three conserved residues (His, His, Asp) for a catalytic non-heme ion were found in His-222, His-227, and Asp-379 (corresponding to His-208, His-213, and Asp-362 of naphthalene dioxygenase of P. putida NCIB 9816). The amino acid sequences of NahAd of strain JF8 showed 99% identity with those of a putative small subunit of aromatic dioxygenase in Aeribacillus pallidus CIC9 (WP_117017433). Those showed 47% identity with those of a small subunit of ring-hydroxylating dioxygenase of polycyclic aromatic hydrocarbons in Mycobacterium sp. CH-2 (PdoB2) (AAZ78218), and 32% identity with those of a naphthalene 1,2-dioxygenase small subunit from P. putida G7 (NahAd) (YP_534823). In a phylogenetic tree based on the amino acid sequences of ring-hydroxylating dioxygenase, NahAc and NahAd of strain JF8 were located in the clades of proteins found in Gram-positive bacteria, respectively (Figure 3B,C). The amino acid sequences of the other ORFs showed similarity with those of meta-cleavage product hydrolases (Orf5B), monooxygenase (Orf6B), dehydrogenases (Orf7B), and gentisate dioxygenases (Orf8B) (Figure 2).
3.3. Gene Expression Analysis
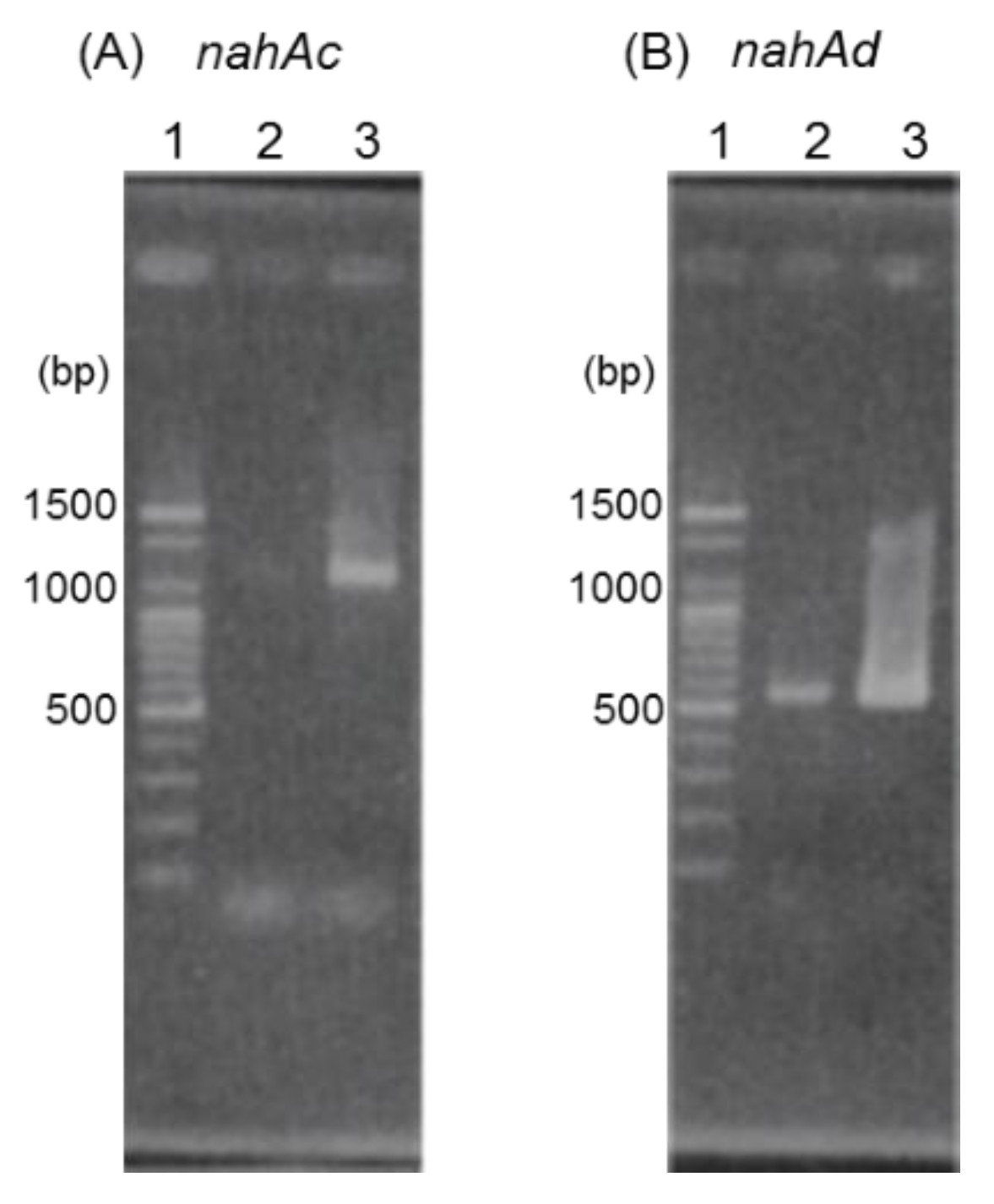
The results of agarose gel electrophoresis of RT-PCR products with RNAs isolated from JF8 grown on LB plate or grown with naphthalene as a vapor were shown in Figure 4. More products of nahAc and nahAd were detected when the naphthalene was added, suggesting that transcription of nahAc and nahAd gene were induced by naphthalene.
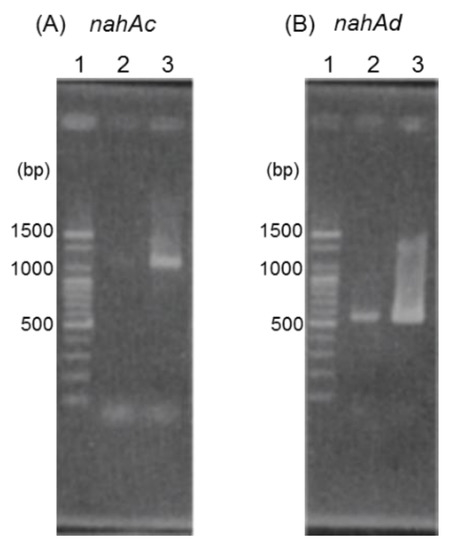
Figure 4.
RT-PCR analyses using primer sets designed to amplify nahAc (A) and nahAd (B). Lane 1, 100 bp DNA ladder; lane 2, RNA extracted from strain JF8 grown on LB plate was used as template; lane 3, RNA extracted from strain JF8 grown on LB plate supplied with naphthalene as a vapor.
3.4. Biotransformation Assay of nahAc and nahAd Gene Products
After expressing of nahAc and nahAd in E. coli, naphthalene was added in a reaction mixture. No metabolite was observed from extracts of the reaction mixture.
4. Discussion
In the present study, the genes related to naphthalene degradation were isolated and characterized from a thermophilic PCB and naphthalene degrader, Geobacillus sp. strain JF8. An enzyme, which dehydrates cis-naphthalene dihydrodiol, was successfully purified and a gene encoding the enzyme, NahB, was identified. The genes encoding terminal components of naphthalene dioxygenase, nahAc and nahAd, were found in the upstream area of nahB. Transcriptions of nahAc and nahAd were induced by naphthalene, indicating that these gene products might be related to naphthalene degradation.
Phylogenetic analysis of NahAc_JF8 and NahAd_JF8 suggested that each of them was classified into a different sub-group from that of large- and small-subunit of PAH-initial dioxygenase found in other Gram-positive bacteria. The group contains hypothetical proteins found in thermophilic bacteria including dibenzofuran- and naphthalene-degrader, Paenibacillus naphthalenovorans 4B1 [20]. Similarly, NahB_JF8 was not clustered into a group of cis-dihydrodiol dehydrogenases derived from mesophilic PAH degraders. The amino acid sequences of NahB_JF8 showed high identity with those of a thermophilic bacterium, Aeribacillus pallidus CIC9, although its function was not elucidated. It should be noted that Aeribacillus pallidus was previously Geobacillus pallidus [21], and thus, these genes could be conserved in the relatively close genus. These results suggest that the naphthalene-degradation pathway of Geobacillus sp. JF8 has evolved through a different route from the previously-known pathways derived from mesophilic bacteria.
In this study, the activity of NahA, a putative terminal dioxygenase of naphthalene, was not detected. This was probably because the NahAc and NahAd were not appropriately expressed in E. coli. Another possible reason was that electron transfer systems for the dioxygenase were not supplied in E. coli. Genes encoding ferredoxin and its reductase could be located around genes encoding terminal dioxygenase, but the strain JF8 did not contain them near nahAc and nahAd. Comparing the amino acid sequences of NahAc of strain JF8, NarAa of Rhodococcus sp. NCIMB 12038, and NahAc of Pseudomonas putida NCIB 9816-4 revealed that the important residues for the enzymatic activity were conserved, which contribute to Rieske-center and active center. In contrast, the residues for electron transfer were not conserved, suggesting that ferredoxin and its reductase might be different from the previously-known ones.
In our previous study, we identified 1,2-dihydroxynaphthalene dioxygenase gene (nahC) in strain JF8 [12]. Recently, the complete genome sequences of JF8 was determined [19]. The genes responsible for catechol metabolism were found in the upstream of nahC from the complete genome sequence of JF8 [22] (Figure 5, nahHLOM). A putative gene encoding 2-hydroxymuconic semialdehyde hydrolase (NahN), which convert 2-hydroxymuconic semialdehyde to 4-oxopent-4-enoate, was found downstream of nahB (Figure 5). The amino acid sequences of the product showed a 75% identity with previously-known 2-hydroxymuconic semialdehyde hydrolase of Hydrogenibacillus schlegelii AL33 (PTQ51003), 32% to NahN of Pseudomonas stutzeri AN10 (AAD02150). It should be noted that a nah gene cluster of JF8 was found in a moderate thermophilic dibenzofuran- and naphthalene-degrader, Paenibacillus naphthalenovorans 4B1 (Figure 5). Gene order and putative amino acid sequence are highly conserved between strains JF8 and 4B1, whose identities were 66–86%. Currently, we could not find putative genes encoding NahD, NahE, and NahF, which degrade meta-cleavage product to salicylate (Figure 1) in the genome sequence of strain JF8. On the other hand, putative gentisate dioxygenase was found (Orf8B, Figure 2) in the downstream area of nahAB genes, suggesting that JF8 could metabolize salicylate via gentisate.

Figure 5.
Alignment of nah gene clusters of strain JF8 with that of Paenibacillus naphthalenovorans 4B1. Coding sequences of each genome are shown as colored arrows.
Metabolic pathways, enzymes, and genes involved in PAH degradation were extensively studied in Gram-negative bacteria [23,24,25]. Genetic and biochemical analyses about PAH-degrader of Gram-positive bacteria were also reported including thermophilic microorganisms [20,26,27,28,29,30,31,32,33]. These facts imply that Gram-positive bacteria can play more important roles in the degradation of high molecular PAHs including biphenyl [34,35,36,37,38,39,40,41]. Further investigation including molecular analysis will provide us more clear information on naphthalene degradation including a lower pathway of thermophilic bacteria.
Author Contributions
Conceptualization, D.M., M.S., T.H., and K.K.; methodology, D.M., A.T., and K.K.; software, L.T.H.T.; validation, M.S. and K.K.; investigation, D.M.; resources, T.H.; data curation, D.M., L.T.H.T., N.H.L., and M.S.; writing—original draft preparation, D.M.; writing—review and editing, M.S., and K.K.; visualization, M.S.; supervision, K.K.; project administration, K.K. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We are grateful to Gouri Mukerjee-Dhar and Minoru Shimura for giving us helpful suggestions. We thank Hozoh Kiyohara of Okayama University of Science for providing pHSG396.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yen, K.M.; Gunsalus, I.C. Plasmid gene organization: Naphthalene/salicylate oxidation. Proc. Natl. Acad. Sci. USA 1982, 79, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A. Homology between nucleotide sequences of promoter regions of nah and sal operons of NAH7 plasmid of Pseudomonas putida. Proc. Natl. Acad. Sci. USA 1986, 83, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Harayama, S.; Rekik, M.; Wasserfallen, A.; Bairoch, A. Evolutionary relationships between catabolic pathways for aromatics: Conservation of gene order and nucleotide sequences of catechol oxidation genes of pWW0 and NAH7 plasmids. Mol. Genet. Genom. 1987, 210, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.J.; Osslund, T.D.; Saunders, R.; Ensley, B.D.; Suggs, S.; Harcourt, A.; Suen, W.C.; Cruden, D.L.; Gibson, D.T.; Zylstra, G.J. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene 1993, 127, 31–37. [Google Scholar] [CrossRef]
- Cane, P.; Williams, P. A restriction map of naphthalene catabolic plasmid pWW60-1 and the location of some of its catabolic genes. Microbiology 1986, 132, 2919–2929. [Google Scholar] [CrossRef]
- Platt, A.; Shingler, V.; Taylor, S.C.; Williams, P.A. The 4-hydroxy-2-oxovalerate aldolase and acetaldehyde dehydrogenase (acylating) encoded by the nahM and nahO genes of the naphthalene catabolic plasmid pWW60-22 provide further evidence of conservation of meta-cleavage pathway gene sequences. Microbiology 1995, 141, 2223–2233. [Google Scholar] [CrossRef]
- Schell, M.A.; Wender, P.E. Identification of the nahR gene product and nucleotide sequences required for its activation of the sal operon. J. Bacteriol. 1986, 166, 9–14. [Google Scholar] [CrossRef]
- Fuenmayor, S.L.; Wild, M.; Boyes, A.L.; Williams, P.A. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J. Bacteriol. 1998, 180, 2522–2530. [Google Scholar]
- Zhou, N.Y.; Al-Dulayymi, J.; Baird, M.S.; Williams, P.A. Salicylate 5-hydroxylase from Ralstonia sp. strain U2: A monooxygenase with close relationships to and shared electron transport proteins with naphthalene dioxygenase. J. Bacteriol. 2002, 184, 1547–1555. [Google Scholar] [CrossRef]
- Shimura, M.; Mukerjee-Dhar, G.; Kimbara, K.; Nagato, H.; Kiyohara, H.; Hatta, T. Isolation and characterization of a thermophilic Bacillus sp. JF8 capable of degrading polychlorinated biphenyls and naphthalene. FEMS Microbiol. Lett. 1999, 178, 87–93. [Google Scholar] [CrossRef]
- Annweiler, E.; Richnow, H.H.; Antranikian, G.; Hebenbrock, S.; Garms, C.; Franke, S.; Francke, W.; Michaelis, W. Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass by the thermophile Bacillus thermoleovorans. Appl. Environ. Microbiol. 2000, 66, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Bubinas, A.; Giedraitytė, G.; Kalėdienė, L.; Nivinskiene, O.; Butkiene, R. Degradation of naphthalene by thermophilic bacteria via a pathway, through protocatechuic acid. Cent. Eur. J. Biol. 2008, 3, 61–68. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Liu, J.; Li, R.; Shen, B. Isolation of a thermophilic bacterium, Geobacillus sp. SH-1, capable of degrading aliphatic hydrocarbons and naphthalene simultaneously, and identification of its naphthalene degrading pathway. Bioresour. Technol. 2012, 124, 83–89. [Google Scholar] [CrossRef]
- Miyazawa, D.; Mukerjee-Dhar, G.; Shimura, M.; Hatta, T.; Kimbara, K. Genes for Mn(ii)-dependent NahC and Fe(ii)-dependent NahH located in close proximity in the thermophilic naphthalene and PCB degrader, Bacillus sp. JF8: Cloning and characterization. Microbiology 2004, 150, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, N.; Iida, T.; Sawada, T.; Yamauchi, K.; Wang, Y.W.; Fukuda, M.; Kiyohara, H. Nucleotide sequences and characterization of genes encoding naphthalene upper pathway of Pseudomonas aeruginosa PAK1 and Pseudomonas putida OUS82. J. Biosci. Bioeng. 1999, 87, 721–731. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Patel, T.R.; Gibson, D.T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J. Bacteriol. 1974, 119, 879–888. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Di Gennaro, P.; Terreni, P.; Masi, G.; Botti, S.; De Ferra, F.; Bestetti, G. Identification and characterization of genes involved in naphthalene degradation in Rhodococcus opacus R7. Appl. Microbiol. Biotechnol. 2010, 87, 297–308. [Google Scholar] [CrossRef]
- Thanh, L.T.H.; Thi, T.V.N.; Shintani, M.; Moriuchi, R.; Dohra, H.; Loc, N.H.; Kimbara, K. Isolation and characterization of a moderate thermophilic Paenibacillus naphthalenovorans strain 4B1 capable of degrading dibenzofuran from dioxin-contaminated soil in Vietnam. J. Biosci. Bioeng. 2019, 128, 571–577. [Google Scholar] [CrossRef]
- Miñana-Galbis, D.; Pinzón, D.L.; Lorén, J.G.; Manresa, À.; Oliart-Ros, R.M. Reclassification of Geobacillus pallidus (Scholz et al. 1988) Banat et al. 2004 as Aeribacillus pallidus gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 1600–1604. [Google Scholar] [CrossRef]
- Shintani, M.; Ohtsubo, Y.; Fukuda, K.; Hosoyama, A.; Ohji, S.; Yamazoe, A.; Fujita, N.; Nagata, Y.; Tsuda, M.; Hatta, T.; et al. Complete genome sequence of the thermophilic polychlorinated biphenyl degrader Geobacillus sp. strain JF8 (NBRC 109937). Genome Announc. 2014, 2, e01213-13. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.R.; Cammack, R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu. Rev. Microbiol. 1992, 46, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, B.; Lee, K.; Carredano, E.; Parales, R.E.; Gibson, D.T.; Eklund, H.; Ramaswamy, S. Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure 1998, 6, 571–586. [Google Scholar] [CrossRef]
- Khan, A.A.; Walia, S.K. Expression, localization, and functional analysis of polychlorinated biphenyl degradation genes cbpABCD of Pseudomonas putida. Appl. Environ. Microbiol. 1991, 57, 1325–1332. [Google Scholar]
- Kim, E.; Aversano, P.J.; Romine, M.F.; Schneider, R.P.; Zylstra, G.J. Homology between genes for aromatic hydrocarbon degradation in surface and deep-subsurface Sphingomonas strains. Appl. Environ. Microbiol. 1996, 62, 1467–1470. [Google Scholar]
- Treadway, S.L.; Yanagimachi, K.S.; Lankenau, E.; Lessard, P.A.; Stephanopoulos, G.; Sinskey, A.J. Isolation and characterization of indene bioconversion genes from Rhodococcus strain I24. Appl. Microbiol. Biotechnol. 1999, 51, 786–793. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, R.F.; Cao, W.W.; Doerge, D.R.; Wennerstrom, D.; Cerniglia, C.E. Molecular cloning, nucleotide sequence, and expression of genes encoding a polycyclic aromatic ring dioxygenase from Mycobacterium sp. strain PYR-1. Appl. Environ. Microbiol. 2001, 67, 3577–3585. [Google Scholar] [CrossRef]
- Wang, R.F.; Wennerstrom, D.; Cao, W.W.; Khan, A.A.; Cerniglia, C.E. Cloning, expression, and characterization of the katG gene, encoding catalase-peroxidase, from the polycyclic aromatic hydrocarbon-degrading bacterium Mycobacterium sp. strain PYR-1. Appl. Environ. Microbiol. 2000, 66, 4300–4304. [Google Scholar] [CrossRef]
- Saito, A.; Iwabuchi, T.; Harayama, S. A novel phenanthrene dioxygenase from Nocardioides sp. strain KP7: Expression in Escherichia coli. J. Bacteriol. 2000, 182, 2134–2141. [Google Scholar] [CrossRef]
- Kasuga, K.; Habe, H.; Chung, J.S.; Yoshida, T.; Nojiri, H.; Yamane, H.; Omori, T. Isolation and characterization of the genes encoding a novel oxygenase component of angular dioxygenase from the gram-positive dibenzofuran-degrader Terrabacter sp. strain DBF63. Biochem. Biophys. Res. Commun. 2001, 283, 195–204. [Google Scholar] [CrossRef]
- Larkin, M.J.; Allen, C.C.; Kulakov, L.A.; Lipscomb, D.A. Purification and characterization of a novel naphthalene dioxygenase from Rhodococcus sp. strain NCIMB12038. J. Bacteriol. 1999, 181, 6200–6204. [Google Scholar] [PubMed]
- Andreoni, V.; Bernasconi, S.; Colombo, M.; van Beilen, J.B.; Cavalca, L. Detection of genes for alkane and naphthalene catabolism in Rhodococcus sp. strain 1BN. Environ. Microbiol. 2000, 2, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Heitkamp, M.A.; Franklin, W.; Cerniglia, C.E. Microbial metabolism of polycyclic aromatic hydrocarbons: Isolation and characterization of a pyrene-degrading bacterium. Appl. Environ. Microbiol. 1988, 54, 2549–2555. [Google Scholar] [PubMed]
- Dean-Ross, D.; Moody, J.D.; Freeman, J.P.; Doerge, D.R.; Cerniglia, C.E. Metabolism of anthracene by a Rhodococcus species. FEMS Microbiol. Lett. 2001, 204, 205–211. [Google Scholar] [CrossRef]
- Boldrin, B.; Tiehm, A.; Fritzsche, C. Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl. Environ. Microbiol. 1993, 59, 1927–1930. [Google Scholar]
- Schneider, J.; Grosser, R.; Jayasimhulu, K.; Xue, W.; Warshawsky, D. Degradation of pyrene, benz[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. strain RJGII-135, isolated from a former coal gasification site. Appl. Environ. Microbiol. 1996, 62, 13–19. [Google Scholar]
- Kanaly, R.A.; Harayama, S. Advances in the field of high-molecular-weight polycyclic aromatic hydrocarbon biodegradation by bacteria. Microb. Biotechnol. 2010, 3, 136–164. [Google Scholar] [CrossRef]
- Cebron, A.; Norini, M.P.; Beguiristain, T.; Leyval, C. Real-time PCR quantification of PAH-ring hydroxylating dioxygenase (pah-rhdalpha) genes from gram positive and gram negative bacteria in soil and sediment samples. J. Microbiol. Methods 2008, 73, 148–159. [Google Scholar] [CrossRef]
- Mehetre, G.T.; Dastager, S.G.; Dharne, M.S. Biodegradation of mixed polycyclic aromatic hydrocarbons by pure and mixed cultures of biosurfactant producing thermophilic and thermo-tolerant bacteria. Sci. Total Environ. 2019, 679, 52–60. [Google Scholar] [CrossRef]
- Wanapaisan, P.; Laothamteep, N.; Vejarano, F.; Chakraborty, J.; Shintani, M.; Muangchinda, C.; Morita, T.; Suzuki-Minakuchi, C.; Inoue, K.; Nojiri, H.; et al. Synergistic degradation of pyrene by five culturable bacteria in a mangrove sediment-derived bacterial consortium. J. Hazard. Mater. 2018, 342, 561–570. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).