Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation of Fruit Processing Coproducts
2.2. Physicochemical Characterization of Fruit Processing Coproducts
2.3. Evaluation of the Protective Effects of Fruit Processing Coproducts on Freeze-Dried Probiotic Lactobacillus Strains
2.3.1. Microorganisms, Inoculum Preparation, and Treatments
2.3.2. Freeze-Drying and Survival of Probiotic Lactobacillus
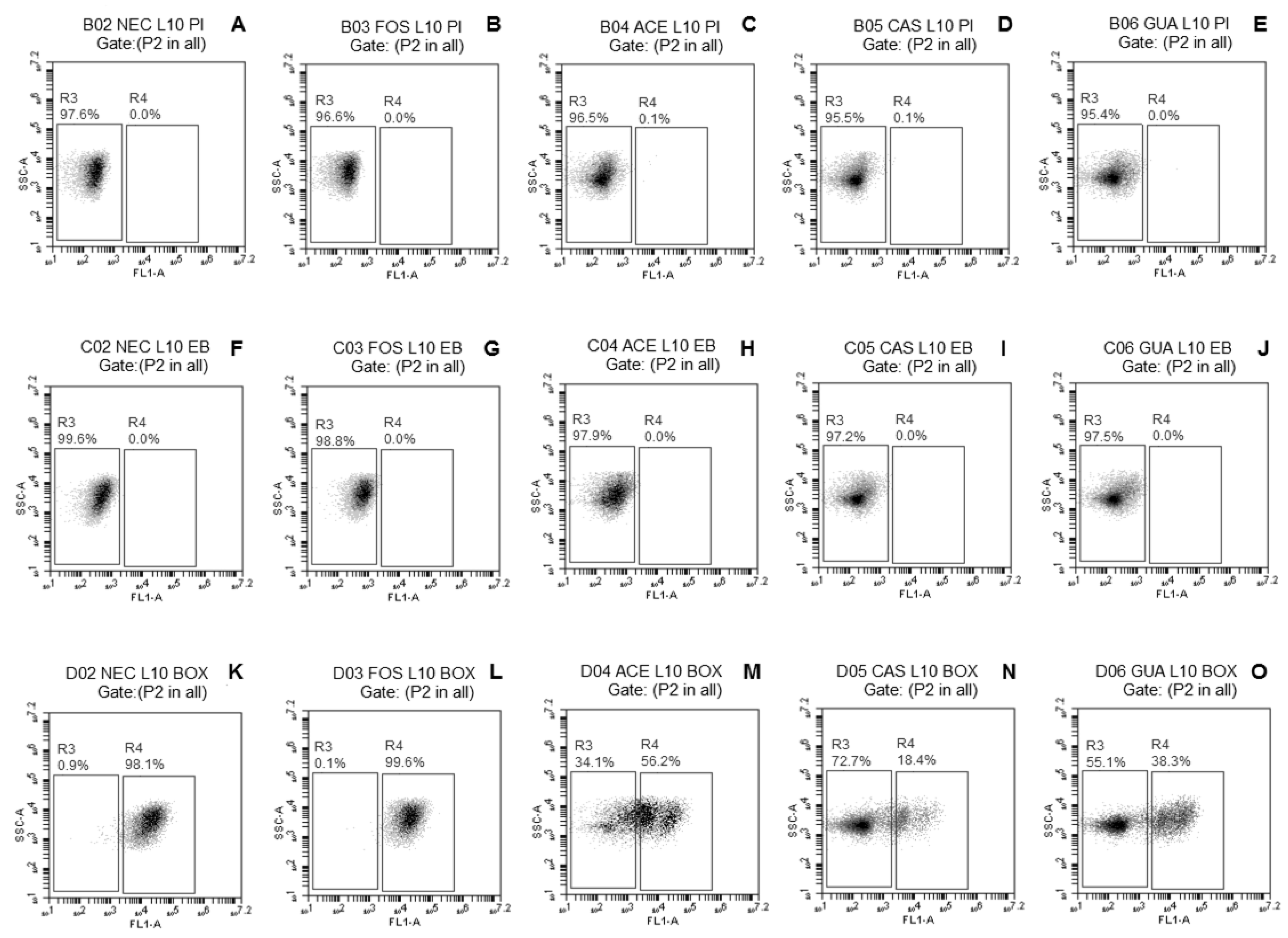
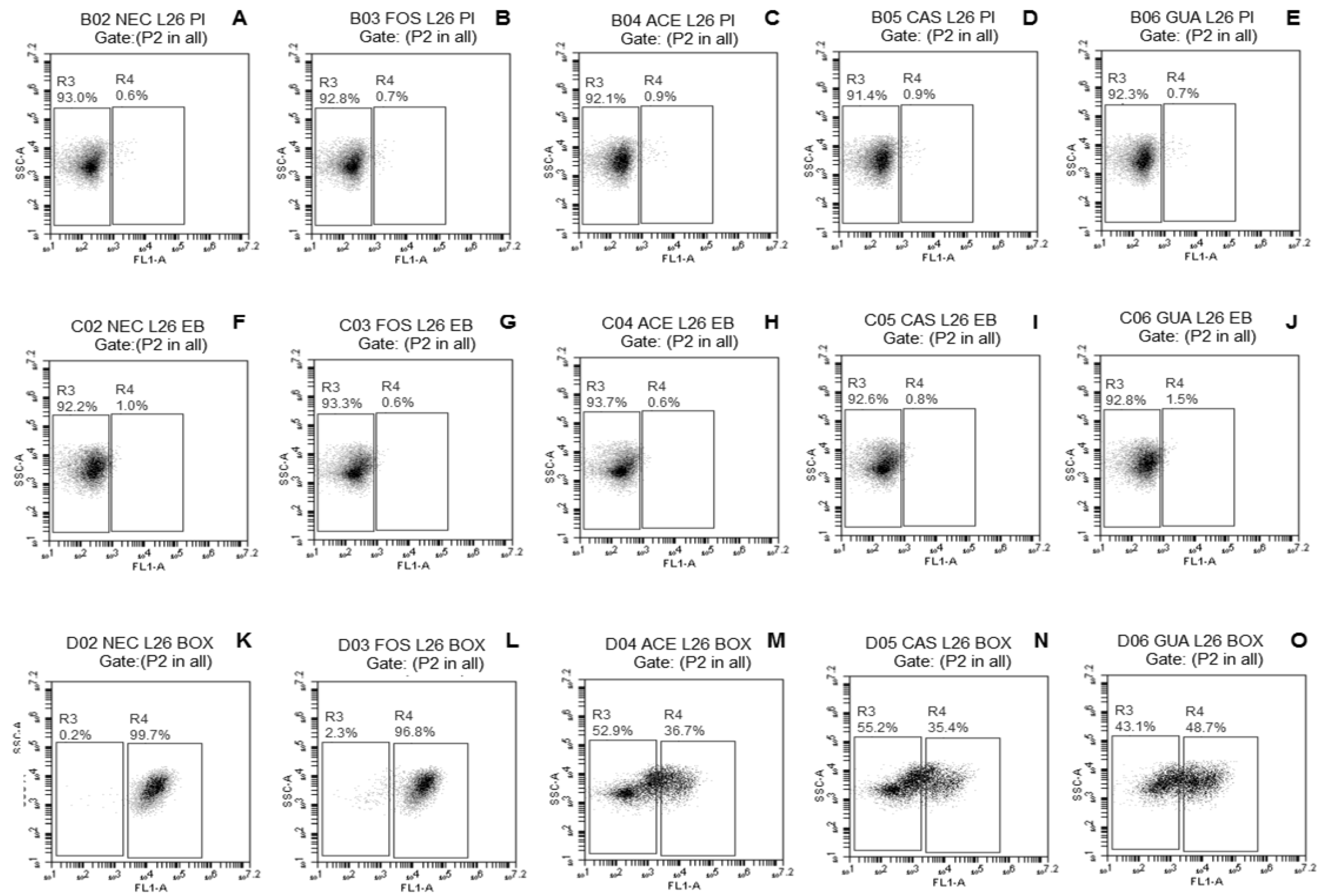
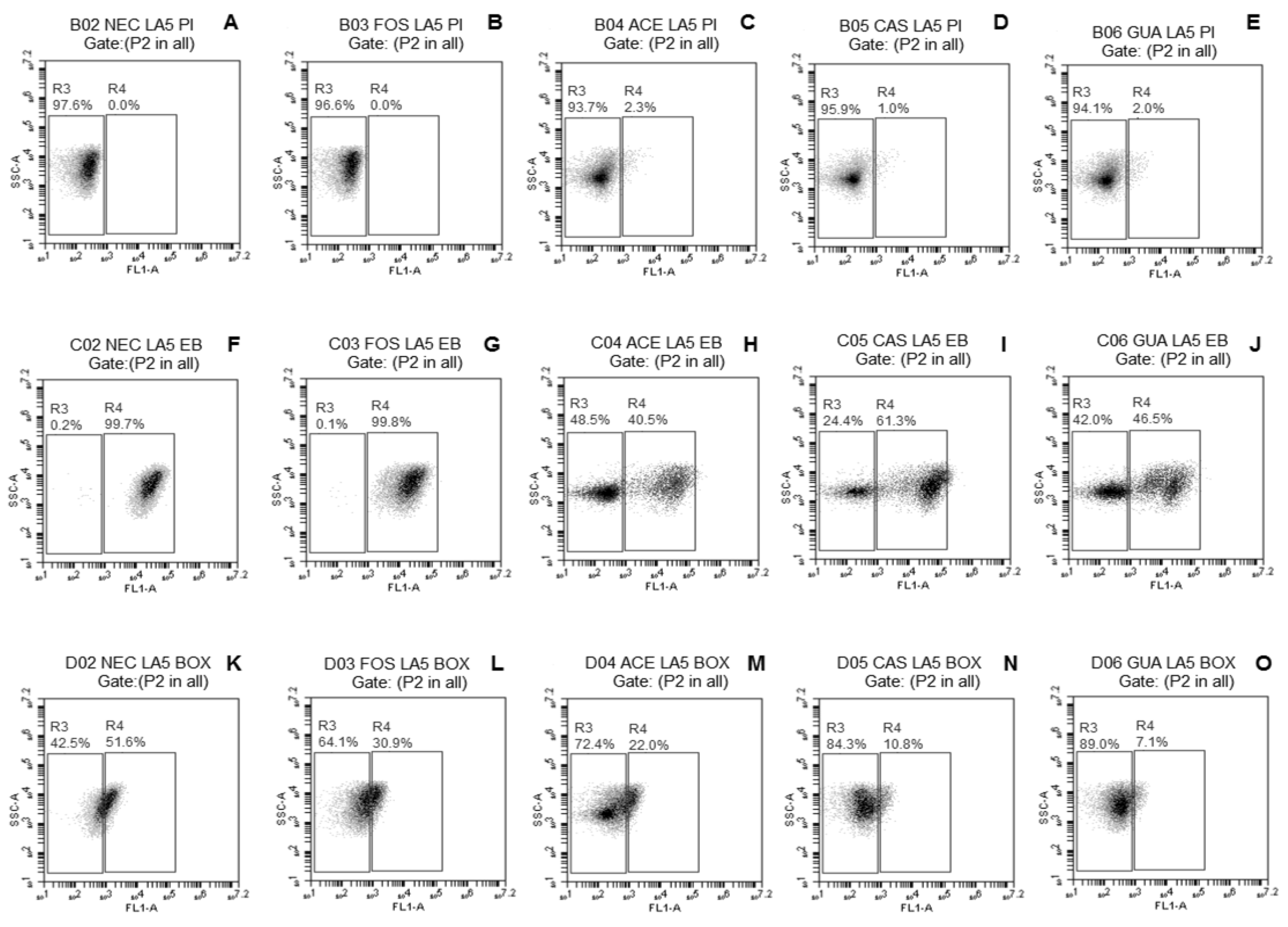
2.3.3. Evaluation of Damage to Membrane Functions of Probiotic Lactobacillus Cells after Freeze-Drying
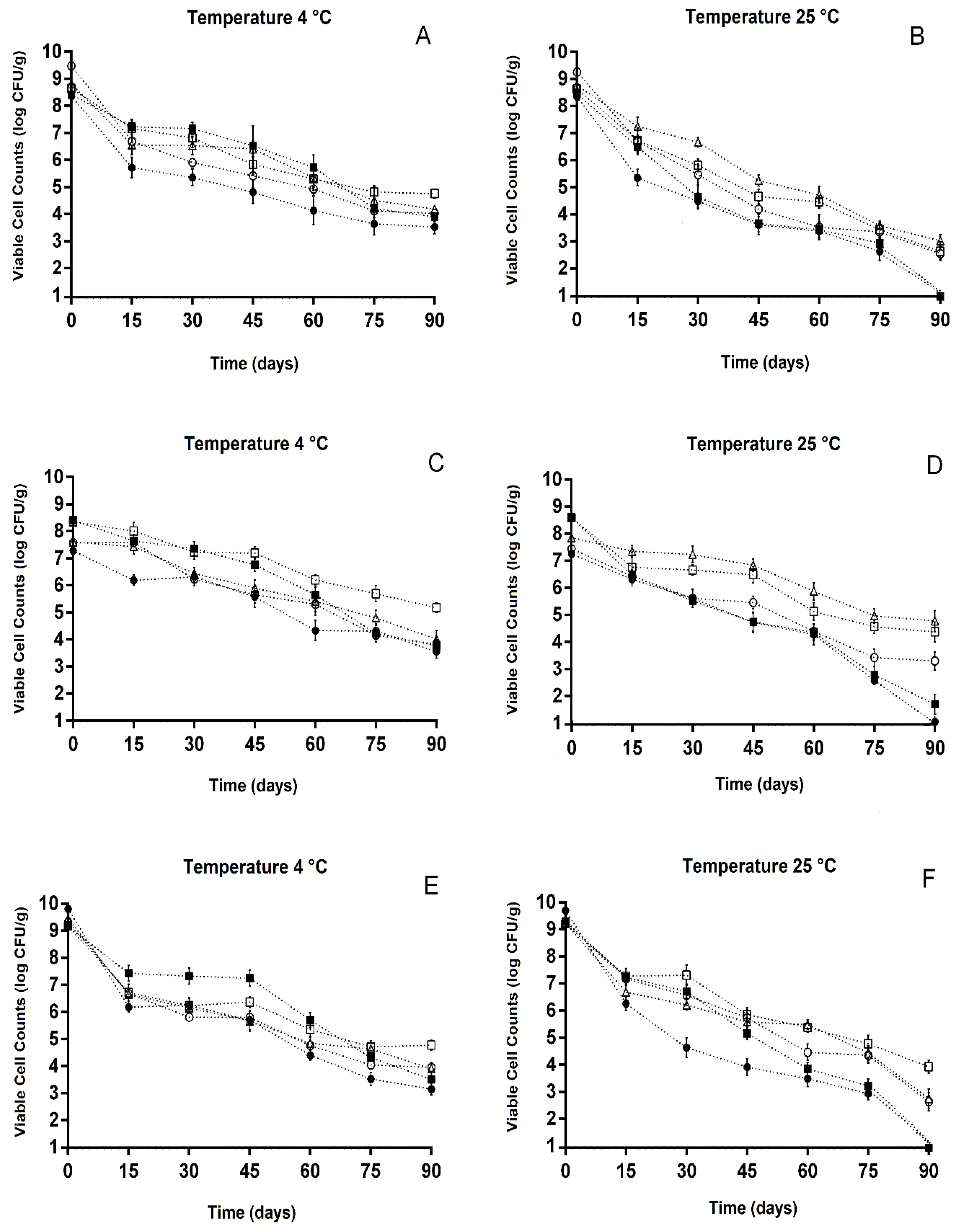
2.3.4. Enumeration of Viable Cells of Freeze-Dried Probiotic Lactobacillus during Storage
2.4. Statistical Analysis
3. Results and Discussion
3.1. Viable Counts of Probiotic Lactobacillus before and after Freeze-Drying
3.2. Evaluation of Damage to Membrane Functions of Probiotic Lactobacillus Cells after Freeze-Drying
3.3. Viable Counts of Freeze-Dried Lactobacillus during Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Aguilar, G.; Villa-Rodriguez, J.; Ayala-Zavala, J.; Yahia, E. Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends Food Sci. Technol. 2010, 21, 475–482. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Infante, J.; Selani, M.M.; Toledo, N.M.V.; Silveira-Diniz, M.F.; Alencar, S.M.; Spoto, M.H.F. Antioxidant activity of agroindustrial residues from tropical fruits. Braz. J. Food Nutr. 2013, 24, 87–91. [Google Scholar]
- Nóbrega, E.M.; Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. The Impact of Hot Air Drying on the Physical-Chemical Characteristics, Bioactive Compounds and Antioxidant Activity of Acerola (Malphigia emarginata) Residue. J. Food Process. Preserv. 2015, 39, 131–141. [Google Scholar] [CrossRef]
- Duarte, F.N.D.; Rodrigues, J.B.; Lima, M.C.; Lima, M.S.; Pacheco, M.T.B.; Pintado, M.M.E.; Aquino, J.S.; Souza, E.L. Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial byproduct on Lactobacillus sp. J. Sci. Food Agric. 2017, 97, 3712–3719. [Google Scholar] [CrossRef]
- Batista, K.S.; Alves, A.F.; Lima, M.D.S.; Silva, L.A.; Lins, P.P.; Gomes, J.A.S.; Silva, A.S.; Toscano, L.T.; Meireles, B.R.L.A.; Cordeiro, A.M.T.M.; et al. Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br. J. Nutr. 2018, 119, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morrellj, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.F.; Araújo, A.O.; Luciano, W.A.; Albuquerque, T.M.R.; Arcanjo, N.M.O.; Madruga, M.S.; Lima, M.S.; Saarela, M.; Souza, E.L. The performance of five fruit-derived and freeze-dried potentially probiotic Lactobacillus strains in apple, orange, and grape juices. J. Sci. Food Agric. 2018, 98, 5000–5010. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Díaz, M.R.; Pataro, A.; Sandonato, N.; Gómez-Zavaglia, A.; Disalvo, E.A. Critical water activity for the preservation of Lactobacillus bulgaricus by vacuum drying. Int. J. Food Microbiol. 2008, 128, 342–347. [Google Scholar] [CrossRef]
- Velly, H.; Bouix, M.; Passot, S.; Penicaud, C.; Beinsteiner, H.; Ghorbal, S.; Lieben, P.; Fonseca, F. Cyclopropanation of unsaturated fatty acids and membrane rigidification improve the freeze-drying resistance of Lactococcus lactis subsp. Lactis TOMSC161. Appl. Microbiol. Biotechnol. 2015, 99, 907–918. [Google Scholar] [CrossRef]
- Nunes, G.L.; Etchepare, M.A.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Flores, E.M.M.; Silva, C.B.; Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT-Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Romano, N.; Schebor, C.; Mobili, P.; Gómez-Zavaglia, A. Role of mono- and oligosaccharides from FOS as stabilizing agents during freeze-drying and storage of Lactobacillus delbrueckii subsp. bulgaricus. Food Res. Int. 2016, 90, 251–258. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Gerbino, E.; Illanes, A.; Gomez-Zavaglia, A. Galacto-oligosaccharides as protective molecules in the preservation of Lactobacillus delbrueckii subsp. bulgaricus. Cryobiology 2011, 62, 123–129. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Dominguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, P.; Castro, H.; Kirby, R. Evidence of membrane lipid oxidation of spray-dried Lactobacillus bulgaricus during storage. Lett. Appl. Microbiol. 1996, 22, 34–38. [Google Scholar] [CrossRef]
- Liu, X.; Yan, X.; Bi, J.; Liu, J.; Zhou, M.; Wu, X.; Chen, Q. Determination of phenolic compounds and antioxidant activities from peel, flesh and seed of guava (Psidium guajava L.). Electrophoresis 2018, 39, 1654–1662. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Gómez-Zavaglia, A. Okara: A nutritionally valuable by-product able to stabilize Lactobacillus plantarum during freeze-drying, spray-drying, and storage. Front. Microbiol. 2017, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Ball, S.; Bullock, S.; Lloyd, L.; Mapp, K.P.; Ewen, A. Analysis of carbohydrates, alcohols, and organic acids by ion-exchange chromatography. In Agilent Hi-Plex Columns Applications Compendium; Agilent Technologies Inc.: Santa Clara, CA, USA, 2011; pp. 1–98. [Google Scholar]
- Padilha, C.V.S.; Miskinis, G.A.; Souza, M.E.A.O.; Pereira, G.E.; Oliveira, D.; Bordignon-Luiz, M.T.; Lima, M.S. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, W.; Chichilo, P.; Reynolds, H. (Eds.) Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; 2005. Current Through Revision 3, 2010, met. 985.29; AOAC: Gaithersburg, MD, USA, 2010; Chapter 45; pp. 101–102. [Google Scholar]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Sousa, M.S.B.; Vieira, L.M. Total phenolics and in vitro antioxidant capacity of tropical fruit pulp wastes. Braz. J. Food Technol. 2011, 14, 202–210. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Sariburun, E.; Sahin, S.; Demir, C.; Turkben, C.; Uylaser, V. Phenolic content and antioxidant activity of raspberry cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef]
- Sousa, S.; Pinto, J.; Pereira, C.; Malcata, F.X.; Pacheco, M.T.B.; Gomes, A.M.; Pintado, M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod. Process. 2015, 95, 96–105. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Carrillo, M.G.; Ferrario, M.; Guerrero, S. Effectiveness of UV-C light assisted by mild heat on Saccharomyces cerevisiae KE 162 inactivation in carrot-orange juice blend studied by flow cytometry and transmission electron microscopy. Food Microbiol. 2018, 73, 1–10. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, S.J.; Kang, D.H. Bactericidal effect of 266 to 279 nm wavelength UVC-LEDs for inactivation of Gram positive and Gram negative foodborne pathogenic bacteria and yeasts. Food Res. Int. 2017, 97, 280–287. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef] [Green Version]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.; Robles-Sanchez, R.; Martinez-Tellez, M.; Olivas, G.; Alvarez-Parrilla, E.; de la Rosa, L. Bioactive compounds in fruits: Health benefits and effect of storage conditions. Postharvest Stewart Rev. 2008, 4, 1–10. [Google Scholar]
- Champagne, C.P.; Møllgaard, H. Production of Probiotic Cultures and Their Addition in Fermented Foods. In Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 513–532. [Google Scholar]
- Champagne, C.P.; Mondou, F.; Raymond, Y.; Roy, D. Effect of polymers and storage temperature on the stability of freeze-dried lactic acid bacteria. Food Res. Int. 1996, 29, 555–562. [Google Scholar] [CrossRef]
| Parameters | Fruit Processing Coproducts | ||
|---|---|---|---|
| Acerola (ACE) | Cashew (CAS) | Guava (GUA) | |
| Simple sugars (g/100 g) | |||
| Fructose | 8.48 ± 0.01 a | 4.80 ± 0.01 b | 3.92 ± 0.01 c |
| Glucose | 5.31 ± 0.01 a | 4.88 ± 0.01 b | 3.17 ± 0.01 c |
| Maltose | 1.52 ± 0.01 b | 1.97 ± 0.01 a | 1.53 ± 0.01 b |
| Dietary fiber (g/100 g) | |||
| Insoluble dietary fiber | 61.16 ± 1.75 a | 47.49 ± 2.26 b | 49.12 ± 1.58 b |
| Soluble dietary fiber | 8.09 ± 0.69 b | 1.74 ± 0.53 c | 33.44 ± 3.63 a |
| Total dietary fiber | 69.25 ± 1.06 b | 49.22 ± 1.73 c | 82.55 ± 2.05 a |
| Phenolic compounds (mg/100 g) | |||
| Flavanols | |||
| Catechin | 3.12 ± 0.00 | ND | 1.95 ± 0.02 |
| Flavanones | |||
| Hesperetin | 1.43 ± 0.01 b | 1.25 ± 0.01 c | 1.61 ± 0.01 a |
| Naringenin | 1.37 ± 0.01 a | 0.42 ± 0.01 b | 0.31 ± 0.01 c |
| Flavonols | |||
| Kaempferol | 1.18 ± 0.01 a | 0.50 ± 0.02 c | 0.81 ± 0.02 b |
| Myricetin | 0.49 ± 0.01 c | 2.71 ± 0.06 a | 0.84 ± 0.00 b |
| Quercitin | 4.16 ± 0.01 a | 0.91 ± 0.02 b | 0.89 ± 0.03 b |
| Rutin | 1.19 ± 0.01 | 0.97 ± 0.02 | ND |
| Hydroxybenzoic acids | |||
| Syringic acid | ND | 0.91 ± 0.07 | 0.52 ± 0.03 |
| Hydroxycinnamic acids | |||
| Caffeic acid | 0.56 ± 0.01 b | 0.55 ± 0.01 b | 1.21 ± 0.01 a |
| p-Coumaric acid | 0.39 ± 0.01 | ND | ND |
| Caftaric acid | 0.92 ± 0.01 b | 1.32 ± 0.01 a | 0.64 ± 0.01 c |
| Chlorogenic acid | 0.35 ± 0.01 b | 0.31 ± 0.01 b | 0.62 ± 0.03 a |
| Polyphenols | |||
| Trans-resveratrol | 1.12 ± 0.02 a | 0.45 ± 0.01 b | 0.32 ± 0.01 c |
| Cis-resveratrol | 1.51 ± 0.07 a | 0.27 ± 0.01 c | 0.91 ± 0.05 b |
| Epicatechin gallate | 0.37 ± 0.01 c | 0.71 ± 0.01 b | 1.22 ± 0.02 a |
| Epicatechin | ND | 1.04 ± 0.05 | 1.25 ± 0.05 |
| Anthocyanins | |||
| Petunidin 3-glucoside | 0.49 ± 0.01 | 1.25 ± 0.05 | ND |
| Pelargonidin 3-glucoside | ND | 1.11 ± 0.01 | ND |
| Procyanidin B1 | ND | 0.62 ± 0.04 | 0.51 ± 0.01 |
| Procyanidin B2 | ND | 1.69 ± 0.09 | 0.43 ± 0.01 |
| Procyanidin A2 | ND | 1.05 ± 0.01 | 1.13 ± 0.01 |
| Total flavonoids (mg EC/100 g) 1 | 79.83 ± 0.23 a | 44.49 ± 0.61 b | 44.09 ± 1.01 b |
| Total phenolics (mg EAG/100 g) 2 | 492.107 ± 0.54 a | 368.520 ± 1.09 b | 304.057 ± 0.94 c |
| FRAP (µmol TEAC/g) 3 | 0.92 ± 0.01 a | 0.88 ± 0.01 b | 0.74 ± 0.01 c |
| ABTS (µmol TEAC/g) 3 | 16.14 ± 0.01 a | 15.29 ± 0.01 b | 14.54 ± 0.01 c |
| Treatments | Strains | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lactobacillus paracasei L-10 | Lactobacillus casei L-26 | Lactobacillus acidophilus LA-05 | |||||||
| Before Freeze-Drying | After Freeze-Drying | Average log Reduction * | Before Freeze-Drying | After Freeze-Drying | Average log Reduction * | Before Freeze-Drying | After Freeze-Drying | Average log Reduction * | |
| NEC | 9.3 ± 0.1 ** | 8.7 ± 0.2 | 0.5 ± 0.1 aC | 10.2 ± 0.1 ** | 7.3 ± 0.4 | 2.9 ± 0.3 aA | 10.3 ± 0.2 ** | 9.4 ± 0.1 | 0.9 ± 0.1 aB |
| FOS | 9.1 ± 0.2 | 8.9 ± 0.1 | 0.2 ± 0.0 bC | 10.2 ± 0.1 ** | 8.9 ± 0.3 | 1.3 ± 0.1 bA | 10.3 ± 0.1 ** | 9.4 ± 0.2 | 0.8 ± 0.0 aB |
| ACE | 9.1 ± 0.2 | 9.0 ± 0.2 | 0.2 ± 0.1 bC | 10.3 ± 0.1 ** | 8.9 ± 0.2 | 1.4 ± 0.3 bA | 10.2 ± 0.2 ** | 9.3 ± 0.1 | 0.9 ± 0.1 aB |
| CAS | 9.5 ± 0.2 ** | 8.9 ± 0.1 | 0.6 ± 0.1 aC | 9.8 ± 0.1 ** | 7.9 ± 0.4 | 2.0 ± 0.3 bA | 10.2 ± 0.2 ** | 9.2 ± 0.1 | 1.0 ± 0.1 aB |
| GUA | 9.3 ± 0.1 ** | 9.1 ± 0.1 | 0.3 ± 0.0 bC | 9.4 ± 0.1 ** | 7.6 ± 0.4 | 1.8 ± 0.3 bA | 10.2 ± 0.1 ** | 9.3 ± 0.2 | 1.0 ± 0.1 aB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, C.M.; Sampaio, K.B.; Menezes, F.N.D.D.; Almeida, E.T.d.C.; Lima, M.d.S.; Viera, V.B.; Garcia, E.F.; Gómez-Zavaglia, A.; de Souza, E.L.; de Oliveira, M.E.G. Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage. Microorganisms 2020, 8, 96. https://doi.org/10.3390/microorganisms8010096
Araújo CM, Sampaio KB, Menezes FNDD, Almeida ETdC, Lima MdS, Viera VB, Garcia EF, Gómez-Zavaglia A, de Souza EL, de Oliveira MEG. Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage. Microorganisms. 2020; 8(1):96. https://doi.org/10.3390/microorganisms8010096
Chicago/Turabian StyleAraújo, Caroliny Mesquita, Karoliny Brito Sampaio, Francisca Nayara Dantas Duarte Menezes, Erika Tayse da Cruz Almeida, Marcos dos Santos Lima, Vanessa Bordin Viera, Estefânia Fernandes Garcia, Andrea Gómez-Zavaglia, Evandro Leite de Souza, and Maria Elieidy Gomes de Oliveira. 2020. "Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage" Microorganisms 8, no. 1: 96. https://doi.org/10.3390/microorganisms8010096
APA StyleAraújo, C. M., Sampaio, K. B., Menezes, F. N. D. D., Almeida, E. T. d. C., Lima, M. d. S., Viera, V. B., Garcia, E. F., Gómez-Zavaglia, A., de Souza, E. L., & de Oliveira, M. E. G. (2020). Protective Effects of Tropical Fruit Processing Coproducts on Probiotic Lactobacillus Strains during Freeze-Drying and Storage. Microorganisms, 8(1), 96. https://doi.org/10.3390/microorganisms8010096








