Evidence for Multi-Organ Infection During Experimental Meningococcal Sepsis due to ST-11 Isolates in Human Transferrin-Transgenic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Experimental Infection in Mice
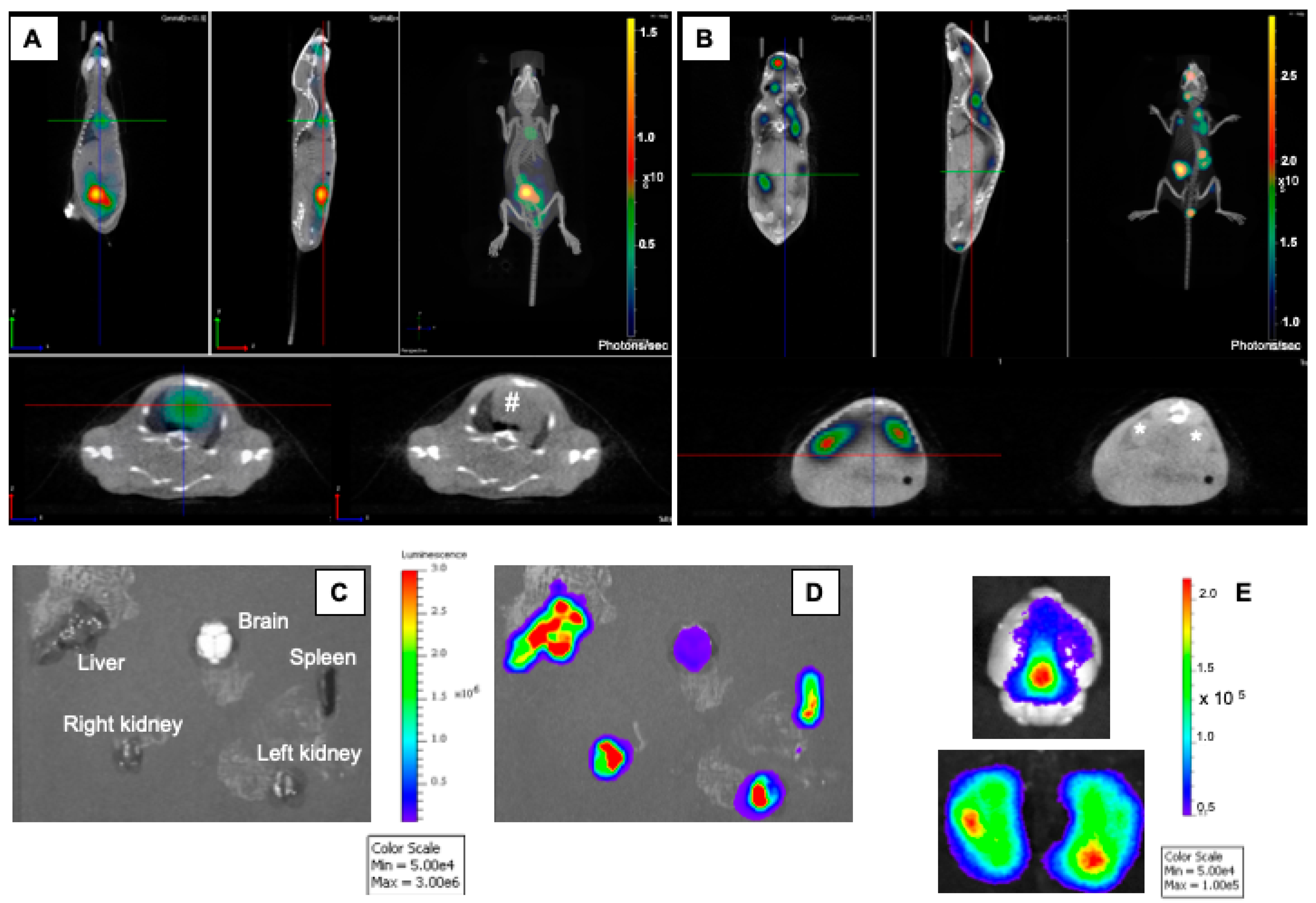
2.3. Dynamic In Vivo Imaging
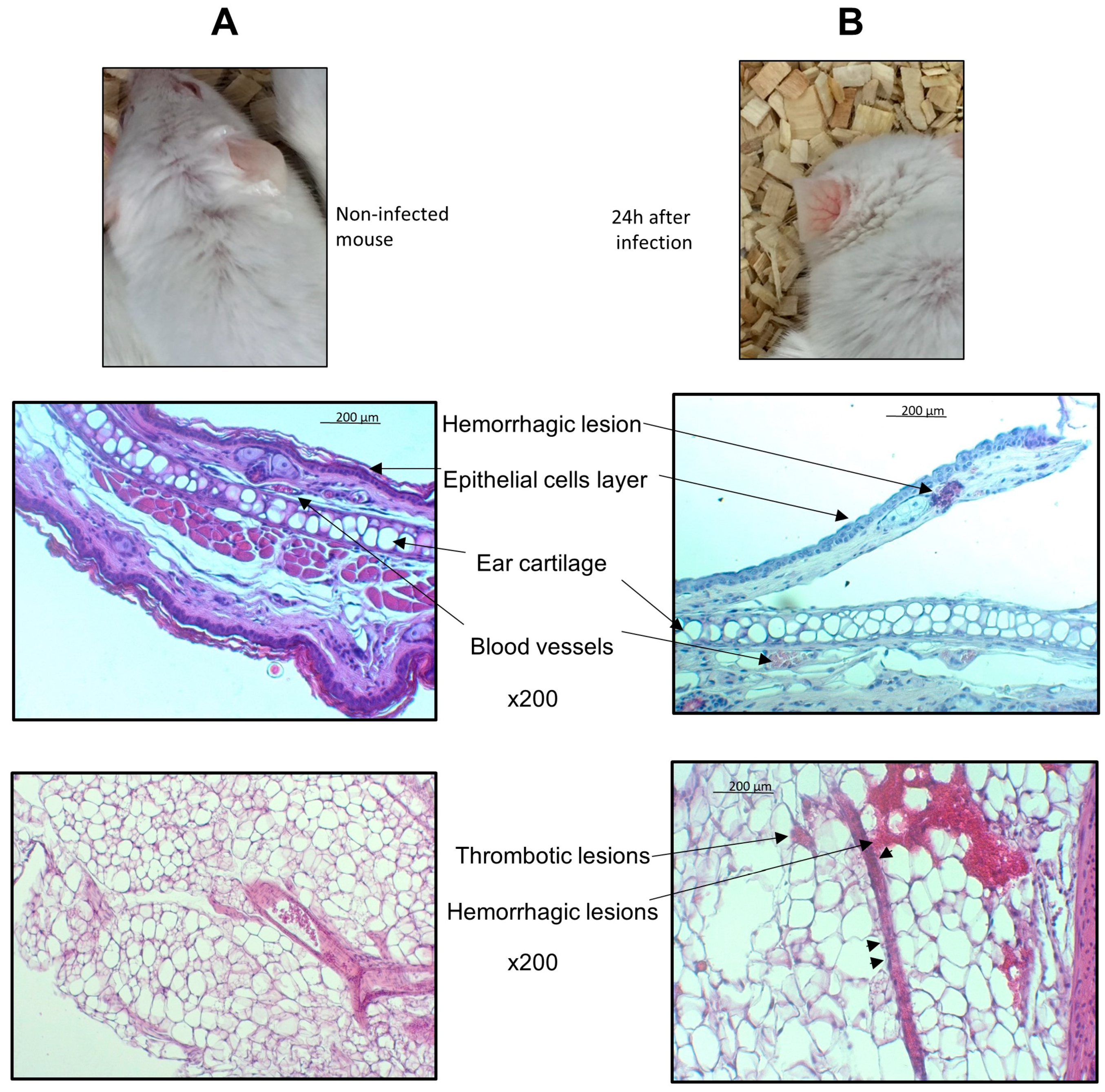
2.4. Histological Analysis
2.5. Ethical Aspects
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandtzaeg, P.; van Deuren, M. Classification and pathogenesis of meningococcal infections. Methods Mol. Biol. 2012, 799, 21–35. [Google Scholar] [CrossRef]
- Caugant, D.A. Genetics and evolution of Neisseria meningitidis: Importance for the epidemiology of meningococcal disease. Infect. Genet. Evol. 2008, 8, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Zarantonelli, M.L.; Lancellotti, M.; Deghmane, A.E.; Giorgini, D.; Hong, E.; Ruckly, C.; Alonso, J.-M.; Taha, M.-K. Hyperinvasive genotypes of Neisseria meningitidis in France. Clin. Microbiol. Infect. 2008, 14, 467–472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deghmane, A.-E.; Veckerlé, C.; Giorgini, D.; Hong, E.; Ruckly, C.; Taha, M.-K. Differential modulation of TNF-alpha-induced apoptosis by Neisseria meningitidis. PLoS Pathog. 2009, 5, e1000405. [Google Scholar] [CrossRef]
- Join-Lambert, O.; Lecuyer, H.; Miller, F.; Lelievre, L.; Jamet, A.; Furio, L.; Schmitt, A.; Pelissier, P.; Fraitag, S.; Coureuil, M.; et al. Meningococcal interaction to microvasculature triggers the tissular lesions of purpura fulminans. J. Infect. Dis. 2013, 208, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Hellerud, B.C.; Olstad, O.K.; Nielsen, E.W.; Trøseid, A.-M.S.; Skadberg, Ø.; Thorgersen, E.B.; Vege, Å.; Mollnes, T.E.; Brandtzæg, P. Massive Organ Inflammation in Experimental and in Clinical Meningococcal Septic Shock. Shock 2015, 44, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Bergounioux, J.; Coureuil, M.; Belli, E.; Ly, M.; Cambillau, M.; Goudin, N.; Nassif, X.; Join-Lambert, O. Experimental Evidence of Bacterial Colonization of Human Coronary Microvasculature and Myocardial Tissue during Meningococcemia. Infect. Immun. 2016, 84, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Johswich, K. Innate immune recognition and inflammation in Neisseria meningitidis infection. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Pajon, R.; Buckwalter, C.M.; Johswich, K.O.; Gray-Owen, S.D.; Granoff, D.M. A native outer membrane vesicle vaccine confers protection against meningococcal colonization in human CEACAM1 transgenic mice. Vaccine 2015, 33, 1317–1323. [Google Scholar] [CrossRef]
- Johansson, L.; Rytkonen, A.; Bergman, P.; Albiger, B.; Källström, H.; Hökfelt, T.; Agerberth, B.; Cattaneo, R.; Jonsson, A.-B. CD46 in meningococcal disease. Science 2003, 301, 373–375. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Sjölinder, M.; Wan, Y.; Sjölinder, H. CD46 accelerates macrophage-mediated host susceptibility to meningococcal sepsis in a murine model. Eur. J. Immunol. 2017, 47, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Lujan, E.; Pajon, R.; Granoff, D.M. Impaired Immunogenicity of Meningococcal Neisserial Surface Protein A in Human Complement Factor H Transgenic Mice. Infect. Immun. 2016, 84, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.-K.; Claus, H.; Lappann, M.; Veyrier, F.J.; Otto, A.; Becher, D.; Deghmane, A.-E.; Frosch, M.; Hellenbrand, W.; Hong, E.; et al. Evolutionary Events Associated with an Outbreak of Meningococcal Disease in Men Who Have Sex with Men. PLoS ONE 2016, 11, e0154047. [Google Scholar] [CrossRef] [PubMed]
- Perkins-Balding, D.; Ratliff-Griffin, M.; Stojiljkovic, I. Iron transport systems in Neisseria meningitidis. Microbiol. Mol. Biol. Rev. 2004, 68, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Zarantonelli, M.-L.; Szatanik, M.; Giorgini, D.; Hong, E.; Huerre, M.; Guillou, F.; Alonso, J.-M.; Taha, M.-K. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect. Immun. 2007, 75, 5609–5614. [Google Scholar] [CrossRef]
- Melican, K.; Michea Veloso, P.; Martin, T.; Bruneval, P.; Duménil, G. Adhesion of Neisseria meningitidis to dermal vessels leads to local vascular damage and purpura in a humanized mouse model. PLoS Pathog. 2013, 9, e1003139. [Google Scholar] [CrossRef]
- Capel, E.; Barnier, J.-P.; Zomer, A.L.; Bole-Feysot, C.; Nussbaumer, T.; Jamet, A.; Lécuyer, H.; Euphrasie, D.; Virion, Z.; Frapy, E.; et al. Peripheral blood vessels are a niche for blood-borne meningococci. Virulence 2017, 8, 1808–1819. [Google Scholar] [CrossRef]
- Bonazzi, D.; Lo Schiavo, V.; Machata, S.; Djafer-Cherif, I.; Nivoit, P.; Manriquez, V.; Tanimoto, H.; Husson, J.; Henry, N.; Chaté, H.; et al. Intermittent Pili-Mediated Forces Fluidize Neisseria meningitidis Aggregates Promoting Vascular Colonization. Cell 2018, 174, 143–155. [Google Scholar] [CrossRef]
- Sevestre, J.; Diene, S.M.; Aouiti-Trabelsi, M.; Deghmane, A.-E.; Tournier, I.; François, P.; Caron, F.; Taha, M.-K. Differential expression of hemoglobin receptor, HmbR, between carriage and invasive isolates of Neisseria meningitidis contributes to virulence: Lessons from a clonal outbreak. Virulence 2018, 9, 923–929. [Google Scholar] [CrossRef]
- Guiddir, T.; Deghmane, A.-E.; Giorgini, D.; Taha, M.-K. Lipocalin 2 in cerebrospinal fluid as a marker of acute bacterial meningitis. BMC Infect. Dis. 2014, 14, 276. [Google Scholar] [CrossRef]
- Szatanik, M.; Hong, E.; Ruckly, C.; Ledroit, M.; Giorgini, D.; Jopek, K.; Nicola, M.-A.; Deghmane, A.-E.; Taha, M.-K. Experimental meningococcal sepsis in congenic transgenic mice expressing human transferrin. PLoS ONE 2011, 6, e22210. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Antunes, A.; Fiette, L.; Deghmane, A.-E.; Taha, M.-K. Impact of corticosteroids on experimental meningococcal sepsis in mice. Steroids 2015, 101, 96–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belkacem, N.; Hong, E.; Antunes, A.; Terrade, A.; Deghmane, A.-E.; Taha, M.-K. Use of Animal Models to Support Revising Meningococcal Breakpoints of β-Lactams. Antimicrob. Agents Chemother. 2016, 60, 4023–4027. [Google Scholar] [CrossRef]
- Levy, M.; Deghmane, A.-E.; Aouiti-Trabelsi, M.; Dauger, S.; Faye, A.; Mariani-Kurkdjian, P.; Taha, M.-K. Analysis of the impact of corticosteroids adjuvant treatment during experimental invasive meningococcal infection in mice. Steroids 2018, 136, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Guiddir, T.; Gros, M.; Hong, E.; Terrade, A.; Denizon, M.; Deghmane, A.-E.; Taha, M.-K. Unusual initial abdominal presentations of invasive meningococcal disease. Clin. Infect. Dis. 2018, 67, 1220–1227. [Google Scholar] [CrossRef]
- Cheddani, H.; Desgabriel, A.-L.; Coffin, E.; Taha, M.-K.; Verdet, C.; Bachmeyer, C.; Flejou, J.-F.; Amiot, X. No Neck Pain: Meningococcemia. Am. J. Med. 2018, 131, 37–40. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, M.; Aouiti Trabelsi, M.; Taha, M.-K. Evidence for Multi-Organ Infection During Experimental Meningococcal Sepsis due to ST-11 Isolates in Human Transferrin-Transgenic Mice. Microorganisms 2020, 8, 1456. https://doi.org/10.3390/microorganisms8101456
Levy M, Aouiti Trabelsi M, Taha M-K. Evidence for Multi-Organ Infection During Experimental Meningococcal Sepsis due to ST-11 Isolates in Human Transferrin-Transgenic Mice. Microorganisms. 2020; 8(10):1456. https://doi.org/10.3390/microorganisms8101456
Chicago/Turabian StyleLevy, Michael, Myriam Aouiti Trabelsi, and Muhamed-Kheir Taha. 2020. "Evidence for Multi-Organ Infection During Experimental Meningococcal Sepsis due to ST-11 Isolates in Human Transferrin-Transgenic Mice" Microorganisms 8, no. 10: 1456. https://doi.org/10.3390/microorganisms8101456
APA StyleLevy, M., Aouiti Trabelsi, M., & Taha, M.-K. (2020). Evidence for Multi-Organ Infection During Experimental Meningococcal Sepsis due to ST-11 Isolates in Human Transferrin-Transgenic Mice. Microorganisms, 8(10), 1456. https://doi.org/10.3390/microorganisms8101456




