Different Bacteroides Species Colonise Human and Chicken Intestinal Tract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Target Species
2.2. Design of Species-Specific Primers
2.3. PCR Conditions
2.4. Human and Chicken Samples
2.5. Clustering of Bacteroides Species
2.6. Genome Analysis, Identification of Human- or Chicken-Specific Genes
2.7. Anaerobic Culture, Growth Media and In Vitro Growth Competition
2.8. Protein Mass Spectrometry
2.9. Statistics
2.10. Ethics Approval and Consent to Participate
3. Results
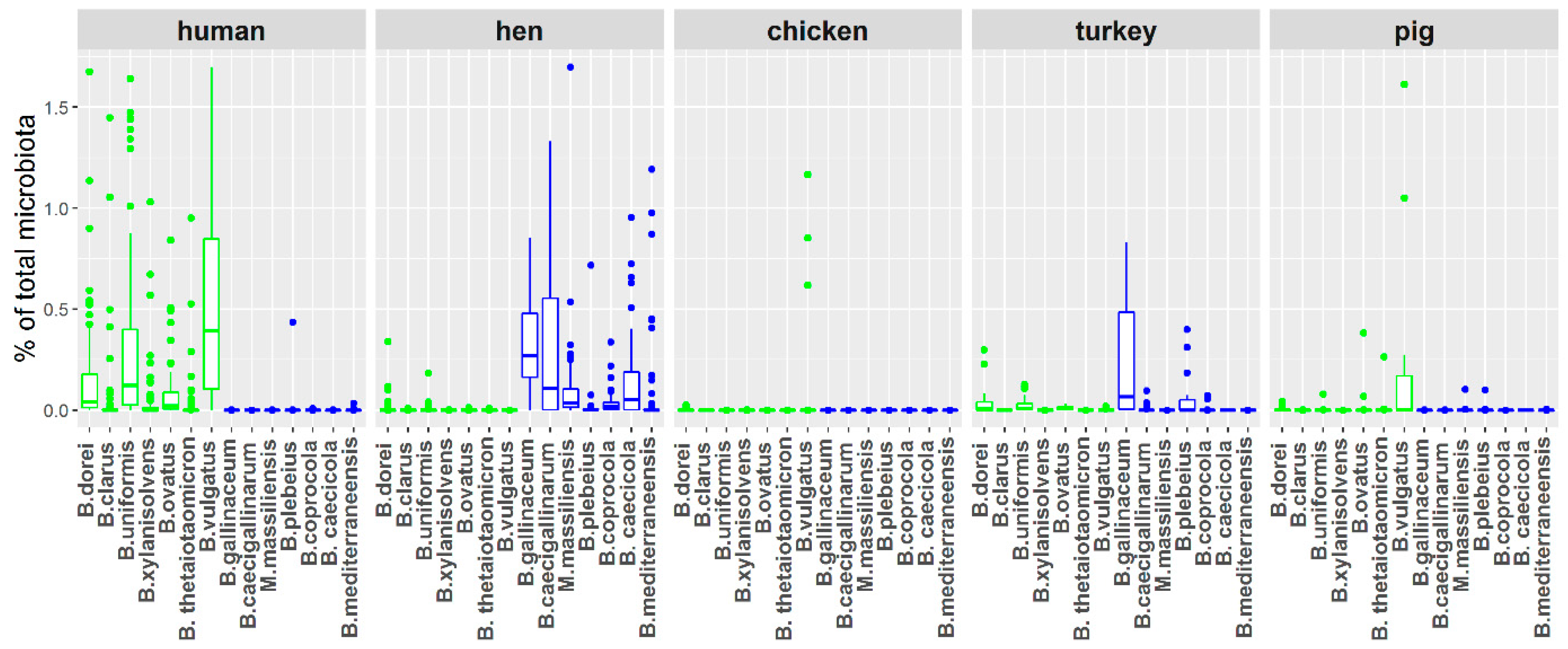
3.1. Distribution of Bacteroides Species in Chicken and Human Samples
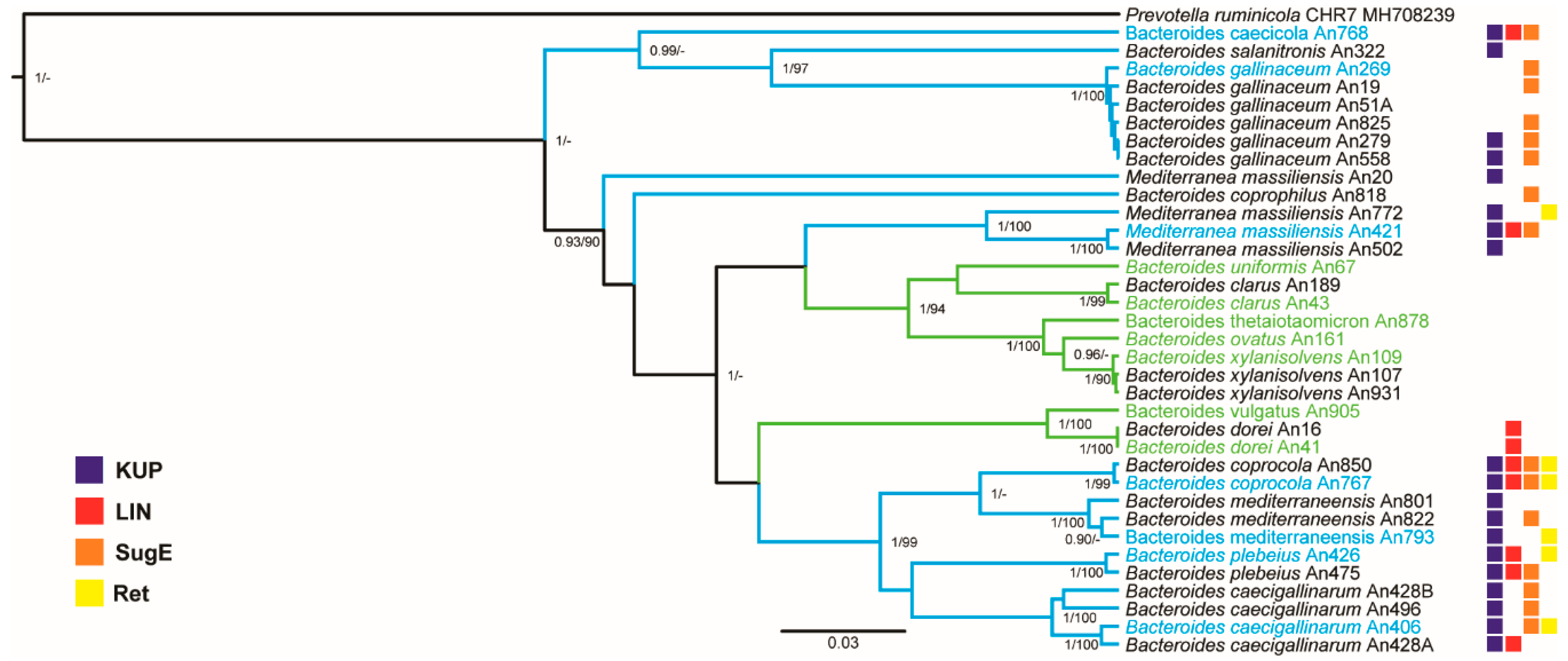
3.2. Clustering of Chicken- and Human-Adapted Bacteroides Species
3.3. Genome Differences between Human- and Chicken-Adapted Bacteroides Species
3.4. Identification of Genes Specific for Human- or Chicken-Adapted Bacteroides Species
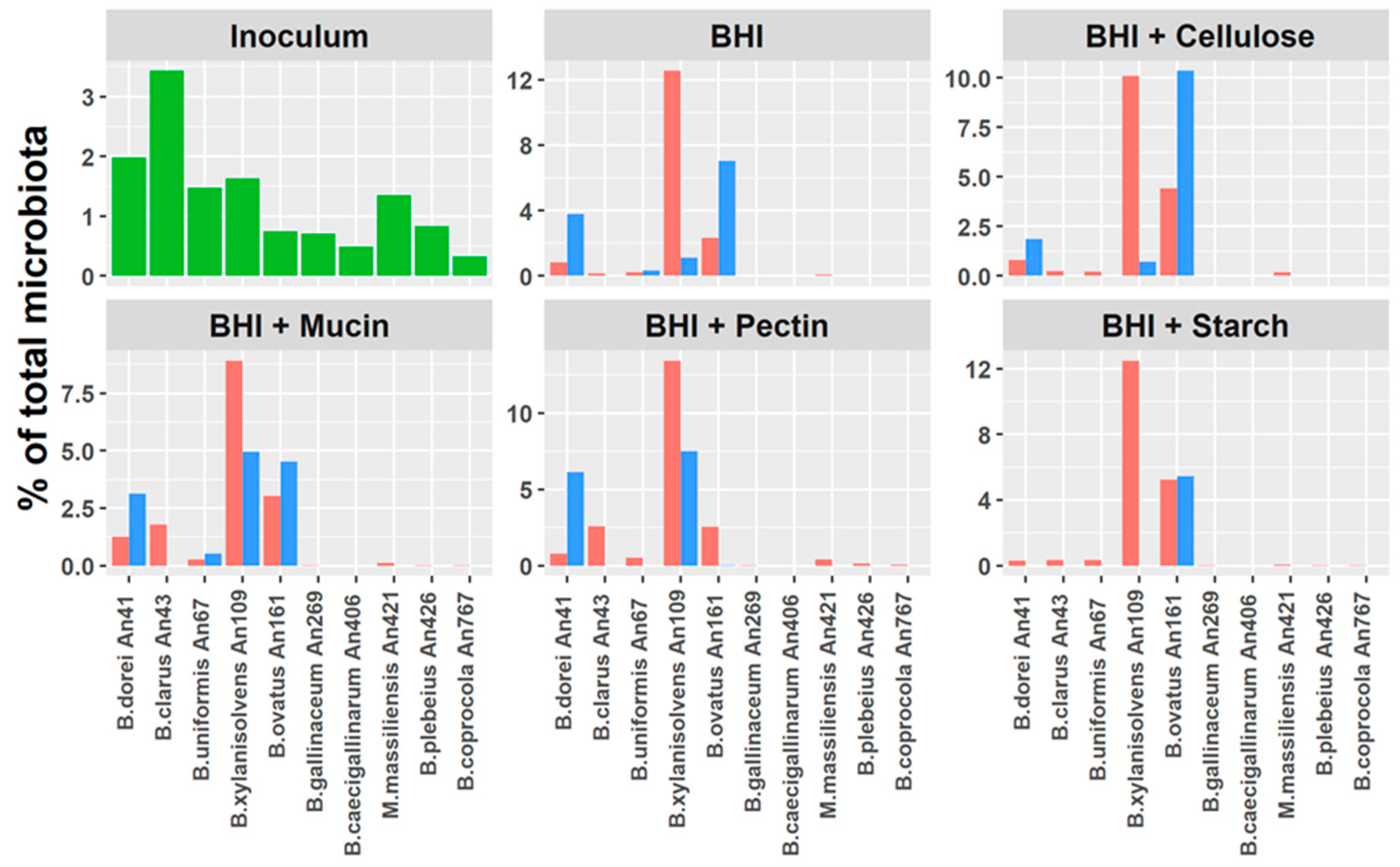
3.5. Do Host Adaptation and Differences in Gene Content Affect Bacteroides Competition?
3.6. Are Genes Specific for Human Bacteroides Species Expressed during Co-Culture In Vitro?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| KUP | K+ (potassium) uptake protein |
| BHI broth | Brain Heart Infusion broth |
| GNAT | Gcn5-related N-acetyltransferase |
References
- Gerzova, L.; Babak, V.; Sedlar, K.; Faldynova, M.; Videnska, P.; Cejkova, D.; Jensen, A.N.; Denis, M.; Kerouanton, A.; Ricci, A.; et al. Characterization of antibiotic resistance gene abundance and microbiota composition in feces of organic and conventional pigs from four EU countries. PLoS ONE 2015, 10, e0132892. [Google Scholar] [CrossRef] [PubMed]
- Kubasova, T.; Davidova-Gerzova, L.; Merlot, E.; Medvecky, M.; Polansky, O.; Gardan-Salmon, D.; Quesnel, H.; Rychlik, I. Housing systems influence gut microbiota composition of sows but not of their piglets. PLoS ONE 2017, 12, e0170051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubasova, T.; Kollarcikova, M.; Crhanova, M.; Karasova, D.; Cejkova, D.; Sebkova, A.; Matiasovicova, J.; Faldynova, M.; Pokorna, A.; Cizek, A.; et al. Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS ONE 2019, 14, e0212446. [Google Scholar] [CrossRef] [PubMed]
- Videnska, P.; Sedlar, K.; Lukac, M.; Faldynova, M.; Gerzova, L.; Cejkova, D.; Sisak, F.; Rychlik, I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS ONE 2014, 9, e115142. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, P.W.; Claesson, M.J. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimaraes, V.; Sokol, H.; Dore, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Medvecky, M.; Cejkova, D.; Polansky, O.; Karasova, D.; Kubasova, T.; Cizek, A.; Rychlik, I. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom. 2018, 19, 561. [Google Scholar] [CrossRef] [Green Version]
- Atherly, T.; Ziemer, C.J. Bacteroides isolated from four mammalian hosts lack host-specific 16S rRNA gene phylogeny and carbon and nitrogen utilization patterns. Microbiologyopen 2014, 3, 225–238. [Google Scholar] [CrossRef]
- Li, M.; Shang, Q.; Li, G.; Wang, X.; Yu, G. Degradation of Marine algae-derived carbohydrates by bacteroidetes isolated from human gut microbiota. Mar. Drugs 2017, 15, 92. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Martinez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, I. Composition and function of chicken gut microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Kubasova, T.; Kollarcikova, M.; Crhanova, M.; Karasova, D.; Cejkova, D.; Sebkova, A.; Matiasovicova, J.; Faldynova, M.; Sisak, F.; Babak, V.; et al. Gut anaerobes capable of chicken caecum colonisation. Microorganisms 2019, 7, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Crhanova, M.; Karasova, D.; Juricova, H.; Matiasovicova, J.; Jahodarova, E.; Kubasova, T.; Seidlerova, Z.; Cizek, A.; Rychlik, I. Systematic culturomics shows that half of chicken caecal microbiota members can be grown in vitro except for two lineages of Clostridiales and a single lineage of Bacteroidetes. Microorganisms 2019, 7, 496. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Tan, H.; Zhai, Q.; Chen, W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res. Int. 2019, 116, 637–644. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Comparative genomics of transport proteins in seven Bacteroides species. PLoS ONE 2018, 13, e0208151. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Foley, M.H.; Gardill, B.R.; Dejean, G.; Schnizlein, M.; Bahr, C.M.E.; Louise Creagh, A.; van Petegem, F.; Koropatkin, N.M.; Brumer, H. Surface glycan-binding proteins are essential for cereal beta-glucan utilization by the human gut symbiont Bacteroides ovatus. Cell. Mol. Life Sci. 2019, 76, 4319–4340. [Google Scholar] [CrossRef]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saputra, S.; Irisawa, T.; Sakamoto, M.; Kitahara, M.; Sulistiani; Yulinery, T.; Ohkuma, M.; Dinoto, A. Bacteroides caecigallinarum sp. nov., isolated from caecum of an Indonesian chicken. Int. J. Syst. Evol. Microbiol. 2015, 65, 4341–4346. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Contreras, B.E.; Moran-Ramos, S.; Villarruel-Vazquez, R.; Macias-Kauffer, L.; Villamil-Ramirez, H.; Leon-Mimila, P.; Vega-Badillo, J.; Sanchez-Munoz, F.; Llanos-Moreno, L.E.; Canizalez-Roman, A.; et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr. Obes. 2018, 13, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Salojarvi, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K.; de Vos, W.M.; Salonen, A. Intake of whole-grain and fiber-rich rye bread versus refined wheat bread does not differentiate intestinal microbiota composition in Finnish adults with metabolic syndrome. J. Nutr. 2013, 143, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Ngom, I.I.; Mailhe, M.; Ricaboni, D.; Vitton, V.; Benezech, A.; Khelaifia, S.; Michelle, C.; Cadoret, F.; Armstrong, N.; Levasseur, A.; et al. Noncontiguous finished genome sequence and description of Mediterranea massiliensis gen. nov., sp. nov., a new member of the Bacteroidaceae family isolated from human colon. New Microbes New Infect. 2018, 21, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Herrero-Fresno, A.; Thofner, I.; Skov, S.; Olsen, J.E. Interaction differences of the avian host-specific Salmonella enterica serovar gallinarum, the host-generalist S. Typhimurium, and the cattle host-adapted S. Dublin with chicken primary macrophage. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [PubMed]
- Lukjancenko, O.; Wassenaar, T.M.; Ussery, D.W. Comparison of 61 sequenced Escherichia coli genomes. Microb. Ecol. 2010, 60, 708–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaspers, E.; Overmann, J. Ecological significance of microdiversity: Identical 16S rRNA gene sequences can be found in bacteria with highly divergent genomes and ecophysiologies. Appl. Environ. Microbiol. 2004, 70, 4831–4839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seedorf, H.; Griffin, N.W.; Ridaura, V.K.; Reyes, A.; Cheng, J.; Rey, F.E.; Smith, M.I.; Simon, G.M.; Scheffrahn, R.H.; Woebken, D.; et al. Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 2014, 159, 253–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamberg, K.; Adamberg, S. Selection of fast and slow growing bacteria from fecal microbiota using continuous culture with changing dilution rate. Microb. Ecol. Health Dis. 2018, 29, 1549922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svihus, B.; Choct, M.; Classen, H.L. Function and nutritional roles of the avian caeca: A review. World Poult. Sci. J. 2013, 69, 249–263. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, environments, and gut microbiota. A preliminary investigation in children living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | An41 * | An109 | An161 | An43 | An67 | An878 | An905 | N. expr.# | Abund. (%) $ |
|---|---|---|---|---|---|---|---|---|---|
| Glutamate decarboxylase | 100 | 84.23 | 84.02 | 84.94 | 84.73 | 83.61 | 97.93 | 55 | 1.054 |
| Glucose-6-phosphate 1-dehydrogenase | 100 | 58.06 | 58.47 | 58.84 | 60.44 | 58.27 | 97.37 | 53 | 0.090 |
| 6-phosphogluconate dehydrogenase, decarboxylating | 100 | 61.65 | 61.23 | 61.6 | 60.55 | 62.5 | 95.4 | 50 | 0.206 |
| Pirin | 100 | 58.12 | 67.52 | 59.4 | 56.84 | 66.67 | 92.74 | 47 | 0.109 |
| Transaldolase | 100 | 85.78 | 86.24 | 85.78 | 85.78 | 86.24 | 98.62 | 44 | 0.084 |
| Nitroreductase family protein | 100 | 64.29 | 63.78 | 60.71 | 61.73 | 66.33 | 95.02 | 32 | 0.039 |
| Endonuclease IV | 100 | 75.95 | 74.05 | 79.01 | 78.46 | 74.05 | 96.18 | 29 | 0.043 |
| Osmosensitive K+ channel histidine kinase KdpD | 100 | 77.13 | 76.6 | 78.49 | 80.65 | 78.07 | 98.93 | 26 | 0.015 |
| Integral membrane protein | 100 | 58.83 | 58.11 | 56.9 | 57.77 | 58.21 | 97.36 | 12 | 0.029 |
| Glutamate/γ-aminobutyrate antiporter | 100 | 83.05 | 83.47 | 80 | 80.62 | 83.47 | 98.12 | 12 | 0.029 |
| Glutamine synthetase type I | 100 | 83.8 | 83.8 | 81 | 82 | 86.44 | 97.98 | 10 | 0.073 |
| YbbM seven transmembrane helix protein | 100 | 74.24 | 73.48 | 73.21 | 69.81 | 74.62 | 98.87 | 9 | 0.018 |
| probable uroporphyrin-III c-methyltransferase | 100 | 58.47 | 60.17 | 56.78 | 61.02 | 57.27 | 97.46 | 5 | 0.009 |
| FIG00936253: hypothetical protein | 100 | 78.35 | 77.32 | 78.35 | 82.72 | 80.41 | 99.07 | 3 | 0.011 |
| Imidazolonepropionase | 100 | 70.05 | 70.39 | 65.78 | 65.53 | 69.86 | 98.55 | 3 | 0.006 |
| Cell division protein MraZ | 100 | 68.42 | 67.67 | 60.87 | 67.67 | 68.42 | 97.83 | 3 | 0.006 |
| Formiminotetrahydrofolate cyclodeaminase | 100 | 54.68 | 56.65 | 55.96 | 57.14 | 55.83 | 98.55 | 2 | 0.005 |
| Urocanate hydratase | 100 | 84.98 | 84.07 | 81.03 | 81.54 | 84.94 | 97.59 | 2 | 0.007 |
| FIG00406657: hypothetical protein | 100 | 61.92 | 61.67 | 59.71 | 58.54 | 60.93 | 97.31 | 2 | 0.007 |
| Glutamate formiminotransferase | 100 | 79.33 | 79.33 | 85.19 | 87.12 | 82.67 | 98.98 | 1 | 0.002 |
| COG1272: Predicted membrane protein hemolysin III | 100 | 58.69 | 58.69 | 55.29 | 54.81 | 59.81 | 98.58 | 1 | 0.002 |
| Potassium-transporting ATPase A chain | 100 | 85.74 | 85.92 | 85.74 | 85.21 | 85.21 | 99.12 | 1 | 0.007 |
| Potassium-transporting ATPase C chain | 100 | 77.13 | 76.6 | 81.82 | 79.03 | 77.25 | 96.83 | 1 | 0.024 |
| D-alanyl-D-alanine dipeptidase | 100 | 68.56 | 66.67 | 66.98 | 70.65 | 65.7 | 98.19 | 1 | 0.002 |
| FIG00407539: hypothetical protein | 100 | 68.12 | 70.44 | 56.59 | 59.02 | 67.79 | 95.69 | n.e. | n.e. |
| Cold shock protein CspA | 100 | 50 | 50 | 51.68 | 58.11 | 53.69 | 99.32 | n.e. | n.e. |
| FIG00407264: hypothetical protein | 100 | 70 | 68.82 | 63.74 | 62.35 | 68.82 | 99.42 | n.e. | n.e. |
| FIG00412566: hypothetical protein | 100 | 56.85 | 56.85 | 54.58 | 54.47 | 55.37 | 95.53 | n.e. | n.e. |
| membrane protein ykgB | 100 | 70.31 | 70.83 | 87.11 | 90.21 | 70.31 | 95.9 | n.e. | n.e. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kollarcikova, M.; Faldynova, M.; Matiasovicova, J.; Jahodarova, E.; Kubasova, T.; Seidlerova, Z.; Babak, V.; Videnska, P.; Cizek, A.; Rychlik, I. Different Bacteroides Species Colonise Human and Chicken Intestinal Tract. Microorganisms 2020, 8, 1483. https://doi.org/10.3390/microorganisms8101483
Kollarcikova M, Faldynova M, Matiasovicova J, Jahodarova E, Kubasova T, Seidlerova Z, Babak V, Videnska P, Cizek A, Rychlik I. Different Bacteroides Species Colonise Human and Chicken Intestinal Tract. Microorganisms. 2020; 8(10):1483. https://doi.org/10.3390/microorganisms8101483
Chicago/Turabian StyleKollarcikova, Miloslava, Marcela Faldynova, Jitka Matiasovicova, Eva Jahodarova, Tereza Kubasova, Zuzana Seidlerova, Vladimir Babak, Petra Videnska, Alois Cizek, and Ivan Rychlik. 2020. "Different Bacteroides Species Colonise Human and Chicken Intestinal Tract" Microorganisms 8, no. 10: 1483. https://doi.org/10.3390/microorganisms8101483
APA StyleKollarcikova, M., Faldynova, M., Matiasovicova, J., Jahodarova, E., Kubasova, T., Seidlerova, Z., Babak, V., Videnska, P., Cizek, A., & Rychlik, I. (2020). Different Bacteroides Species Colonise Human and Chicken Intestinal Tract. Microorganisms, 8(10), 1483. https://doi.org/10.3390/microorganisms8101483





