1. Introduction
Bovine mastitis (BM) is the most prevalent disease affecting the dairy industry worldwide, and it largely impacts both the productivity and quality of dairy products, which lead to huge economical loss [
1].
Staphylococcus spp. strains are considered the most common pathogen for BM [
2]. In a survey conducted from 2015 to 2017, the isolation rate of
Streptococcus spp. in 6305 samples from Shandong, China, was as high as 57.9% [
3]. In addition to that,
E. coli is also involved in BM induction, where an epidemiological study showed that
E. coli is present in 14.4% of affected dairy cows [
4]. In recent years, antibiotics have been used as the major treatment for BM, but overuse of antibiotics may leave residues in dairy products, which promote antibiotic resistance [
5]. As a widely used antibiotic drug, cefotaxime was shown to have little improvement in cure rate, bacterial clearance, or bacterial load in Gram-negative bacteria-induced BM [
6]. While others have reported that in Gram-positive bacterial infection-induced BM, antibiotic treatment decreases somatic cell count (scc) in milk products, the overall quality is still affected [
7]. In addition, after mastitis treatment with gentamicin, which is a very effective antibiotic drug, it leads to the emergence of multidrug-resistant strains, as well as increasing the risk of the presence of this drug in the milk [
8]. Regarding this, it is critical to develop a new class of drugs that are effective in treating both Gram-positive and Gram-negative bacteria-induced BM.
Inflammasomes are supramolecular cellular complexes driving multiple innate immune responses, which include inflammatory caspases activation, proinflammatory interleukin 1 beta (IL-1β) and IL-18 maturation, as well as pyroptosis [
9]. Recent studies have shown that transfected bacterial endotoxin LPS triggers noncanonical inflammasome activation [
10]. NLRP6, a nucleotide-binding oligomerization domain (NOD)-like receptor family member, can also form inflammasome upon recognition of intestinal microbes [
11]. Emerging evidence of interactions between inflammasomes and bacterial ligands emphasized the importance of inflammasome activation in regulating host immune response against bacterial infection; however, the specific mechanism of inflammasome activation in BM remains to be elucidated.
Inflammasome activation is generally considered beneficial for bacterial clearance; however, exacerbating activation of inflammasomes may cause serious side effects [
12]. Previous studies have shown that inflammation and cell damage are critical in BM pathogenesis [
13], but attenuation of ASC-independent NLRP3 inflammasome activation, on the other hand, significantly ameliorates BM severity [
14]. Therefore, targeting inflammasome activity should be a potential solution in treating BM.
In recent years, many strategies have been developed to treat BM by enhancing the host immune response. Specifically, overexpression of antimicrobial peptides in the mammary gland was shown to be effective, which indicated the potential of developing antimicrobial peptide drugs in treating BM [
15]. With the lack of adaptive immunity, insects have developed two efficient innate immune systems in defending themselves against attack outside: Phagocytosis and encapsulation by specialized hemocytes; direct killing by enzymes and antimicrobial peptides secreted in hemolymph [
16]. Earlier studies found that injection of bacteria into pupae of cecropia moth induces the secretion of antimicrobial molecules in hemolymph [
17]. It was followed by successful extraction of two kinds of antimicrobial peptides from
Zophobas atratus (
Z. atratus), and their activity in killing both Gram-positive and Gram-negative bacteria was demonstrated as well [
18].
In the present study, we performed a similar bacterial injecting strategy to induce antimicrobial compounds in the hemolymph of Z. morio, a widely extant species in China that belongs to the same genus with Z. atratu. The safety and antibacterial activity of hemolymph were assessed through a series of in vitro models. We found that Z. morio hemolymph is effective in both the direct killing of BM-inducing pathogens and downregulation of inflammasome genes expression and/or activity, which leads to the attenuation of BM severity. Overall, our study highlighted the potential of Z. morio hemolymph as a novel drug candidate in treating BM.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Escherichia coli (E. coli, CVCC1450), E. coli (ATCC25922), Staphylococcus aureus (S. aureus, ATCC25923), Proteus vulgaris (P. vulgaris, CVCC1971), Clostridium perfringens (C. perfringens, CVCC1125), Streptococcus suis (S. suis, CVCC3307), and Salmonella typhimurium (S. typhimurium, ATCC14028) were purchased from the China Institute of Veterinary Drug Center (Beijing, China) and cultured in the lab with luria-bertani (LB) broth (Oxoid, Basingstoke, England). Klebsiella pneumoniae (K. pneumoniae) and Staphylococcus simulans (S. simulans No.11582) were isolated from milk samples of dairy cows with subclinical mastitis from a local farm and cultured in the lab with caton-adjusted mueller-hinton agar (CAMH-A) or caton-adjusted mueller-hinton broth (CAMH-B) (Oxoid, Basingstoke, England).
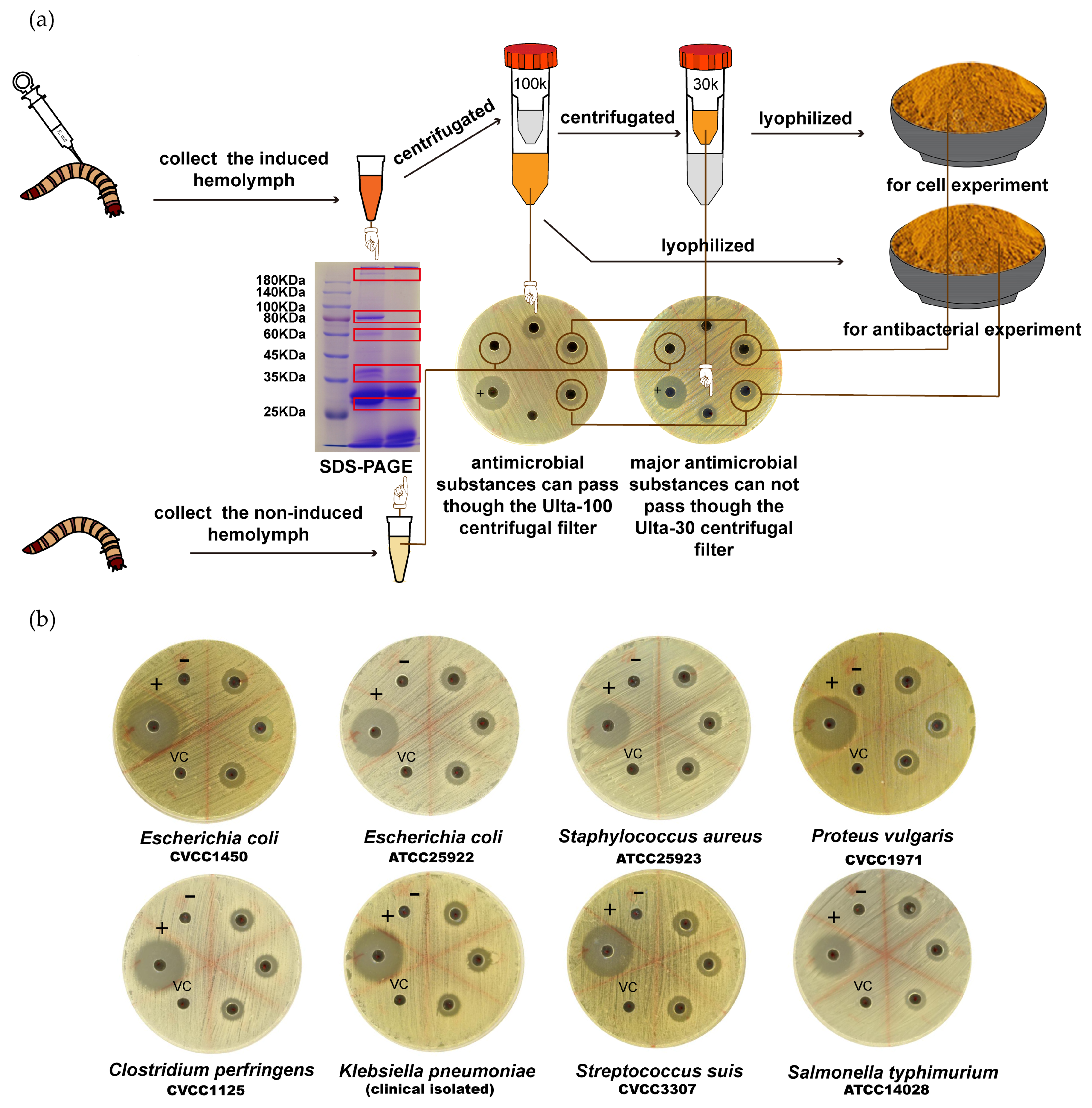
2.2. Z. morio Immunization and Hemolymph Collection
As previously described [
18], 3rd instar larvae of
Z. morio were injected with 1 μL of heat-killed, overnight culture of
E. coli or other bacterial strains (approximately 1 × 10
7 cells per injection). Then, 24 h later, injected insects were chilled for 1 min in ice-cold water, and approximately 30 μL of hemolymph was recovered by sectioning the metathoracic leg and gently squeezing the abdomen cavity. Harvested hemolymph was pooled in precooled plastic tubes and boiled for 10 min. After centrifugation at 20,000×
g for 30 min at 4 °C, the cell-free hemolymph was collected and filled through a 0.2 μm filter. Hemolymph from larvae without infection was also collected as a negative control. For quality test, 10 mg of hemolymph was run in 10% SDS-PAGE and analyzed.
After qualification, cell-free hemolymph was frozen at −80 °C for 1 h and then sublimated for 6 h under a pressure of 0.5 mbar and trap temperature of −40 °C. Lyophilized hemolymph was stored at −20 °C for long-term storage and re-dissolved in an appropriate volume of sterile water for the inhibition zone tests and biostability determination.
For cell assays, qualified, cell-free hemolymph was centrifugated (5000× g, 15 min) with an Amicon Ultra-100 centrifugal filter (Millipore, MA, USA). The filtrated hemolymph was centrifugated (5000× g, 15 min) by an Amicon Ultra-30 centrifugal filter (Millipore, MA, USA), and the supernatant hemolymph was used for the cell experiment. For long-term storage, supernatant was lyophilized as described above.
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Evaluations
A bacterial susceptibility assay was performed to primarily compare the susceptibility of various bacterial strains against
Z. morio hemolymph. As previously described [
18], 6 mm-diameter wells were cut out in sterile petri dishes with 7.5 mL of CAMH-A medium, and 20 μL of hemolymph was added in each well. Later, log-phase bacteria were evenly coated on the plate (approximately 1 × 10
6 cells/plate). Plates were incubated overnight at 37 °C, and the diameters of the clear zones were recorded.
E. coli ATCC25922 served as a reference control. Three experiments were carried out in duplicate.
For MIC determination, each bacteria strain (1 × 108 CFU/mL or OD600 of 0.5) was diluted 1:1000 in CAMH-B medium and added to a 96-well plate (100 μL/well). Then, 100 μL of serially diluted hemolymph (ultra-filtrated and lyophilized) was added. The MIC value was recorded after 18 h of incubation. E. coli ATCC25922 was used as a reference control. Three experiments were carried out in duplicate.
The MBC of Z. morio hemolymph was determined after MIC. A 10 μL sample from each well of the MIC plates was collected and transferred into a fresh CAMH-A plate. Plates were incubated at 37 °C for 18 to 24 h, and the MBC value was recorded. E. coli ATCC25922 was used as the reference control. Three experiments were carried out in duplicate.
2.4. Biostability Determination of Z. morio Hemolymph
2.4.1. Effect of High Temperature on the Bioactivity of Z. morio Hemolymph
To assess the effect of high temperature on the antibacterial activity of Z. morio hemolymph, hemolymph samples were boiled in a water bath for 5 to 30 min. In addition, 20 μL of boiled hemolymph was added into the CAMH-A plate with E. coli (ATCC25922) and incubated overnight at 37 °C. The diameter of clear zones was recorded. Three experiments were carried out in duplicate.
2.4.2. Effect of Repeated Freeze–Thaw Cycle on the Bioactivity of Z. morio Hemolymph
Hemolymph samples were frozen and thawed 6 times. Then, 20 μL of treated hemolymph was added into the CAMH-A plate with E. coli (ATCC25922) and incubated overnight at 37 °C. The diameter of clear zones was recorded. Three experiments were carried out in duplicate.
2.4.3. Effect of Dissolving in Cow mIlk on the Bioactivity of Z. morio Hemolymph
Lyophilized Z. morio hemolymph samples were dissolved in the same volume of milk where it was extracted from. Further, 20 μL of dissolved hemolymph was added into the CAMH-A plate with E. coli (ATCC25922) and incubated overnight at 37 °C. The diameter of clear zones was recorded. Three experiments were carried out in duplicate.
2.4.4. Effect of UV Exposure on the Bioactivity of Z. morio Hemolymph
Here, 500 μL of hemolymph was added into 24-well cell culture plates and exposed under UV light for 5 to 40 min. Then, 20 μL of UV-treated hemolymph was added into the CAMH-A plate with E. coli (ATCC25922) and incubated overnight at 37 °C. The diameter of clear zones was recorded. Three experiments were carried out in duplicate.
2.5. Time Course Determination of Z. morio Hemolymph’s Inhibitory Activity
Overnight-cultured E. coli CVCC1450 or S. aureus ATCC25923 was diluted in CAMH-B media (1 × 105 CFU/mL) with 1 MIC of Z. morio hemolymph (0.5 mg/mL for E. coli CVCC1450 and 1 mg/mL for S. aureus ATCC25923). In addition, 1 MIC of gentamicin (0.5 μg/mL) was used as a control drug in both assays. Bacteria were cultured at 37 °C with constant shaking and the OD595 was recorded at 1, 2, 3, 4, 5, 6, 7, or 8 h after drug addition. Three experiments were carried out in duplicate.
2.6. Time Course Determination of Z. morio Hemolymph’s Bactericidal Activity
E. coli strains CVCC1450 and S. aureus ATCC25923 were used in this assay. After overnight culture, bacteria were diluted 1:1000 in 1 mL CAMH-B medium and cultured subsequently at 37 °C with constant shaking, and 10 MIC of Z. morio hemolymph (5 mg/mL for E. coli CVCC1450 and 10 mg/mL for S. aureus ATCC25923) was added 4 or 8 h later. Then, 10 MIC of gentamicin (5 μg/mL) and enrofloxacin (1.25 μg/mL) were used as control drugs in the E. coli group, and gentamicin (5 μg/mL), enrofloxacin (2.5 μg/mL), and Ampicillin (20 μg/mL) were used as control drugs in the S. aureus group. Next, 100 μL of bacteria culture was collected at 2, 4, 6, 8, 10, 12, 14, 16, 18, or 20 h after drug addition and evenly coated on CAMH-A plates after appropriate folds of dilution. Plates were incubated at 37 °C overnight and the number of colonies were counted. Three experiments were carried out in duplicate.
2.7. Scanning Electron Microscopy
As previously described [
19], bacteria in the exponential phase were diluted in phosphate-buffered saline (PBS) to reach a final concentration of 10
8 CFU/mL. Bacterial samples were then treated with 1 MBC of
Z. morio hemolymph (1 mg/mL for
E. coli CVCC1450 and 1 mg/mL for
S. aureus ATCC25923) at 37 °C for 30 min. After that, bacterial samples were collected by centrifugation (5000×
g, 10 min) and fixed according to the following procedure. Cells were fixed at 4 °C overnight with 3% glutaraldehyde in 1 M phosphate buffer (pH 7.1). After washing with PBS, samples were incubated with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer before dehydration in a graduated ethanol series (30%, 50%, 70%, 90%, 95%, and 100%), followed by 100% acetone. Samples were critical-point dried with liquid carbon dioxide using a CPD 030 critical point dryer (BAL-TEC, Witten, Germany) and then sputter-coated with 20 nm gold particles using a SCD 005 (BAL-TEC). The cells were then examined under a Quanta 200 FEG field-emission scanning electron microscope (FEI, Eindhoven, The Netherlands). Three experiments were carried out in duplicate.
2.8. Bacterial Membrane Permeability Assay
E. coli strains CVCC1450 and
S. aureus ATCC25923 were used in this assay. As previously described [
19,
20], bacteria were cultured to reach an OD
600 value between 0.4 and 0.5. After centrifugation (10,000×
g, 1 min), bacterial pellets were suspended in PBS to reach approximately 5 × 10
7 CFU/mL. Suspensions were treated with various concentrations of
Z. morio hemolymph for 1 h. Bacterial lysates were collected by centrifugation (5000×
g, 10 min) and incubated with 10 μM of propidium iodide (PI) at 37 °C for 30 min in the dark. Samples were excited at 535 nm and OD
615 were recorded. Bacterial lysates produced by ultrasonic treatment (300 W, 20 min) were used as positive control. For negative control, intact bacterial suspensions were incubated with PI; for blank control, only PI solution was used. Relative fluorescence intensity (RFI) was calculated as below:
Three experiments were carried out in duplicate.
2.9. Hemolytic Activity Determination
As previously described [
20], sheep erythrocytes were collected from fresh sheep blood by centrifugation (2000×
g, 3 min, 4 °C). Erythrocytes were washed three times with PBS and adjusted to a stock concentration of 20%. For the hemolytic assay, 100 μL of erythrocyte stock was mixed with the same volume of
Z. morio hemolymph (various concentrations starting from 0.2 mg/mL) and diluted with PBS to reach a total volume of 1 mL. Samples were incubated at 37 °C for 2 h. After centrifugation (2000×
g, 3 min, 4 °C), the supernatants were removed and erythrocytes were hemolyzed by adding the same volume of 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA). The absorbance of the hemolyzed erythrocytes supernatants at 576 nm was measured. The level of hemolysis was considered as 0% for untreated erythrocytes samples and 100% for 0.1% Triton X-100 buffer. Three experiments were carried out in duplicate.
2.10. Adhesion Assay
MAC-T cells are a cell line derived from the bovine mammary epithelium, which were transferred with simian virus 40 (SV-40) T antigen [
21] (a gift from Ying Yu, China Agricultural University). They were cultured in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium (1:1) supplemented with 5% fetal bovine serum, 100 U/mL of penicillin, and 0.1 mg/mL of streptomycin at 37 °C in an atmosphere of 5% CO
2.
E. coli CVCC1450 or S. simulans No.11582 was cultured in LB broth overnight at 37 °C with constant shaking and subcultured 1:100 in fresh LB broth for an additional 3 h until reaching the mid-log phase (OD600 = 0.5).
For the adhesion assay, as previously described [
22], MAC-T cells (3 × 10
5 cells/well) were seeded into a 6-well cell culture plate (Corning, Inc., Corning City, NY, USA). Confluent cell monolayers were pretreated with 4 MIC of hemolymph (2 mg/mL for
E. coli CVCC1450 and 4 mg/mL for
S. simulans No.11582) for 1 h and then exposed to
E. coli CVCC1450 (3 × 10
7 CFU) or
S. simulans No.11582 (3 × 10
7 CFU). In a similar experimental setting, cell monolayers were treated with hemolymph and bacteria simultaneously. Then, 2 h after bacteria stimulation, cell monolayers were washed four times with PBS to remove nonadherent bacteria and treated with 0.05% trypsin for 10 min at 37 °C. Cells were harvested by centrifugation (4000×
g, 10 min) and lysed with 100 μL of 0.2% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) buffer. CFUs of
E. coli or
S. simulans were determined by LB agar plating. The adhesion rate of bacteria was determined by dividing the number of adhered bacteria treated with hemolymph to the number of adhered bacteria in the control group [
23].
2.11. Internalization Assay
As previously described [
22], MAC-T cells (3 × 10
5 cells/well) were seeded into a 6-well cell culture. Confluent cell monolayers were pretreated with 4 MIC of hemolymph (4 mg/mL) for 1 h and then exposed to
S. simulans No.11582 (3 × 10
7 CFU). In a similar experimental setting, cell monolayers were treated with hemolymph and bacteria simultaneously. Internalizing events of
S. simulans No.11582 were recorded after 2 h of co-incubation with
Z. morio hemolymph followed by 2 h of culture with DMEM supplemented with gentamicin (100 μg/mL). After washing, digestion, and lysing, the supernatants were diluted and coated on a CAMH-A agar plate, and the CFU number was counted 18 h later.
2.12. Western Blot
MAC-T cells (3 × 10
5 cells/well) were seeded into a 6-well cell culture plate and treated with
E. coli CVCC1450 (3 × 10
7 CFU) or
S. simulans No.11582 (3 × 10
7 CFU), in the presence or absence of
Z. morio hemolymph (2 mg/mL for
E. coli CVCC1450 and 4 mg/mL for
S. simulans No.11582). Then, 8 h later, the whole cell lysis of each sample was collected and protein concentrations were determined with a bicinchoninic acid (BCA) protein assay kit (Pierce Chemical Co., Rockford, IL, USA), as previously described [
22]. Primary antibodies including rabbit polyclonal anti-NLRP3 (19771-1-AP, 1:500 dilution; Protein Tech Group, Wuhan, Hubei, China), rabbit polyclonal anti-caspase-1 (ab179515, 1:500 dilution; Abcam, Cambridge, UK), goat polyclonal anti-NLRP6 (SC50639, 1:500 dilution; Santa Cruz Biotechnology, CA, USA), and mouse anti-GAPDH mAb (60004-1-Ig, 1:500 dilution; Protein Tech Group Wuhan, Hubei, China); and secondary antibodies including horseradish peroxidase-conjugated affinipure goat anti-mouse IgG (SA00001-1, 1:5000 dilution; Protein Tech Group Wuhan, Hubei, China) and goat anti-rabbit IgG (SA00001-2, 1:8000 dilution; Protein Tech Group Wuhan, Hubei, China) were used. Densitometric values of Western blot images were obtained from three independent experiments using Image J software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA). Results are presented as the ratio of NLRP3, NLRP6, or caspase-1 intensity to GAPDH.
2.13. Cytotoxicity Determination
A cytotoxicity assay was performed based on the Enhanced Cell Counting Kit-8 (CCK-8) (Beyotime, Beijing, China). For detecting the pyroptosis induced by E. coli or S. simulans, MAC-T cells (5000 cells/well) were seeded into a 96-well cell culture plate and treated with E. coli or S. simulans (5 × 105 CFU) in the presence or absence of Z. morio hemolymph (2 mg/mL for E. coli CVCC1450 and 4 mg/mL for S. simulans No.11582). Then, 18 or 24 h after stimulation, cells were washed with PBS and cultured in DMEM supplemented with 2% FBS and 10 μL of enhanced CCK-8 solution. After 2 h of culture, OD450 was measured. For detecting the cytotoxicity of Z. morio hemolymph, MAC-T cells (5000 cells/well) were seeded into a 96-well cell culture plate and treated with various concentrations of Z. morio hemolymph. Then, 2 h later, cells were washed with PBS and cultured in DMEM supplemented with 2% FBS and 10 μL of enhanced CCK-8 solution. After 2 h of culture, OD450 was measured.
2.14. Enzyme-Linked Immunosorbent Assay (ELISA)
MAC-T cell supernatants were collected 18 and 24 h after E. coli or S. simulans No.11582 infection, with or without Z. morio hemolymph (2 mg/mL for E. coli CVCC1450 and 4 mg/mL for S. simulans No.11582). Commercially available ELISA kits specific for bovine IL-1β (DG90995Q) and bovine IL-18 (DG91524Q; Beijing Dongge Biotechnology Co., Beijing, China) were used to detect the concentration of IL-1β and IL-18 in supernatants, respectively.
2.15. Caspase Substrates Cleavage Assay
MAC-T cells (3 × 105 cells/well) were seeded into a 6-well cell culture plate and treated with E. coli CVCC1450 (3 × 107 CFU) or S. simulans No.11582 (3 × 107 CFU), in the presence or absence of Z. morio hemolymph (2 mg/mL for E. coli CVCC1450 and 4 mg/mL for S. simulans No.11582). After stimulation for 8 h, MAC-T cells were lysed with caspase assay buffer (50 mM HEPES pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA, and 10% glycerol), and supernatants were collected after centrifugation (21,000 g, 2 min) and mixed with Ac-YVAD-pNA (YTB1001, BioLabBJ Company Ltd., Beijing, China) or Ac-LEVD-pNA (YTB1004, BioLabBJ Company Ltd., Beijing, China) (final concentration 200 μM) in a 96-well plate. The OD value was measured at 405 nm before or after 2 h of incubation at 37 °C. The level of increase in OD405 indicates the cleaving activity of caspases.
2.16. Statistical Analysis
Statistical analysis was performed by using GraphPad Prism7 software (version 7, GraphPad Software Inc., San Diego, CA, USA). For two-group comparisons with Gaussian distribution, a two-tailed unpaired t-test with Welch’s correction was applied when the variances of two groups were proved equal by the F test. For two-group comparisons with non-Gaussian distribution, a Mann–Whitney test was applied. For multigroup comparisons with Gaussian distribution, one-way ANOVA with Tukey-Kramer’s multiple-comparison test was used after the homogeneity of variance was confirmed by Bartlett’s test. For multigroup comparisons with non-Gaussian distribution, a Kruskal–Wallis test with Dunn’s test was used. p values of 0.05 or less were the threshold for statistical significance. p-values: * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
It is well-known that antibiotic peptides are widely expressed in most insect species [
25].
Harmonia axyridis, an Asian lady beetle, was reported to express an abundant level of various kinds of antimicrobial peptides [
26]. In our study, as compared to the control,
E. coli-induced hemolymph extracts showed multiple additional bands in SDS-PAGE analysis. Therefore, it is reasonable to assume the high diversity of antimicrobial molecules in
E. coli-induced
Z.
morio hemolymph. In addition, as reported previously, there were three different antimicrobial compounds identified in
Z. atratus (another species closely related to
Z. morio)—coleoptericin, defensins B, and defensins C [
18]—so it is reasonable to assume that similar compounds existed
Z. morio as well. Ultrafiltration separation and SDS-PAGE analysis suggested that the approximate molecular weight of effective antimicrobial compounds is between 30 and 100 kDa. With improved techniques like 2-D electrophoresis or high-performance liquid chromatography (HPLC), more compounds with antibacterial activity would be identified. More importantly,
E. coli-induced
Z. morio hemolymph showed a broad antibacterial profile against
E. coli,
S. aureus,
K. pneumoniae, as well as other commonly seen pathogens in BM, which suggests its potential capability as a future drug candidate.
Our data show that
Z. morio hemolymph is effective in killing
E. coli or
S. aureus, in addition to gentamicin and enrofloxacin. SEM and membrane permeability results revealed the bactericidal activity of hemolymph, which is consistent with the role of other arthropod-derived antimicrobial peptides, as previously reported [
20]. In general,
Z. morio hemolymph is worthwhile to be developed in both preventing and curing BM.
From the perspective of drug development, biostability is undoubtedly an important factor in addition to effectivity. Gramicidin A, a proved antimicrobial peptide drug clinically, can only be used topically due to its hemolytic activity [
27]. As a common side-effect, hemolysis largely compromised the application of most antimicrobial peptides [
19,
28]. Although considered an in vitro environment, it is believed that the inactivity of hemolysis is also a pre-requirement for all drugs targeting lactiferous ducts. Our data demonstrate that
Z. morio hemolymph has no obvious hemolytic activity or cytotoxicity in the range of effective concentrations. Besides, the biostability of
Z. morio hemolymph under various extreme conditions was also assured. Overall, these results strongly suggest that
Z. morio hemolymph would be a promising candidate for antimicrobial drugs.
As described above,
E. coli and
S. aureus are two of the major pathogens inducing BM, and
E. coli CVCC1450 is the most commonly used strain in BM studies [
29,
30].
S. simulans, a non-
aureus staphylococci species, is also responsible for subclinical BM [
31]. In addition,
S. simulans was reported to induce osteoarticular and dermatic infection in human [
32]. Our data show that
S. simulans has the most cytotoxicity in various Staphylococcus species tested. Therefore, in order to investigate the protective role of
Z. morio hemolymph in mammary epithelial cells under infection, we used both
E. coli CVCC1450 and
S. simulans (
Supplementary Figure S3).
Regarding the molecular mechanism of
Z. morio hemolymph’s protective effect during bacterial infection, we found that
Z. morio hemolymph efficiently downregulates the expression of inflammasome-related genes including NLRP3 and NLRP6, which are reported as important players in bacterial sensing and inflammasome activation [
10,
11]. Besides,
Z. morio hemolymph treatment also attenuates cleavage of caspase-1 and caspase-4, and the secretion of mature IL-1β and IL-18 was also inhibited. These data illustrated the regulating mechanisms of
Z. morio hemolymph. Thus, future work on compound purification and functional determination will provide a deeper insight on molecular interactions and signal transduction. Overall, our study highlights the potential role of
E. coli-induced
Z. morio hemolymph in BM protection, which promotes the investigation of antimicrobial activity from natural extracts and the development of new treatments in response to bacterial infection-induced BM.