Vaccines against Meningococcal Diseases
Abstract
:1. Introduction
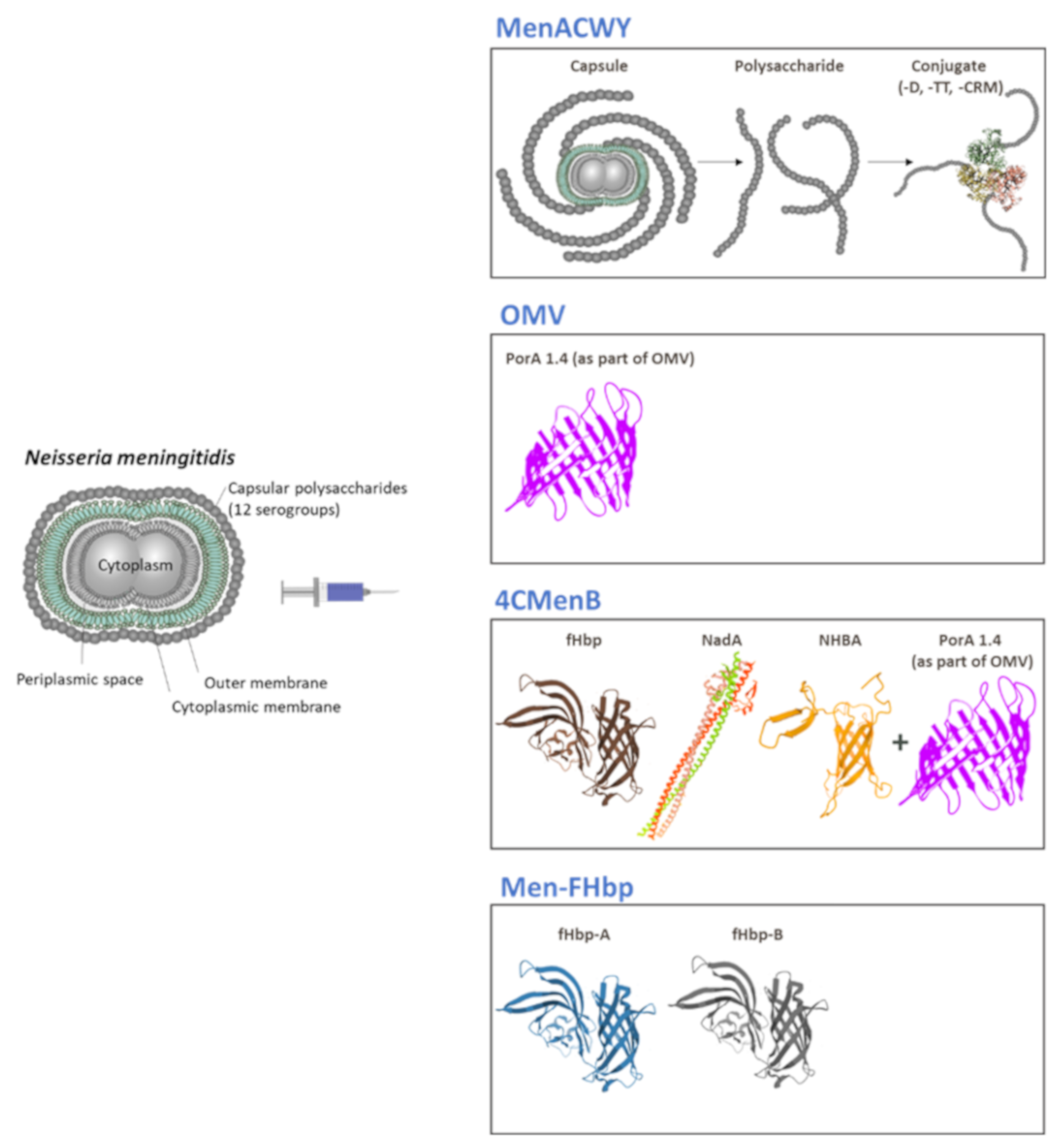
2. Meningococcal Polysaccharide-Conjugate Vaccines
2.1. Monovalent Conjugate Vaccines
2.1.1. Meningococcal Serogroup C Conjugate Vaccines
2.1.2. Meningococcal Serogroup A Conjugate Vaccines
2.2. Multivalent Conjugate Vaccines
3. Meningococcal Protein-Based Vaccines
3.1. Outer Membrane Vesicles (OMV)-Based Vaccines
3.2. 4CMenB Vaccine
3.3. MenB-FHbp Vaccine
4. Outbreaks
5. Future Perspectives
6. Conclusions
Trademarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pace, D.; Pollard, A.J. Meningococcal disease: Clinical presentation and sequelae. Vaccine 2012, 30 (Suppl. S2), B3–B9. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Meningococcal Meningitis. Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/meningococcal-meningitis (accessed on 22 April 2020).
- Jafri, R.Z.; Ali, A.; Messonnier, N.E.; Tevi-Benissan, C.; Durrheim, D.; Eskola, J.; Fermon, F.; Klugman, K.P.; Ramsay, M.; Sow, S.; et al. Global epidemiology of invasive meningococcal disease. Popul. Health Metr. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, L.H.; Trotter, C.L.; Ramsay, M.E. Global epidemiology of meningococcal disease. Vaccine 2009, 27 (Suppl. S2), B51–B63. [Google Scholar] [CrossRef] [PubMed]
- Pelton, S.I. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J. Adolesc. Health 2016, 59, S3–S11. [Google Scholar] [CrossRef] [Green Version]
- Christensen, H.; May, M.; Bowen, L.; Hickman, M.; Trotter, C.L. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 853–861. [Google Scholar] [CrossRef]
- Vetter, V.; Baxter, R.; Denizer, G.; Sáfadi, M.A.; Silfverdal, S.A.; Vyse, A.; Borrow, R. Routinely vaccinating adolescents against meningococcus: Targeting transmission & disease. Expert Rev. Vaccines 2016, 15, 641–658. [Google Scholar] [CrossRef]
- Vuocolo, S.; Balmer, P.; Gruber, W.C.; Jansen, K.U.; Anderson, A.S.; Perez, J.L.; York, L.J. Vaccination strategies for the prevention of meningococcal disease. Hum. Vaccines Immunother. 2018, 14, 1203–1215. [Google Scholar] [CrossRef]
- Martinón-Torres, F. Deciphering the burden of meningococcal disease: Conventional and under-recognized elements. J. Adolesc. Health 2016, 59, S12–S20. [Google Scholar] [CrossRef] [Green Version]
- Dretler, A.W.; Rouphael, N.G.; Stephens, D.S. Progress toward the global control of Neisseria meningitidis: 21st century vaccines, current guidelines, and challenges for future vaccine development. Hum. Vaccines Immunother. 2018, 14, 1146–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, R.; Bai, X.; Borrow, R.; Caugant, D.A.; Carlos, J.; Ceyhan, M.; Christensen, H.; Climent, Y.; De Wals, P.; Dinleyici, E.C.; et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: Epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev. Vaccines 2019, 18, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshavan, P.; Pellegrini, M.; Vadivelu-Pechai, K.; Nissen, M. An update of clinical experience with the quadrivalent meningococcal ACWY-CRM conjugate vaccine. Expert Rev. Vaccines 2018, 17, 865–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masignani, V.; Pizza, M.; Moxon, E.R. The development of a vaccine against meningococcus B using reverse vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Abad, R.; Trotter, C.; van der Klis, F.R.; Vazquez, J.A. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 2013, 31, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Salisbury, D.; Ramsay, M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: A success story. Vaccine 2001, 20, S58–S67. [Google Scholar] [CrossRef]
- Goldschneider, I.; Gotschlich, E.C.; Artenstein, M.S. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1969, 129, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Andrews, N.; Goldblatt, D.; Miller, E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: Reevaluation of correlates of protection. Infect. Immun. 2001, 69, 1568–1573. [Google Scholar] [CrossRef] [Green Version]
- Campbell, H.; Borrow, R.; Salisbury, D.; Miller, E. Meningococcal C conjugate vaccine: The experience in England and Wales. Vaccine 2009, 27 (Suppl. S2), B20–B29. [Google Scholar] [CrossRef]
- Findlow, H.; Campbell, H.; Lucidarme, J.; Andrews, N.; Linley, E.; Ladhani, S.; Borrow, R. Serogroup C Neisseria meningitidis disease epidemiology, seroprevalence, vaccine effectiveness and waning immunity, England, 1998/99 to 2015/16. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Bijlsma, M.W.; Brouwer, M.C.; Spanjaard, L.; van de Beek, D.; van der Ende, A. A decade of herd protection after introduction of meningococcal serogroup C conjugate vaccination. Clin. Infect. Dis. 2014, 59, 1216–1221. [Google Scholar] [CrossRef] [Green Version]
- De Wals, P.; Deceuninck, G.; Lefebvre, B.; Boulianne, N.; De Serres, G. Effectiveness of serogroup C meningococcal conjugate vaccine: A 7-year follow-up in Quebec, Canada. Pediatr. Infect. Dis. J. 2011, 30, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Scheifele, D.W.; Halperin, S.A.; Vaudry, W.; Le Saux, N.; Tsang, R.; Bettinger, J.A. The impact of the meningococcal serogroup C conjugate vaccine in Canada between 2002 and 2012. Clin. Infect. Dis. 2014, 59, 1208–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, G.L.; Wang, H.; Lahra, M.; Booy, R.; Mc, I.P. Meningococcal disease epidemiology in Australia 10 years after implementation of a national conjugate meningococcal C immunization programme. Epidemiol. Infect. 2016, 144, 2382–2391. [Google Scholar] [CrossRef] [Green Version]
- Parent du Chatelet, I.; Deghmane, A.E.; Antona, D.; Hong, E.; Fonteneau, L.; Taha, M.K.; Lévy-Bruhl, D. Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006–2015. J. Infect. 2017, 74, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Alarcón, P.; Carlos, J.; Caugant, D.A.; Christensen, H.; Debbag, R.; De Wals, P.; Echániz-Aviles, G.; Findlow, J.; Head, C.; et al. The Global Meningococcal Initiative: Global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev. Vaccines 2017, 16, 313–328. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.M.; Mesaros, N.; Van Der Wielen, M.; Baine, Y. Conjugate meningococcal vaccines development: GSK Biologicals experience. Adv. Prev. Med. 2011, 2011, 846756. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 52–54. [Google Scholar]
- Campbell, H.; Edelstein, M.; Andrews, N.; Borrow, R.; Ramsay, M.; Ladhani, S. Emergency meningococcal ACWY vaccination program for teenagers to control group W meningococcal disease, England, 2015–2016. Emerg. Infect. Dis. 2017, 23, 1184–1187. [Google Scholar] [CrossRef] [Green Version]
- Borrow, R.; Caugant, D.A.; Ceyhan, M.; Christensen, H.; Dinleyici, E.C.; Findlow, J.; Glennie, L.; Von Gottberg, A.; Kechrid, A.; Vazquez Moreno, J.; et al. Meningococcal disease in the Middle East and Africa: Findings and updates from the Global Meningococcal Initiative. J. Infect. 2017, 75, 1–11. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Meningococcal A conjugate vaccine: Updated guidance, February 2015. Wkly. Epidemiol. Rec. 2015, 90, 57–62. [Google Scholar]
- World Health Organization. Meningococcal A Conjugate 10 Dose Presentation. Available online: https://www.who.int/immunization_standards/vaccine_quality/PQ_197_MenAconjugate_10dose_SII/en/ (accessed on 15 June 2020).
- Trotter, C.L.; Lingani, C.; Fernandez, K.; Cooper, L.V.; Bita, A.; Tevi-Benissan, C.; Ronveaux, O.; Preziosi, M.P.; Stuart, J.M. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–2015: An analysis of surveillance data. Lancet Infect. Dis. 2017, 17, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Mueller, J.E. Long-term effectiveness of MenAfriVac. Lancet Infect. Dis. 2019, 19, 228–229. [Google Scholar] [CrossRef]
- White, M.; Idoko, O.; Sow, S.; Diallo, A.; Kampmann, B.; Borrow, R.; Trotter, C. Antibody kinetics following vaccination with MenAfriVac: An analysis of serological data from randomised trials. Lancet Infect. Dis. 2019, 19, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, P.A.; Diomande, F.; Ba, A.K.; Sanou, I.; Ouedraogo, A.S.; Ouedraogo, R.; Sangare, L.; Kandolo, D.; Ake, F.; Saga, I.M.; et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin. Infect. Dis. 2013, 56, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daugla, D.M.; Gami, J.P.; Gamougam, K.; Naibei, N.; Mbainadji, L.; Narbe, M.; Toralta, J.; Kodbesse, B.; Ngadoua, C.; Coldiron, M.E.; et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: A community study. Lancet 2014, 383, 40–47. [Google Scholar] [CrossRef] [Green Version]
- MenAfriCar Consortium. Household transmission of Neisseria meningitidis in the African meningitis belt: A longitudinal cohort study. Lancet Glob. Health 2016, 4, e989–e995. [Google Scholar] [CrossRef] [Green Version]
- MenAfriCar Consortium. The diversity of meningococcal carriage across the African meningitis belt and the impact of vaccination with a group A meningococcal conjugate vaccine. J. Infect. Dis. 2015, 212, 1298–1307. [Google Scholar] [CrossRef] [Green Version]
- Mustapha, M.M.; Marsh, J.W.; Harrison, L.H. Global epidemiology of capsular group W meningococcal disease (1970–2015): Multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine 2016, 34, 1515–1523. [Google Scholar] [CrossRef]
- Booy, R.; Gentile, A.; Nissen, M.; Whelan, J.; Abitbol, V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum. Vaccines Immunother. 2019, 15, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Campbell, H.; Saliba, V.; Borrow, R.; Ramsay, M.; Ladhani, S.N. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. Eurosurveillance 2015, 20. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Neuzil, K.M.; Boyce, C.R.; Pasetti, M.F.; Reymann, M.K.; Martellet, L.; Hosken, N.; LaForce, F.M.; Dhere, R.M.; Pisal, S.S.; et al. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: A phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect. Dis. 2018, 18, 1088–1096. [Google Scholar] [CrossRef] [Green Version]
- Cendron, L.; Veggi, D.; Girardi, E.; Zanotti, G. Structure of the uncomplexed Neisseria meningitidis factor H-binding protein fHbp (rLP2086). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 531–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, A.; Dello Iacono, L.; Maruggi, G.; Benucci, B.; Merola, M.; Lo Surdo, P.; López-Sagaseta, J.; Pizza, M.; Malito, E.; Bottomley, M.J. NadA3 structures reveal undecad coiled coils and LOX1 binding regions competed by meningococcus B vaccine-elicited human antibodies. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, V.; Musi, V.; de Chiara, C.; Veggi, D.; Serruto, D.; Scarselli, M.; Kelly, G.; Pizza, M.; Pastore, A. Structure of the C-terminal domain of Neisseria heparin binding antigen (NHBA), one of the main antigens of a novel vaccine against Neisseria meningitidis. J. Biol. Chem. 2011, 286, 41767–41775. [Google Scholar] [CrossRef] [Green Version]
- Sehnal, D.; Rose, A.; Kovca, J.; Burley, S.K.; Velankar, S. Mol*: Towards a common library and tools for web molecular graphics. In MolVa: Workshop on Molecular Graphics and Visual Analysis of Molecular Data; The Eurographics Association: Brno, Czech Republic, 2018. [Google Scholar] [CrossRef]
- Derrick, J.P.; Urwin, R.; Suker, J.; Feavers, I.M.; Maiden, M.C. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 1999, 67, 2406–2413. [Google Scholar] [CrossRef] [Green Version]
- Cohn, A.C.; MacNeil, J.R.; Clark, T.A.; Ortega-Sanchez, I.R.; Briere, E.Z.; Meissner, H.C.; Baker, C.J.; Messonnier, N.E. Prevention and control of meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2013, 62, 1–28. [Google Scholar]
- Presa, J.; Findlow, J.; Vojicic, J.; Williams, S.; Serra, L. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: A review. Infect. Dis. 2019, 8, 307–333. [Google Scholar] [CrossRef] [Green Version]
- Robertson, C.A.; Greenberg, D.P.; Hedrick, J.; Pichichero, M.; Decker, M.D.; Saunders, M. Safety and immunogenicity of a booster dose of meningococcal (groups A, C, W, and Y) polysaccharide diphtheria toxoid conjugate vaccine. Vaccine 2016, 34, 5273–5278. [Google Scholar] [CrossRef] [Green Version]
- Robertson, C.A.; Hedrick, J.; Bassily, E.; Greenberg, D.P. Persistence of bactericidal antibodies 4 years after a booster dose of quadrivalent meningococcal diphtheria toxoid conjugate vaccine (MenACWY-D). Vaccine 2019, 37, 1016–1020. [Google Scholar] [CrossRef]
- Macneil, J.R.; Cohn, A.C.; Zell, E.R.; Schmink, S.; Miller, E.; Clark, T.; Messonnier, N.E. Early estimate of the effectiveness of quadrivalent meningococcal conjugate vaccine. Pediatr. Infect. Dis. J. 2011, 30, 451–455. [Google Scholar] [CrossRef]
- European Medicines Agency. Menveo Product Information. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/menveo (accessed on 30 July 2020).
- MacNeil, J.R.; Rubin, L.; McNamara, L.; Briere, E.C.; Clark, T.A.; Cohn, A.C. Use of MenACWY-CRM vaccine in children aged 2 through 23 months at increased risk for meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 527–530. [Google Scholar] [PubMed]
- MENVEO® Meningococcal (Groups A, C., Y and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine. Full Prescribing Information, GSK. Available online: https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Menveo/pdf/MENVEO.PDF (accessed on 30 July 2020).
- Argentina: Ministry of Health. National Immunisation Calendar. Available online: http://www.msal.gob.ar/images/stories/ryc/graficos/0000001210cnt-2018-10_calendario-nacional-vacunacion.pdf (accessed on 17 July 2020).
- Switzerland: Federal Office of Public Health. Swiss Vaccination Schedule Synopsis. Available online: https://www.infovac.ch/docs/public/-main/synopsis-schweizerischer-impfplan-2020.pdf (accessed on 17 July 2020).
- Johnston, W.; Essink, B.; Kirstein, J.; Forleo-Neto, E.; Percell, S.; Han, L.; Keshavan, P.; Smolenov, I. Comparative assessment of a single dose and a 2-dose vaccination series of a quadrivalent meningococcal CRM-conjugate vaccine (MenACWY-CRM) in children 2–10 years of age. Pediatr. Infect. Dis. J. 2016, 35, e19–e27. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Gupta, A.; Jeanfreau, R.; Klein, N.P.; Reisinger, K.; Walter, E.; Bedell, L.; Gill, C.; Dull, P.M. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2–10 years of age. Vaccine 2010, 28, 7865–7872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, S.L.; Christensen, S.; Verma, B.; Xie, F.; Keshavan, P.; Dull, P.M.; Smolenov, I. Antibody persistence 5 years after vaccination at 2 to 10 years of age with Quadrivalent MenACWY-CRM conjugate vaccine, and responses to a booster vaccination. Vaccine 2015, 33, 2175–2182. [Google Scholar] [CrossRef] [Green Version]
- Tipton, M.; Daly, W.; Senders, S.; Block, S.L.; Lattanzi, M.; Mzolo, T.; Barbi, S.; Pellegrini, M.; Keshavan, P. MenACWY-CRM conjugate vaccine booster dose given 4–6 years after priming: Results from a phase IIIb, multicenter, open label study in adolescents and adults. Vaccine 2019, 37, 6171–6179. [Google Scholar] [CrossRef]
- Read, R.C.; Baxter, D.; Chadwick, D.R.; Faust, S.N.; Finn, A.; Gordon, S.B.; Heath, P.T.; Lewis, D.J.; Pollard, A.J.; Turner, D.P.; et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: An observer-blind, phase 3 randomised clinical trial. Lancet 2014, 384, 2123–2131. [Google Scholar] [CrossRef]
- Im, J.H.; Woo, H.; Ha, B.M.; Lee, J.S.; Chung, M.H.; Jung, J. Effectiveness of a single dose of the quadrivalent meningococcal conjugate vaccine, MenACWY-CRM, in the Korean Armed Forces. Vaccine 2020, 38, 730–732. [Google Scholar] [CrossRef]
- European Medicines Agency. Nimenrix. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/nimenrix-epar-product-information_en.pdf (accessed on 31 July 2020).
- Serra, L.C.; York, L.J.; Balmer, P.; Webber, C. Meningococcal group A, C, W, and Y tetanus toxoid conjugate vaccine: A review of clinical data in adolescents. J. Adolesc. Health 2018, 63, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Sanofi Press Release. FDA Approves MenQuadfiTM, the Latest Innovation in Meningococcal (MenACWY) Vaccination. Available online: https://www.sanofi.com/-/media/Project/One-Sanofi-Web/Websites/Global/Sanofi-COM/Home/media-room/press-releases/2020/2020-04-24-07-00-00-2021445-en.pdf (accessed on 26 April 2020).
- Vesikari, T.; Borrow, R.; Forsten, A.; Findlow, H.; Dhingra, M.S.; Jordanov, E. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: A Phase II randomized study. Hum. Vaccines Immunother. 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.-J.; Hedrick, J.; Christensen, S.; Pan, J.; Jordanov, E.; Dhingra, M.S. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine 2020, 38, 3560–3569. [Google Scholar] [CrossRef]
- Kirstein, J.; Pina, M.; Pan, J.; Jordanov, E.; Dhingra, M.S. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adults 56 years of age and older: A Phase II randomized study. Hum. Vaccines Immunother. 2020, 16, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Áñez, G.; Hedrick, J.; Simon, M.W.; Christensen, S.; Jeanfreau, R.; Yau, E.; Pan, J.; Jordanov, E.; Dhingra, M.S. Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: A Phase III randomized study. Hum. Vaccines Immunother. 2020, 16, 1292–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ACIP. ACIP June 2020 Meeting Videos: Welcome & Introductions; Meningococcal Vaccine; Influenza Vaccines. Available online: https://www.cdc.gov/vaccines/acip/meetings/live-mtg-2020-6.html (accessed on 22 July 2020).
- Toneatto, D.; Pizza, M.; Masignani, V.; Rappuoli, R. Emerging experience with meningococcal serogroup B protein vaccines. Expert Rev. Vaccines 2017, 16, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.; Oster, P.; Arnold, R.; Tatley, M.V.; Naess, L.M.; Aaberge, I.S.; Galloway, Y.; McNicholas, A.; O’Hallahan, J.; Rosenqvist, E.; et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): Lessons from past programs and implications for the future. Hum. Vaccines Immunother. 2013, 9, 1241–1253. [Google Scholar] [CrossRef] [Green Version]
- Caron, F.; du Chatelet, I.P.; Leroy, J.P.; Ruckly, C.; Blanchard, M.; Bohic, N.; Massy, N.; Morer, I.; Floret, D.; Delbos, V.; et al. From tailor-made to ready-to-wear meningococcal B vaccines: Longitudinal study of a clonal meningococcal B outbreak. Lancet Infect. Dis. 2011, 11, 455–463. [Google Scholar] [CrossRef]
- Sevestre, J.; Hong, E.; Delbos, V.; Terrade, A.; Mallet, E.; Deghmane, A.E.; Lemee, L.; Taha, M.K.; Caron, F. Durability of immunogenicity and strain coverage of MenBvac, a meningococcal vaccine based on outer membrane vesicles: Lessons of the Normandy campaign. Vaccine 2017, 35, 4029–4033. [Google Scholar] [CrossRef] [Green Version]
- Muzzi, A.; Brozzi, A.; Serino, L.; Bodini, M.; Abad, R.; Caugant, D.; Comanducci, M.; Lemos, A.P.; Gorla, M.C.; Křížová, P.; et al. Genetic Meningococcal Antigen Typing System (gMATS): A genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine 2019, 37, 991–1000. [Google Scholar] [CrossRef]
- Martinón-Torres, F.; Nolan, T.; Toneatto, D.; Banzhoff, A. Persistence of the immune response after 4CMenB vaccination, and the response to an additional booster dose in infants, children, adolescents, and young adults. Hum. Vaccines Immunother. 2019, 15, 2940–2951. [Google Scholar] [CrossRef] [Green Version]
- Nolan, T.; Santolaya, M.E.; de Looze, F.; Marshall, H.; Richmond, P.; Henein, S.; Rheault, P.; Heaton, K.; Perrett, K.P.; Garfield, H.; et al. Antibody persistence and booster response in adolescents and young adults 4 and 7.5 years after immunization with 4CMenB vaccine. Vaccine 2019, 37, 1209–1218. [Google Scholar] [CrossRef]
- Marshall, H.S.; McMillan, M.; Koehler, A.P.; Lawrence, A.; Sullivan, T.R.; MacLennan, J.M.; Maiden, M.C.J.; Ladhani, S.N.; Ramsay, M.E.; Trotter, C.; et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N. Engl. J. Med. 2020, 382, 318–327. [Google Scholar] [CrossRef]
- Government of Andorra. Vaccination Schedule. Available online: https://www.salut.ad/images/stories/Salut/pdfs/temes_salut/Calendari_vacunacions.pdf (accessed on 22 June 2020).
- HSE 2018. Immunisation Schedule. Available online: https://www.hse.ie/eng/health/immunisation/pubinfo/pcischedule/immschedule/ (accessed on 22 July 2020).
- Piano Nazionale Prevenzione Vaccinale. PNPV 2017–2019. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 22 July 2020).
- Ministry of Health of the Republic of Lithuania. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/f4a925d0f50f11e79a1bc86190c2f01a?positionInSearchResults=0&searchModelUUID=1561434a-b283-4be2-87f5-4f556ad37c32 (accessed on 22 July 2020).
- MacNeil, J.R.; Rubin, L.; Folaranmi, T.; Ortega-Sanchez, I.R.; Patel, M.; Martin, S.W. Use of serogroup B meningococcal vaccines in adolescents and young adults: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1171–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, S.R.; Andrews, N.J.; Beebeejaun, K.; Campbell, H.; Ribeiro, S.; Ward, C.; White, J.M.; Borrow, R.; Ramsay, M.E.; Ladhani, S.N. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: A national observational cohort study. Lancet 2016, 388, 2775–2782. [Google Scholar] [CrossRef] [Green Version]
- Ladhani, S.N.; Andrews, N.; Parikh, S.R.; Campbell, H.; White, J.; Edelstein, M.; Bai, X.; Lucidarme, J.; Borrow, R.; Ramsay, M.E. Vaccination of Infants with Meningococcal Group B Vaccine (4CMenB) in England. N. Engl. J. Med. 2020, 382, 309–317. [Google Scholar] [CrossRef] [PubMed]
- De Wals, P.; Deceuninck, G.; Lefebvre, B.; Tsang, R.; Law, D.; De Serres, G.; Gilca, V.; Gilca, R.; Boulianne, N. Impact of an immunization campaign to control an increased incidence of serogroup B meningococcal disease in one region of Quebec, Canada. Clin. Infect. Dis. 2017, 64, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Deceuninck, G.; Lefebvre, B.; Tsang, R.; Betala-Belinga, J.F.; De Serres, G.; De Wals, P. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine 2019, 37, 4243–4245. [Google Scholar] [CrossRef]
- Marshall, H.S.; Lally, N.; Flood, L.; Phillips, P. First statewide meningococcal B vaccine program in infants, children and adolescents: Evidence for implementation in South Australia. Med. J. Aust. 2020, 212, 89–93. [Google Scholar] [CrossRef]
- Basta, N.E.; Mahmoud, A.A.; Wolfson, J.; Ploss, A.; Heller, B.L.; Hanna, S.; Johnsen, P.; Izzo, R.; Grenfell, B.T.; Findlow, J.; et al. Immunogenicity of a meningococcal B vaccine during a university outbreak. N. Engl. J. Med. 2016, 375, 220–228. [Google Scholar] [CrossRef]
- Lujan, E.; Winter, K.; Rovaris, J.; Liu, Q.; Granoff, D.M. Serum bactericidal antibody responses of students immunized with a meningococcal serogroup B vaccine in response to an outbreak on a university campus. Clin. Infect. Dis. 2017, 65, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Whelan, J.; Bambini, S.; Biolchi, A.; Brunelli, B.; Robert-Du Ry van Beest Holle, M. Outbreaks of meningococcal B infection and the 4CMenB vaccine: Historical and future perspectives. Expert Rev. Vaccines 2015, 14, 713–736. [Google Scholar] [CrossRef]
- Soeters, H.M.; McNamara, L.A.; Blain, A.E.; Whaley, M.; MacNeil, J.R.; Hariri, S.; Mbaeyi, S.A. For the Serogroup B Meningococcal Disease University Outbreak Group. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg. Infect. Dis. 2019, 25, 434–440. [Google Scholar] [CrossRef] [Green Version]
- Findlow, J.; Nuttens, C.; Kriz, P. Introduction of a second MenB vaccine into Europe—Needs and opportunities for public health. Expert Rev. Vaccines 2019, 18, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.L.; Absalon, J.; Beeslaar, J.; Balmer, P.; Jansen, K.U.; Jones, T.R.; Harris, S.; York, L.J.; Jiang, Q.; Radley, D.; et al. From research to licensure and beyond: Clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev. Vaccines 2018, 17, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Beeslaar, J.; Absalon, J.; Balmer, P.; Srivastava, A.; Maansson, R.; York, L.J.; Perez, J.L. Clinical data supporting a 2-dose schedule of MenB-FHbp, a bivalent meningococcal serogroup B vaccine, in adolescents and young adults. Vaccine 2018, 36, 4004–4013. [Google Scholar] [CrossRef] [PubMed]
- Biagini, M.; Spinsanti, M.; De Angelis, G.; Tomei, S.; Ferlenghi, I.; Scarselli, M.; Rigat, F.; Messuti, N.; Biolchi, A.; Muzzi, A.; et al. Expression of factor H binding protein in meningococcal strains can vary at least 15-fold and is genetically determined. Proc. Natl. Acad. Sci. USA 2016, 113, 2714–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesikari, T.; Ostergaard, L.; Beeslaar, J.; Absalon, J.; Eiden, J.J.; Jansen, K.U.; Jones, T.R.; Harris, S.L.; Maansson, R.; Munson, S.; et al. Persistence and 4-year boosting of the bactericidal response elicited by two- and three-dose schedules of MenB-FHbp: A phase 3 extension study in adolescents. Vaccine 2019, 37, 1710–1719. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Giuliani, M.M.; Biolchi, A.; Pizza, M.; Beebeejaun, K.; Lucidarme, J.; Findlow, J.; Ramsay, M.E.; Borrow, R. Effectiveness of meningococcal B vaccine against endemic hypervirulent Neisseria meningitidis W Strain, England. Emerg. Infect. Dis. 2016, 22, 309–311. [Google Scholar] [CrossRef] [Green Version]
- Hong, E.; Giuliani, M.M.; Deghmane, A.E.; Comanducci, M.; Brunelli, B.; Dull, P.; Pizza, M.; Taha, M.K. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine 2013, 31, 1113–1116. [Google Scholar] [CrossRef]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; McArdle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef]
- Petousis-Harris, H.; Radcliff, F.J. Exploitation of Neisseria meningitidis group B OMV vaccines against N. gonorrhoeae to inform the development and deployment of effective gonorrhea vaccines. Front. Immunol. 2019, 10, 683. [Google Scholar] [CrossRef] [Green Version]
- Isitt, C.; Cosgrove, C.A.; Ramsay, M.E.; Ladhani, S.N. Success of 4CMenB in preventing meningococcal disease: Evidence from real-world experience. Arch. Dis. Child 2020. [Google Scholar] [CrossRef]
- Block, S.L.; Szenborn, L.; Daly, W.; Jackowska, T.; D’Agostino, D.; Han, L.; Dull, P.M.; Smolenov, I. A comparative evaluation of two investigational meningococcal ABCWY vaccine formulations: Results of a phase 2 randomized, controlled trial. Vaccine 2015, 33, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Saez-Llorens, X.; Aguilera Vaca, D.C.; Abarca, K.; Maho, E.; Graña, M.G.; Heijnen, E.; Smolenov, I.; Dull, P.M. Immunogenicity and safety of investigational vaccine formulations against meningococcal serogroups A, B, C, W, and Y in healthy adolescents. Hum. Vaccines Immunother. 2015, 11, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez-Llorens, X.; Aguilera Vaca, D.C.; Abarca, K.; Maho, E.; Han, L.; Smolenov, I.; Dull, P. Persistence of meningococcal antibodies and response to a third dose after a two-dose vaccination series with investigational MenABCWY vaccine formulations in adolescents. Pediatr. Infect. Dis. J. 2015, 34, e264–e278. [Google Scholar] [CrossRef] [PubMed]
- Saez-Llorens, X.; Beltran-Rodriguez, J.; Novoa Pizarro, J.M.; Mensi, I.; Keshavan, P.; Toneatto, D. Four-year antibody persistence and response to a booster dose of a pentavalent MenABCWY vaccine administered to healthy adolescents and young adults. Hum. Vaccines Immunother. 2018, 14, 1161–1174. [Google Scholar] [CrossRef]
- Szenborn, L.; Block, S.L.; Jackowska, T.; Konior, R.; D’Agostino, D.; Smolenov, I.; Toneatto, D.; Welsch, J.A. Immune responses to booster vaccination with meningococcal ABCWY vaccine after primary vaccination with either investigational or licensed vaccines: A Phase 2 randomized study. Pediatr. Infect. Dis. J. 2018, 37, 475–482. [Google Scholar] [CrossRef]
- Welsch, J.A.; Senders, S.; Essink, B.; Klein, T.; Smolenov, I.; Pedotti, P.; Barbi, S.; Verma, B.; Toneatto, D. Breadth of coverage against a panel of 110 invasive disease isolates, immunogenicity and safety for 2 and 3 doses of an investigational MenABCWY vaccine in US adolescents—Results from a randomized, controlled, observer-blind phase II study. Vaccine 2018, 36, 5309–5317. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Meningococcal Outbreaks. Available online: https://www.cdc.gov/meningococcal/outbreaks/index.html (accessed on 5 June 2020).
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Wkly. Epidemiol. Rec. 2011, 86, 521–539. [Google Scholar]
- Van Kessel, F.; van den Ende, C.; Oordt-Speets, A.M.; Kyaw, M.H. Outbreaks of meningococcal meningitis in non-African countries over the last 50 years: A systematic review. J. Glob. Health 2019, 9, 010411. [Google Scholar] [CrossRef]
- Gala, R.P.; D’Souza, M.; Zughaier, S.M. Evaluation of various adjuvant nanoparticulate formulations for meningococcal capsular polysaccharide-based vaccine. Vaccine 2016, 34, 3260–3267. [Google Scholar] [CrossRef]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA is a versatile platform to design effective multivalent combination vaccines. Vaccines 2020, 8, 540. [Google Scholar] [CrossRef]
- World Health Organization. Defeating Meningitis by 2030. Available online: https://www.who.int/initiatives/defeating-meningitis-by-2030 (accessed on 21 August 2020).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizza, M.; Bekkat-Berkani, R.; Rappuoli, R. Vaccines against Meningococcal Diseases. Microorganisms 2020, 8, 1521. https://doi.org/10.3390/microorganisms8101521
Pizza M, Bekkat-Berkani R, Rappuoli R. Vaccines against Meningococcal Diseases. Microorganisms. 2020; 8(10):1521. https://doi.org/10.3390/microorganisms8101521
Chicago/Turabian StylePizza, Mariagrazia, Rafik Bekkat-Berkani, and Rino Rappuoli. 2020. "Vaccines against Meningococcal Diseases" Microorganisms 8, no. 10: 1521. https://doi.org/10.3390/microorganisms8101521
APA StylePizza, M., Bekkat-Berkani, R., & Rappuoli, R. (2020). Vaccines against Meningococcal Diseases. Microorganisms, 8(10), 1521. https://doi.org/10.3390/microorganisms8101521





