Wine Yeast Peroxiredoxin TSA1 Plays a Role in Growth, Stress Response and Trehalose Metabolism in Biomass Propagation
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Media
2.2. Growth Conditions
2.3. Redox Parameters
2.4. Metabolite Determinations
3. Results
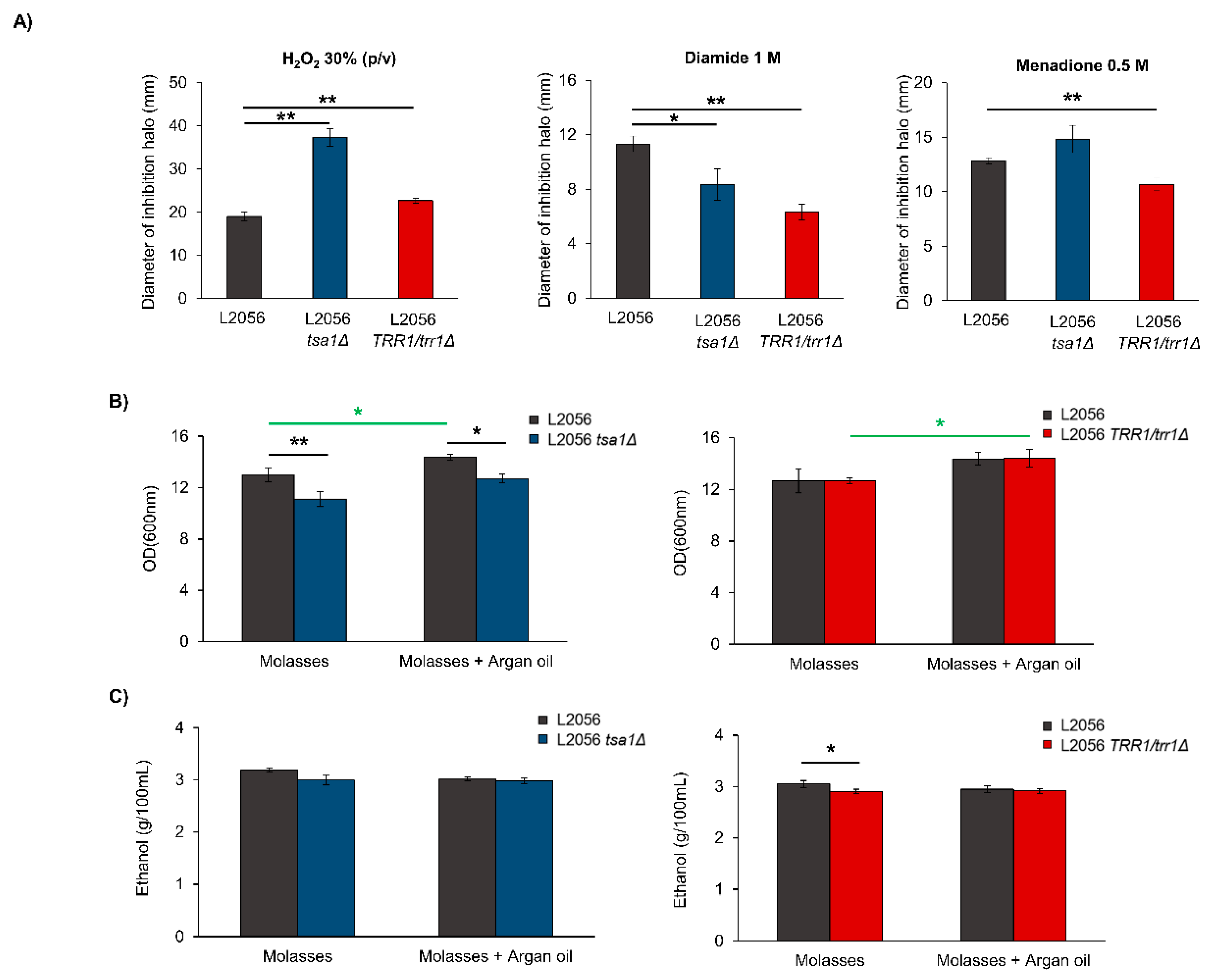
3.1. TSA1 is the Most Relevant Peroxiredoxin for Growth
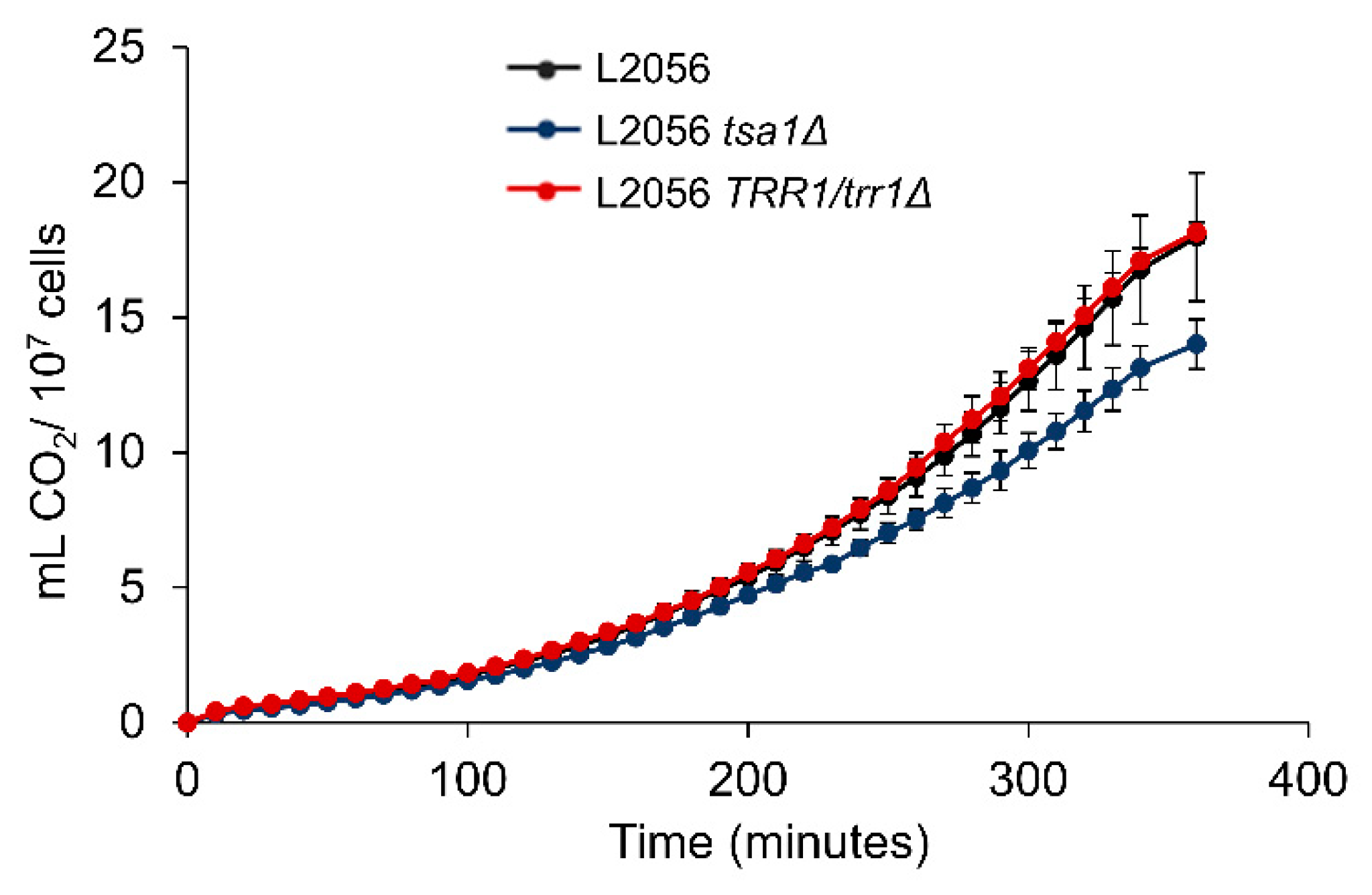
3.2. Lack of Peroxiredoxin TSA1 Diminishes Growth in Molasses
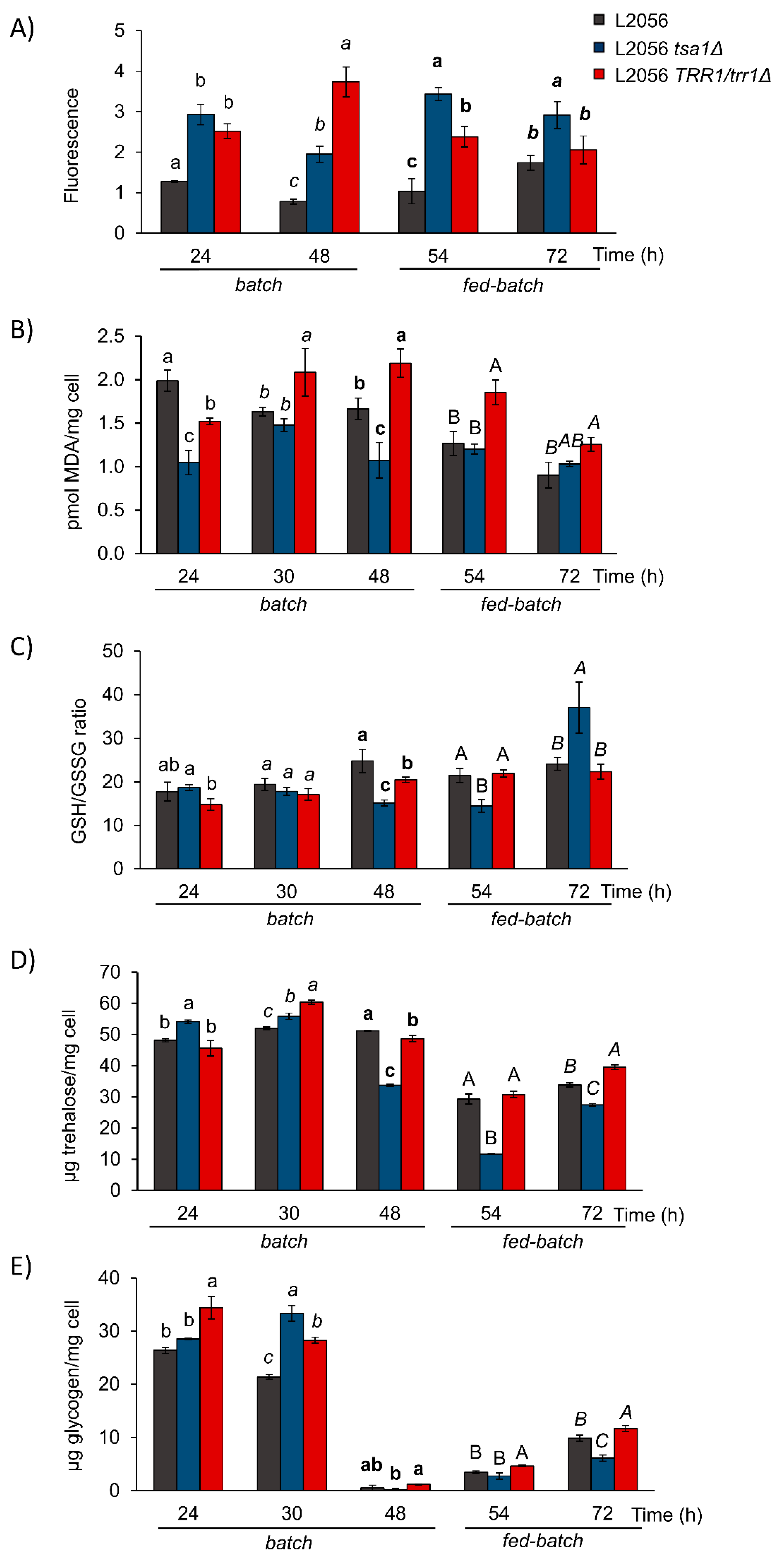
3.3. Peroxiredoxin TSA1 Plays a Major Role in Redox Balance during Growth on Molasses
3.4. TSA1 Impacts the Metabolic Transition from Fermentation to Respiration in Industrial Biomass Propagation Conditions
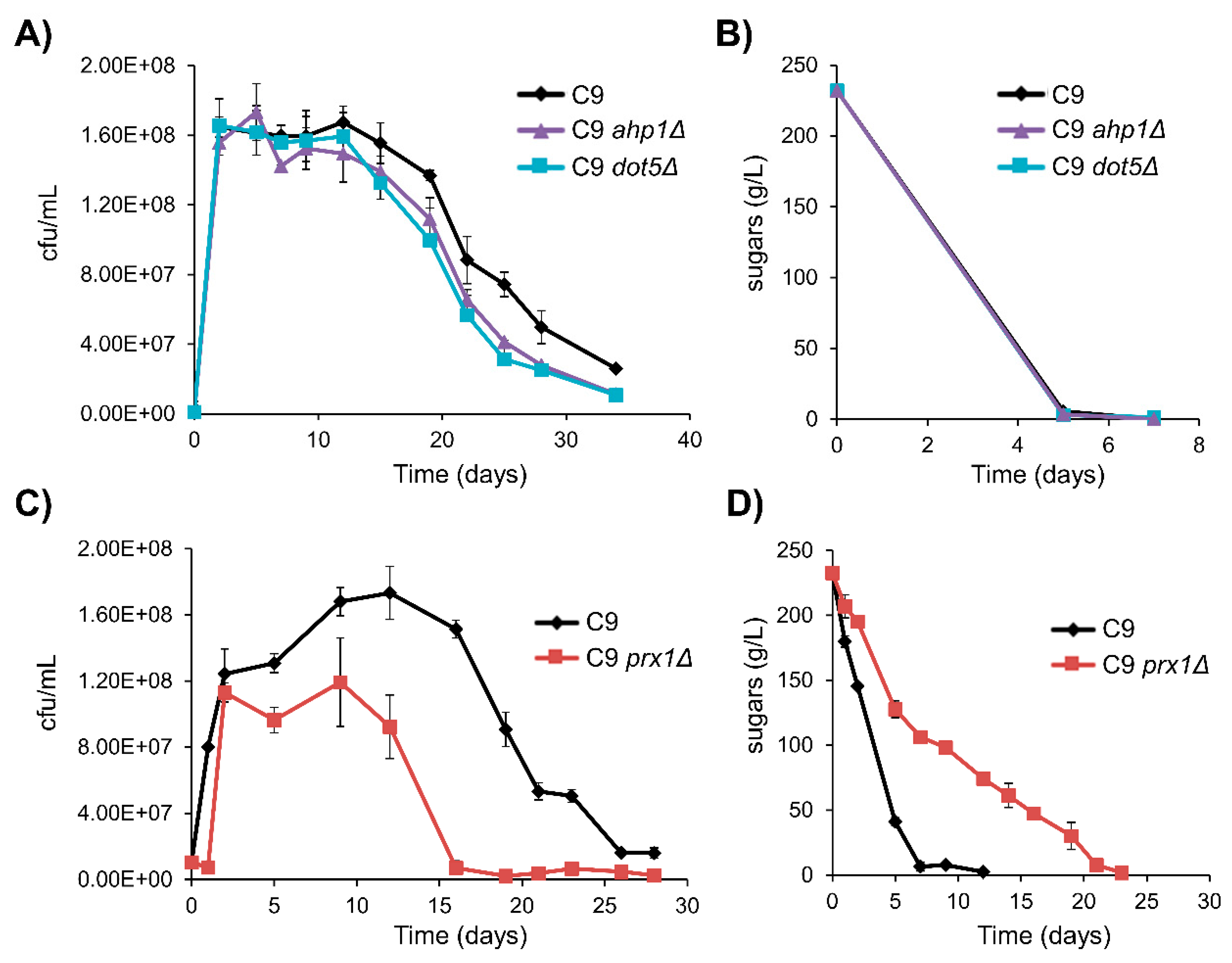
3.5. TSA1 Does Not Play a Determinant Role during Vinification
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matallana, E.; Aranda, A. Biotechnological impact of stress response on wine yeast. Lett. Appl. Microbiol. 2017, 64, 103–110. [Google Scholar] [CrossRef] [PubMed]
- García-Ríos, E.; Guillamón, J.M. Mechanisms of Yeast Adaptation to Wine Fermentations. Prog. Mol. Subcell. Biol. 2019, 58, 37–59. [Google Scholar] [PubMed]
- Pérez-Torrado, R.; Gamero, E.; Gómez-Pastor, R.; Garre, E.; Aranda, A.; Matallana, E. Yeast biomass, an optimised product with myriad applications in the food industry. Trends Food Sci. Technol. 2015, 46, 167–175. [Google Scholar] [CrossRef]
- Perez-Torrado, R.; Gomez-Pastor, R.; Larsson, C.; Matallana, E. Fermentative capacity of dry active wine yeast requires a specific oxidative stress response during industrial biomass growth. Appl. Microbiol. Biotechnol. 2009, 81, 951–960. [Google Scholar] [CrossRef]
- Garre, E.; Raginel, F.; Palacios, A.; Julien, A.; Matallana, E. Oxidative stress responses and lipid peroxidation damage are induced during dehydration in the production of dry active wine yeasts. Int. J. Food Microbiol. 2010, 136, 295–303. [Google Scholar] [CrossRef]
- Franca, M.B.; Panek, A.D.; Eleutherio, E.C. Oxidative stress and its effects during dehydration. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 621–631. [Google Scholar] [CrossRef]
- Tapia, H.; Koshland, D.E. Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr. Biol. 2014, 24, 2758–2766. [Google Scholar] [CrossRef]
- Gamero-Sandemetrio, E.; Torrellas, M.; Rabena, M.T.; Gomez-Pastor, R.; Aranda, A.; Matallana, E. Food-grade argan oil supplementation in molasses enhances fermentative performance and antioxidant defenses of active dry wine yeast. AMB Express. 2015, 5, 75. [Google Scholar] [CrossRef]
- Gamero-Sandemetrio, E.; Gamero-Sandemetrio, E.; Gómez-Pastor, R.; Aranda, A.; Matallana, E. Validation and biochemical characterisation of beneficial argan oil treatment in biomass propagation for industrial active dry yeast production. Innov. Food Sci. Emerg. Technol. 2019, 51, 156–166. [Google Scholar] [CrossRef]
- Gamero-Sandemetrio, E.; Paya-Tormo, L.; Gomez-Pastor, R.; Aranda, A.; Matallana, E. Non-canonical regulation of glutathione and trehalose biosynthesis characterizes non-Saccharomyces wine yeasts with poor performance in active dry yeast production. Microb. Cell 2018, 5, 184–197. [Google Scholar] [CrossRef]
- Rossignol, T.; Dulau, L.; Julien, A.; Blondin, B. Genome-wide monitoring of wine yeast gene expression during alcoholic fermentation. Yeast 2003, 20, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Orozco, H.; Picazo, C.; Matallana, E. Yeast Life Span and its Impact on Food Fermentations. Fermentation 2019, 5, 37. [Google Scholar] [CrossRef]
- Conrad, M.; Schothorst, J.; Kankipati, H.N.; Van Zeebroeck, G.; Rubio-Texeira, M.; Thevelein, J.M. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2014, 38, 254–299. [Google Scholar] [CrossRef]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.; Ros, J.; Belli, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta 2008, 1780, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.B.; Delaunay-Moisan, A.; Outten, C.E.; Igbaria, A. Functions and cellular compartmentation of the thioredoxin and glutathione pathways in yeast. Antioxid. Redox Signal. 2013, 18, 1699–1711. [Google Scholar] [CrossRef]
- Iraqui, I.; Kienda, G.; Soeur, J.; Faye, G.; Baldacci, G.; Kolodner, R.D.; Huang, M.E. Peroxiredoxin Tsa1 is the key peroxidase suppressing genome instability and protecting against cell death in Saccharomyces cerevisiae. PLoS Genet. 2009, 5, e1000524. [Google Scholar] [CrossRef]
- Wong, C.M.; Zhou, Y.; Ng, R.W.M.; Kung, H.F.; Jin, D.Y. Cooperation of yeast peroxiredoxins Tsa1p and Tsa2p in the cellular defense against oxidative and nitrosative stress. J. Biol. Chem. 2002, 277, 5385–5394. [Google Scholar] [CrossRef]
- Picazo, C.; McDonagh, B.; Peinado, J.; Barcena, J.A.; Matallana, E.; Aranda, A. Saccharomyces cerevisiae Cytosolic Thioredoxins Control Glycolysis, Lipid Metabolism, and Protein Biosynthesis under Wine-Making Conditions. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Picazo, C.; Matallana, E.; Aranda, A. Yeast thioredoxin reductase Trr1p controls TORC1-regulated processes. Sci. Rep. 2018, 8, 16500. [Google Scholar] [CrossRef]
- Toledano, M.B.; Huang, B. Microbial 2-Cys peroxiredoxins: Insights into their complex physiological roles. Mol. Cells 2016, 39, 31. [Google Scholar] [PubMed]
- Jang, H.H.; Lee, K.O.; Chi, Y.H.; Jung, B.G.; Park, S.K.; Park, J.H.; Choi, Y.O. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 2004, 117, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Trotter, E.W.; Rand, J.D.; Vickerstaff, J.; Grant, C.M. The yeast Tsa1 peroxiredoxin is a ribosome-associated antioxidant. Biochem. J. 2008, 412, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hanzén, S.; Vielfort, K.; Yang, J.; Roger, F.; Andersson, V.; Zamarbide-Forés, S.; Liu, B. Lifespan Control by Redox-Dependent Recruitment of Chaperones to Misfolded Proteins. Cell 2016, 166, 140–151. [Google Scholar] [CrossRef]
- Guldener, U.; Heck, S.; Fielder, T.; Beinhauer, J.; Hegemann, J.H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996, 24, 2519–2524. [Google Scholar] [CrossRef]
- Delneri, D.; Tomlin, G.C.; Wixon, J.L.; Hutter, A.; Sefton, M.; Louis, E.J.; Oliver, S.G. Exploring redundancy in the yeast genome: An improved strategy for use of the cre-loxP system. Gene 2000, 252, 127–135. [Google Scholar] [CrossRef]
- Gietz, R.D.; Woods, R.A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzym. 2002, 350, 87–96. [Google Scholar]
- Lino, F.S.D.O.; Basso, T.O.; Sommer, M.O.A. A synthetic medium to simulate sugarcane molasses. Biotechnol. Biofuels 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Schnierda, T.; Bauer, F.F.; Divol, B.; van Rensburg, E.; Görgens, J.F. Optimization of carbon and nitrogen medium components for biomass production using non-Saccharomyces wine yeasts. Lett. Appl. Microbiol. 2014, 58, 478–485. [Google Scholar] [CrossRef]
- Nakata, H.; Tamura, M.; Shintani, T.; Gomi, K. Evaluation of baker’s yeast strains exhibiting significant growth on Japanese beet molasses and compound analysis of the molasses types. J. Biosci. Bioeng. 2014, 117, 715–719. [Google Scholar] [CrossRef]
- Riou, C.; Nicaud, J.M.; Barre, P.; Gaillardin, C. Stationary-phase gene expression in Saccharomyces cerevisiae during wine fermentation. Yeast 1997, 13, 903–915. [Google Scholar] [CrossRef]
- Klinger, H.; Rinnerthaler, M.; Lam, Y.T.; Laun, P.; Heeren, G.; Klocker, A.; Breitenbach, M. Quantitation of (a)symmetric inheritance of functional and of oxidatively damaged mitochondrial aconitase in the cell division of old yeast mother cells. Exp. Gerontol. 2010, 45, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Parrou, J.L.; Francois, J. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal. Biochem. 1997, 248, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Gamero-Sandemetrio, E.; Gomez-Pastor, R.; Matallana, E. Antioxidant defense parameters as predictive biomarkers for fermentative capacity of active dried wine yeast. Biotechnol. J. 2014, 9, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Gómez-Pastor, R.; Pérez-Torrado, R.; Cabiscol, E.; Ros, J.; Matallana, E. Reduction of oxidative cellular damage by overexpression of the thioredoxin TRX2 gene improves yield and quality of wine yeast dry active biomass. Microb. Cell Fact. 2010, 9, 9. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Robyt, J.F.; Whelan, W.J. Reducing value methods for maltodextrins. I. Chain-length dependence of alkaline 3,5-dinitrosalicylate and chain-length independence of alkaline copper. Anal. Biochem. 1972, 45, 510–516. [Google Scholar] [CrossRef]
- Walker, M.E.; Gardner, J.M.; Vystavelova, A.; McBryde, C.; de Barros Lopes, M.; Jiranek, V. Application of the reuseable, KanMX selectable marker to industrial yeast: Construction and evaluation of heterothallic wine strains of Saccharomyces cerevisiae, possessing minimal foreign DNA sequences. FEMS Yeast Res. 2003, 4, 339–347. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Véronneau, S.; Arkin, A.P. Functional profiling of the Saccharomyces cerevisiae genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef]
- Perez-Torrado, R.; Bruno-Barcena, J.M.; Matallana, E. Monitoring stress-related genes during the process of biomass propagation of Saccharomyces cerevisiae strains used for wine making. Appl. Environ. Microbiol. 2005, 71, 6831–6837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacDiarmid, C.W.; Taggart, J.; Kerdsomboon, K.; Kubisiak, M.; Panascharoen, S.; Schelble, K.; Eide, D.J. Peroxiredoxin chaperone activity is critical for protein homeostasis in zinc-deficient yeast. J. Biol. Chem. 2013, 288, 31313–31327. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.; Xiong, X.; Sohn, K.; Schröppel, K.; Brunner, H.; Rupp, S. The moonlighting protein Tsa1p is implicated in oxidative stress response and in cell wall biogenesis in Candida albicans. Mol. Microbiol. 2005, 57, 1318–1341. [Google Scholar] [CrossRef] [PubMed]
- Bodvard, K.; Peeters, K.; Roger, F.; Romanov, N.; Igbaria, A.; Welkenhuysen, N.; Molin, M. Light-sensing via hydrogen peroxide and a peroxiredoxin. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Maywood, E.S. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef]
- Irokawa, H.; Tachibana, T.; Watanabe, T.; Matsuyama, Y.; Motohashi, H.; Ogasawara, A.; Kuge, S. Redox-dependent Regulation of Gluconeogenesis by a Novel Mechanism Mediated by a Peroxidatic Cysteine of Peroxiredoxin. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrigós, V.; Picazo, C.; Matallana, E.; Aranda, A. Wine Yeast Peroxiredoxin TSA1 Plays a Role in Growth, Stress Response and Trehalose Metabolism in Biomass Propagation. Microorganisms 2020, 8, 1537. https://doi.org/10.3390/microorganisms8101537
Garrigós V, Picazo C, Matallana E, Aranda A. Wine Yeast Peroxiredoxin TSA1 Plays a Role in Growth, Stress Response and Trehalose Metabolism in Biomass Propagation. Microorganisms. 2020; 8(10):1537. https://doi.org/10.3390/microorganisms8101537
Chicago/Turabian StyleGarrigós, Víctor, Cecilia Picazo, Emilia Matallana, and Agustín Aranda. 2020. "Wine Yeast Peroxiredoxin TSA1 Plays a Role in Growth, Stress Response and Trehalose Metabolism in Biomass Propagation" Microorganisms 8, no. 10: 1537. https://doi.org/10.3390/microorganisms8101537
APA StyleGarrigós, V., Picazo, C., Matallana, E., & Aranda, A. (2020). Wine Yeast Peroxiredoxin TSA1 Plays a Role in Growth, Stress Response and Trehalose Metabolism in Biomass Propagation. Microorganisms, 8(10), 1537. https://doi.org/10.3390/microorganisms8101537







