Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Isolation and Purification
2.2. DNA Extraction, Phylogenetic Marker Gene Amplification, and Sequencing
2.3. Microbial Inoculum Preparation
2.4. Determination of Indole-3- Acetic acid Secretion
2.5. Seed Treatment and Seedling Growth Conditions
2.6. Determination of Proline Contents
2.7. Photosynthetic Parameter Measurements
2.8. Statistical Analysis
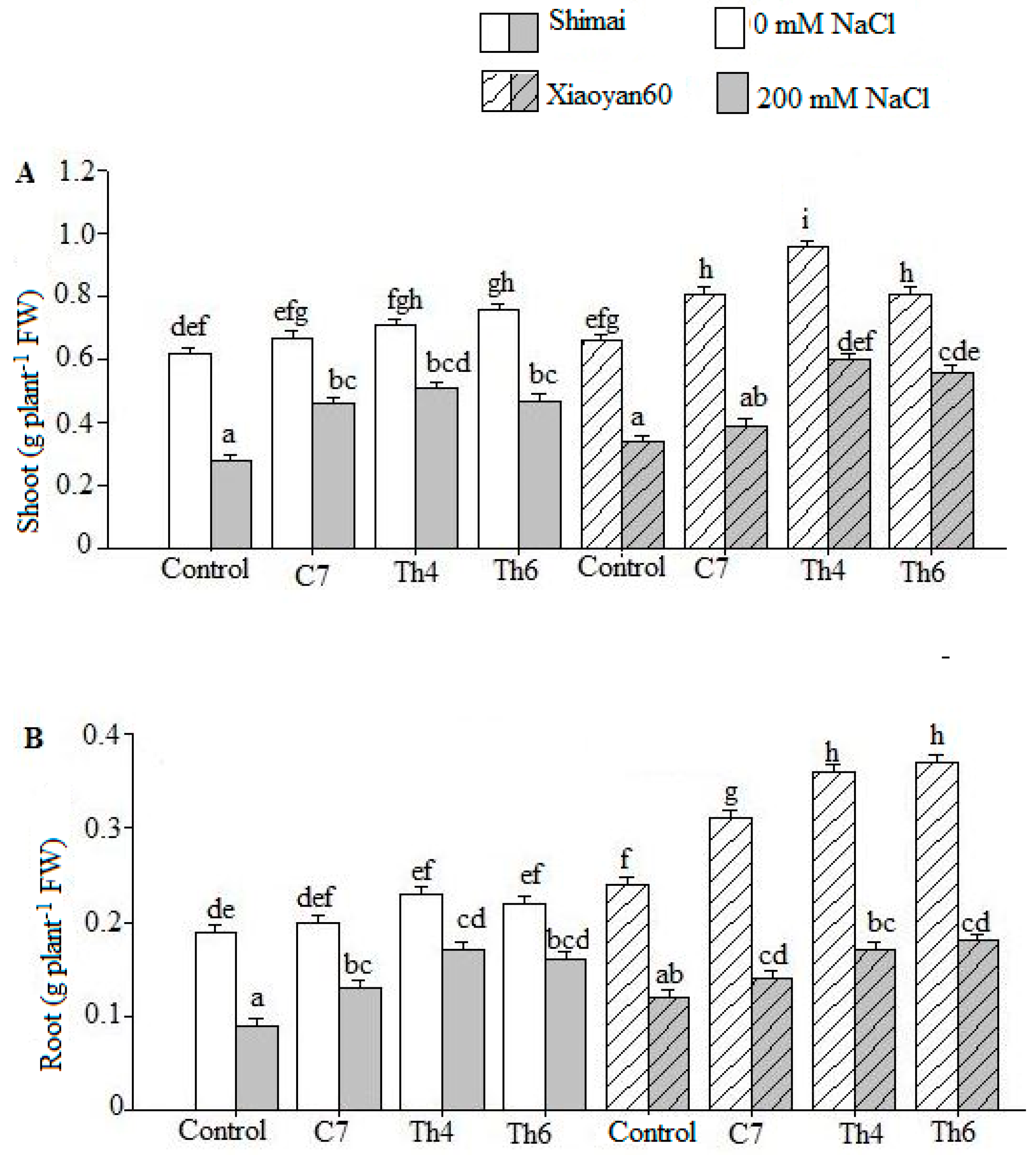
3. Results
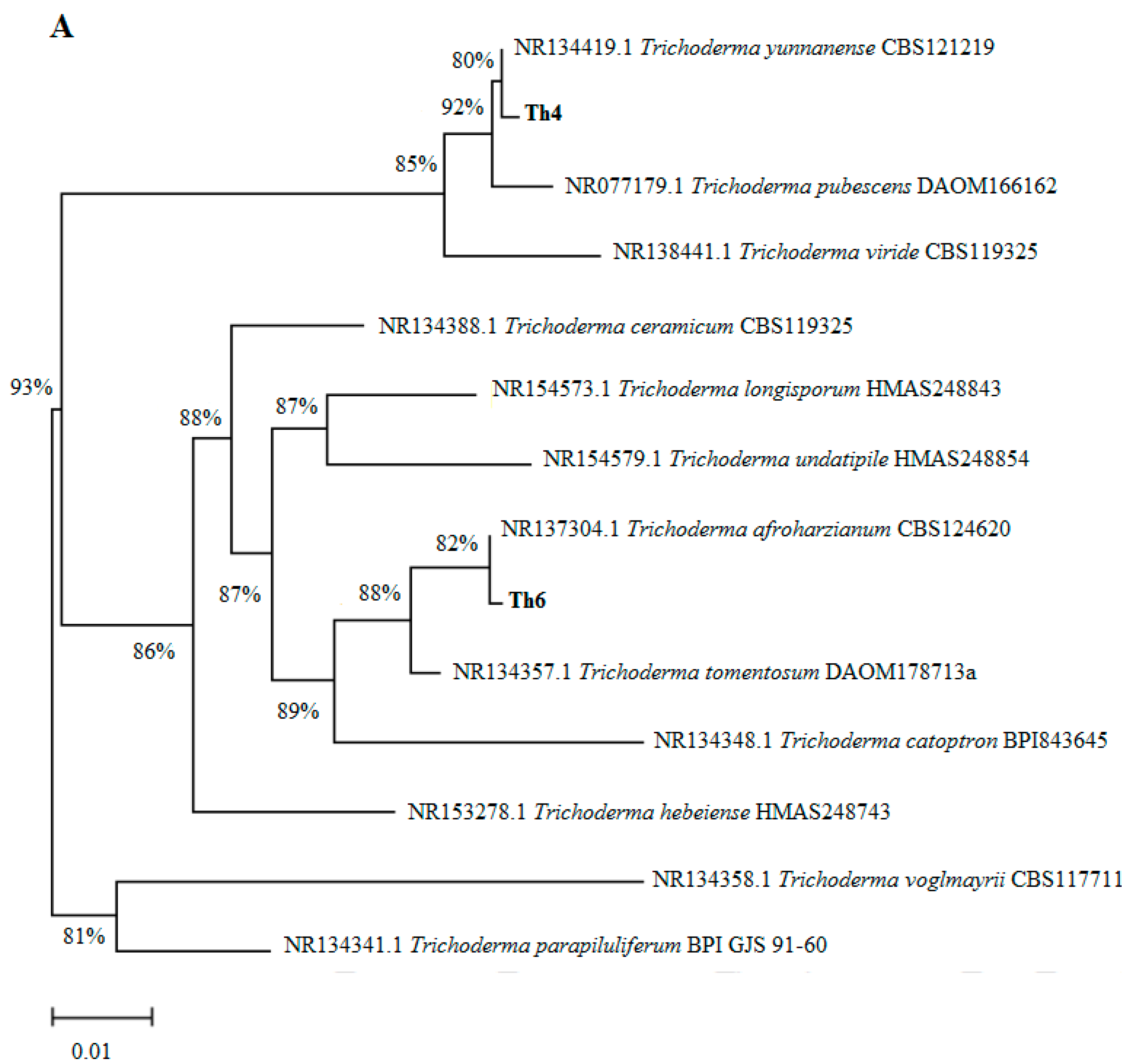
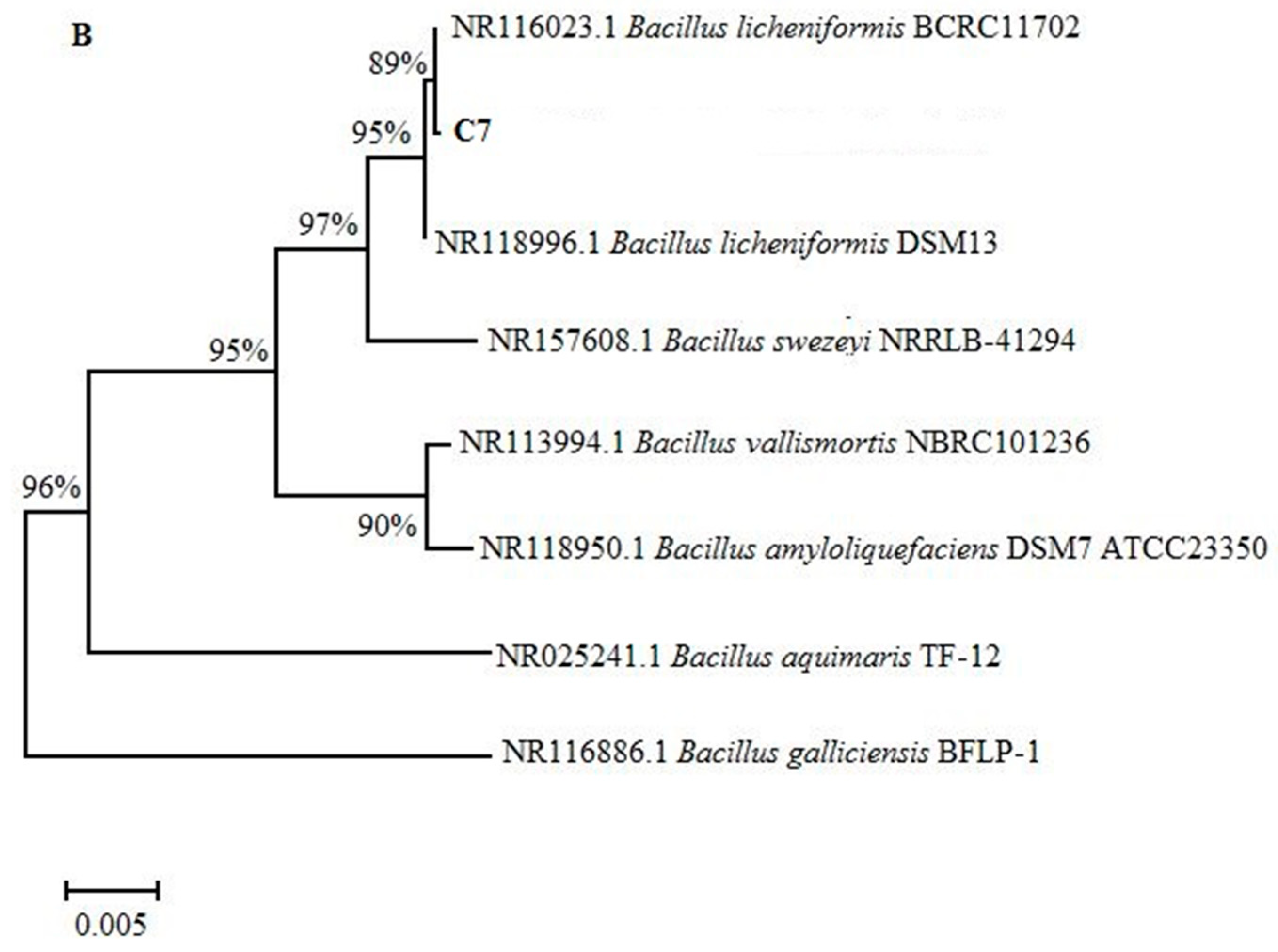
3.1. Phylogenetic Analyses and Determination of Indole-3-Acetic Acid (IAA) Secretion
3.2. Proline Contents
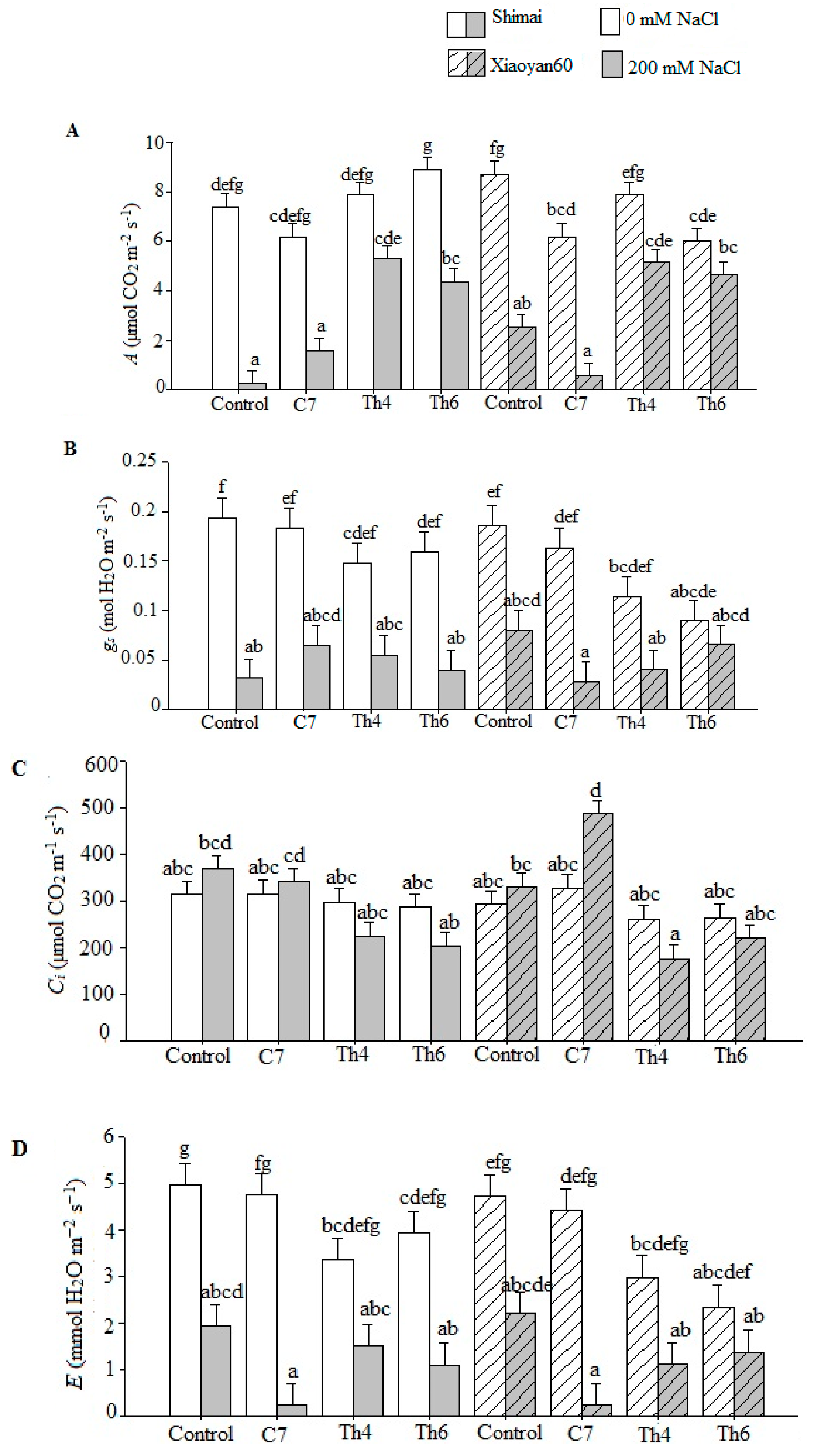
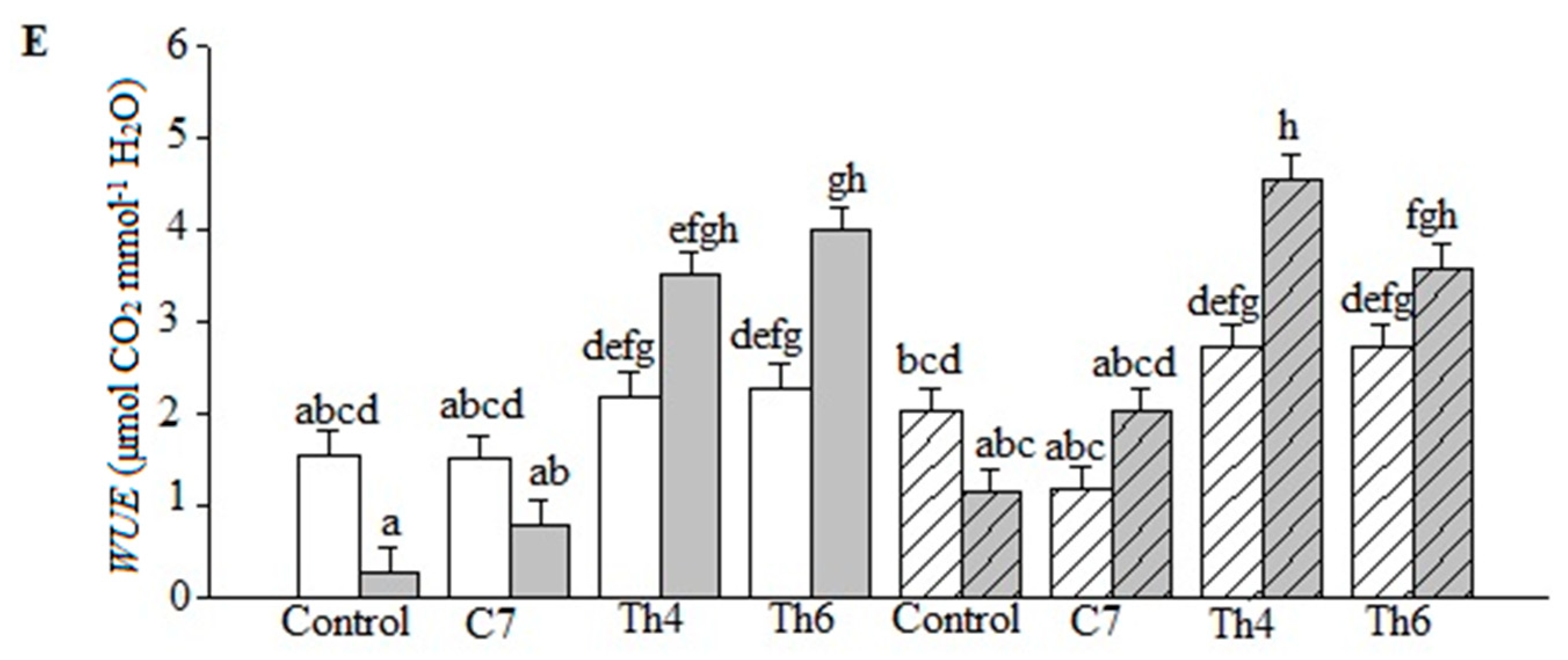
3.3. Photosynthetic Parameters
3.4. Principal Component Analysis of Cultivar-Specific Photosynthetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isayenkov, S.V.; Maathus, F. Plant salinity stress: Many unanswered questions remain. Front. Plant. Sci. 2017, 10, 80. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; de Sousa, L. Global mapping of soil salinity change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity, and extreme temperatures: Toward genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Liu, F.; Mo, X.; Zhang, S.; Chen, F.; Li, D. Gas exchange characteristics and their influencing factors for halophytic plant communities on west coast of Bohai Sea. PLoS ONE 2020, 15, e0229047. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Ancín, M.; Fakhet, D.; González-Torralba, J.; Gámez, A.L.; Seminario, A.; Soba, D.; Ben Mariem, S.; Garriga, M.; Aranjuelo, I. Photosynthetic Metabolism under Stressful Growth Conditions as a Bases for Crop Breeding and Yield Improvement. Plants 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Niinemets, U.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M.; Rodriguez, P.L.; et al. Diffusional conductance to CO2 as a target for increasing photosynthesis and photosynthetic water use efficiency. Photosyn. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, H.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop. J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Shabala, L.; Mackay, A.; Tian, Y.; Jacobsen, S.E.; Zhou, D.; Shabala, S. Oxidative stress protection and stomatal patterning as components of salinity tolerance mechanism in quinoa (Chenopodium quinoa). Physiol. Plant. 2012, 146, 26–38. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.I.; Mynard, G.; Raman, A.; Hodgkins, D.; Mitchell, D.; Nicol, H. Salt effects on proline and glycine betaine levels and photosynthetic performance in Melilptus Siculus, Tecticorni pergranulata and Thinopyrum ponticum measured in simulated saline conditions. Funct Plant Biol. 2016, 43, 254. [Google Scholar] [CrossRef]
- Renzhao, M.; Fitzpatrick, R.W.; Xiaojing, L.; Davis, P.J. Chemical properties of selected soils from the North China Plain. In Regional Water and Soil Assessment for Managing Sustainable Agriculture in China and Australia; McVicar, T.R., Li, R., Walker, J., Fizpatrick, R.W., Liu, C., Eds.; ACIAR Monograph: Canberra, Australia, 2002; Volume 84, pp. 173–186. [Google Scholar]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Li, Z.S.; Zheng, Q.; Li, H. Review and new progress in wheat wide hybridization for improving the resistance to biotic and abiotic stresses. In Advances in Wheat Genetics: From Genome to Field; Ogihara, Y., Takumi, S., Hand, H., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to plant–microbe interactions provide opportunities to improve resistance breeding against root diseases in grain legumes. Plant. Cell Environ. 2018, 42, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M.; Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields: A review. Crit. Rev. Biotechnol. 2019, 39, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Mitter, B.; Brader, G.; Pfaffenbichler, N.; Sessitsch, A. Next-generation microbiome applications for crop production-limitations and the need of knowledge-based solutions. Curr. Opin. Microbiol. 2019, 49, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Dodd, I.C.; Perez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, A.; Srivastava, P.; Choudhary, K.K.; Dikshit, A. Plant growth-promoting microorganisms in sustainable agriculture. In Role of Plant. Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Ajay, K., Amit, K., Singh, K., Kumar, C., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant. Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Gan, Y. Seed treatment with Trichoderma longibrachiatum T6 promotes wheat seedling growth under NaCl stress through activating the enzymatic and non-enzymatic antioxidant defense systems. Int. J. Mol. Sci. 2019, 20, 3729. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Elsayed, F.A.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant. Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. A Halotolerant Bacterium Bacillus licheniformis HSW-16 Augments Induced Systemic Tolerance to Salt Stress in Wheat Plant (Triticum aestivum). Front. Plant. Sci. 2016, 7, 1890. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant. Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book 2010, 8, e0140. [Google Scholar] [CrossRef] [PubMed]
- Chakdar, H.; Borse, D.N.; Verma, S.; Choudhary, P.; Das, S. Microbial management of crop salinity stress: Mechanisms, applications, and prospects. In Salt Stress, Microbes, and Plant. Interactions: Mechanisms and Molecular Approaches; Akhtar, M., Ed.; Springer: Singapore, 2019; Volume 2. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant. Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbial. 2018, 9, 2525. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant. Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant. Biol. 2019, 19, 1–18. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Ma, Y.; Vosátka, M.; Freitas, H. Editorial: Beneficial microbes alleviate climatic stresses in plants. Front. Plant. Sci. 2019, 10, 595. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H. Seed coating with arbuscular mycorrhizal fungi as an ecotechnological approach for sustainable agricultural production of common wheat (Triticum aestivum L.). J. Toxicol. Environ. Health Part A 2016, 79, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Carvalho, M.F.; Magalhães, C.; Janoušková, M.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating with inocula of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria for nutritional enhancement of maize under different fertilization regimes. Arch. Agron. Soil Sci. 2019, 65, 31–43. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Li, X. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J. Plant. Physiol. 2009, 166, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.J.; Terrile, M.C.; Windels, D.; Lombardo, M.C.; Bartoli, C.G.; Vazquez, F.; Casalongué, C.A. MiR393 regulation of auxin signaling and redox-related components during acclimation to salinity in Arabidopsis. PLoS ONE 2014, 9, e107678. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant. Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 6, 667. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Tian, Y.; Guo, K.; Sun, H.; Liu, X.; Chu, H.; Liu, B. Long- term phytoremediation of coastal saline soil reveals plant species-specific patterns of microbial community recruitment. mSystems 2020, 5, e00741-19. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I.; Henis, Y. A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 1981, 9, 59–67. [Google Scholar] [CrossRef]
- Yang, L.; Bian, X.; Yang, R.; Zhou, C.; Tang, B. Assessment of organic amendments for improving coastal saline soil. Land Degrad. Dev. 2018, 29. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Zarrin, M.; Ganj, F.; Faramarzi, S. Analysis of the rDNA internal transcribed spacer region of the Fusarium species by polymerase chain reaction-restriction fragment length polymorphism. Biomed. Rep. 2016, 4, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.D. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Mendoza, A.; Clouston, A.; Li, J.-H.; Nieto-Jacobo, M.F.; Cummings, N.; Steyaert, J.; Hill, R. Isolation and mass production of Trichoderma. Methods Mol. Biol. 2016, 1477. [Google Scholar] [CrossRef]
- Bach, J.N.; Bramkamp, M. Flotillins functionally organize the bacterial membrane. Mol. Microbiol. 2013, 88, 1205–1217. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic Acid. Plant. Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef]
- Wang, Y.; Xi, W.; Wang, Z.; Wang, B.; Xu, X.; Han, M.; Zhou, S.; Zhang, Y. Contribution of ear photosynthesis to grain yield under rainfed and irrigation conditions for winter wheat cultivars released in the past 30 years in North China Plain. J. Integr. Agric. 2016, 15, 2247–2256. [Google Scholar] [CrossRef]
- Singh, P.C.; Nautiyal, C.S. A novel method to prepare concentrated conidial biomass formulation of Trichoderma harzianum for seed application. J. Appl. Microb. 2012, 113, 1442–1450. [Google Scholar] [CrossRef]
- Swaminathan, J.; van Koten, C.; Henderson, H.V.; Jackson, T.A.; Wilson, M.J. Formulations for delivering Trichoderma atroviridae spores as seed coatings, effects of temperature and relative humidity on storage stability. J. Appl. Microb. 2016, 120, 425–431. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivum L.) by seed biopriming with salinity tolerant isolates of Trichoderma harzianum. Plant. Soil 2011, 347, 387–400. [Google Scholar] [CrossRef]
- Bado, S.; Forster, B.P.; Ghanim, A.M.A.; Jankowicz-Cieslak, J.; Berthold, G.; Luxiang, L. Protocol for screening for salt tolerance in rice. In Protocols for Pre-Field Screening of Mutants for Salt Tolerance in Rice, Wheat, and Barley; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Viterbo, A.; Harel, M.; Horwitz, B.A.; Chet, I.; Mukherjee, P.K. Trichoderma mitogen-activated protein kinase signaling is involved in induction of plant systemic resistance. Appl. Environ. Microbiol. 2005, 71, 6241–6246. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant. Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Boyy, G.; Ellison, S.; Heilberger, R. Package ‘Car: Companion to Applied Regression. Available online: http://cran-r.project.org/web/packages/car/car.pdf (accessed on 10 June 2020).
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Software 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A.; ur-Rehman, S. Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J. Integr. Agric. 2006, 48, 181–189. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. J. Integr. Plant. Biol. 2007, 49, 1003–1015. [Google Scholar] [CrossRef]
- Leveau, J.H.J.; Lindow, S.E. Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl. Environ. Microbiol. 2005, 71, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant. Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Stijn, S.; Jos, V.; Roseline, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant. Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 2013, 8, e62085. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Wang, H.; Shao, H.-B. Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front. Plant. Sci. 2015, 6, 792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved salinity tolerance of arachis hypogaea (L.) by the interaction of halotolerant plant growth promoting rhizobacteria. J. Plant. Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline accumulation in plants. Plant. Sign. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Cetner, M.D.; Samborska, I.A.; Lukasik, I.; Oukarroum, A.; Rusinowski, S.; Pietkiewicz, S.; Świątek, M.; Dąbrowski, P. Effective microorganisms’ impact on photosynthetic activity of Arabidopsis plant grown under salinity stress conditions Land Reclamation. Ann. Wars. Univ. Life Sci.-SGGW 2016, 48, 153–163. [Google Scholar] [CrossRef]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant. Physiol. 2009, 151, 792–808. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervantes-Díaz, L. Bacillus spp. inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil. J. Agri. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Gomaa, E.Z. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Vergara, A.G.; López-Bucio, J. Trichoderma modulates stomatal aperture and leaf transpiration through an abscisic acid-dependent mechanism in arabidopsis. J. Plant Growth Regul. 2015, 34, 425–432. [Google Scholar] [CrossRef]
- Signorelli, S. The fermentation analogy: A point of view for understanding the intriguing role of proline accumulation in stressed plants. Front. Plant. Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant. Cell Environ. 2013, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant. Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Oancea, F. Effects of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef]

| Isolates | Salt stress (NaCl) | |
|---|---|---|
| 0 mM | 200 mM | |
| C7 | 1.03 ± 0.02 b | 1.18 ± 0.02 c |
| Th4 | 0.11 ± 0.020 a | 0.11 ± 0.02 a |
| Th6 | 0.09 ± 0.02 a | 0.05 ± 0.02 a |
| A | |||
|---|---|---|---|
| Cultivars | Seed Treatment | µmol Proline g−1 FW | |
| 0 mM NaCl | 200 mM NaCl | ||
| Shimai | Uncoated | 2.16 ± 1.24 a | 67.98 ± 1.24 e |
| C7 | 0.97 ± 1.24 a | 56.38 ± 1.24 d | |
| Th4 | 2.67 ± 1.24 a | 59.23 ± 1.24 d | |
| Th6 | 1.59 ± 1.24 a | 61.09 ± 1.24 d | |
| Xiaoyan60 | Uncoated | 12.76 ±1.24 b | 28.07 ± 1.24 c |
| C7 | 0.55 ±1.24 a | 69.18 ± 1.24 e | |
| Th4 | 1.50 ± 1.24 a | 17.20 ± 1.24 b | |
| Th6 | 1.28 ±1.24 a | 3.50 ± 1.24 a | |
| B | |||
| Treatments | F | ||
| C | 560.93 *** | ||
| M | 122.04 *** | ||
| S | 4666.77 *** | ||
| C × M | 149.81 *** | ||
| C × S | 739.18 *** | ||
| M × S | 122.49 *** | ||
| C × M × S | 168.61 ** | ||
| Treatment | Photosynthetic Parameters | ||||
|---|---|---|---|---|---|
| A | gs | Ci | E | WUE | |
| C | 0.60 ns | 2.64 ns | 0.00 ns | 1.67 ns | 10.43 ** |
| M | 25.29 *** | 2.65 ns | 18.69 *** | 6.53 ** | 73.30 *** |
| S | 258.58 *** | 117.77 *** | 0.00 ns | 138.18 *** | 9.66 ** |
| C × M | 8.36 *** | 1.67 ns | 3.54 * | 0.39 ns | 1.97 ns |
| C × S | 4.42 * | 2.90 ns | 1.46 ns | 2.14 ns | 1.23 ns |
| M × S | 13.63 *** | 2.92 * | 8.17 *** | 6.41** | 22.59 *** |
| C × M × S | 2.18 ns | 2.04 ns | 1.39 ns | 0.78 ns | 2.42 ns |
| Treatments | F | |
|---|---|---|
| Shoot | Root | |
| C | 50.391 *** | 228.01 *** |
| M | 69.13 *** | 65.31 *** |
| S | 666.32 *** | 846.81 *** |
| C × M | 6.40 ** | 5.47 ** |
| C × S | 11.35 ** | 141.61 *** |
| M × S | 1.33 ns | 3.47 * |
| C × M × S | 7.05 *** | 14.43 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress. Microorganisms 2020, 8, 1565. https://doi.org/10.3390/microorganisms8101565
Oljira AM, Hussain T, Waghmode TR, Zhao H, Sun H, Liu X, Wang X, Liu B. Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress. Microorganisms. 2020; 8(10):1565. https://doi.org/10.3390/microorganisms8101565
Chicago/Turabian StyleOljira, Abraham Mulu, Tabassum Hussain, Tatoba R. Waghmode, Huicheng Zhao, Hongyong Sun, Xiaojing Liu, Xinzhen Wang, and Binbin Liu. 2020. "Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress" Microorganisms 8, no. 10: 1565. https://doi.org/10.3390/microorganisms8101565
APA StyleOljira, A. M., Hussain, T., Waghmode, T. R., Zhao, H., Sun, H., Liu, X., Wang, X., & Liu, B. (2020). Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress. Microorganisms, 8(10), 1565. https://doi.org/10.3390/microorganisms8101565






