Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
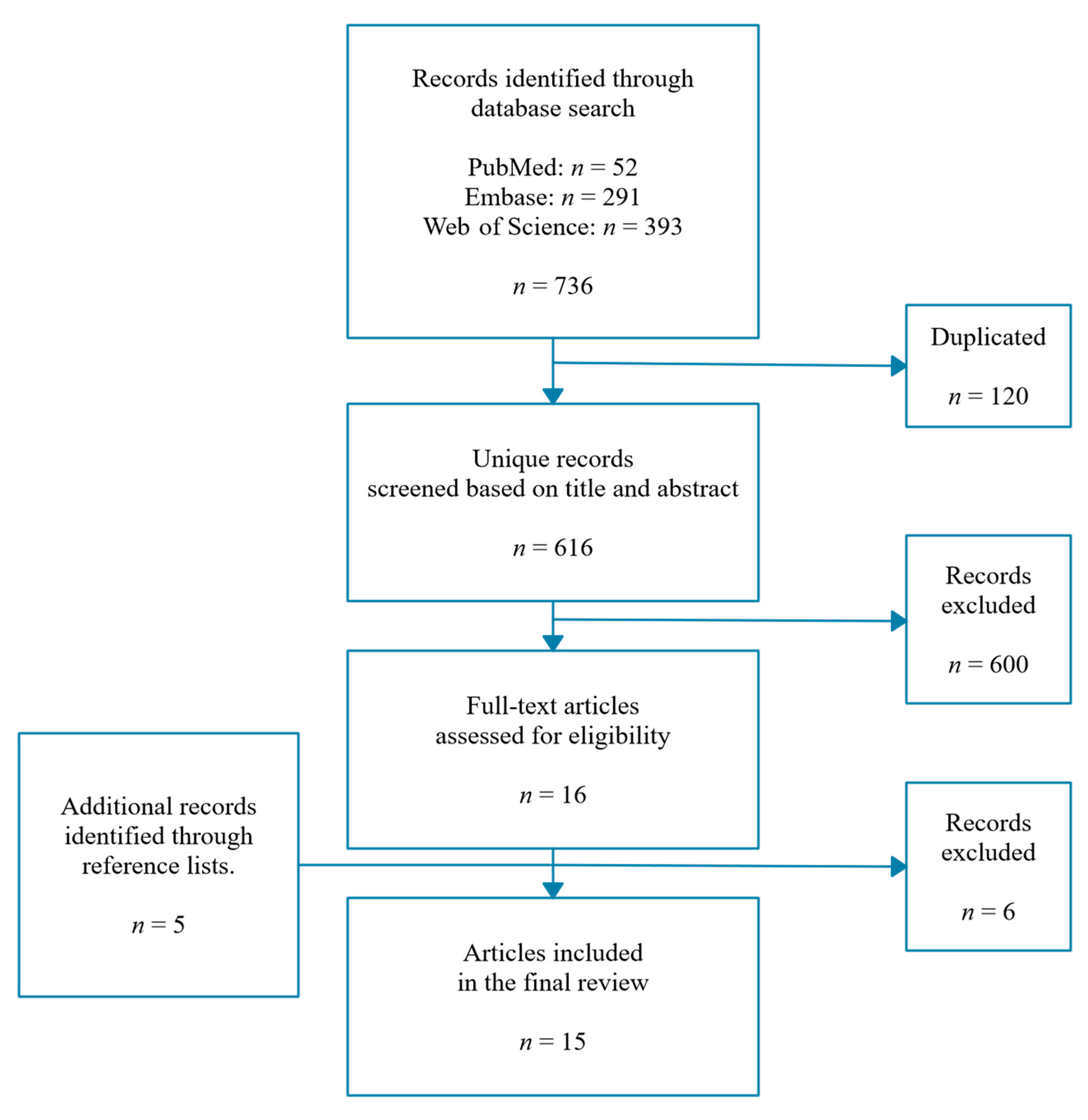
2.2. Data Extraction
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Crohn’s Disease |
| CRC | Colorectal Cancer |
| DGGE | Denaturing Gradient Gel Electrophoresis |
| FISH | Fluorescence In Situ Hybridization |
| FODMAP | Fermentable Oligo-, Di-, Monosaccharides, and Polyols |
| Funct. GI | Functional Intestinal Disorders |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable Bowel Syndrome |
| OL | Open Label |
| PP | Per protocol |
| qPCR | quantitative Polymerase Chain Reaction |
| RCT | Randomized Controlled Trial |
References
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a More Comprehensive Concept for Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to Promote Abundance of Akkermansia Muciniphila, an Emerging Probiotics in the Gut, Evidence from Dietary Intervention Studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Verhoog, S.; Taneri, P.E.; Díaz, Z.M.R.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia Muciniphila and Faecalibacterium Prausnitzii: A Systematic Review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.R.; Shepherd, S.J. Personal View: Food for Thought-Western Lifestyle and Susceptibility to Crohn’s Disease. The FODMAP Hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Controversies and Reality of the FODMAP Diet for Patients with Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef] [Green Version]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef]
- Durchschein, F.; Petritsch, W.; Hammer, H.F. Diet Therapy for Inflammatory Bowel Diseases: The Established and the New. World J. Gastroenterol. 2016, 22, 2179–2194. [Google Scholar] [CrossRef]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low Fermentable, Oligo-, Di-, Mono-Saccharides and Polyol Diet in the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.-T.; Heitkemper, M.M.; Zia, J. Effects of Low-FODMAPS Diet on Irritable Bowel Syndrome Symptoms and Gut Microbiome. Gastroenterol. Nurs. 2019, 42, 150–158. [Google Scholar] [CrossRef]
- Gibson, P.R. Use of the Low-FODMAP Diet in Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2017, 32, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, Double-Blind, Placebo-Controlled Trial of Fructo- Oligosaccharides in Active Crohn’s Disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, Microbiological, and Immunological Effects of Fructo-Oligosaccharide in Patients with Crohn’s Disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T.; et al. Effects of Prebiotics on Immunologic Indicators and Intestinal Microbiota Structure in Perioperative Colorectal Cancer Patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef]
- Huaman, J.W.; Mego, M.; Manichanh, C.; Cañellas, N.; Cañueto, D.; Segurola, H.; Jansana, M.; Malagelada, C.; Accarino, A.; Vulevic, J.; et al. Effects of Prebiotics vs a Diet Low in FODMAPs in Patients With Functional Gut Disorders. Gastroenterology 2018, 155, 1004–1007. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs Alter Symptoms and the Metabolome of Patients with IBS: A Randomised Controlled Trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical Trial: The Effects of a Trans-Galactooligosaccharide Prebiotic on Faecal Microbiota and Symptoms in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Joossens, M.; De Preter, V.; Ballet, V.; Verbeke, K.; Rutgeerts, P.; Vermeire, S. Effect of Oligofructose-Enriched Inulin (OF-IN) on Bacterial Composition and Disease Activity of Patients with Crohn’s Disease: Results from a Double-Blinded Randomised Controlled Trial. Gut 2012, 61, 958. [Google Scholar] [CrossRef]
- Valcheva, R.; Koleva, P.; Martínez, I.; Walter, J.; Gänzle, M.G.; Dieleman, L.A. Inulin-Type Fructans Improve Active Ulcerative Colitis Associated with Microbiota Changes and Increased Short-Chain Fatty Acids Levels. Gut Microbes 2019, 10, 334–357. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets That Differ in Their FODMAP Content Alter the Colonic Luminal Microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-Term Irritable Bowel Syndrome Symptom Control with Reintroduction of Selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [Green Version]
- Bennet, S.M.P.; Bohn, L.; Storsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Tornblom, H.; Ohman, L.; Simren, M. Multivariate Modelling of Faecal Bacterial Profiles of Patients with IBS Predicts Responsiveness to a Diet Low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of Inulin on the Human Gut Microbiota: Stimulation of Bifidobacterium Adolescentis and Faecalibacterium Prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Wijayabahu, A.T.; Waugh, S.G.; Ukhanova, M.; Mai, V. Dietary Raisin Intake Has Limited Effect on Gut Microbiota Composition in Adult Volunteers. Nutr. J. 2019, 18, 14. [Google Scholar] [CrossRef]
- Fernando, W.M.U.; Hill, J.E.; Zello, G.A.; Tyler, R.T.; Dahl, W.J.; Van Kessel, A.G. Diets Supplemented with Chickpea or Its Main Oligosaccharide Component Raffinose Modify Faecal Microbial Composition in Healthy Adults. Benef. Microbes 2010, 1, 197–207. [Google Scholar] [CrossRef] [Green Version]
- West, N.P.; Christophersen, C.T.; Pyne, D.B.; Cripps, A.W.; Conlon, M.A.; Topping, D.L.; Kang, S.; McSweeney, C.S.; Fricker, P.A.; Aguirre, D.; et al. Butyrylated Starch Increases Colonic Butyrate Concentration but Has Limited Effects on Immunity in Healthy Physically Active Individuals. Exerc. Immunol. Rev. 2013, 19, 102–119. [Google Scholar]
- Tochio, T.; Kadota, Y.; Tanaka, T.; Koga, Y. 1-Kestose, the Smallest Fructooligosaccharide Component, Which Efficiently Stimulates Faecalibacterium Prausnitzii as Well as Bifidobacteria in Humans. Foods 2018, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Stasse-Wolthuis, M. The Bifidogenic Effect of Inulin and Oligofructose and Its Consequences for Gut Health. Eur. J. Clin. Nutr. 2009, 63, 1277–1289. [Google Scholar] [CrossRef]
- De Preter, V.; Vanhoutte, T.; Huys, G.; Swings, J.; Rutgeerts, P.; Verbeke, K. Baseline Microbiota Activity and Initial Bifidobacteria Counts Influence Responses to Prebiotic Dosing in Healthy Subjects. Aliment. Pharmacol. Ther. 2008, 27, 504–513. [Google Scholar] [CrossRef]
- Rycroft, C.E.; Jones, M.R.; Gibson, G.R.; Rastall, R.A. A Comparative in Vitro Evaluation of the Fermentation Properties of Prebiotic Oligosaccharides. J. Appl. Microbiol. 2001, 91, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Buffie, C.G.; Pamer, E.G. Microbiota-Mediated Colonization Resistance against Intestinal Pathogens. Nat. Rev. Immun. 2013, 13, 790–801. [Google Scholar] [CrossRef] [Green Version]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the Gut Microbiota in Intestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Molecular Communication with the Immune System. Front. Microbiol. 2017, 8, 2345. [Google Scholar] [CrossRef] [Green Version]
- Medina, M.; Izquierdo, E.; Ennahar, S.; Sanz, Y. Differential Immunomodulatory Properties of Bifidobacterium Logum Strains: Relevance to Probiotic Selection and Clinical Applications. Clin. Exp. Immunol. 2007, 150, 531–538. [Google Scholar] [CrossRef]
- Jalanka-Tuovinen, J.; Salonen, A.; Nikkilä, J.; Immonen, O.; Kekkonen, R.; Lahti, L.; Palva, A.; de Vos, W.M. Intestinal Microbiota in Healthy Adults: Temporal Analysis Reveals Individual and Common Core and Relation to Intestinal Symptoms. PLoS ONE 2011, 6, e23035. [Google Scholar] [CrossRef]
- Pratt, C.; Campbell, M.D. The Effect of Bifidobacterium on Reducing Symptomatic Abdominal Pain in Patients with Irritable Bowel Syndrome: A Systematic Review. Probiotics Antimicrob. Proteins 2020, 12, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Hernandez, O.; Muthaiyan, A.; Moreno, F.J.; Montilla, A.; Sanz, M.L.; Ricke, S.C. Effect of Prebiotic Carbohydrates on the Growth and Tolerance of Lactobacillus. Food Microbiol. 2012, 30, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium Species as Probiotics: Potentials and Challenges. J. Anim. Sci. Biotechnol. 2020, 11. [Google Scholar] [CrossRef]
- Martín, R.; Bermúdez-Humarán, L.G.; Langella, P. Searching for the Bacterial Effector: The Example of the Multi-Skilled Commensal Bacterium Faecalibacterium Prausnitzii. Front. Microbiol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional Characterization of Novel Faecalibacterium Prausnitzii Strains Isolated from Healthy Volunteers: A Step Forward in the Use of F. Prausnitzii as a Next-Generation Probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic Inulin-Type Fructans Induce Specific Changes in the Human Gut Microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloan, T.J.; Jalanka, J.; Major, G.A.D.; Krishnasamy, S.; Pritchard, S.; Abdelrazig, S.; Korpela, K.; Singh, G.; Mulvenna, C.; Hoad, C.L.; et al. A Low FODMAP Diet Is Associated with Changes in the Microbiota and Reduction in Breath Hydrogen but Not Colonic Volume in Healthy Subjects. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Laue, H.; Denger, K.; Cook, A.M. Taurine Reduction in Anaerobic Respiration of Bilophila Wadsworthia RZATAU. Appl. Environ. Microbiol. 1997, 63, 2016–2021. [Google Scholar] [CrossRef] [Green Version]
- Baron, E.J. Bilophila Wadsworthia: A Unique Gram-Negative Anaerobic Rod. Anaerobe 1997, 3, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, S.; Vencesclau, C.; Fernandez, C.; Valente, F.; Pereira, I. Hydrogen as an energy source for the human pathogen Bilophila wadsworthia. Antonie van Leeuwenhoek 2008, 93, 381–390. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrota, A.; Erdman, S.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10-/- Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; Da Costa, G.; Van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila Wadsworthia Aggravates High Fat Diet Induced Metabolic Dysfunctions in Mice. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Finegold, S.; Summanen, P.; Gerardo, S.H.; Baron, E. Clinical Importance of Bilophila Wadsworthia. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 1058–1063. [Google Scholar] [CrossRef]
- Kasten, M.J.; Rosenblatt, J.E.; Gustafson, D.R. Bilophila Wadsworthia Bacteremia in Two Patients with Hepatic Abscesses. J. Clin. Microbiol. 1992, 30, 2502–2503. [Google Scholar] [CrossRef] [Green Version]
- Bernard, D.; Verschraegen, G.; Claeys, G.; Lauwers, S.; Rosseel, P. Bilophila Wadsworthia Bacteremia in a Patient with Gangrenous Appendicitis. Clin. Infect. Dis. 1994, 18, 1023–1024. [Google Scholar] [CrossRef]
- Mosca, A.; D’Alagni, M.; Del Prete, R.; De Michele, G.P.; Summanen, P.H.; Finegold, S.M.; Miragliotta, G. Preliminary Evidence of Endotoxic Activity of Bilophila Wadsworthia. Anaerobe 1995, 1, 21–24. [Google Scholar] [CrossRef]
- Gerardo, S.H.; Garcia, M.M.; Wexler, H.M.; Finegold, S.M. Adherence of Bilophila Wadsworthia to Cultured Human Embryonic Intestinal Cells. Anaerobe 1998, 4, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.J.; Summanen, P.; Downes, J.; Roberts, M.C.; Wexler, H.; Finegold, S.M. Bilophila Wadsworthia, Gen. Nov. and Sp. Nov., a Unique Gram-Negative Anaerobic Rod Recovered from Appendicitis Specimens and Human Faeces. J. Gen. Microbiol. 1989, 135, 3405–3411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautwein, E.A.; Rieckhoff, D.; Erbersdobler, H.F. Dietary Inulin Lowers Plasma Cholesterol and Triacylglycerol and Alters Biliary Bile Acid Profile in Hamsters. J. Nutr. 1998, 128, 1937–1943. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Berean, K.J.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Intestinal Gases: Influence on Gut Disorders and the Role of Dietary Manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. [Google Scholar] [CrossRef]
- Gibson, P.R.; Halmos, E.P.; Muir, J.G. Review Article: FODMAPS, Prebiotics and Gut Health-the FODMAP Hypothesis Revisited. Aliment. Pharmacol. Ther. 2020, 52, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut Microbiota Richness Promotes Its Stability upon Increased Dietary Fibre Intake in Healthy Adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef]
- Mcdonald, D.; Hyde, E.; Debelius, J.W.; Morton, J.T.; Gonzalez, A.; Ackermann, G.; Aksenov, A.A.; Behsaz, B.; Brennan, C.; Chen, Y.; et al. American Gut: An Open Platform for Citizen Science Microbiome Research. mSystems 2018, 3, e00031-18. [Google Scholar] [CrossRef] [Green Version]
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The Human Microbiome in Evolution. BMC Biol. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.a.; Bewtra, M.; Knights, D.; Walters, W.a.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Simpson, H.L.; Campbell, B.J. Review Article: Dietary Fibre-Microbiota Interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, M.; Cummings, J.H. The Microbial Contribution to Human Fecal Mass. J. Med. Microbiol. 1980, 13, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Bingham, S.A.; Heaton, K.W.; Eastwood, M.A. Fecal Weight, Colon Cancer Risk, and Dietary Intake of Nonstarch Polysaccharides (Dietary Fiber). Gastroenterology 1992, 103, 1783–1789. [Google Scholar] [CrossRef]
- Haack, V.S.; Marlett, J.A.; Kurasawa, S. Plant Residue and Bacteria as Bases for Increased Stool Weight Accompanying Consumption of Higher Dietary Fiber Diets. J. Am. Coll. Nutr. 2000, 19, 426–433. [Google Scholar] [CrossRef]
- De Vries, J.; Miller, P.E.; Verbeke, K. Effects of Cereal Fiber on Bowel Function: A Systematic Review of Intervention Trials. World J. Gastroenterol. 2015, 21, 8952–8963. [Google Scholar] [CrossRef]
- De Vries, J.; Le Bourgot, C.; Calame, W.; Respondek, F. Effects of β-Fructans Fiber on Bowel Function: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 91. [Google Scholar] [CrossRef] [Green Version]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Costello, E.K.; Berg-lyons, D.; Gonzalez, A.; Stombaugh, J.; Knights, D.; Gajer, P.; Ravel, J.; Fierer, N.; et al. Moving Pictures of the Human Microbiome. Genome Biol. 2011, 12, R50. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, S.M.; Kearney, S.M.; Smillie, C.S.; Alm, E.J. Two Dynamic Regimes in the Human Gut Microbiome. PLoS Comput. Biol. 2017, 13, e1005364. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and Diet-Responsive Groups of Bacteria within the Human Colonic Microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H. The Shrinking Human Gut Microbiome. Curr Opin in Microbiol. 2017, 38, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rausch, P.; Rühlemann, M.; Hermes, B.M.; Doms, S.; Dagan, T.; Dierking, K.; Domin, H.; Fraune, S.; Von Frieling, J.; Hentschel, U.; et al. Comparative Analysis of Amplicon and Metagenomic Sequencing Methods Reveals Key Features in the Evolution of Animal Metaorganisms. Microbiome 2019, 7, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeputte, D.; Kathagen, G.; D’hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative Microbiome Profiling Links Gut Community Variation to Microbial Load. Nature 2017, 551, 507. [Google Scholar] [CrossRef]
- Tkacz, A.; Hortala, M.; Poole, P.S. Absolute Quantitation of Microbiota Abundance in Environmental Samples. Microbiome 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, J.T.; Marotz, C.; Washburne, A.; Silverman, J.; Zaramela, L.S.; Edlund, A.; Zengler, K.; Knight, R. Establishing Microbial Composition Measurement Standards with Reference Frames. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Stämmler, F.; Gläsner, J.; Hiergeist, A.; Holler, E.; Weber, D.; Oefner, P.J.; Gessner, A.; Spang, R. Adjusting Microbiome Profiles for Differences in Microbial Load by Spike-in Bacteria. Microbiome 2016, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Di Tourlousse, M.; Ohashi, A.; Sekiguchi, Y. Sample Tracking in Microbiome Community Profiling Assays Using Synthetic 16S RRNA Gene Spike-in Controls. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Props, R.; Kerckhof, F.M.; Rubbens, P.; De Vrieze, J.; Sanabria, E.H.; Waegeman, W.; Monsieurs, P.; Hammes, F.; Boon, N. Absolute Quantification of Microbial Taxon Abundances. ISME J. 2017, 11, 584–587. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, A.; Parlov, M.; Hu, P.; Schnell, D.; Wei, X.; Tiesman, J.P. Spike-in Genomic DNA for Validating Performance of Metagenomics Workflows. Biotechniques 2018, 65, 315–321. [Google Scholar] [CrossRef]
- Jian, C.; Luukkonen, P.; Yki-Järvinen, H.; Salonen, A.; Korpela, K. Quantitative PCR Provides a Simple and Accessible Method for Quantitative Microbiota Profiling. PLoS ONE 2020, 15, e0227285. [Google Scholar] [CrossRef] [Green Version]
- De Preter, V.; Verbeke, K. Metabolomics as a Diagnostic Tool in Gastroenterology. World J. Gastrointest. Pharmacol. Ther. 2013, 4, 97. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The Fecal Metabolome as a Functional Readout of the Gut Microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best Practices for Analysing Microbiomes. Nat. Rev. Genet. 2018, 16, 410–422. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, D.; Tito, R.Y.; Vanleeuwen, R.; Falony, G.; Raes, J. Practical Considerations for Large-Scale Gut Microbiome Studies. FEMS Microbiol. Rev. 2017, 41 (Suppl. S1), S154–S167. [Google Scholar] [CrossRef]
- Amos, G.C.A.; Logan, A.; Anwar, S.; Fritzsche, M.; Mate, R.; Bleazard, T.; Rijpkema, S. Developing Standards for the Microbiome Field. Microbiome 2020, 8, 98. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Ravel, J.; Fricke, W.F. “Available upon Request”: Not Good Enough for Microbiome Data! Microbiome 2018, 6, 8. [Google Scholar] [CrossRef]
- Mego, M.; Manichanh, C.; Accarino, A.; Campos, D.; Pozuelo, M.; Varela, E.; Vulevic, J.; Tzortzis, G.; Gibson, G.; Guarner, F.; et al. Metabolic Adaptation of Colonic Microbiota to Galactooligosaccharides: A Proof-of-Concept-Study. Aliment. Pharmacol. Ther. 2017, 45, 670–680. [Google Scholar] [CrossRef] [Green Version]
- Mego, M.; Accarino, A.; Tzortzis, G.; Vulevic, J.; Gibson, G.; Guarner, F.; Azpiroz, F. Colonic Gas Homeostasis: Mechanisms of Adaptation Following HOST-G904 Galactooligosaccharide Use in Humans. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Dolan, R.; Chey, W.D.; Eswaran, S. The Role of Diet in the Management of Irritable Bowel Syndrome: A Focus on FODMAPs. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 607–615. [Google Scholar] [CrossRef]
- Wilson, B.; Whelan, K. Prebiotic Inulin-Type Fructans and Galacto-Oligosaccharides: Definition, Specificity, Function, and Application in Gastrointestinal Disorders. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 64–68. [Google Scholar] [CrossRef] [Green Version]
- The human microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Imhann, F.; Brandsma, E.; Jankipersadsing, S.A.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Valeur, J.; Roseth, A.G.; Knudsen, T.; Malmstrom, G.H.; Fiennes, J.T.; Midtvedt, T.; Berstad, A. Fecal Fermentation in Irritable Bowel Syndrome: Influence of Dietary Restriction of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols. Digestion 2016, 94, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Cope, J.L.; Hollister, E.B.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Versalovic, J.; Shulman, R.J. Randomised Clinical Trial: Gut Microbiome Biomarkers Are Associated with Clinical Response to a Low FODMAP Diet in Children with the Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2015, 42, 418–427. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandeputte, D.; Joossens, M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms 2020, 8, 1638. https://doi.org/10.3390/microorganisms8111638
Vandeputte D, Joossens M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms. 2020; 8(11):1638. https://doi.org/10.3390/microorganisms8111638
Chicago/Turabian StyleVandeputte, Doris, and Marie Joossens. 2020. "Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review" Microorganisms 8, no. 11: 1638. https://doi.org/10.3390/microorganisms8111638
APA StyleVandeputte, D., & Joossens, M. (2020). Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms, 8(11), 1638. https://doi.org/10.3390/microorganisms8111638





