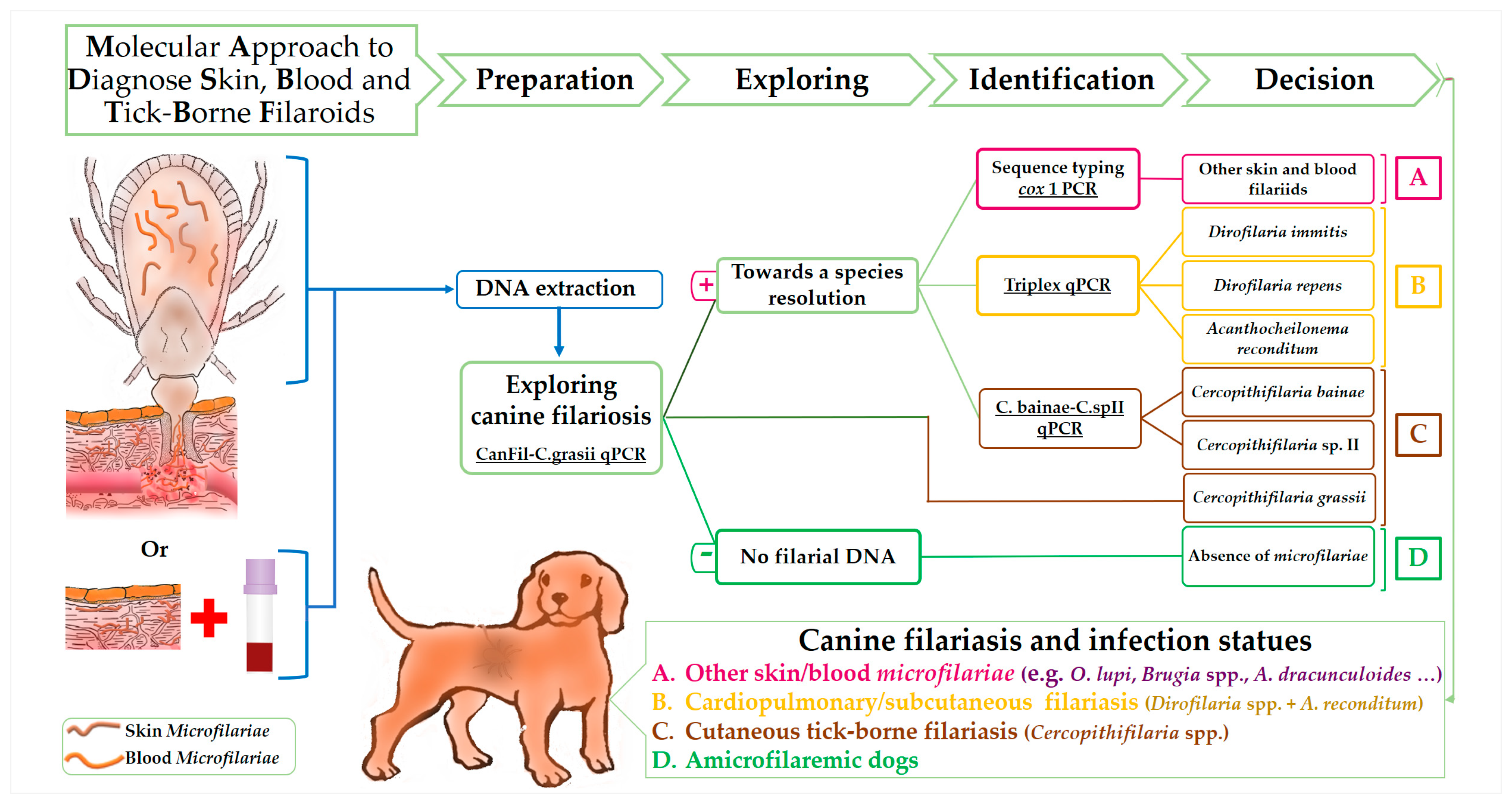
Molecular Approach for the Diagnosis of Blood and Skin Canine Filarioids
Abstract
1. Introduction
2. Materiel and Methods
2.1. Design Protocol and Specificity-Based Principles of the Duplex Real Time qPCRs
2.2. Run Protocols
2.3. Assays Validation
2.3.1. Specificity Validation
2.3.2. Limit of Detection and Efficiency Assessment
2.3.3. Microfilariae Quantification Protocol
2.4. Set Up of a Molecular Approach for the Diagnosis of Blood and Skin Filarioids
2.4.1. Samples Collection
2.4.2. Ethics Approval and Consent to Participate
2.4.3. Diagnostic Approach Standardization on Biological Samples
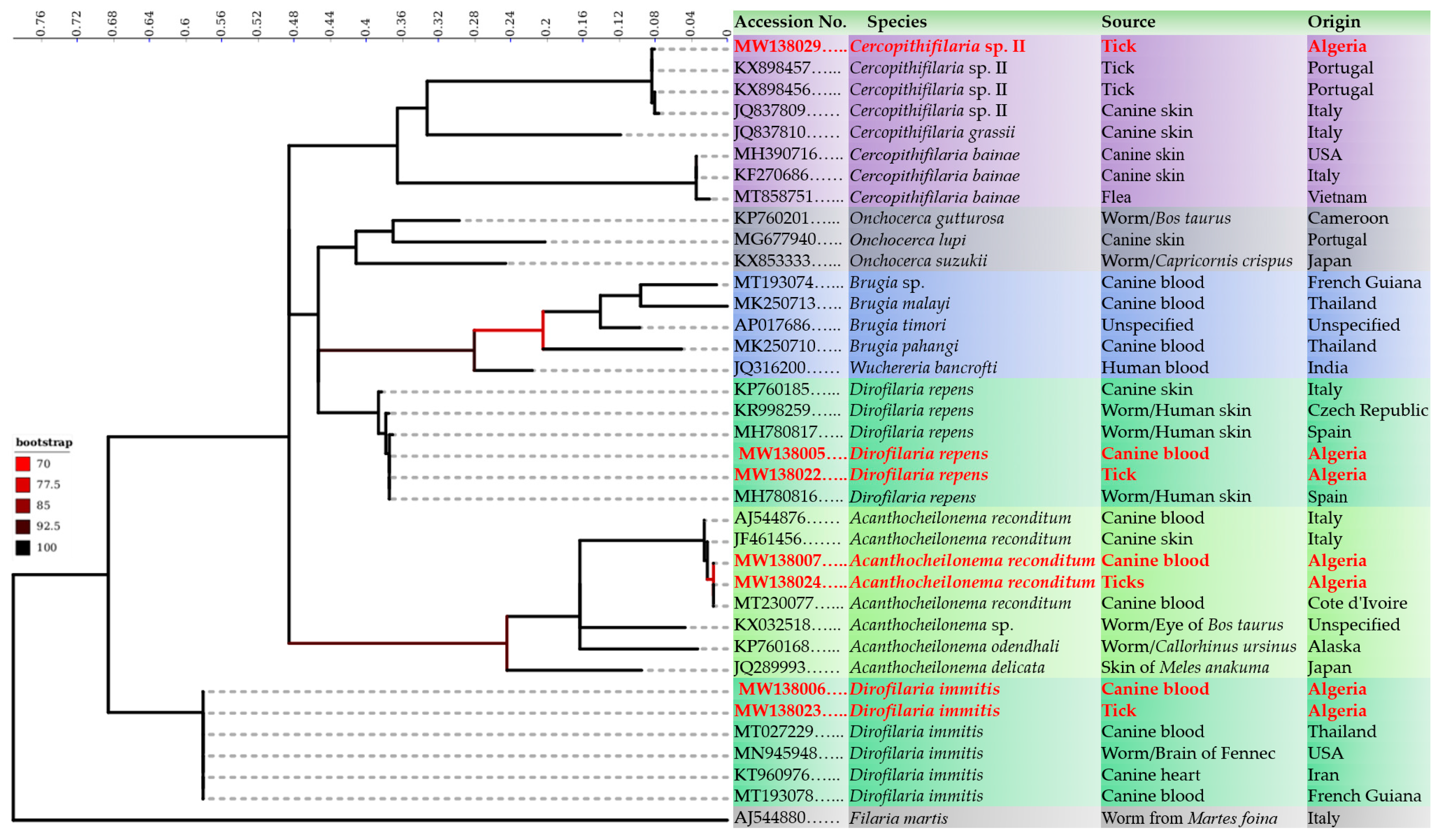
3. Results
Analytical Sensitivity and Assay Performance Characteristics
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission; C.A.B. International: Wallingford, UK, 1992; p. 578. [Google Scholar]
- Ravindran, R.; Varghese, S.; Nair, S.N.; Balan, V.M.; Lakshmanan, B.; Ashruf, R.M.; Kumar, S.S.; Gopalan, A.K.K.; Nair, A.S.; Chandrasekhar, L. Canine filarial infections in a human Brugia malayi endemic area of India. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Latrofa, M.S.; Annoscia, G.; Colella, V.; Cavalera, M.A.; Maia, C.; Martin, C.; Šlapeta, J.; Otranto, D. A real-time PCR tool for the surveillance of zoonotic Onchocerca lupi in dogs, cats and potential vectors. PLoS Negl. Trop. Dis. 2018, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Brianti, E.; Abramo, F.; Gaglio, G.; Napoli, E.; Latrofa, M.S.; Ramos, R.A.; Dantas-Torres, F.; Bain, O. Cutaneous distribution and localization of Cercopithifilaria sp. microfilariae in dogs. Vet. Parasitol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Laidoudi, Y.; Davoust, B.; Niang, E.H.A.; Varloud, M.; Fenollar, F.; Mediannikov, O. Development of a multiplex qPCR-based approach for the diagnosis of Dirofilaria immitis, D. repens and Acanthocheilonema reconditum. Parasites Vectors 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Sréter, T.; Széll, Z. Onchocercosis: A newly recognized disease in dogs. Vet. Parasitol. 2008, 151, 1–13. [Google Scholar] [CrossRef]
- Otranto, D.; Brianti, E.; Dantas-Torres, F.; Miro, G.; Latrofa, M.S.; Mutafchiev, Y.; Bain, O. Species diversity of dermal microfilariae of the genus Cercopithifilaria infesting dogs in the Mediterranean region. Parasitology 2013, 140, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Cortes, H.C.E.; Cardoso, L.; Giannelli, A.; Latrofa, M.S.; Dantas-Torres, F.; Otranto, D. Diversity of Cercopithifilaria species in dogs from Portugal. Parasites Vectors 2014, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cantey, P.T.; Weeks, J.; Edwards, M.; Rao, S.; Ostovar, G.A.; Dehority, W.; Alzona, M.; Swoboda, S.; Christiaens, B.; Ballan, W. The emergence of zoonotic Onchocerca lupi infection in the United States—A case-series. Clin. Infect. Dis. 2016, 62, 778–783. [Google Scholar] [CrossRef]
- Otranto, D.; Brianti, E.; Latrofa, M.; Annoscia, G.; Weigl, S.; Lia, R.; Gaglio, G.; Napoli, E.; Giannetto, S.; Papadopoulos, E.; et al. On a Cercopithifilaria sp. transmitted by Rhipicephalus sanguineus: A neglected, but widespread filarioid of dogs. Parasites Vectors 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Rodonaja, T.E. A new species of Nematode, Onchocerca lupi n. sp., from Canis lupus cubanensis. Soobscenija Akademii Nauk Gruzinskoj SSR 1967, 45, 715–719. [Google Scholar]
- Otranto, D.; Giannelli, A.; Trumble, N.S.; Chavkin, M.; Kennard, G.; Latrofa, M.S.; Bowman, D.D.; Dantas-Torres, F.; Eberhard, M.L. Clinical case presentation and a review of the literature of canine onchocercosis by Onchocerca lupi in the United States. Parasites Vectors 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Hermosilla, C.; Hetzel, U.; Bausch, M.; Grübl, J.; Bauer, C. First autochthonous case of canine ocular onchocercosis in Germany. Vet. Rec. 2005, 156, 450–451. [Google Scholar] [CrossRef] [PubMed]
- Széll, Z.; Erdélyi, I.; Sréter, T.; Albert, M.; Varga, I. Canine ocular onchocercosis in Hungary. Vet. Parasitol. 2001, 97, 245–251. [Google Scholar] [CrossRef]
- Labelle, A.L.; Daniels, J.B.; Dix, M.; Labelle, P. Onchocerca lupi causing ocular disease in two cats. Vet. Ophthalmol. 2011, 14. [Google Scholar] [CrossRef]
- Labelle, A.L.; Maddox, C.W.; Daniels, J.B.; Lanka, S.; Eggett, T.E.; Dubielzig, R.R.; Labelle, P. Canine ocular onchocercosis in the United States is associated with Onchocerca lupi. Vet. Parasitol. 2013, 193. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Annoscia, G.; Latrofa, M.S.; Pereira, A.; Giannelli, A.; Pedroso, L.; Otranto, D. Onchocerca lupi nematode in cat, Portugal. Emerg. Infect. Dis. 2015, 21, 2252–2253. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F.; Giannelli, A.; Latrofa, M.S.; Papadopoulos, E.; Cardoso, L.; Cortes, H. Zoonotic Onchocerca lupi infection in dogs, Greece and Portugal, 2011–2012. Emerg. Infect. Dis. 2013, 19, 2000–2003. [Google Scholar] [CrossRef]
- Miró, G.; Montoya, A.; Checa, R.; Gálvez, R.; Mínguez, J.J.; Marino, V.; Otranto, D. First detection of Onchocerca lupi infection in dogs in southern Spain. Parasites Vectors 2016, 9. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Giannelli, A.; Abramo, F.; Ignjatović Ćupina, A.; Petrić, D.; Cardoso, L.; Mutafchiev, Y.; Cortes, H. Cutaneous Distribution and Circadian Rhythm of Onchocerca lupi Microfilariae in Dogs. PLoS Negl. Trop. Dis. 2013. [Google Scholar] [CrossRef]
- Bain, O.; Aeschlimann, A.; Chatelanat, P. Présence, chez des tiques de la région de Genève, de larves infestantes qui pourraient se rapporter à la filaire de chien Dipetalonema grassii. Ann. Parasitol. Hum. Comparée 1982. [Google Scholar] [CrossRef]
- Pampiglione, S.; Canestri Trotti, G.; Marchetti, S. Ritrovamento di Diptalonema grassii (Noè, 1907) in Rhipicephalus sanguineus su cane in Italia e descrizione di alcuni suoi stadi larvali. Parassitologia 1983, 25, 316. [Google Scholar]
- Otranto, D.; Varcasia, A.; Solinas, C.; Scala, A.; Brianti, E.; Dantas-Torres, F.; Annoscia, G.; Martin, C.; Mutafchiev, Y.; Bain, O. Redescription of Cercopithifilaria bainae Almeida & Vicente, 1984 (Spirurida, Onchocercidae) from a dog in Sardinia, Italy. Parasites Vectors 2013. [Google Scholar] [CrossRef]
- Otranto, D.; Latrofa, M.S.; Brianti, E.; Annoscia, G.; Parisi, A.; Dantas-Torres, F.; Bain, O.; Gasser, R.B. An assessment of genetic variability in the mitochondrial cytochrome c oxidase subunit 1 gene of Cercopithifilaria sp. (Spirurida, Onchocercidae) from dog and Rhipicephalus sanguineus populations. Mol. Cell. Probes 2012. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Varcasia, A.; Brianti, E.; Giannetto, S.; Pipia, A.P.; Columbano, N.; Tosciri, G.; Dantas-Torres, F.; Garippa, G.; Otranto, D.; et al. Cercopithifilaria spp. in dogs in Sardinia Island (Italy). Parasitol. Res. 2014. [Google Scholar] [CrossRef]
- Ferri, E.; Barbuto, M.; Bain, O.; Galimberti, A.; Uni, S.; Guerrero, R.; Ferté, H.; Bandi, C.; Martin, C.; Casiraghi, M. Integrated taxonomy: Traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda). Front. Zool. 2009, 6, 1–12. [Google Scholar] [CrossRef]
- SantaLucia, J. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc. Natl. Acad. Sci. USA 1998. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Bio-Rad Lab. Real-Time PCR Applications Guide; Bio-Rad Lab. Inc.: Hercules, CA, USA, 2006; Volume 41. [Google Scholar]
- Medkour, H.; Laidoudi, Y.; Lafri, I.; Davoust, B.; Mekroud, A.; Bitam, I.; Mediannikov, O. Canine vector-borne protozoa: Molecular and serological investigation for Leishmania spp., Trypanosoma spp., Babesia spp., and Hepatozoon spp. in dogs from Northern Algeria. Vet. Parasitol. Reg. Stud. Rep. 2020. [Google Scholar] [CrossRef]
- Bessas, A.; Leulmi, H.; Bitam, I.; Zaidi, S.; Ait-Oudhia, K.; Raoult, D.; Parola, P. Molecular evidence of vector-borne pathogens in dogs and cats and their ectoparasites in Algiers, Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 23–28. [Google Scholar] [CrossRef]
- Halos, L.; Jamal, T.; Vial, L.; Maillard, R.; Suau, A.; Le Menach, A.; Boulouis, H.J.; Vayssier-Taussat, M. Determination of an efficient and reliable method for DNA extraction from ticks. Vet. Res. 2004, 35. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47. [Google Scholar] [CrossRef] [PubMed]
- Yule, G.U. On the methods of measuring association between two attributes. J. R. Stat. Soc. 1912, 75, 579–652. [Google Scholar] [CrossRef]
- Bonett, D.G.; Price, R.M. Statistical inference for generalized Yule coefficients in 2 × 2 contingency tables. Sociol. Methods Res. 2007, 35, 429–446. [Google Scholar] [CrossRef]
- Warrens, M.J. On association coefficients for 2 × 2 tables and properties that do not depend on the marginal distributions. Psychometrika 2008, 73, 777. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasites Vectors 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Overview on Dirofilaria immitis in the Americas, with notes on other filarial worms infecting dogs. Vet. Parasitol. 2020, 282. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Breitschwerdt, E.B. Managing canine vector-borne diseases of zoonotic concern: Part one. Trends Parasitol. 2009, 25, 157–163. [Google Scholar] [CrossRef]
- Lineberry, M.W.; Sundstrom, K.D.; Little, S.E.; Stayton, E.M.; Allen, K.E. Detection of Cercopithifilaria bainae infection in shelter dogs and ticks in Oklahoma, USA. Parasites Vectors 2020, 13. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Mari, J.; Tahir, D.; Watier-grillot, S. Detection of Canine Vector-Borne Filariasis and Their Wolbachia Endosymbionts in French Guiana. Microorganisms 2020, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.; Nascimento, F.S.; Mathison, B.A.; Bishop, H.; Bradbury, R.S.; Cama, V.A.; da Silva, A.J. Duplex Real-Time PCR Assay for Clinical Differentiation of Onchocerca lupi and Onchocerca volvulus. Am. J. Trop. Med. Hyg. 2020. [Google Scholar] [CrossRef]
- Rojas, A.; Rojas, D.; Montenegro, V.M.; Baneth, G. Detection of Dirofilaria immitis and other arthropod-borne filarioids by an HRM real-time qPCR, blood-concentrating techniques and a serological assay in dogs from Costa Rica. Parasites Vectors 2015, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Namrata, P.; Miller, J.M.; Shilpa, M.; Reddy, P.R.; Bandoski, C.; Rossi, M.J.; Sapi, E. Filarial nematode infection in Ixodes scapularis ticks collected from Southern Connecticut. Vet. Sci. 2014, 1, 5–15. [Google Scholar] [CrossRef]
- Beaver, P.C.; Burgdorfer, W. A microfilaria of exceptional size from the ixodid tick, Ixodes dammini, from Shelter Island, New York. J. Parasitol. 1984, 70. [Google Scholar] [CrossRef]
- Voigt, C.C.; Peschel, U.; Wibbelt, G.; Frölich, K. An alternative, less invasive blood sample collection technique for serologic studies utilizing Triatomine bugs (Heteroptera; Insecta). J. Wildl. Dis. 2006, 42. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani PA, M.; Irwin, P.J.; Gatne, M.; Coleman, G.T.; McInnes, L.M.; Traub, R.J. A survey of canine filarial diseases of veterinary and public health significance in India. Parasites Vectors 2010, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Magi, M.; Guardone, L.; Prati, M.C.; Tozzini, G.; Torracca, B.; Monni, G.; Macchioni, F. Canine filarial infections in Tuscany, central Italy. J. Helminthol. 2012, 86, 113. [Google Scholar] [CrossRef]
- Latrofa, M.S.; Dantas-Torres, F.; Giannelli, A.; Otranto, D. Molecular detection of tick-borne pathogens in Rhipicephalus sanguineus group ticks. Ticks Tick Borne Dis. 2014, 5. [Google Scholar] [CrossRef]
- Meriem-Hind, B.-M.; Mohamed, M. Prevalence of canine Dirofilaria immitis infection in the city of Algiers, Algeria. Afr. J. Agric. Res. 2009, 4, 1097–1100. [Google Scholar]
- Tahir, D.; Damene, H.; Davoust, B.; Parola, P. First molecular detection of Dirofilaria immitis (Spirurida: Onchocercidae) infection in dogs from Northern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51. [Google Scholar] [CrossRef]
- Henning, T.C.; Orr, J.M.; Smith, J.D.; Arias, J.R.; Rasgon, J.L.; Norris, D.E. Discovery of filarial nematode DNA in Amblyomma americanum in Northern Virginia. Ticks Tick Borne Dis. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
| Assay Name | Sequences Names | Sequences | Specificity |
|---|---|---|---|
| CanFil-C. grassii | C. Fil.354f | GATCGTAATTTTARTACYTCTTTTTATGA | Filaria/C. grassii |
| Can-fil.411p | 6VIC-TATCAGCATTTGTTTTGGTTTTT-TAMRA | ||
| C. grassii.433p | 6FAM-GGAAGGGTGGTAATCCTCTTCTTT-TAMRA | ||
| C. Fil.564r | CAGCAATCCAAATAGAAGCAAA | ||
| C. bainae-C.spII | T-Fil-62f | TTGTCTTTTTGGTTTACTTTTGTGG | C. bainae/Cercopithifilaria sp. II |
| C. bai.121p | 6FAM-AGGGGGTGCTGGTAGCAGG-TAMRA | ||
| C. sp. II.116p | 6VIC-GTTGGTAGAGGCCCTGGGAGT-TAMRA | ||
| T-Fil-337r | GAAGTCAAATAAGAAGTRCAAACAAACA |
| Panels of Tested DNA from Filarioids | CanFil-C. grasii | C. bainae-C. sp II | ||||
|---|---|---|---|---|---|---|
| Species Name | Specimens | Tested Samples (n) | Filarial DNA | C. grassii | C. bainae | C. sp. II |
| C. bainae | Adult worms | 1 | 1 | Negative | 1 | Negative |
| Larva F1 “Microfilaria” | 2 | 2 | Negative | 2 | Negative | |
| Infected ticks | 5 | 5 | Negative | 5 | Negative | |
| Cercopithifilaria sp. II | Larva F1 “Microfilaria” | 1 | 1 | Negative | Negative | 1 |
| C. bainae + C.sp. II | Mixed DNA | 3 | 3 | Negative | 3 | 3 |
| C. grassii | Larva F1 “Microfilaria” | 1 | 1 | 1 | Negative | Negative |
| C. grassii + C. bainae | Mixed DNA | 3 | 3 | 3 | 3 | Negative |
| C. grassii + C.sp. II | Mixed DNA | 2 | 2 | 2 | Negative | 2 |
| O. lupi | Larva F1 “Microfilaria” | 1 | 1 | Negative | Negative | Negative |
| Infected skin | 6 | 6 | Negative | Negative | Negative | |
| D. immitis | Adult worms | 2 | 2 | Negative | Negative | Negative |
| D. repens | Adult worms | 1 | 1 | Negative | Negative | Negative |
| A. reconditum | Blood microfilaria | 1 | 1 | Negative | Negative | Negative |
| T. callipaeda | Adult worms | 2 | 2 | Negative | Negative | Negative |
| S. digitata | Adult worms | 2 | 2 | Negative | Negative | Negative |
| Mansonella sp. | Blood microfilaria | 1 | 1 | Negative | Negative | Negative |
| M. perstens | Blood microfilaria | 1 | 1 | Negative | Negative | Negative |
| W. bancrofiti | Blood microfilaria | 2 | 2 | Negative | Negative | Negative |
| Loa loa | Blood microfilaria | 1 | 1 | Negative | Negative | Negative |
| Brugia sp. | Infected mosquitoes | 3 | 3 | Negative | Negative | Negative |
| B. malayi. | Infected mosquitoes | 3 | 3 | Negative | Negative | Negative |
| Filarial free samples | Ticks | 5 | Negative | Negative | Negative | Negative |
| Dog skin | 5 | Negative | Negative | Negative | Negative | |
| Assays | DNA Target | Efficiency (%) | Coefficient of Determination (R2) | Slope | Y-Intercept | |
|---|---|---|---|---|---|---|
| CanFil-C.grasii | Single-species DNA | Onchocerca lupi | 103.2 | 0.994 | −3.247 | 40.541 |
| Cercopithifilaria grassii | 99.3 | 0.999 | −3.38 | 41.018 | ||
| Pooled DNAs | O. lupi | 103.8 | 0.993 | −3.235 | 43.74 | |
| C. grassii | 99.3 | 0.996 | −3.34 | 45.107 | ||
| C. bainae-C.sp.II | Single-species DNA | Cercopithifilaria bainae | 100.3 | 0.996 | −3.314 | 43.902 |
| Cercopithifilaria sp. II. | 100.3 | 0.997 | −3.315 | 45.792 | ||
| Pooled DNAs | C. bainae | 99.5 | 0.995 | −3.334 | 44.918 | |
| Cercopithifilaria sp. II. | 104.9 | 0.994 | −3.21 | 43.694 | ||
| SQ mfs/mL | SQ Per qPCR Reaction from mfs/5µL | Ct Mean | E-RFU | SCRS |
|---|---|---|---|---|
| 4.7 × 10+2 | 2.35 × 100 | 24.26 | 1554 | [E = 96.9%] [S = −3.398] [Y.int = 33.2] [R2 = 0.998] |
| 4.7 × 10+1 | 2.35 × 10−1 | 27.35 | 1363 | |
| 4.7 × 100 | 2.35 × 10−2 | 31.03 | 1046 | |
| 4.7 × 10−1 | 2.35 × 10−3 | 34.02 | 596 | |
| 4.7 × 10−2 | 2.35 × 10−4 | 37.92 | 175 | |
| 4.7 × 10−3 | 2.35 × 10−5 | N/A | N/A | |
| 4.7 × 10−4 | 2.35 × 10−6 | N/A | N/A | |
| 4.7 × 10−5 | 2.35 × 10−7 | N/A | N/A | |
| 4.7 × 10−6 | 2.35 × 10−8 | N/A | N/A | |
| 4.7 × 10−7 | 2.35 × 10−9 | N/A | N/A | |
| Cut Off Value | 38.0 | 161 | ||
| Negative Control | N/A | 6.09 | ||
| Filarial Species | Multiplex qPCR | PCR/Sequencing | ||
|---|---|---|---|---|
| Blood | Tick | Blood | Tick | |
| Single-species DNA | ||||
| D. immitis | 14 | 9 | 14 | 9 |
| D. repens | 2 | 2 | 2 | 2 |
| A. reconditum | 1 | 1 | 1 | 1 |
| C. bainae | 0 | 0 | 0 | 0 |
| Cercopithifilaria sp. II | 0 | 2 | 0 | 2 |
| Total | 17 | 14 | 17 | 14 |
| Multi-species DNA | ||||
| D. immitis and D. repens | 2 | 1 | 2 ur | 1 ur |
| D. immitis and A. reconditum | 3 | 3 | 3 ur | 3 ur |
| D. immitis and C. bainae | 0 | 4 | 0 | 4 ur |
| D. immitis and Cercopithifilaria sp. II | 0 | 1 | 0 | 1 ur |
| D. immitis, D. repens and C. bainae | 0 | 1 | 0 | 1 ur |
| Total | 5 | 10 | 5 ur | 10 ur |
| Performances (in % Unless Specified) | Multiplex qPCRs Approach | Sequence Typing Approach | ||
|---|---|---|---|---|
| Ticks | Blood | Ticks | Blood | |
| True positive (n = 19) | 19 | 17 | 14 | 17 |
| True negative (n = 48) | 48 | 48 | 48 | 48 |
| False positive (n) | 0 | 0 | 0 | 0 |
| False negative (n) | 0 | 2 | 5 | 2 |
| Total | 67 | 67 | 67 | 67 |
| Sensitivity | 100 | 89.47 | 73.68 | 89.47 |
| Specificity | 100 | 100 | 100 | 100 |
| Predictive positive value (PPV) | 100 | 100 | 100 | 100 |
| Predictive negative value (PNV) | 100 | 96 | 90.57 | 96 |
| False positive rate | 0 | 0 | 0 | 0 |
| False negative rate | 0 | 4 | 9.43 | 4 |
| Correct classifcation | 28.36 | 28.36 | 28.36 | 28.36 |
| Prevalence | 28.36 | 25.37 | 20.9 | 25.37 |
| Youden index | 1 | 0.86 | 0.74 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laidoudi, Y.; Bedjaoui, S.; Medkour, H.; Latrofa, M.S.; Mekroud, A.; Bitam, I.; Davoust, B.; Otranto, D.; Mediannikov, O. Molecular Approach for the Diagnosis of Blood and Skin Canine Filarioids. Microorganisms 2020, 8, 1671. https://doi.org/10.3390/microorganisms8111671
Laidoudi Y, Bedjaoui S, Medkour H, Latrofa MS, Mekroud A, Bitam I, Davoust B, Otranto D, Mediannikov O. Molecular Approach for the Diagnosis of Blood and Skin Canine Filarioids. Microorganisms. 2020; 8(11):1671. https://doi.org/10.3390/microorganisms8111671
Chicago/Turabian StyleLaidoudi, Younes, Samia Bedjaoui, Hacène Medkour, Maria Stefania Latrofa, Abdeslam Mekroud, Idir Bitam, Bernard Davoust, Domenico Otranto, and Oleg Mediannikov. 2020. "Molecular Approach for the Diagnosis of Blood and Skin Canine Filarioids" Microorganisms 8, no. 11: 1671. https://doi.org/10.3390/microorganisms8111671
APA StyleLaidoudi, Y., Bedjaoui, S., Medkour, H., Latrofa, M. S., Mekroud, A., Bitam, I., Davoust, B., Otranto, D., & Mediannikov, O. (2020). Molecular Approach for the Diagnosis of Blood and Skin Canine Filarioids. Microorganisms, 8(11), 1671. https://doi.org/10.3390/microorganisms8111671






