Killer Yeasts for the Biological Control of Postharvest Fungal Crop Diseases
Abstract
1. Introduction
2. Counteractive Measures to Control Fungal Diseases
2.1. Preharvest Stage
2.2. Postharvest Stage
2.3. Biological Control
3. Yeasts as Biocontrol Agents
3.1. The Yeast Killer Phenotype
3.2. Genetics of the Killer Phenotype
4. Application of Killer Yeasts as Biocontrol Agents
4.1. Preharvest Application of Killer Yeast
4.2. Postharvest Application of Killer Yeasts
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of pesticides: A brief review. Front. Biosci. 2008, 13, 1240–1249. [Google Scholar] [CrossRef]
- Jankowska, M.; Kaczynski, P.; Hrynko, I.; Lozowicka, B. Dissipation of six fungicides in greenhouse-grown tomatoes with processing and health risk. Environ. Sci. Pollut. Res. 2016, 23, 11885–11900. [Google Scholar] [CrossRef] [PubMed]
- Muri, S.D.; van der Voet, H.; Boon, P.E.; van Klaveren, J.D.; Brüschweiler, B.J. Comparison of human health risks resulting from exposure to fungicides and mycotoxins via food. Food Chem. Toxicol. 2009, 47, 2963–2974. [Google Scholar] [CrossRef] [PubMed]
- Wightwick, A.; Walters, R.; Allinson, G.; Reichman, S.; Menzies, N. Environmental risks of fungicides used in horticultural production systems. In Fungicides; Carisse, O., Ed.; InTech: Rijeka, Croatia, 2009; pp. 273–304. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? 2nd ed.; Fungicide Resistance Action Committee: Brussels, Belgium, 2007; p. 48. [Google Scholar]
- Eckert, J.W.; Sievert, J.R.; Ratnayake, M. Reduction of imazalil effectiveness against citrus green mold in California packinghouses by resistant biotypes of Penicillium digitatum. Plant Dis. 1994, 78, 971–974. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef]
- Giles, K.L.; McCornack, B.P.; Royer, T.A.; Elliott, N.C. Incorporating biological control into IPM decision making. Curr. Opin. Insect Sci. 2017, 20, 84–89. [Google Scholar] [CrossRef]
- Matyjaszczyk, E. Products containing microorganisms as a tool in integrated pest management and the rules of their market placement in the European Union. Pest Manag. Sci. 2015, 71, 1201–1206. [Google Scholar] [CrossRef]
- Owen, M.D.; Beckie, H.J.; Leeson, J.Y.; Norsworthy, J.K.; Steckel, L.E. Integrated pest management and weed management in the United States and Canada. Pest Manag. Sci. 2018, 71, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.T.; Suckling, D.M.; Wearing, C.H. Past, present, and future of integrated control of apple pests: The New Zealand experience. Annu. Rev. Entomol. 2017, 62, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Barkai-Golan, R. Postharvest Diseases of Fruits and Vegetables: Development and Control, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2001; p. 418. [Google Scholar]
- Timmer, L.W.; Je, F. The effect of rainfall, drainage, tree spacing and fungicide application on the incidence of citrus brown rot. Phytopathology 1975, 65, 241–242. [Google Scholar] [CrossRef]
- Prusky, D.; Fuchs, Y.; Yanko, U. Assessment of latent infections as a basis for control of postharvest disease of mango. Plant Dis. 1983, 67, 816–818. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Artés, F.; Gómez, P.; Aguayo, E.; Escalona, V.; Artés-Hernández, F. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharvest Biol. Technol. 2009, 51, 287–296. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; Adel, A., Ed.; Kader: California, CA, USA, 2002. [Google Scholar]
- Zhang, H.Y.; Fu, C.X.; Zheng, X.D.; He, D.; Shan, L.J.; Jhon, X. Effect of Cryptococcus laurentii (Kufferath) Skinner in combination with sodium bicarbonate on biocontrol of postharvest green mold decay of citrus fruits. Bot. Bull. Acad. Sin. 2004, 45, 159–164. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.E.; Cohen, L.; Weiss, B.; Touitou, D.; Eilam, Y.; Chalutz, E. Influence of CaCl2 on Penicillium digitatum, grapefruit peel tissue, and biocontrol activity of Pichia guilliermondii. Phytopathology 1997, 87, 310–315. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Chalutz, E.; Eilam, Y. Effects of Ca2+ and Mg2+ on Botrytis cinerea and Penicillium expansum in vitro and on the biocontrol activity of Candida oleophila. Plant Pathol. J. 1995, 44, 1016–1024. [Google Scholar] [CrossRef]
- Emmert, E.A.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Schaible, U.E.; Kaufmann, S.H. A nutritive view on the host–pathogen interplay. Trends Microbiol. 2005, 13, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Tech. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Wisniewski, M.; Wilson, C.; Droby, S.; Chalutz, E.; El Ghaouth, A.; Stevens, C. Postharvest biocontrol: New concepts and applications. In Biological Control: A Global Perspective; International: Cambridge, MA, USA, 2007; pp. 262–273. [Google Scholar]
- Calderon, C.E.; Rotem, N.; Harris, R.; Vela-Corcía, D.; Levy, M. Pseudozyma aphidis activates reactive oxygen species production, programmed cell death and morphological alterations in the necrotrophic fungus Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Gafni, A.; Calderon, C.E.; Harris, R.; Buxdorf, K.; Dafa-Berger, A.; Zeilinger-Reichert, E.; Levy, M. Biological control of the cucurbit powdery mildew pathogen Podosphaera xanthii by means of the epiphytic fungus Pseudozyma aphidis and parasitism as a mode of action. Front. Plant Sci. 2015, 6, 132. [Google Scholar] [CrossRef]
- Arrarte, E.; Garmendia, G.; Rossini, C.; Wisniewski, M.; Vero, S. Volatile organic compounds produced by Antarctic strains of Candida sake play a role in the control of postharvest pathogens of apples. Biol. Control 2017, 109, 14–20. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Huang, R.; Li, G.Q.; Zhang, J.; Yang, L.; Che, H.J.; Jiang, D.H.; Huang, H.C. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology 2011, 101, 859–869. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Chalutz, E.; Ben-Arie, R.; Droby, S.; Cohen, L.; Weiss, B.; Wilson, C.L. Yeasts as biocontrol agents of postharvest diseases of fruits. Phytoparasit. Isr. J. Plant Prot. Sci. 1988, 16, 69. [Google Scholar]
- Janisiewicz, W.J. Postharvest biological control of blue mold on apples. Phytopathology 1987, 77, 481–485. [Google Scholar] [CrossRef]
- Janisiewicz, W.J. Biocontrol of postharvest diseases of apples with antagonist mixtures. Phytopathology 1988, 78, 194–198. [Google Scholar] [CrossRef]
- Perez, M.F.; Ibarreche, J.P.; Isas, A.S.; Sepulveda, M.; Ramallo, J.; Dib, J.R. Antagonistic yeasts for the biological control of Penicillium digitatum on lemons stored under export conditions. Biol. Control 2017, 115, 135–140. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; González, M.B.; Bentancur, O.; Wisniewski, M. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus× domestica). FEMS Yeast Res. 2013, 13, 189–199. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Magliani, W.; Conti, S.; Gerloni, M.; Bertolotti, D.; Polonelli, L. Yeast killer systems. Clin. Microbiol. Rev. 1997, 10, 369–400. [Google Scholar] [CrossRef]
- Meinhardt, F.; Klassen, R. Yeast killer toxins: Fundamentals and applications. In Physiology and Genetics; Springer: Berlin/Heidelberg, Germany, 2009; pp. 107–130. [Google Scholar]
- Schaffrath, R.; Meinhardt, F.; Klassen, R. Yeast killer toxins: Fundamentals and applications. In Physiology and Genetics; Springer: Cham, Switzerland, 2018; pp. 87–118. [Google Scholar]
- Starmer, W.T.; Ganter, P.F.; Aberdeen, V.; Lachance, M.A.; Phaff, H.J. The ecological role of killer yeasts in natural communities of yeasts. Can. J. Microbiol. 1987, 33, 783–796. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Breinig, F. The viral killer system in yeast: From molecular biology to application. FEMS Microbiol. Rev. 2002, 26, 257–276. [Google Scholar] [CrossRef]
- Kaufmann, G. Anticodon nucleases. Trends Biochem. Sci. 2000, 25, 70–74. [Google Scholar] [CrossRef]
- Konisky, J. Colicins and other bacteriocins with established modes of action. Ann. Rev. Microbiol. 1982, 36, 122–145. [Google Scholar] [CrossRef] [PubMed]
- Satwika, D.; Klassen, R.; Meinhardt, F. Anticodon nuclease encoding virus-like elements in yeast. Appl. Microbial. Biot. 2012, 96, 345–356. [Google Scholar] [CrossRef]
- Bevan, E.A.; Makower, M. The physiological basis of the killer character in yeast. Proc. XIth Int. Congr. Genet 1963, 1, 202–203. [Google Scholar]
- Woods, D.R.; Bevan, E.A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. Microbiology 1968, 51, 115–126. [Google Scholar] [CrossRef]
- Ciani, M.; Fatichenti, F. Killer Toxin of Kluyveromyces phaffii DBVPG 6076 as a Biopreservative Agent to Control apiculate wine yeasts. Appl. Environ. Microbiol. 2001, 67, 3058–3063. [Google Scholar] [CrossRef]
- Goretti, M.; Turchetti, B.; Buratta, M.; Branda, E.; Corazzi, L.; Vaughan-Martini, A.; Buzzini, P. In vitro antimycotic activity of a Williopsis saturnus killer protein against food spoilage yeasts. Int. J. Food Microbiol. 2009, 131, 178–182. [Google Scholar] [CrossRef]
- Somers, J.M.; Bevan, E.A. The inheritance of the killer character in yeast. Genet. Res. 1969, 13, 71–83. [Google Scholar] [CrossRef]
- Adler, J.; Wood, H.A.; Bozarth, R.F. Virus-like particles from killer, neutral, and sensitive strains of Saccharomyces cerevisiae. J. Virol. 1976, 17, 472–476. [Google Scholar] [CrossRef]
- Berry, E.A.; Bevan, E.A. A new species of double-stranded RNA from yeast. Nature 1972, 239, 279–280. [Google Scholar] [CrossRef]
- Herring, A.J.; Bevan, E.A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J. Gen. Virol. 1974, 22, 387–394. [Google Scholar] [CrossRef]
- Vodkin, M.H.; Fink, G.R. A nucleic acid associated with a killer strain of yeast. Proc. Natl. Acad. Sci. USA 1973, 70, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Vodkin, M.H.; Katterman, F.; Fink, G.R. Yeast killer mutants with altered double-stranded ribonucleic acid. J. Bacteriol. 1974, 117, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Klassen, R.; Schaffrath, R.; Buzzini, P.; Philip Ganter, P.F. Antagonistic interactions and killer yeasts. In Yeasts in Natural Ecosystems: Ecology; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 229–275. [Google Scholar] [CrossRef]
- Philliskirk, G.; Young, T.W. The occurrence of killer character in yeasts of various genera. Antonie Leeuwenhoek 1975, 41, 147. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Breinig, F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006, 4, 212–221. [Google Scholar] [CrossRef]
- Kast, A.; Voges, R.; Schroth, M.; Schaffrath, R.; Klassen, R.; Meinhardt, F. Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression. PLoS Genet. 2015, 11, e1005005. [Google Scholar] [CrossRef]
- Young, T.W.; Yagiu, M. A comparison of the killer character in different yeasts and its classification. Antonie Leeuwenhoek 1978, 44, 59–77. [Google Scholar] [CrossRef]
- Dignard, D.; Whiteway, M.; Germain, D.; Tessier, D.; Thomas, D.Y. Expression in yeast of a cDNA copy of the K2 killer toxin gene. Mol. Gen. Genet. 1991, 227, 127–136. [Google Scholar] [CrossRef]
- Lukša, J.; Podoliankaitė, M.; Vepštaitė, I.; Strazdaitė-Žielienė, Ž.; Urbonavičius, J.; Servienė, E. Yeast β-1, 6-glucan is a primary target for the Saccharomyces cerevisiae K2 toxin. Eukaryot. Cell 2015, 14, 406–414. [Google Scholar] [CrossRef]
- Orentaite, I.; Poranen, M.M.; Oksanen, H.M.; Daugelavicius, R.; Bamford, D.H. K2 killer toxin-induced physiological changes in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2016, 16, 3. [Google Scholar] [CrossRef]
- Santos, A.; Marquina, D.; Leal, J.A.; Peinado, J.M. Biology of killer yeasts. Int. J. Microbiol. 2002, 5, 65–71. [Google Scholar] [CrossRef]
- Muccilli, S.; Wemhoff, S.; Restuccia, C.; Meinhardt, F. Exoglucanase-encoding genes from three Wickerhamomyces anomalus killer strains isolated from olive brine. Yeast 2013, 30, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Schaffrath, R.; Meacock, P.A.; Meinhardt, F. Yeast killer plasmid pGKL2: Molecular analysis of UCS5, a cytoplasmic promoter element essential for ORF5 gene function. Mol. Gen. Genet. 1996, 250, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, F.; Schaffrath, R. Extranuclear inheritance: Cytoplasmic linear double-stranded DNA killer elements of the dairy yeast Kluyveromyces lactis. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2001; Volume 62, pp. 51–70. [Google Scholar] [CrossRef]
- Klassen, R.; Teichert, S.; Meinhardt, F. Novel yeast killer toxins provoke S-phase arrest and DNA damage checkpoint activation. Mol. Microbiol. 2004, 53, 263–273. [Google Scholar] [CrossRef]
- Ocampo-Suarez, I.B.; López, Z.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A.; Knauth, P. Are biological control agents, isolated from tropical fruits, harmless to potential consumers? Food Chem. Toxicol. 2017, 109, 1055–1062. [Google Scholar] [CrossRef]
- Ellis, M.A.; Erincik, O. Anthracnose of grape. In Agriculture and Natural Resources; Keith, L.S., Ed.; The Ohio State University: Columbus, OH, USA, 2008; pp. 1–3. [Google Scholar]
- Magarey, R.D.; Emmett, R.W.; Magarey, P.A.; Franz, P.R. Evaluation of control of grape anthracnose caused by Elsinoe ampelina by preinfection fungicides. Australas Plant Pathol. 1993, 22, 48–52. [Google Scholar] [CrossRef]
- Liu, Z.; Du, S.; Ren, Y.; Liu, Y. Biocontrol ability of killer yeasts (Saccharomyces cerevisiae) isolated from wine against Colletotrichum gloeosporioides on grape. J. Basic Microbiol. 2018, 58, 60–67. [Google Scholar] [CrossRef]
- Santos, A.; Marquina, D. Killer toxin of Pichia membranifaciens and its possible use as a biocontrol agent against grey mould disease of grapevine. Microbiology 2004, 150, 2527–2534. [Google Scholar] [CrossRef]
- Lopes, M.R.; Klein, M.N.; Ferraz, L.P.; da Silva, A.C.; Kupper, K.C. Saccharomyces cerevisiae: A novel and efficient biological control agent for Colletotrichum acutatum during pre-harvest. Microbiol. Res. 2015, 175, 93–99. [Google Scholar] [CrossRef]
- Viñas, I. Principios básicos de la patología de poscosecha. Frut 1990, 5, 285–292. [Google Scholar]
- Sánchez-Torres, P.; Tuset, J.J. Molecular insights into fungicide resistance in sensitive and resistant Penicillium digitatum strains infecting citrus. Postharvest Biol. Technol. 2011, 59, 159–165. [Google Scholar] [CrossRef]
- Perez, M.F.; Contreras, L.; Garnica, N.M.; Fernández-Zenoff, M.V.; Farías, M.E.; Sepulveda, M. Native Killer Yeasts as Biocontrol Agents of Postharvest Fungal Diseases in Lemons. PLoS ONE 2016, 11, e0165590. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.F.; Díaz, M.A.; Pereyra, M.M.; Córdoba, J.M.; Isas, A.S.; Sepúlveda, M.; Dib, J.R. Biocontrol features of Clavispora lusitaniae against Penicillium digitatum on lemons. Postharvest Biol. Technol. 2019, 155, 57–64. [Google Scholar] [CrossRef]
- Pereyra, M.M.; Díaz, M.A.; Meinhardt, F.; Dib, J.R. Effect of stress factors associated with postharvest citrus conditions on the viability and biocontrol activity of Clavispora lusitaniae strain 146. PLoS ONE 2020, 15, e0239432. [Google Scholar] [CrossRef]
- Díaz, M.A.; Pereyra, M.M.; Santander, F.F.S.; Perez, M.F.; Córdoba, J.M.; Alhussein, M.; Dib, J.R. Protection of Citrus Fruits from Postharvest Infection with Penicillium digitatum and Degradation of Patulin by Biocontrol Yeast Clavispora lusitaniae 146. Microorganisms 2020, 8, 1477. [Google Scholar] [CrossRef]
- Platania, C.; Restuccia, C.; Muccilli, S.; Cirvilleri, G. Efficacy of killer yeasts in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis). Food Microbiol. 2012, 30, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Muccilli, S.; Wemhoff, S.; Restuccia, C.; Meinhardt, F. Molecular genetics of Pichia anomala killer strains isolated from naturally fermented olive brine. J. Biotechnol. 2010, 150, 302. [Google Scholar] [CrossRef]
- Da Cunha, T.; Ferraz, L.P.; Wehr, P.P.; Kupper, K.C. Antifungal activity and action mechanisms of yeasts isolates from citrus against Penicillium italicum. Int. J. Food Microbiol. 2018, 276, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, M.; Żarowska, B.; Restuccia, C.; Cirvilleri, G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017, 61, 93–101. [Google Scholar] [CrossRef]
- Corbacı, C.; Ucar, F.B.; Yalcin, H.T. Isolation and characterization of yeasts associated with Turkish-style homemade dairy products and their potential as starter cultures. Afr. J. Microbiol. Res. 2012, 6, 534–542. [Google Scholar] [CrossRef]
- Çorbacı, C.; Uçar, F.B. Purification, characterization and in vivo biocontrol efficiency of killer toxins from Debaryomyces hansenii strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Macioszek, V.K.; Lawrence, C.B.; Kononowicz, A.K. Infection cycle of Alternaria brassicicola on Brassica oleracea leaves under growth room conditions. Plant Pathol. J. 2018, 67, 1088–1096. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Velaquez-Del Valle, M.G.; Hernandez-Lauzardoa, A.N.; Barka, E.A. The Rhizopus stolonifer-Tomato interaction. In Plant-Microbe Interactions; Ait Barka, E., Clément, C., Eds.; Research Signpost: Kerala, India, 2008; pp. 269–289. [Google Scholar]
- Lima, J.R.; Goncalves, L.R.B.; Brandão, L.R.; Rosa, C.A.; Viana, F.M.P. Isolation, identification and activity in vitro of killer yeasts against Colletotrichum gloeosporioides isolated from tropical fruits. J. Basic Microbiol. 2012, 52, 1–10. [Google Scholar] [CrossRef]
- Lima, J.R.; Gondim, D.M.; Oliveira, J.T.A.; Oliveira, F.S.; Gonçalves, L.R.; Viana, F.M. Use of killer yeast in the management of postharvest papaya anthracnose. Postharvest Biol. Technol. 2013, 83, 58–64. [Google Scholar] [CrossRef]
- Di Francesco, A.; Mari, M. Monilinia species of fruit decay: A comparison between biological and epidemiological data. Ital. J. Mycol. 2018, 47, 13–23. [Google Scholar] [CrossRef]
- Madbouly, A.K.; Elyousr, K.A.A.; Ismail, I.M. Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biol. Control 2020, 144, 104239. [Google Scholar] [CrossRef]
- Czarnecka, M.; Żarowska, B.; Połomska, X.; Restuccia, C.; Cirvilleri, G. Role of biocontrol yeasts Debaryomyces hansenii and Wickerhamomyces anomalus in plants’ defence mechanisms against Monilinia fructicola in apple fruits. Food Microbiol. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Bertuzzi, T.; Languasco, L.; Giorni, P.; Kozakiewicz, Z. Occurrence of ochratoxin A-producing fungi in grapes grown in Italy. J. Food Prot. 2003, 66, 633–636. [Google Scholar] [CrossRef]
- Zimmerli, B.; Dick, R. Ochratoxin A in table wine and grape juice: Occurrence and risk assessment. Food Addit. Contam. 1996, 13, 655–668. [Google Scholar] [CrossRef]
- Bleve, G.; Grieco, F.; Cozzi, G.; Logrieco, A.; Visconti, A. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int. J. Food Microbiol. 2006, 108, 204–209. [Google Scholar] [CrossRef]
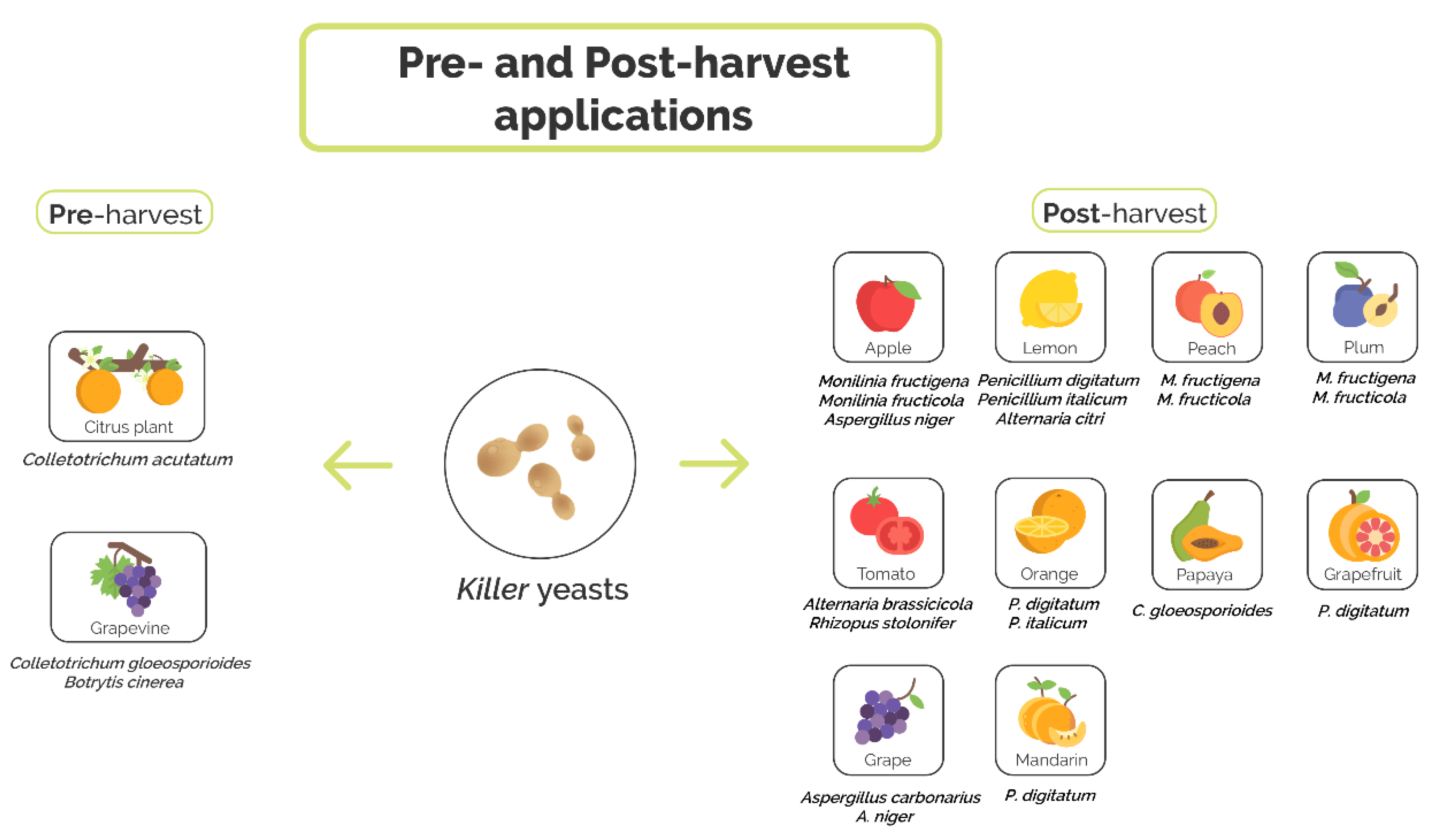


| Application Stage | Fruit | Pathogen | Antagonist Killer Yeast | Accessory Functions | References |
|---|---|---|---|---|---|
| Preharvest | Grapevine | C. gloeosporioides | S. cerevisiae GA8, CK and L24 | Production of antifungal volatile compounds (S. cerevisiae GA8) and production of hydrolytic enzymes (β-1,3-glucanase and chitinase) | [77] |
| B. cinerea | P. membranifaciens CYC 1106 | N.D. | [78] | ||
| Citrus | C. acutatum | S. cerevisiae ACB-AR1, ACB-KD1, ACB-CAT1, ACB-BG1, ACB-K1, and ACB-PE2 | Competition for nutrients, inhibition of spore germination, production of hydrolytic enzymes (β-1,3-glucanase and chitinase) and production of antifungal volatile compounds (S. cerevisiae ACB-CAT1, ACB-BG1) | [79] | |
| Postharvest | Lemon | P. digitatum and P. italicum | P. fermentans 27 and 28, W. anomalus 56, C. lusitaniae 146 | Competition for nutrient and space | [39,82,83,84,85] |
| A. citri | D. hansenii TEM8 and TEM17 | N.D. | [90,91] | ||
| Orange | P. digitatum | W. anomalus BS91 and BS92 | Mycoparasitism, production of hydrolytic enzymes (β-1,3-glucanase) | [70,86,87] | |
| C. lusitaniae 146 | Competition for nutrient and space | [85] | |||
| P. italicum | C. stellimalicola ACBL-04, ACBL-05, ACBL-08 and S. cereviciae ACBL-11 | Inhibition of spore germination, production of hydrolytic enzymes (chitinase) | [88] | ||
| Mandarin | P. digitatum | C. lusitaniae 146 | Competition for nutrient and space | [85] | |
| Grapefruit | P. digitatum | C. lusitaniae 146 | Competition for nutrient and space | [85] | |
| Peach | M. fructigena and M. fructicola | D. hansenii KI2a and W. anomalus BS91 | Production of hydrolytic enzymes (β-1,3-glucanase) and production of antifungal volatile compounds | [89] | |
| Plum | M. fructigena and M. fructicola | D. hansenii KI2a and W. anomalus BS91 | Production of hydrolytic enzymes (β-1,3-glucanase) and production of antifungal volatile compounds (W. anomalus BS91) | [89] | |
| Tomato | A. brassicicola and R. stolonifer | D. hansenii TEM8 and TEM17 | N.D. | [90,91] | |
| Papaya | C. gloeosporioides | W. anomalus 422 and M. guilliermondii 443 | Competition for nutrients and space, mycoparasitism and production of hydrolytic enzymes (β-1,3-glucanase) | [94,95] | |
| Apple | M. fructigena | S. vanrijiae, G. geotrichum, P. kudriavzevii, D. hansenii and R. glutinis | Production of hydrolytic enzymes (chitinase, β-1,3-glucanase, pectinase, and protease) | [97] | |
| M. fructicola | W. anomalus BS91, D. hansenii 4II4b and KI2a | Induction of resistance plant | [98] | ||
| A. niger | D. hansenii TEM8 and TEM17 | N.D. | [90,91] | ||
| Grape | A. carbonarius and A. niger | I. orientalis 2C2 and 16C2 | Competition for nutrients | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, M.A.; Pereyra, M.M.; Picón-Montenegro, E.; Meinhardt, F.; Dib, J.R. Killer Yeasts for the Biological Control of Postharvest Fungal Crop Diseases. Microorganisms 2020, 8, 1680. https://doi.org/10.3390/microorganisms8111680
Díaz MA, Pereyra MM, Picón-Montenegro E, Meinhardt F, Dib JR. Killer Yeasts for the Biological Control of Postharvest Fungal Crop Diseases. Microorganisms. 2020; 8(11):1680. https://doi.org/10.3390/microorganisms8111680
Chicago/Turabian StyleDíaz, Mariana Andrea, Martina María Pereyra, Ernesto Picón-Montenegro, Friedhelm Meinhardt, and Julián Rafael Dib. 2020. "Killer Yeasts for the Biological Control of Postharvest Fungal Crop Diseases" Microorganisms 8, no. 11: 1680. https://doi.org/10.3390/microorganisms8111680
APA StyleDíaz, M. A., Pereyra, M. M., Picón-Montenegro, E., Meinhardt, F., & Dib, J. R. (2020). Killer Yeasts for the Biological Control of Postharvest Fungal Crop Diseases. Microorganisms, 8(11), 1680. https://doi.org/10.3390/microorganisms8111680





