New Technologies for Influenza Vaccines
Abstract
1. Introduction
2. Influenza Virus Surveillance for Vaccine Preparation
3. Recently Registered Non-Egg Technologies
3.1. Cell Culture Vaccines
3.2. Recombinant Protein
4. New Technologies
4.1. Nucleic Acid Technologies
4.1.1. DNA
4.1.2. RNA
4.2. Viral Vectors
4.2.1. Modified Vaccinia Ankara (MVA) and Pox Viruses
4.2.2. Adenovirus Based Vaccines
4.3. Structure/Computational Design, COBRA
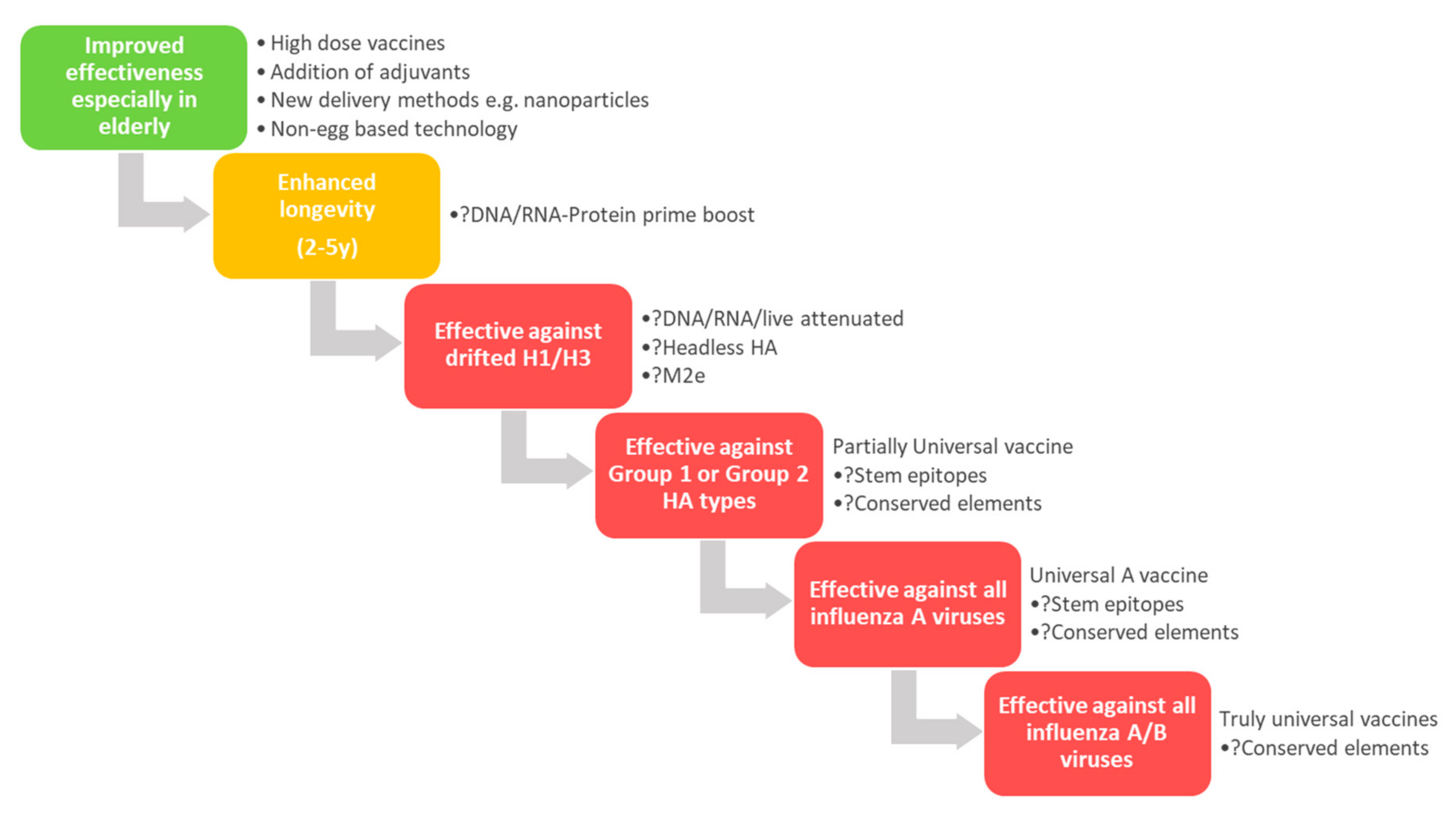
5. Universal Influenza Vaccines
5.1. New Epitopes for Rational Immunogen Design
5.2. Modulating Immunogen Glycosylation and Glycan Engineering
5.3. New Vaccine Platforms for Broader (Universal) Protection
5.4. Other Viral Proteins as Targets for Universal Vaccines
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molinari, N.-A.M.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef]
- Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 20 July 2020).
- Influenza (Flu) Who is at High Risk for Flu Complications. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 24 August 2020).
- Influenza (Flu) Past Pandemics. Available online: https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html (accessed on 24 August 2020).
- Sutton, T.C. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Cocchi, I.; Fumagalli, M.; Zuccotti, G. Influenza Vaccination: Effectiveness, Indications, and Limits in the Pediatric Population. Front Pediatr. 2019, 7, 317. [Google Scholar] [CrossRef]
- Hannoun, C. The evolving history of influenza viruses and influenza vaccines. Expert Rev. Vaccines 2013, 12, 9. [Google Scholar]
- Barberis, I.; Myles, P.; Ault, S.K.; Bragazzi, N.L.; Martini, M. History and evolution of influenza control through vaccination: From the first monovalent vaccine to universal vaccines. J. Prev. Med. Hyg. 2016, 57, E115–E120. [Google Scholar]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Barr, I.G.; Donis, R.O.; Katz, J.M.; McCauley, J.W.; Odagiri, T.; Trusheim, H.; Tsai, T.F.; Wentworth, D.E. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: A step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Budd, A.P.; Wentworth, D.E.; Blanton, L.; Elal, A.I.A.; Alabi, N.; Barnes, J.; Brammer, L.; Burns, E.; Cummings, C.N.; Davis, T.; et al. Update: Influenza Activity-United States, October 1, 2017-February 3, 2018. Mmwr. Morb. Mortal. Wkly. Rep. 2018, 67, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Gaglani, M.; Pruszynski, J.; Murthy, K.; Clipper, L.; Robertson, A.; Reis, M.; Chung, J.R.; Piedra, P.A.; Avadhanula, V.; Nowalk, M.P.; et al. Influenza Vaccine Effectiveness Against 2009 Pandemic Influenza A(H1N1) Virus Differed by Vaccine Type During 2013–2014 in the United States. J. Infect. Dis. 2016, 213, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Chung, J.R.; Jackson, L.A.; Phillips, C.H.; Benoit, J.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; et al. Influenza Vaccine Effectiveness in the United States during the 2015–2016 Season. N. Engl. J. Med. 2017, 377, 534–543. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Bright, H.; Mallory, R. Letter to the editor: Potential causes of the decreased effectiveness of the influenza A(H1N1)pdm09 strain in live attenuated influenza vaccines. Euro. Surveill. 2016, 21, 30394. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Kelvin, A.A.; Zambon, M. Influenza imprinting in childhood and the influence on vaccine response later in life. Euro. Surveill. 2019, 24, 1900720. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Biologicals Influenza. Available online: https://www.who.int/biologicals/vaccines/influenza/en/ (accessed on 30 October 2020).
- BiondVax’s CEO Comments on Impact of COVID-19 Pandemic on the Company’s Ongoing Phase 3 Clinical Trial. Available online: https://www.biondvax.com/2020/03/biondvaxs-ceo-comments-on-impact-of-covid-19-pandemic-on-the-companys-ongoing-phase-3-clinical-trial/ (accessed on 20 October 2020).
- Positive Results from Phase IIb Field Study of FLU-v Vaccine (FLU-v 003), which has been Developed by Imutex Limited, hVIVO’s 49% Joint Venture with the SEEK Group FLU-v is a First-in-Class ‘Universal’, Broad Spectrum, Standalone, Influenza Vaccine Candidate and the Results have Now been Published in a Peer Review Journal. Available online: https://www.pharmiweb.com/press-release/2020-03-10/positive-results-from-phase-iib-field-study-of-flu-v-vaccine-flu-v-003-which-has-been-developed-by-imutex-limited-hvivos-49-joint-venture-with-t (accessed on 6 November 2020).
- Novavax’ NanoFlu Achieves All Primary Endpoints In Phase 3 Clinical Trial. Available online: https://ir.novavax.com/news-releases/news-release-details/novavax-nanoflu-achieves-all-primary-endpoints-phase-3-clinical (accessed on 6 November 2020).
- OSIVAX Announces Completion of Enrolment in its Phase 2a Clinical Trial of OVX836 Universal Influenza Vaccine Candidate. Available online: http://www.osivax.com/osivax-announces-completion-of-enrolment-in-its-phase-2a-clinical-trial-of-ovx836-universal-influenza-vaccine-candidate.html (accessed on 6 November 2020).
- Medicago Announces Phase 3 Study of VLP Quadrivalent Influenza Vaccine. Available online: https://www.prnewswire.com/news-releases/medicago-announces-phase-3-study-of-vlp-quadrivalent-influenza-vaccine-647905263.html (accessed on 6 November 2020).
- A Study to Evaluate the Safety and Immunogenicity of Quadrivalent Influenza Vaccine [NCT03718468]. Available online: https://clinicaltrials.gov/ct2/show/NCT03718468 (accessed on 6 November 2020).
- Influenza A Virus H5N1 Vaccine—NuGenerex Immuno-Oncology. Available online: https://adisinsight.springer.com/drugs/800026774 (accessed on 6 November 2020).
- Available online: https://codagenix.com (accessed on 6 November 2020).
- FluGen Success Brings the Reality of Universal Influenza Vaccines Closer. Available online: https://www.pharmaceutical-technology.com/comment/flugen-success-brings-the-reality-of-universal-influenza-vaccines-closer/ (accessed on 6 November 2020).
- Vivaldi Biosciences’ Phase 2 Influenza Vaccine Shows Broad Cross-Protection and Superiority in Nonclinical Study. Available online: https://www.vivaldibiosciences.com/news-2/vivaldi-biosciences-phase-2-influenza-vaccine-shows-broad-cross-protection-and-superiority-in-nonclinical-study (accessed on 6 November 2020).
- Biopharmaceuticals. Available online: https://www.polymun.com/biopharmaceuticals/reference-projects/ (accessed on 6 November 2020).
- Phase 2 Clinical Results for Vaccitech’s Universal Influenza A Vaccine. Available online: https://www.vaccitech.co.uk/phase-2-clinical-results-for-vaccitech-universal-influenza/ (accessed on 6 November 2020).
- Results from Influenza Challenge Study Published in Lancet Infectious Diseases. Available online: https://investors.vaxart.com/news-releases/news-release-details/results-influenza-challenge-study-published-lancet-infectious (accessed on 6 November 2020).
- NasoVAX TM Intranasal Flu Vaccine with Diverse Immune Response. Available online: https://altimmune.com/nasovax/ (accessed on 6 November 2020).
- Pandemic Influenza Virus Vaccine—AlphaVax. Available online: https://adisinsight.springer.com/drugs/800030591 (accessed on 6 November 2020).
- Seasonal Influenza Vaccine. Available online: https://www.bluewillow.com/vaccine-pipeline/seasonal-influenza-vaccine/ (accessed on 6 November 2020).
- Initiation of Phase I Clinical Trial for Seasonal Influenza HA Vaccine Sublingual Tablet. Available online: https://www.nitto.com/au/en/press/2016/1102.jsp (accessed on 6 November 2020).
- Gorse, G.J.; Grimes, S.; Buck, H.; Mulla, H.; White, P.; Graham, I.; Treanor, J.J.; Hill, H.; May, J.; Blackburn, P. Enhanced Potency and Durability of Antibody Response to Seasonal Trivalent Inactivated Influenza Vaccine (TIV) Combined with a Novel Water-in-Oil Adjuvant System at Reduced Hemagglutinin (HA) Doses. Open Forum Infect. Dis. 2017, 4, S455. [Google Scholar] [CrossRef][Green Version]
- Calzas, C.; Chevalier, C. Innovative Mucosal Vaccine Formulations Against Influenza A Virus Infections. Front. Immunol. 2019, 10, 1605. [Google Scholar] [CrossRef]
- Eurocine Vaccines announces Phase 1/2 study of Immunose Flu vaccine. Available online: https://www.oindpnews.com/2018/01/eurocine-vaccines-announces-phase-1-2-study-of-immunose-flu-vaccine/ (accessed on 6 November 2020).
- Pan, S.-C.; Hsieh, S.-M.; Lin, C.-F.; Hsu, Y.-S.; Chang, M.; Chang, S.-C. A randomized, double-blind, controlled clinical trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with adjuvant LTh(αK): A phase I study. Vaccine 2019, 37, 1994–2003. [Google Scholar] [CrossRef]
- Moderna Publishes Influenza Vaccine Clinical Trial Data. Available online: https://www.biospace.com/article/moderna-releases-phase-i-data-from-2-influenza-trials/ (accessed on 6 November 2020).
- Available online: https://www.inovio.com (accessed on 6 November 2020).
- Available online: https://www.mymetics.com/vaccine-pipeline/intra-nasal-influenza/ (accessed on 6 November 2020).
- Influenza A Virus H1N1 Vaccine—CEL-SCI. Available online: https://adisinsight.springer.com/drugs/800030749 (accessed on 6 November 2020).
- WHO. Cell culture as a substrate for the production of influenza vaccines: Memorandum from a WHO meeting. Bull. World Health Organ. 1995, 73, 431–435. [Google Scholar]
- Perez Rubio, A.; Eiros, J.M. Cell culture-derived flu vaccine: Present and future. Hum. Vaccines Immunother. 2018, 14, 1874–1882. [Google Scholar] [CrossRef]
- Flu Vaccine and People with Egg Allergies. Available online: https://www.cdc.gov/flu/prevent/egg-allergies.htm (accessed on 25 September 2020).
- De Vries, R.D.; Herfst, S.; Richard, M. Avian Influenza A Virus Pandemic Preparedness and Vaccine Development. Vaccines (Basel) 2018, 6, 46. [Google Scholar] [CrossRef]
- Webby, R.J.; Perez, D.R.; Coleman, J.S.; Guan, Y.; Knight, J.H.; Govorkova, E.A.; McClain-Moss, L.R.; Peiris, J.S.; Rehg, J.E.; EI Tuomanen, M.D.; et al. Responsiveness to a pandemic alert: Use of reverse genetics for rapid development of influenza vaccines. Lancet 2004, 363, 1099–1103. [Google Scholar] [CrossRef]
- Lamb, Y.N. Cell-Based Quadrivalent Inactivated Influenza Virus Vaccine (Flucelvax® Tetra/Flucelvax Quadrivalent®): A Review in the Prevention of Influenza. Drugs 2019, 79, 1337–1348. [Google Scholar] [CrossRef]
- Park, Y.W.; Kim, Y.H.; Jung, H.U.; Jeong, O.S.; Hong, E.J.; Kim, H.; Lee, J. Comparison of antigenic mutation during egg and cell passage cultivation of H3N2 influenza virus. Clin. Exp. Vaccine Res. 2020, 9, 56–63. [Google Scholar] [CrossRef]
- Korea’s First Cell-Culture Influenza Vaccine ‘SKYCellflu’ Released. Available online: https://www.fiercepharma.com/pharma-asia/korea-s-first-cell-culture-influenza-vaccine-skycellflu-released (accessed on 24 August 2020).
- Available online: https://www.mims.com/thailand/drug/info/skycellflu%20quadrivalent%20prefilled%20syringe (accessed on 8 September 2020).
- Meyer, W.J.; Wood, J.M.; Major, D.; Robertson, J.S.; Webster, R.G.; Katz, J.M. Influence of host cell-mediated variation on the international surveillance of influenza A (H3N2) viruses. Virology 1993, 196, 130–137. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; De Serres, G.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.A.; Fonseca, K.; Winter, A.-L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012–13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS ONE 2014, 9, e92153. [Google Scholar] [CrossRef]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative effectivenss of the cell-cultured quadrivalent influenza vaccine compared to standard, egg-dervied quadrivalent influenza vaccines in preventing influenza-like illness in 2017–2018. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Yeolekar, L.R.; Guilfoyle, K.; Ganguly, M.; Tyagi, P.; Stittelaar, K.J.; van Amerongen, G.; Dhere, R.M.; BerlandaScorza, F.; Mahmood, K. Immunogenicity and efficacy comparison of MDCK cell-based and egg-based live attenuated influenza vaccines of H5 and H7 subtypes in ferrets. Vaccine 2020, 38, 6280–6290. [Google Scholar] [CrossRef]
- Blanco-Lobo, P.; Nogales, A.; Rodríguez, L.; Martínez-Sobrido, L. Novel Approaches for The Development of Live Attenuated Influenza Vaccines. Viruses 2019, 11, 190. [Google Scholar] [CrossRef]
- Cox, M.M.J.; Izikson, R.; Post, P.; Dunkle, L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Adv. Vaccines 2015, 3, 97–108. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J.; Team, P.S. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef]
- Pillet, S.; Couillard, J.; Trépanier, S.; Poulin, J.F.; Yassine-Diab, B.; Guy, B.; Ward, B.J.; Landry, N. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-Two randomized Phase II clinical trials in 18 to 49 and ≥50 years old adults. PLoS ONE 2019, 14, e0216533. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Seguin, A.; Pillet, P.; Trepanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficact, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (>65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Notification of Changes in the, U.S. Development Plan of VLP Vaccine for Seasonal Influenza Prevfention (MT-2271) and An Impairment Loss (Non-Recurring Item). Available online: https://www.mt-pharma.co.jp/e/release/nr/2020/pdf/e_MTPC200428.pdf (accessed on 20 October 2020).
- Efficacy of a Plant-derived Quadrivalent VLP Vaccine in the Elderly NCT03739112. Available online: https://clinicaltrials.gov/ct2/show/NCT03739112 (accessed on 4 September 2020).
- Giurgea, L.T.; Taubenberger, J.K.; Memoli, M.J. Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine. Vaccines (Basel) 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.L.; McCluskie, M.J. DNA vaccines for viral diseases. Microbes Infect. 1999, 1, 7–21. [Google Scholar]
- Robinson, H.L.; Pertmer, T.M. DNA vaccines for viral infections: Basic studies and applications. Adv. Virus. Res. 2000, 55, 1–74. [Google Scholar]
- Haynes, J.R.; McCabe, D.E.; Swain, W.F.; Widera, G.; Fuller, J.T. Particle-mediated nucleic acid immunization. J. Biotechnol. 1996, 44, 37–42. [Google Scholar] [CrossRef]
- Mir, L.M.; Moller, P.H.; André, F.; Gehl, J. Electric pulse-mediated gene delivery to various animal tissues. Adv. Genet. 2005, 54, 83–114. [Google Scholar]
- Kim, J.H.; Jacob, J. DNA vaccines against influenza viruses. Curr. Top. Microbiol. Immunol. 2009, 333, 197–210. [Google Scholar] [PubMed]
- Lee, L.Y.Y.; Izzard, L. A Review of DNA Vaccines Against Influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef]
- Montgomery, D.L.; Shiver, J.W.; Leander, K.R.; Perry, H.C.; Friedman, A.; Martinez, D.; Ulmer, J.B.; Donnelly, J.J.; Liu, M.A. Heterologous and homologous protection against influenza A by DNA vaccination: Optimization of DNA vectors. DNA Cell Biol. 1993, 12, 777–783. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Friedman, A.; Ulmer, J.B.; Liu, M.A. Further protection against antigenic drift of influenza virus in a ferret model by DNA vaccination. Vaccine 1997, 15, 865–868. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Friedman, A.; Martinez, D.; Montgomery, D.L.; Shiver, J.W.; Motzel, S.L.; Ulmer, J.B.; Liu, M.A. Preclinical efficacy of a prototype DNA vaccine: Enhanced protection against antigenic drift in influenza virus. Nat. Med. 1995, 1, 583–587. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Donnelly, J.J.; Parker, S.E.; Rhodes, G.H.; Felgner, P.L.; Dwarki, V.J.; Gromkowski, S.H.; Deck, R.R.; DeWitt, C.M.; Friedman, A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993, 259, 1745–1749. [Google Scholar] [CrossRef]
- Li, L.; Saade, F. The future of human DNA vaccines. J. Biotechnol. 2012, 162, 171–182. [Google Scholar] [CrossRef]
- Faurez, F.; Le Moigne, V.; Gravier, R.; Jestin, A. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine 2010, 28, 3888–3895. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; Hu, Z.; Gordon, I.J.; Enama, M.E.; Hendel, C.S.; McTamney, P.M.; Pearce, M.B.; Yassine, H.M.; Boyington, J.C.; Bailer, R.; et al. VRC 306 Study Team., DNA priming and influenza vaccine immunogenicity: Two phase 1 open label randomised clinical trials. Lancet 2011, 11, 916–924. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; Belshe, R.; Bernstein, D.I.; Edupuganti, S.; Patel, S.M.; Renehan, P.; Zajdowicz, T.; Schwartz, R.; Koup, R.; Bailer, R.T.; et al. VRC 701 study team, DNA priming for seasonal influenza vaccine: A phase 1b double-blind randomized clinical trial. PLoS ONE 2015, 10, e0125914. [Google Scholar] [CrossRef]
- Houser, K.V.; Bellamy, A.R.; May, J.; Enama, M.E.; Sarwar, U.; Larkin, B.; Bailer, R.T.; Koup, R.; Paskel, M.; Subbarao, K.; et al. the VRC 702 study team DNA vaccine priming for seasonal influenza vaccine in children and adolescents 6 to 17 years of age: A phase 1 randomized clinical trial. PLoS ONE 2018, 13, e0206837. [Google Scholar] [CrossRef]
- Fleeton, M.N.; Chen, M.; Berglund, P.; Rhodes, G.; Parker, S.E.; Murphy, M.; Atkins, G.J.; Liljeström, P. Self-replicative RNA vaccines elicit protection against influenza A virus, respiratory syncytial virus, and a tickborne encephalitis virus. J. Infect Dis. 2001, 183, 1395–1398. [Google Scholar] [CrossRef]
- Brito, L.A.; Maione, D.; Uematsu, Y.; Giovani, C.; Berlanda Scorza, F.; Otten, G.R.; Yu, D.; Mandl, C.W.; Mason, P.W.; Dormitzer, P.R.; et al. Self-amplifying mRNA vaccines. Adv. Genet. 2015, 89, 179–233. [Google Scholar]
- Martinon, F.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.G.; Lévy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.J.; Stitz, L. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Kranz, L.M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; Grunwitz, C.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol. Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2017, 2, 29. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; James, T.; Amilcar Mick, R.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef]
- Lin, A.; Liang, F.; Thompson, E.A.; Vono, M.; Ols, S.; Lindgren, G.; Hassett, K.; Salter, H.; Ciaramella, G. Rhesus Macaque Myeloid-Derived Suppressor Cells Demonstrate T Cell Inhibitory Functions and Are Transiently Increased after Vaccination. J. Immunol. 2018, 2000, 286–294. [Google Scholar] [CrossRef]
- Lindgren, G.; Ols, S.; Liang, F.; Thompson, E.A.; Lin, A.; Hellgren, F.; Bahl, K.; John, S.; Yuzhakov, O.; Hassett, K.J.; et al. Induction of Robust B Cell Responses after Influenza mRNA Vaccination Is Accompanied by Circulating Hemagglutinin-Specific ICOS+ PD-1+ CXCR3+ T Follicular Helper Cells. Front. Immunol. 2017, 8, 1539. [Google Scholar] [CrossRef] [PubMed]
- Berlanda Scorza, F.; Pardi, N. New Kids on the Block: RNA-Based Influenza Virus Vaccines. Vaccines (Basel) 2018, 6, 20. [Google Scholar] [CrossRef]
- Hekele, A.; Bertholet, S.; Archer, J.; Gibson, D.G.; Palladino, G.; Brito, L.A.; Otten, G.R.; Philip, R. Rapidly produced SAM(®) vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microbes. Infect. 2013, 2, e52. [Google Scholar] [CrossRef]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef]
- Magini, D.; Giovani, C.; Mangiavacchi, S.; Maccari, S.; Cecchi, R.; Ulmer, J.B.; De Gregorio, E.; Geall, A.J.; Brazzoli, M.; Bertholet, S. Self-Amplifying mRNA Vaccines Expressing Multiple Conserved Influenza Antigens Confer Protection against Homologous and Heterosubtypic Viral Challenge. PLoS ONE 2016, 11, e0161193. [Google Scholar] [CrossRef]
- McCullough, K.C.; Bassi, I.; Milona, P.; Suter, R.; Thomann-Harwood, L.; Englezou, P.; Démoulins, T.; Ruggli, N. Self-replicating Replicon-RNA Delivery to Dendritic Cells by Chitosan-nanoparticles for Translation In Vitro and In Vivo. Mol. Nucleic. Acids. 2014, 3, e173. [Google Scholar] [CrossRef]
- Démoulins, T.; Milona, P.; Englezou, P.C.; Ebensen, T.; Schulze, K.; Suter, R.; Pichon, C.; Midoux, P.; Guzmán, C.A.; Ruggli, N.; et al. Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine 2016, 12, 711–722. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef]
- Moderna’s Work on a COVID-19 Vaccine Candidate. Available online: https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19 (accessed on 25 September 2020).
- Imperial COVID-19 Vaccine Trial. Available online: https://www.imperial.ac.uk/covid-19-vaccine-trial/ (accessed on 25 September 2020).
- Draper, S.J.; Gilbert, S.C. Utilizing poxviral vectored vaccines for antibody induction-progress and prospects. Vaccine 2013, 31, 4223–4230. [Google Scholar] [CrossRef]
- Berthoud, T.K.; Lillie, P.J.; Hwenda, L.; Collins, K.A.; Ewer, K.J.; Milicic, A.; Poyntz, H.C.; Lambe, T.; Fletcher, H.A.; Hill, A.V.S.; et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin. Infect. Dis. 2011, 52, 1–7. [Google Scholar] [CrossRef]
- Swayze, H.; Allen, J.; Folegatti, P.; Yu, L.M.; Gilbert, S.; Hill, A.; Ellis, C.; Butler, C.C. A phase IIb study to determine the safety and efficacy of candidate INfluenza Vaccine MVA-NP+M1 in combination with licensed Ina CTivated infl Uenza vaccine in adult S aged 65 years and above (INVICTUS): A study protocol. F1000Research 2019, 8, 719. [Google Scholar] [CrossRef]
- Portfolio Pipeline. Available online: https://www.vaccitech.co.uk/pipeline/ (accessed on 18 August 2020).
- Coughlan, L.; Gilbert, S. Adenoviral vectors as novel vaccines for influenza. J. Pharm. Pharm. 2015, 67, 382–399. [Google Scholar] [CrossRef]
- Liebowitz, S.; Gottlieb, K.; Kolhatkar, N.S.; Garg, S.J.; Asher, J.M.; Nazareno, J.; Kim, K.; McIlwain, D.R. TSN Efficacy, immunogenicity, and safety of an oral influenza vaccine: A placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect Dis. 2020, 20, 435–444. [Google Scholar] [CrossRef]
- Liebowitz, D.; Brandl, J.R.; Garg, S.J.; Tucker, S.N. High titre neutralising antibodies to influenza after oral tablet immunisation: A phase 1, randomised, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 1041–1048. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Dieffenbach, C.; Corey, L. Use of adenovirus type-5 vectored vaccines: A cautionary tale. Lancet 2020, 396, E68–E69. [Google Scholar] [CrossRef]
- Giles, B.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef]
- Sautto, G.A.; Ross, T.M. Towards a universal influenza vaccine: Different approaches for one goal. Virol. J. 2018, 15, 17. [Google Scholar] [CrossRef]
- Wei, C.J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug. Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Naficy, A.; Nachbagauer, R.; Berlanda-Scorza, F.; Feser, J.; Wilson, P.C.; Solórzano, A.; Van der Wielen, M.; Walter, E.B.; Albrecht, R.A.; et al. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: Interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 2020, 20, 80–91. [Google Scholar] [CrossRef]
- Thomson, C.A.; Wang, Y.; Jackson, L.M.; Olson, M.; Wang, W.; Liavonchanka, A.; Keleta, L.; Silva, V.; Diederich, S.; Jones, R.B.; et al. Pandemic H1N1 Influenza Infection and Vaccination in Humans Induces Cross-Protective Antibodies that Target the Hemagglutinin Stem. Front. Immunol. 2012, 3, 87. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Wohlbold, T.D.; Hirsh, A.; Hai, R.; Sjursen, H.; Palese, P.; Cox, R.J.; Krammer, F. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J. Virol. 2014, 88, 13260–13268. [Google Scholar] [CrossRef]
- Andrews, S.F.; Joyce, M.G.; Chambers, M.J.; Gillespie, R.A.; Kanekiyo, M.; Leung, K.; Yang, E.S.; Tsybovsky, Y.; Wheatley, A.K.; Crank, M.C.; et al. Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans. Sci. Immunol. 2017, 2, eaan2676. [Google Scholar] [CrossRef]
- Sui, J.; Sheehan, J.; Hwang, W.C.; Bankston, L.A.; Burchett, S.K.; Huang, C.Y.; Liddington, R.C.; Beigel, J.H.; Marasco, W.A. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin. Infect. Dis. 2011, 52, 1003–1009. [Google Scholar] [CrossRef]
- Mesin, L.; Schiepers, A.; Ersching, J.; Barbulescu, A.; Cavazzoni, C.B.; Angelini, A.; Okada, T.; Kurosaki, T.; Victora, G.D. Restricted Clonality and Limited Germinal Center Reentry Characterize Memory B Cell Reactivation by Boosting. Cell 2020, 180, 92–106. [Google Scholar] [CrossRef]
- Davis, C.W.; Jackson, K.J.L.; McCausland, M.M.; Darce, J.; Chang, C.; Linderman, S.L.; Chennareddy, C.; Gerkin, R.; Brown, S.J.; Wrammert, J.; et al. Influenza vaccine-induced human bone marrow plasma cells decline within a year after vaccination. Science 2020, 370, 237–241. [Google Scholar] [CrossRef]
- Pappas, L.; Foglierini, M.; Piccoli, L.; Kallewaard, N.L.; Turrini, F.; Silacci, C.; Fernandez-Rodriguez, B.; Agatic, G.; Giacchetto-Sasselli, I.; Pellicciotta, G.; et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 2014, 516, 418–422. [Google Scholar] [CrossRef]
- Friesen, R.H.E.; Lee, P.S.; Stoop, E.J.M.; Hoffman, R.M.B.; Ekiert, D.C.; Bhabha, G.; Yu, W.; Juraszek, J.; Koudstaal, W.; Jongeneelen, M.; et al. A common solution to group 2 influenza virus neutralization. Proc. Natl. Acad. Sci. USA 2014, 1111, 445–450. [Google Scholar] [CrossRef]
- Ekiert, D.C.; Friesen, R.H.E.; Bhabha, G.; Kwaks, T.; Jongeneelen, M.; Yu, W.; Ophorst, C.; Cox, F.; Korse, H.J.W.M.; Brandenburg, B.; et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 2011, 333, 843–850. [Google Scholar] [CrossRef]
- Joyce, M.G.; Wheatley, A.K.; Thomas, P.V.; Chuang, G.W.; Soto, C.; Bailer, R.T.; Druz, A.; Georgiev, I.S.; Gillespie, R.A.; Kanekiyo, M.; et al. Adrian B McDermott 10 Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell 2016, 166, 609–623. [Google Scholar] [CrossRef]
- Corti, D.; Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333, 850–856. [Google Scholar] [CrossRef]
- Dreyfus, C.; Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongeneelen, M.; et al. Friesen Highly conserved protective epitopes on influenza B viruses. Science 2012, 337, 1343–1348. [Google Scholar] [CrossRef]
- McCarthy, K.R.; Kuraoka, M.; Do, K.T.; McGee, C.E.; KTSempowski, G.D.; Schmidt, A.G.; Kelsoe, G.; Harrison, S.C. Memory B Cells that Cross-React with Group 1 and Group 2 Influenza A Viruses Are Abundant in Adult Human Repertoires. Immunity 2018, 48, 174–184. [Google Scholar] [CrossRef]
- Ekiert, D.C.; Kashyap, A.K.; Steel, J.; Rubrum, A.; Bhabha, G.; Khayat, R.; Lee, J.H.; Dillon, M.A.; O’Neil, R.E.; Faynboym, A.M.; et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 2012, 489, 526–532. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; Leung, K. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef]
- Raymond, D.D.; Bajic, G.; Ferdman, J.; Suphaphiphat, P.; Settembre, E.C.; Moody, M.A.; Schmidt, A.G.; Harrison, S.C. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl. Acad. Sci. USA 2018, 115, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Paul, S.S.; Mo, X.; Yuan, J.Y.R.; Tan, Y.J. The Vestigial Esterase Domain of Haemagglutinin of H5N1 Avian Influenza A Virus: Antigenicity and Contribution to Viral Pathogenesis. Vaccines (Basel) 2018, 6, 53. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.X.; Koutsakos, M.; Jegaskanda, S.; Esterbauer, R.; Tilmanis, D.; Aban, M.; Kedzierska, K.; Hurt, A.C.; Kent, S.J. Cross-lineage protection by human antibodies binding the influenza B hemagglutinin. Nat. Commun. 2019, 10, 324. [Google Scholar] [CrossRef]
- Arunkumar, G.A.; Ioannou, A.; Wohlbold, T.J.; Meade, P.; Aslam, S.; Amanat, F.; Ayllon, J.; García-Sastre, A.; Krammer, F. Broadly Cross-Reactive, Nonneutralizing Antibodies against Influenza B Virus Hemagglutinin Demonstrate Effector Function-Dependent Protection against Lethal Viral Challenge in Mice. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Bangaru, S.; Lang, S.; Schotsaert, M.; Vanderven, H.A.; Zhu, X.; Kose, N.; Bombardi, R.; Finn, J.A.; Kent, S.J.; Gilchuk, P.; et al. A Site of Vulnerability on the Influenza Virus Hemagglutinin Head Domain Trimer Interface. Cell 2019, 177, 1136–1152. [Google Scholar] [CrossRef]
- Watanabe, A.; McCarthy, K.R.; Kuraoka, M.; Schmidt, A.G.; Adachi, Y.; Onodera, T.; Tonouchi, K.; Caradonna, T.M.; Bajic, G.; Song, S.; et al. Antibodies to a Conserved Influenza Head Interface Epitope Protect by an IgG Subtype-Dependent Mechanism. Cell 2019, 177, 1124–1135. [Google Scholar] [CrossRef]
- Stadlbauer, D.; Zhu, X.; McMahon, M.; Turner, J.S.; Wohlbold, T.J.; Schmitz, A.J.; Strohmeier, S.; Yu, W.; Nachbagauer, R.; Mudd, P.A.; et al. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 2019, 366, 499–504. [Google Scholar] [CrossRef]
- Tokatlian, T.; Read, B.J.; Jones, C.A.; Kulp, D.W.; Menis, S.; Chang, J.Y.H.; Steichen, J.M.; Kumari, S.; Allen, J.D.; Dane, E.L.; et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science 2019, 363, 649–654. [Google Scholar] [CrossRef]
- Liao, H.Y.; Wang, S.C.; Ko, Y.A.; Lin, K.I.; Ma, C.; Cheng, T.J.R. Chimeric hemagglutinin vaccine elicits broadly protective CD4 and CD8 T cell responses against multiple influenza strains and subtypes. Proc. Natl. Acad. Sci. USA 2020, 117, 17757–17763. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Hutchinson, G.B.; Boyington, J.C.; Moin, S.M.; Gillespie, R.A.; Tsybovsky, Y.; Stephens, T.; Vaile, J.R.; Lederhofer, J.; Corbett, K.S.; et al. Glycan repositioning of influenza hemagglutinin stem facilitates the elicitation of protective cross-group antibody responses. Nat. Commun. 2020, 11, 791. [Google Scholar] [CrossRef]
- Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Immunological basis for enhanced immunity of nanoparticle vaccines. Expert Rev. Vaccines 2019, 18, 269–280. [Google Scholar] [CrossRef]
- Georgiev, I.S.; Joyce, M.G.; Chen, R.E.; Leung, K.; McKee, K.; Druz, A.; Van Galen, J.G.; Kanekiyo, M.; Tsybovsky, Y.; Yang, E.S.; et al. Two-Component Ferritin Nanoparticles for Multimerization of Diverse Trimeric Antigens. ACS Infect Dis. 2018, 4, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Antanasijevic, A.; Fallas, J.A.; Sheffler, W.; Copps, J.; Ellis, D.; Hutchinson, G.B.; Moyer, A.; Yasmeen, A.; Park, Y.J.; et al. Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. Elife 2020, 9, e57659. [Google Scholar] [CrossRef] [PubMed]
- Kolpe, A.; Fiers, W.; Saelens, X. M2-based influenza vaccines: Recent advances and clinical potential. Expert Rev. Vaccines 2017, 16, 123–136. [Google Scholar] [CrossRef]
- McMichael, A.J.; Gotch, F.M.; Noble, G.R.; Beare, P.A. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 1983, 309, 13–17. [Google Scholar] [CrossRef]
- Koutsakos, M.; Loh, L.; Clemens, E.B.; Sant, S.; Nüssing, S.; Fox, A.; Chung, A.W.; Laurie, K.L.; Hurt, A.C.; Rockman, S.; et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci. Transl. Med. 2018, 10, eaan8405. [Google Scholar] [CrossRef]
| Company | Phase | Administration | Reference |
|---|---|---|---|
| Recombinant | |||
| BiondVax | Phase III | Oral | [19] |
| Imutex | Phase II | SC | [20] |
| Recombinant—VLP | |||
| Novavax | Phase III | IM | [21] |
| Osivax | Phase II | IM | [22] |
| Medicago | Phase III/discontinued | IM | [23] |
| Medigen | Phase II | IM | [24] |
| Recombinant—H5 protein fragment | |||
| Generex | Phase I | Oral | [25] |
| Live attenuated | |||
| Codagenix | Phase I | Nasal | [26] |
| FluGen | Phase II | Nasal | [27] |
| Vivaldi | Phase II | Nasal | [28] |
| Polymun | Phase I | Nasal | [29] |
| Vector—adenovirus | |||
| Vaccitech | Phase II | IM | [30] |
| Vaxart | Phase II | Oral | [31] |
| Altimmune | Phase II | Nasal | [32] |
| Vector—alphavirus | |||
| AlphaVax | Phase II | IM | [33] |
| Adjuvant—novel | |||
| BlueWillow | Phase I | Nasal | [34] |
| Nitto Denko | Phase I | Sublingual | [35] |
| Mercia | Phase II | IM | [36] |
| Adjuvant—toxin | |||
| Mucosis | Phase I | Nasal | [37] |
| Eurocine | Phase I/II | Nasal | [38] |
| Advagene | Phase II | Nasal | [39] |
| mRNA | |||
| Moderna Therapeutics | Phase I | IM | [40] |
| DNA vaccine | |||
| Inovio | Phase I | IM | [41] |
| Virosomes | |||
| Mymetics | Phase II | Nasal | [42] |
| Dendritic cells | |||
| CEL-SCI | Phase I | IM | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rockman, S.; Laurie, K.L.; Parkes, S.; Wheatley, A.; Barr, I.G. New Technologies for Influenza Vaccines. Microorganisms 2020, 8, 1745. https://doi.org/10.3390/microorganisms8111745
Rockman S, Laurie KL, Parkes S, Wheatley A, Barr IG. New Technologies for Influenza Vaccines. Microorganisms. 2020; 8(11):1745. https://doi.org/10.3390/microorganisms8111745
Chicago/Turabian StyleRockman, Steven, Karen L. Laurie, Simone Parkes, Adam Wheatley, and Ian G. Barr. 2020. "New Technologies for Influenza Vaccines" Microorganisms 8, no. 11: 1745. https://doi.org/10.3390/microorganisms8111745
APA StyleRockman, S., Laurie, K. L., Parkes, S., Wheatley, A., & Barr, I. G. (2020). New Technologies for Influenza Vaccines. Microorganisms, 8(11), 1745. https://doi.org/10.3390/microorganisms8111745






