Enteroviruses from Humans and Great Apes in the Republic of Congo: Recombination within Enterovirus C Serotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
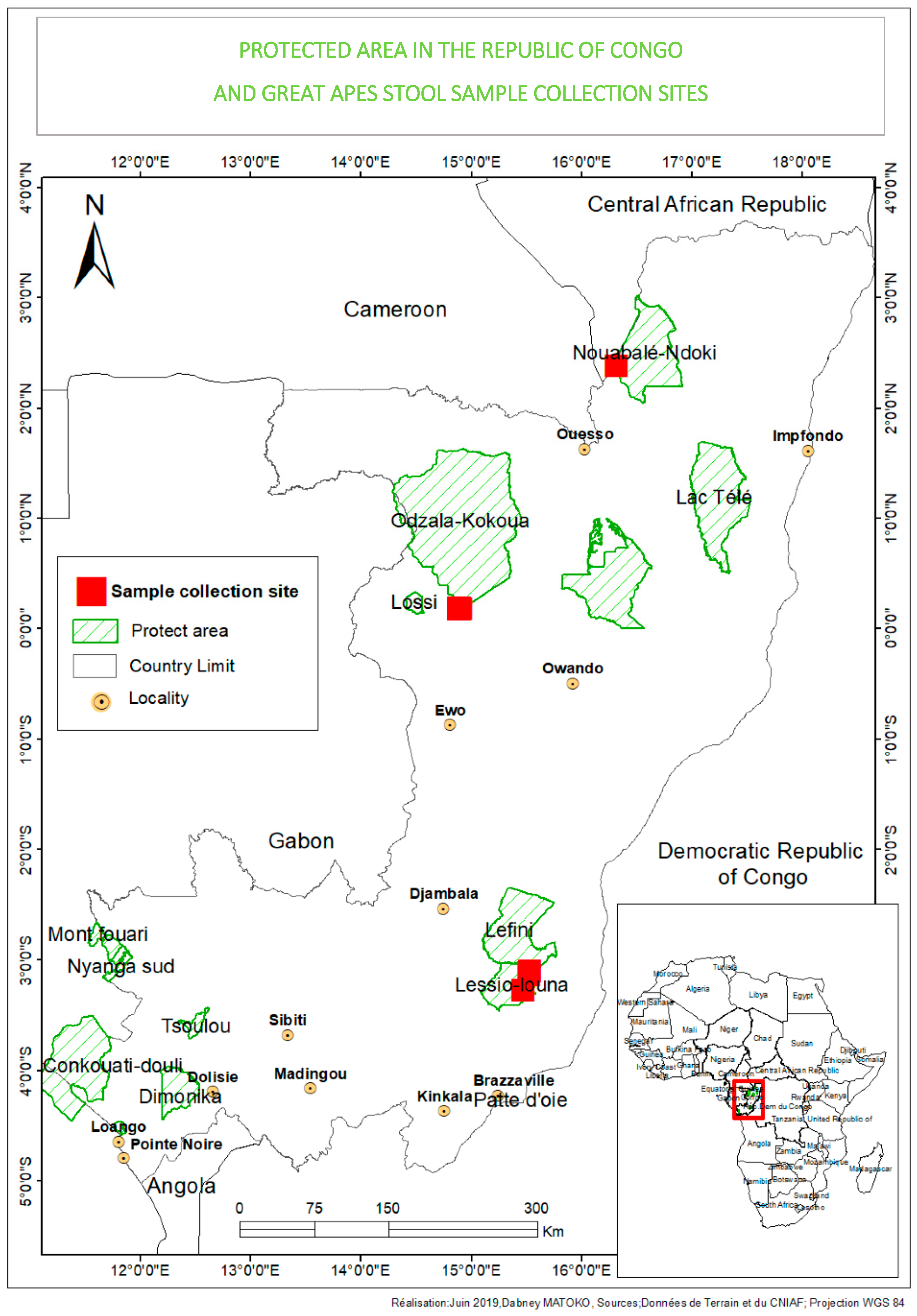
2.2. Sample Collection and Study Area
2.3. RNA Extraction and Molecular Detection of Enteroviruses by RT-qPCR
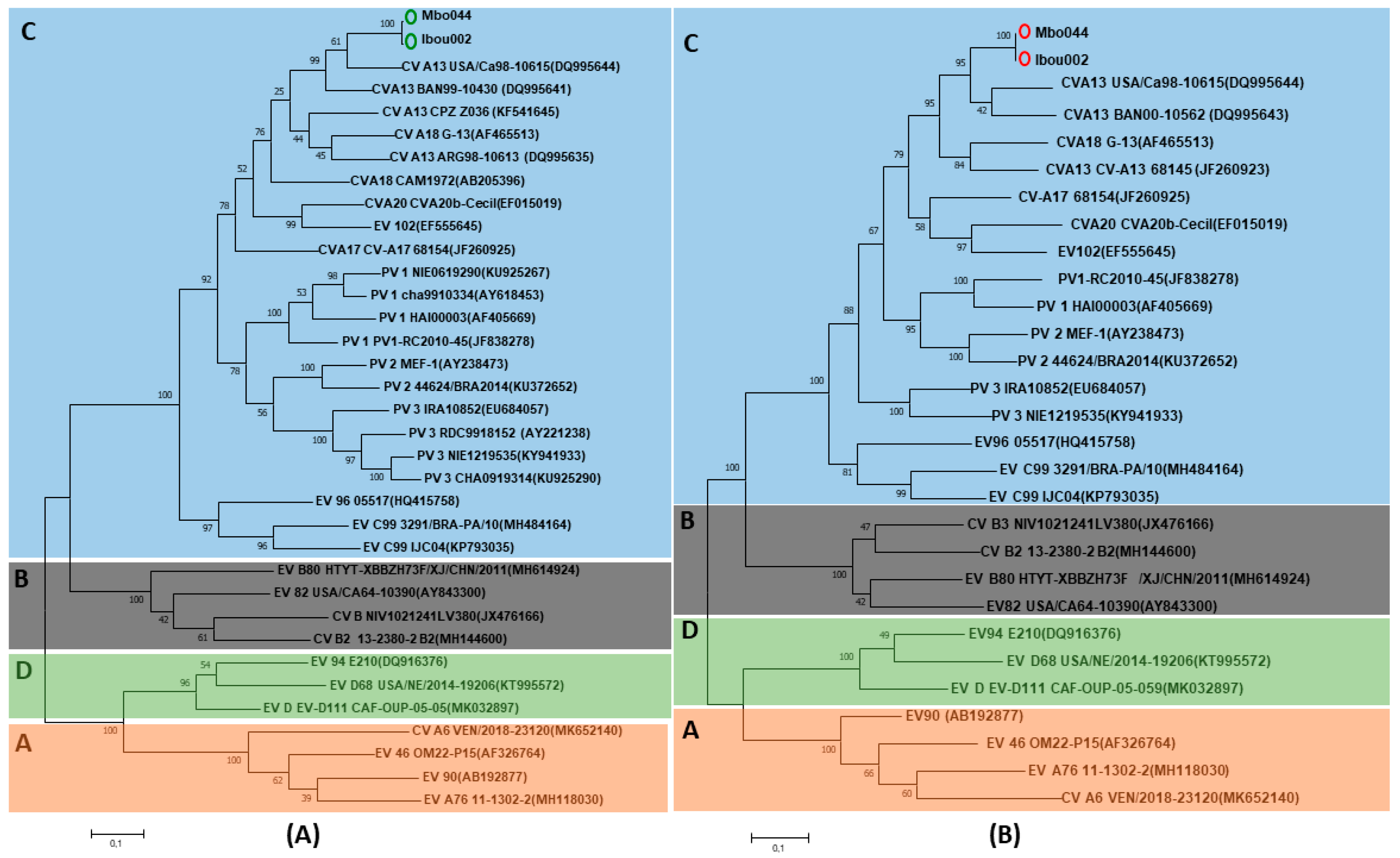
2.4. Molecular Characterization of Enteroviruses and Phylogenetic Analysis
2.5. Cell Culture and Viral Isolation
2.6. Complete Genome Amplification of EVs
2.7. Bioinformatic Analysis NGS
2.8. Identification of Great Ape Species
3. Results
3.1. Origin of Feces and Differentiation of Great Ape Individuals
3.2. Molecular Detection and Typing of Human and Great Ape Enteroviruses
3.3. Isolation and Genomic Analysis of Enterovirus Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palacios, G.; Oberste, M. Enteroviruses as agents of emerging infectious diseases. J. Neurovirol. 2005, 11, 424–433. [Google Scholar] [CrossRef]
- Boros, A.; Pankovics, P.; Knowles, N.J.; Reuter, G. Natural interspecies recombinant bovine/porcine enterovirus in sheep. J. Gen. Virol. 2012, 3, 1941–1951. [Google Scholar] [CrossRef]
- Tapparel, C.; Cordey, S.; Van Belle, S.; Turin, L.; Lee, W.-M.; Regamey, N.; Meylan, P.; Muhlemann, K.; Gobbini, F.; Kaiser, L. New Molecular Detection Tools Adapted to Emerging Rhinoviruses. J. Clin. Microbiol. 2009, 47, 1742–1749. [Google Scholar] [CrossRef]
- Midgley, C.M.; Watson, J.T.; Nix, W.A.; Curns, A.T.; Rogers, S.L.; Brown, B.A.; Conover, C.; Dominguez, S.R.; Feikin, D.R.; Gray, S.; et al. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): A descriptive epidemiological investigation. Lancet Respir. Med. 2015, 3, 879–887. [Google Scholar] [CrossRef]
- Hyypia, T.; Hovi, T.; Knowles, N.J.; Stanway, G. Classification of enteroviruses based on molecular and biological properties. J. Gen. Virol. 1997, 78, 1–11. [Google Scholar] [CrossRef]
- Lauber, C.; Gorbalenya, A.E. Toward Genetics-Based Virus Taxonomy: Comparative Analysis of a Genetics-Based Classification and the Taxonomy of Picornaviruses. J. Virol. 2012, 86, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A.; Jiang, B.; Maher, K.; Strobert, E.; Oberste, M.S. Identification of Enteroviruses in Naturally Infected Captive Primates. J. Clin. Microbiol. 2008, 46, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, S.; Hesse, R.A.; Hause, B.M. First Identification and Characterization of Porcine Enterovirus G in the United States. PLoS ONE 2014, 9, e97517. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xing, Z.; Gai, X.; Li, S.; San, Z.; Wang, X. Identification of a Novel Enterovirus E Isolates HY12 from Cattle with Severe Respiratory and Enteric Diseases. PLoS ONE 2014, 9, e97730. [Google Scholar] [CrossRef] [PubMed]
- Sadeuh-Mba, S.A.; Bessaud, M.; Massenet, D.; Joffret, M.-L.; Endegue, M.-C.; Njouom, R.; Reynes, J.-M.; Rousset, D.; Delpeyroux, F. High Frequency and Diversity of Species C Enteroviruses in Cameroon and Neighboring Countries. J. Clin. Microbiol. 2013, 51, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yoshida, H.; Ding, Z.; Tao, Z.; Zhang, J.; Tian, B.; Zhao, Z.; Zhang, L. Molecular Epidemiology and Recombination of Human Enteroviruses from AFP surveillance in Yunnan, China from 2006 to 2010. Sci. Rep. 2014, 4, 6058. [Google Scholar] [CrossRef] [PubMed]
- Smura, T.; Blomqvist, S.; Vuorinen, T.; Ivanova, O.; Samoilovich, E.; Al-Hello, H.; Savolainen-Kopra, C.; Hovi, T.; Roivainen, M. Recombination in the Evolution of Enterovirus C Species Sub-Group that Contains Types CVA-21, CVA-24, EV-C95, EV-C96 and EV-C99. PLoS ONE 2014, 9, e94579. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Ciacka, A.; Witek, A.; Kuryk, L.; Zuk-Wasek, A. Environmental Surveillance of Non-polio Enteroviruses in Poland, 2011. Food Environ. Virol. 2015, 7, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Combelas, N.; Holmblat, B.; Joffret, M.; Colbère-garapin, F.; Delpeyroux, F. Recombination between Poliovirus and Coxsackie a Viruses of Species C: A Model of Viral Genetic Plasticity and Emergence. Viruses 2011, 3, 1460–1484. [Google Scholar] [CrossRef]
- Kuryk, L.; Wieczorek, M.; Diedrich, S.; Bottcher, S.; Witek, A.; Litwinska, B. Genetic Analysis of Poliovirus Strains Isolated from Sewage in Poland. J. Med. Virol. 2013, 86, 1243–1248. [Google Scholar] [CrossRef]
- Sadeuh-Mba, S.A.; Kavunga-Membo, H.; Joffret, M.-L.; Yogolelo, R.; Endegue-Zanga, M.C.; Bessaud, M.; Njouom, R.; Muyembe-Tamfu, J.-J.; Delpeyroux, F. Genetic landscape and macro-evolution of co-circulating Coxsackieviruses A and Vaccine-derived Polioviruses in the Democratic Republic of Congo, 2008–2013. PLoS Negl. Trop. Dis. 2019, 13, e0007335. [Google Scholar] [CrossRef]
- Oberste, M.S.; Maher, K.; Michele, S.M.; Uddin, M.; Pallansch, M.A. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species Human enterovirus A Printed in Great Britain. J. Gen. Virol. 2005, 86, 445–451. [Google Scholar] [CrossRef]
- Bessaud, M.; Pillet, S.; Ibrahim, W.; Joffret, M.-L.; Pozzetto, B.; Delpeyroux, F.; Gouandjika-Vasilache, I. Molecular Characterization of Human Enteroviruses in the Central African Republic: Uncovering Wide Diversity and Identification of a New Human Enterovirus A71 Genogroup. J. Clin. Microbiol. 2012, 50, 1650–1658. [Google Scholar] [CrossRef]
- Sadeuh-Mba, S.A.; Bessaud, M.; Joffret, M.-L.; Endegue Zanga, M.-C.; Balanant, J.; Ngole, E.M.; Njouom, R.; Reynes, J.-M.; Delpeyroux, F.; Rousset, D. Characterization of Enteroviruses from Non-Human Primates in Cameroon Revealed Virus Types Widespread in Humans along with Candidate New Types and Species. PLoS Negl. Trop. Dis. 2014, 8, e3052. [Google Scholar] [CrossRef]
- Harvala, H.; McIntyre, C.L.; Imai, N.; Clasper, L.; Djoko, C.F.; Lebreton, M.; Vermeulen, M.; Saville, A.; Mutapi, F.; Tamoufe, U.; et al. High Seroprevalence of Enterovirus Infections in Apes and Old World Monkeys. Emerg. Infect Dis. 2012, 18, 283–286. [Google Scholar] [CrossRef]
- Mombo, I.M.; Berthet, N.; Lukashev, A.N.; Bleicker, T.; Brünink, S.; Léger, L.; Atencia, R.; Cox, D.; Bouchier, C.; Durand, P.; et al. First Detection of an Enterovirus C99 in a Captive Chimpanzee with Acute Flaccid Paralysis, from the Tchimpounga Chimpanzee Rehabilitation Center, Republic of Congo. PLoS ONE 2015, 10, e0136700. [Google Scholar] [CrossRef] [PubMed]
- Mombo, I.M.; Lukashev, A.N.; Bleicker, T.; Brünink, S.; Berthet, N.; Maganga, G.D.; Durand, P.; Arnathau, C.; Boundenga, L.; Ngoubangoye, B.; et al. African Non-Human Primates Host Diverse Enteroviruses. PLoS ONE 2017, 12, e0169067. [Google Scholar] [CrossRef] [PubMed]
- UNIC. Regional Action Plan for the Conservation of Western Lowland Gorillas and Central Chimpanzees 2015–2025; ID: 45257; IUCN: Gland, Switzerland, 2014; ISBN 9782831717029. [Google Scholar]
- Ninove, L.; Nougairède, A.; Gazin, C.; Thirion, L.; Delogu, I.; Zandotti, C.; Charrel, R.; De Lamballerie, X. RNA and DNA bacteriophages as molecular diagnosis controls in clinical virology: A comprehensive study of more than 45,000 routine PCR tests. PLoS ONE 2011, 6, e16142. [Google Scholar] [CrossRef] [PubMed]
- Leitch, E.C.; Harvala, H.; Robertson, I.; Ubillos, I.; Templeton, K.; Simmonds, P. Direct identification of human enterovirus serotypes in cerebrospinal fluid by amplification and sequencing of the VP1 region. J. Clin. Virol. 2009, 44, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Saadi, H.; Reteno, D.-G.I.; Colson, P.; Aherfi, S.; Minodier, P.; Pagnier, I.; Raoult, D.; La Scola, B. Shan Virus: A New Mimivirus Isolated from the Stool of a Tunisian Patient with Pneumonia. Intervirology 2013, 56, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Xu, G.C.; Xu, T.J.; Zhu, R.; Zhang, Y.; Li, S.Q.; Wang, H.W.; Li, J.T. LR-Gapcloser: A tiling path-based gap closer that uses long reads to complete genome assembly. Gigascience 2018, 8, giy157. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Lipman, D.J. Protein database searches for multiple alignments. Proc. Natl. Acad. Sci. USA 1990, 87, 5509–5513. [Google Scholar] [CrossRef]
- Wroblewski, E.E.; Norman, P.J.; Guethlein, L.A.; Rudicell, R.S.; Ramirez, M.A.; Li, Y.; Hahn, B.H.; Pusey, A.E.; Parham, P. Signature Patterns of MHC Diversity in Three Gombe Communities of Wild Chimpanzees Reflect Fitness in Reproduction and Immune Defense against SIVcpz. PLoS Biol. 2015, 13, e1002144. [Google Scholar] [CrossRef] [PubMed]
- Hans, J.B.; Bergl, R.A.; Vigilant, L. Gorilla MHC class I gene and sequence variation in a comparative context. Immunogenetics 2017, 69, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Maibach, V.; Hans, J.B.; Hvilsom, C.; Marques-bonet, T. MHC class I diversity in chimpanzees and bonobos. Immunogenetics 2017, 69, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Hamad, I.; Sokhna, C.; Raoult, D.; Bittar, F. Molecular Detection of Eukaryotes in a Single Human Stool Sample from Senegal. PLoS ONE 2012, 7, e40888. [Google Scholar] [CrossRef]
- Hamad, I.; Keita, M.B.; Peeters, M.; Delaporte, E.; Raoult, D.; Bittar, F. Pathogenic eukaryotes in gut microbiota of western lowland gorillas as revealed by molecular survey. Sci. Rep. 2014, 4, 6417. [Google Scholar] [CrossRef]
- Lee, I.; Kim, Y.O.; Park, S.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Padidam, M.; Sawyer, S.; Fauquet, C.M. Possible Emergence of New Geminiviruses by Frequent Recombination. Virology 1999, 225, 218–225. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Benschop, K.S.M.; Van Der Avoort, H.G.; Jusic, E.; Vennema, H.; Van Binnendijk, R.; Duizer, E. Polio and Measles Down the Drain: Environmental Enterovirus Surveillance in the Netherlands, 2005 to 2015. Appl. Environ. Microbiol. 2017, 83, e00558-17. [Google Scholar] [CrossRef]
- Bessaud, M.; Joffret, M.-L.; Holmblat, B.; Razafindratsimandresy, R.; Delpeyroux, F. Genetic Relationship between Cocirculating Human Enteroviruses Species C. PLoS ONE 2011, 6, e24823. [Google Scholar] [CrossRef]
- Cabrerizo, M.; Tarragó, D.; Muñoz-Almagro, C.; Del Amo, E.; Domínguez-Gil, M.; Eiros, J.M.; López-Miragaya, I.; Pérez, C.; Reina, J.; Otero, A.; et al. Molecular epidemiology of enterovirus 71, coxsackievirus A16 and A6 associated with hand, foot and mouth disease in Spain. Clin. Microbiol. Infect. 2014, 20, O150–O156. [Google Scholar] [CrossRef] [PubMed]
- Di Cristanziano, V.; Böttcher, S.; Diedrich, S.; Timmen-Wego, M.; Knops, E.; Lübke, N.; Kaiser, R.; Pfister, H.; Kaboré, Y.; D’Alfonso, R. Detection and characterization of enteroviruses and parechoviruses in healthy people living in the South of Côte d’Ivoire. J. Clin. Virol. 2015, 71, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Drexler, J.F.; Caron, M.; Lukashev, A.; Nkoghe, D.; Gonzalez, J.P.; Drosten, C. Type 1 wild poliovirus and putative enterovirus 109 in an outbreak of acute flaccid paralysis in Congo. Eurosurveillance 2010, 15, 19723. [Google Scholar] [CrossRef] [PubMed]
- Lukashev, A.N.; Drexler, J.F.; Kotova, V.O.; Amjaga, E.N.; Reznik, V.I.; Gmyl, A.P.; Grard, G.; Taty, R.T.; Trotsenko, O.E.; Leroy, E.M.; et al. Novel serotypes 105 and 116 are members of distinct subgroups of Human enterovirus C. J. Gen. Virol. 2012, 93, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Rakoto-Andrianarivelo, M.; Rousset, D.; Razafindratsimandresy, R.; Chevaliez, S.; Guillot, S.; Balanant, J.; Delpeyroux, F. High Frequency of Human Enterovirus Species C Circulation in Madagascar. J. Clin. Microbiol. 2005, 43, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, N.M.; Mor, S.K.; Mohammed, M.E.M.; Bastawecy, I.M.; Fakhry, H.M.; Youssef, C.R.B.; Abouzeid, N.Z.; Goyal, S.M. Isolation and molecular characterization of bovine enteroviruses in Egypt. Vet. J. 2015, 206, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Rakoto-Andrianarivelo, M.; Gumede, N.; Jegouic, S.; Balanant, J.; Andriamamonjy, S.N.; Rabemanantsoa, S.; Birmingham, M.; Randriamanalina, B.; Nkolomoni, L.; Venter, M.; et al. Reemergence of Recombinant Vaccine-Derived Poliovirus Outbreak in Madagascar. J. Infect. Dis. 2008, 197, 1427–1435. [Google Scholar] [CrossRef]
- Adeniji, J.A.; Cephas Faleye, T.O. Enterovirus C strains circulating in Nigeria and their contribution to the emergence of recombinant circulating vaccine-derived polioviruses. Arch. Virol. 2015, 160, 675–683. [Google Scholar] [CrossRef]
- Donbraye, E.; Olasunkanmi, O.I.; Opabode, B.A.; Ishola, T.R.; Cephas Faleye, T.O.; Adetwumi, M.O.; Aldeniji, J.A. Abundance of enterovirus C in RD-L20B cell culture-negative stool samples from acute flaccid paralysis cases in Nigeria is geographically defined. J. Med. Microbiol. 2018, 67, 854–865. [Google Scholar] [CrossRef]
- Burns, C.C.; Shaw, J.; Jorba, J.; Bukbuk, D.; Adu, F.; Gumede, N.; Pate, A. Multiple Independent Emergences of Type 2 Vaccine-Derived Polioviruses during a Large Outbreak in Northern Nigeria. J. Virol. 2013, 87, 4907–4922. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Shulman, L.M. Polioviruses and other Enteroviruses. In Global Water Pathogen Project; Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: East Lansing, MI, USA; UNESCO: Paris, France, 2017; Available online: http://www.waterpathogens.org/book/polioviruses-and-other-enteroviruses (accessed on 11 May 2020).
- Arita, M.; Shimizu, H.; Nagata, N.; Ami, Y.; Suzaki, Y.; Sata, T.; Iwasaki, T.; Miyamura, T. Temperature-sensitive mutants of enterovirus 71 show attenuation in cynomolgus monkeys. J. Gen. Virol. 2005, 86 Pt 5, 1391–1401. [Google Scholar] [CrossRef]


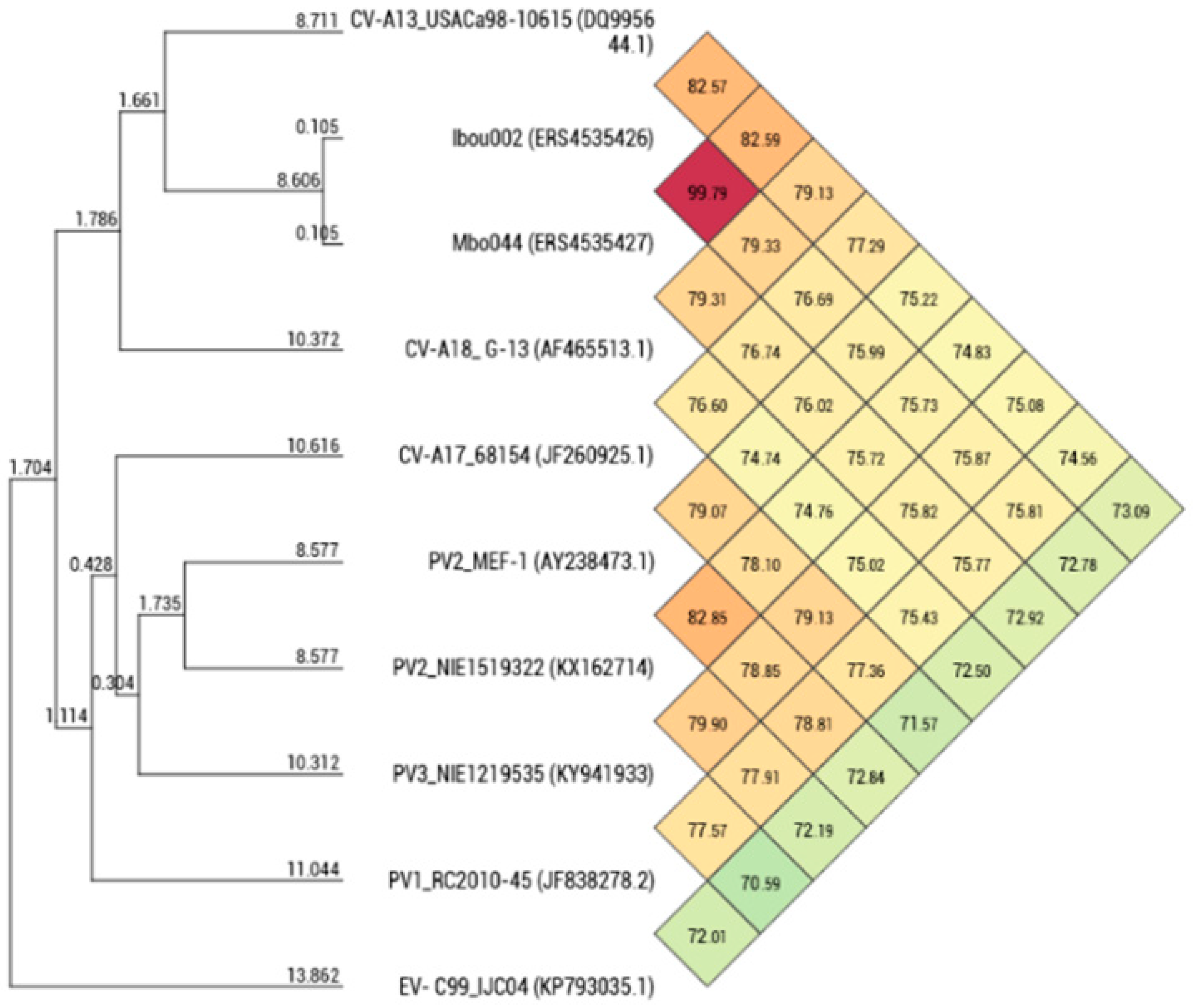


| Region | Amino Acid Identity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | CV-A13 (DQ995644) | CV-A17 (JF260925) | CV-A18 (AF465513) | CV-A20 (EF015019) | EV-C99 (KP793035) | PV1 (JF838278) | PV2 (KX162714) | PV3 (KY941933) | |
| 5’-UTR | 708 | 89.24 | 91.27 | 91.13 | 91.40 | - | 85.53 | 93.41 | 91.77 |
| VP4 | 207 | 84.54 | 84.18 | 91.13 | 81.59 | 80.10 | 79.80 | 78.17 | 81.07 |
| VP2 | 813 | 82.90 | 73.13 | 83.00 | 74.02 | 72.94 | - | 74.04 | 73.43 |
| VP3 | 714 | 82.49 | 76.36 | 78.11 | 74.71 | 74.86 | 74.43 | 75.69 | 74.31 |
| VP1 | 924 | 83.03 | - | 75.87 | - | 74.22 | 72.40 | 69.42 | 70.51 |
| 2A | 444 | 82.17 | - | - | 72.81 | 72.21 | 74.04 | 73.38 | 72.54 |
| 2B | 291 | 80.07 | - | 78.56 | 73.29 | 72.16 | 74.38 | 75.53 | 75.09 |
| 2C | 922 | 89.23 | - | 73.08 | - | 77.48 | 76.92 | 76.87 | 76.25 |
| 3A | 329 | 82.76 | 81.05 | 77.97 | 80.84 | 80.80 | 81.93 | 82.73 | 82.00 |
| 3B | 66 | 85.79 | 89.83 | - | 88.14 | 89 | 88.14 | 88.52 | 90.16 |
| 3C | 549 | 85.32 | - | - | 85.98 | 85.06 | 86.16 | 86.25 | 85.61 |
| 3D | 1263 | 85.10 | - | - | 85.74 | 86.39 | 86.66 | 83.29 | 85.74 |
| Mbo044 (LR796218) | 7230 | 82.80 | 77.43 | 78.91 | 77.63 | 77.83 | 78.57 | 77.63 | 77.69 |
| Ibou002 (LR796210) | 7230 | 82.77 | 77.44 | 78.88 | 77.58 | 77.80 | 78.52 | 77.64 | 77.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amona, I.; Medkour, H.; Akiana, J.; Davoust, B.; Tall, M.L.; Grimaldier, C.; Gazin, C.; Zandotti, C.; Levasseur, A.; Scola, B.L.; et al. Enteroviruses from Humans and Great Apes in the Republic of Congo: Recombination within Enterovirus C Serotypes. Microorganisms 2020, 8, 1779. https://doi.org/10.3390/microorganisms8111779
Amona I, Medkour H, Akiana J, Davoust B, Tall ML, Grimaldier C, Gazin C, Zandotti C, Levasseur A, Scola BL, et al. Enteroviruses from Humans and Great Apes in the Republic of Congo: Recombination within Enterovirus C Serotypes. Microorganisms. 2020; 8(11):1779. https://doi.org/10.3390/microorganisms8111779
Chicago/Turabian StyleAmona, Inestin, Hacène Medkour, Jean Akiana, Bernard Davoust, Mamadou Lamine Tall, Clio Grimaldier, Celine Gazin, Christine Zandotti, Anthony Levasseur, Bernard La Scola, and et al. 2020. "Enteroviruses from Humans and Great Apes in the Republic of Congo: Recombination within Enterovirus C Serotypes" Microorganisms 8, no. 11: 1779. https://doi.org/10.3390/microorganisms8111779
APA StyleAmona, I., Medkour, H., Akiana, J., Davoust, B., Tall, M. L., Grimaldier, C., Gazin, C., Zandotti, C., Levasseur, A., Scola, B. L., Raoult, D., Fenollar, F., Banga-Mboko, H., & Mediannikov, O. (2020). Enteroviruses from Humans and Great Apes in the Republic of Congo: Recombination within Enterovirus C Serotypes. Microorganisms, 8(11), 1779. https://doi.org/10.3390/microorganisms8111779






